Abstract
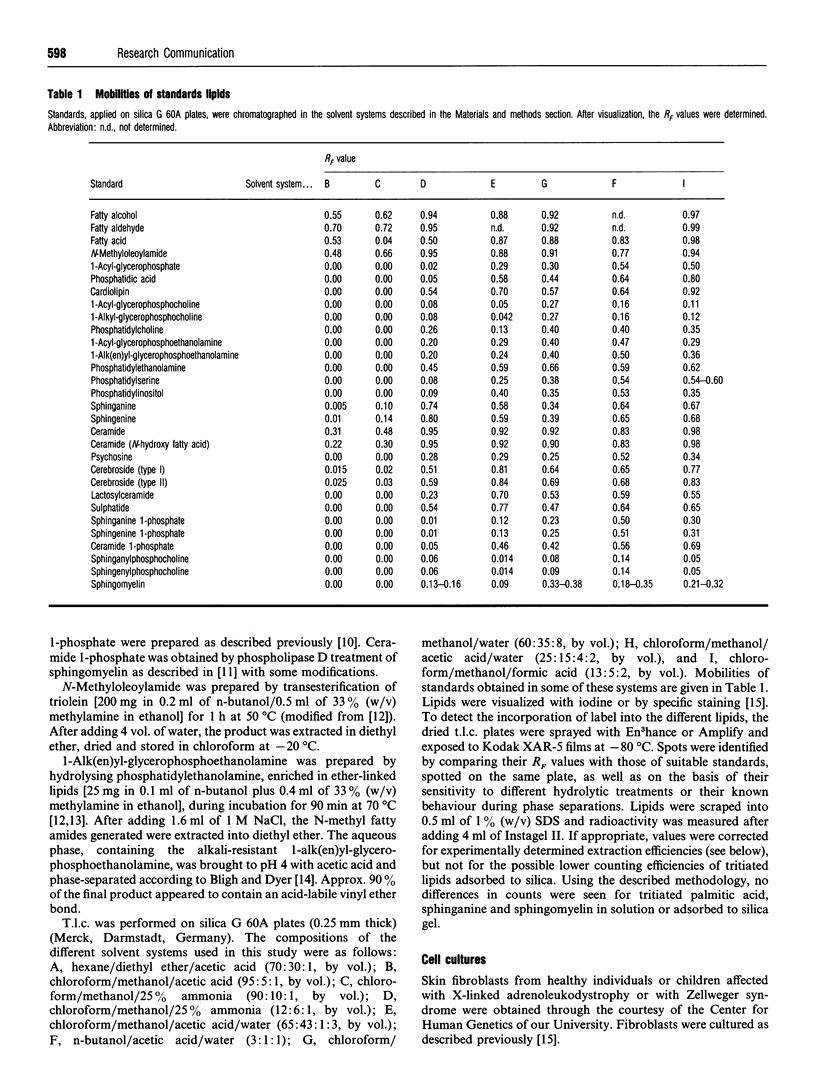
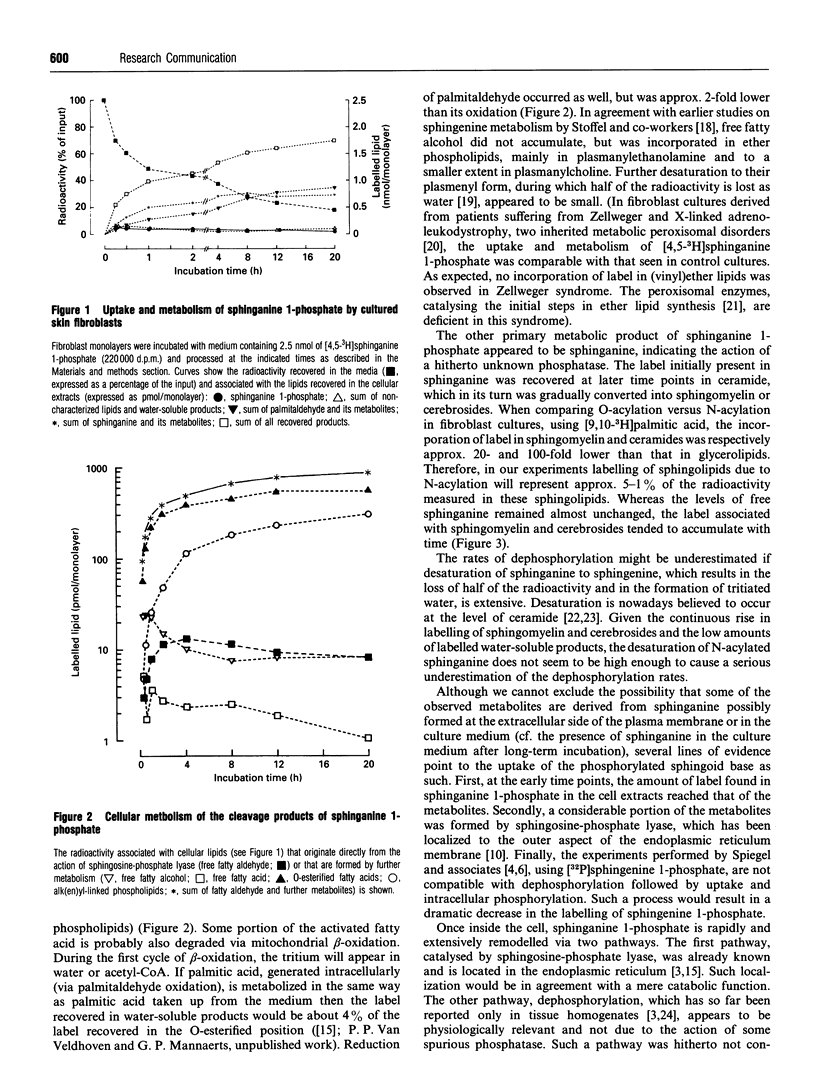
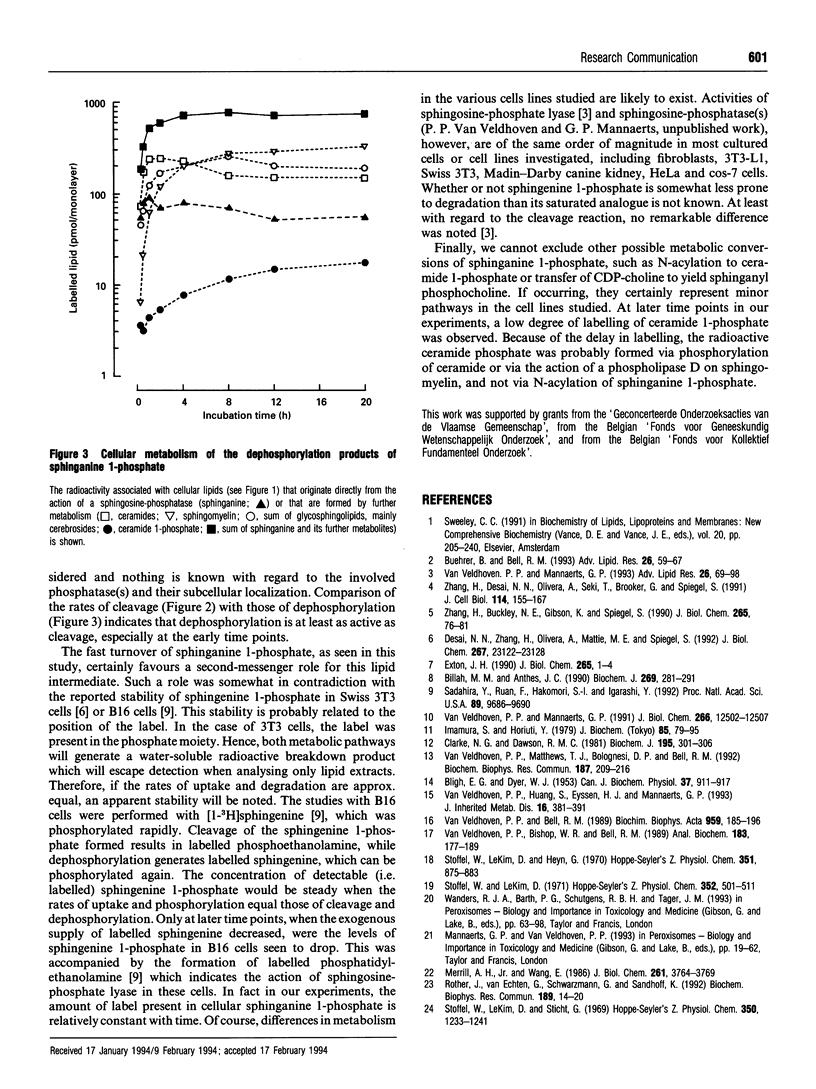
On addition of [4,5-3H]sphinganine 1-phosphate to human fibroblast monolayers, the label was efficiently removed from the culture medium. In contrast with the reported stability of phosphorylated sphingenine in 3T3 cells [Desai, Zhang, Olivera, Mattie and Spiegel (1992). J. Biol. Chem. 267, 23122-23128] and B16 melanoma cells [Sadahira, Ruan, Hakomuri and Igarashi (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 9686-9690], sphinganine 1-phosphate appeared to be subjected to a fast and extensive metabolism in fibroblasts, the major pathways being cleavage and dephosphorylation. The first of these pathways, catalysed by sphingosine-phosphate lyase, resulted in the formation of labelled palmitaldehyde, which was recovered, mainly after oxidation, in glycerophospholipids in an ester bond. A smaller part of the palmitaldehyde was reduced and incorporated in alk(en)ylphospholipids. Dephosphorylation of spinganine 1-phosphate, a hitherto overlooked pathway catalysed by an unknown phosphatase(s), gave rise to sphinganine, which was converted by N-acylation into ceramide and then incorporated in spingomyelin and glycosphingolipids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehrer B. M., Bell R. M. Sphingosine kinase: properties and cellular functions. Adv Lipid Res. 1993;26:59–67. [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. N., Zhang H., Olivera A., Mattie M. E., Spiegel S. Sphingosine-1-phosphate, a metabolite of sphingosine, increases phosphatidic acid levels by phospholipase D activation. J Biol Chem. 1992 Nov 15;267(32):23122–23128. [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Imamura S., Horiuti Y. Purification of Streptomyces chromofuscus phospholipase D by hydrophobic affinity chromatography on palmitoyl cellulose. J Biochem. 1979 Jan;85(1):79–95. doi: 10.1093/oxfordjournals.jbchem.a132334. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Wang E. Biosynthesis of long-chain (sphingoid) bases from serine by LM cells. Evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganine(s). J Biol Chem. 1986 Mar 15;261(8):3764–3769. [PubMed] [Google Scholar]
- Rother J., van Echten G., Schwarzmann G., Sandhoff K. Biosynthesis of sphingolipids: dihydroceramide and not sphinganine is desaturated by cultured cells. Biochem Biophys Res Commun. 1992 Nov 30;189(1):14–