Abstract
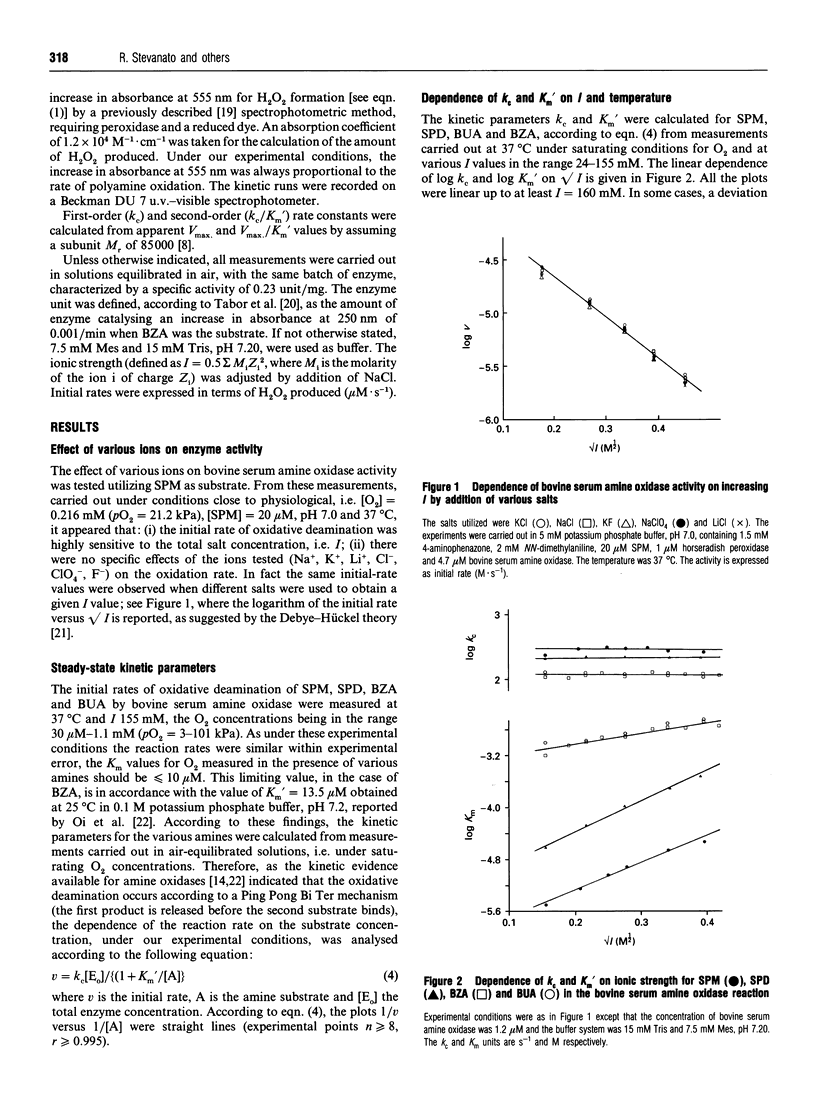
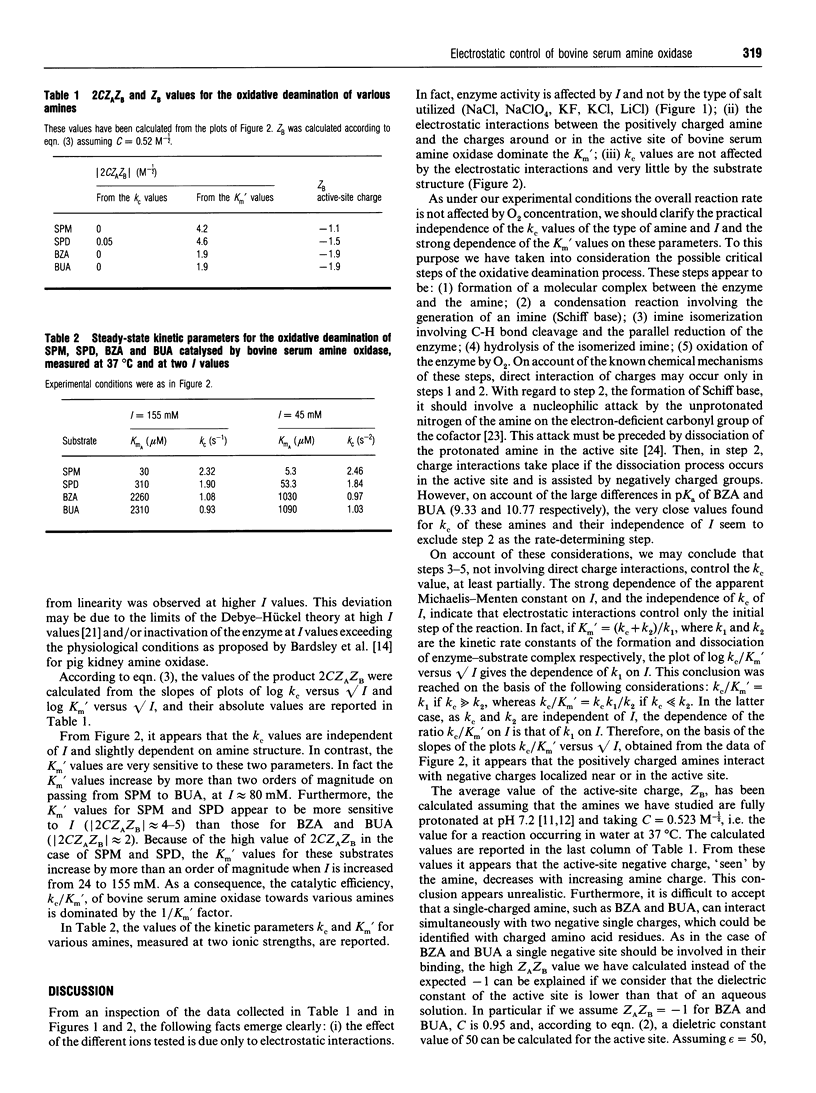
The ionic-strength-dependence of steady-state kinetic parameters (kc and Km') for non-biogenic (benzylamine, butylamine) and biogenic (spermine, spermidine) amines has been measured in the bovine serum amine oxidase reaction. The catalytic rate constant (kc) values are similar (0.9-2.5 s-1) for all the substrates studied and are almost constant over the experimental ionic strength range (24-155 mM). In contrast, Km' values are in the range 6-2300 microM and undergo a 4-12-fold increase with increasing ionic strength, parallelled by a decrease in catalytic efficiency. From an analysis of the kc and Km' values and their dependence on ionic strength, we conclude that more than one negative site is involved in the binding of these amines and that the relative dielectric constant of the binding site is lower than that of aqueous solutions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardsley W. G., Crabbe M. J., Shindler J. S., Ashford J. S. Oxidation of p-dimethylaminomethylbenzylamine by pig kidney diamine oxidase. A new method for spectrophotometric assay. Biochem J. 1972 May;127(5):875–879. doi: 10.1042/bj1270875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S. Kinetics of the diamine oxidase reaction. Biochem J. 1973 Mar;131(3):459–469. doi: 10.1042/bj1310459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffoni F. Nature of the organic cofactor of pig plasma benzylamine oxidase. Biochim Biophys Acta. 1990 Aug 1;1040(1):77–83. doi: 10.1016/0167-4838(90)90148-9. [DOI] [PubMed] [Google Scholar]
- Farnum M., Palcic M., Klinman J. P. pH dependence of deuterium isotope effects and tritium exchange in the bovine plasma amine oxidase reaction: a role for single-base catalysis in amine oxidation and imine exchange. Biochemistry. 1986 Apr 22;25(8):1898–1904. doi: 10.1021/bi00356a010. [DOI] [PubMed] [Google Scholar]
- Grant K. L., Klinman J. P. Evidence that both protium and deuterium undergo significant tunneling in the reaction catalyzed by bovine serum amine oxidase. Biochemistry. 1989 Aug 8;28(16):6597–6605. doi: 10.1021/bi00442a010. [DOI] [PubMed] [Google Scholar]
- Janes S. M., Mu D., Wemmer D., Smith A. J., Kaur S., Maltby D., Burlingame A. L., Klinman J. P. A new redox cofactor in eukaryotic enzymes: 6-hydroxydopa at the active site of bovine serum amine oxidase. Science. 1990 May 25;248(4958):981–987. doi: 10.1126/science.2111581. [DOI] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Mondovì B., Riccio P. Copper amine oxidases: structure and function. Adv Inorg Biochem. 1984;6:225–244. [PubMed] [Google Scholar]
- Mondovì B., Turini P., Befani O., Sabatini S. Purification of bovine plasma amine oxidase. Methods Enzymol. 1983;94:314–318. doi: 10.1016/s0076-6879(83)94056-9. [DOI] [PubMed] [Google Scholar]
- Oi S., Inamasu M., Yasunobu K. T. Mechanistic studies of beef plasma amine oxidase. Biochemistry. 1970 Aug 18;9(17):3378–3383. doi: 10.1021/bi00819a013. [DOI] [PubMed] [Google Scholar]
- Palcic M. M., Klinman J. P. Isotopic probes yield microscopic constants: separation of binding energy from catalytic efficiency in the bovine plasma amine oxidase reaction. Biochemistry. 1983 Dec 6;22(25):5957–5966. doi: 10.1021/bi00294a040. [DOI] [PubMed] [Google Scholar]
- Stevanato R., Porchia M., Befani O., Mondovi B., Rigo A. Characterization of free and immobilized amine oxidases. Biotechnol Appl Biochem. 1989 Jun;11(3):266–272. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. MONOAMINE OXIDASE. IV. NATURE OF THE SECOND PROSTHETIC GROUP OF PLASMA MONOAMINE OXIDASE. J Biol Chem. 1963 Aug;238:2669–2675. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. Monoamine oxidase. I. Purification, crystallization, and properties of plasma monoamine oxidase. J Biol Chem. 1962 May;237:1511–1516. [PubMed] [Google Scholar]


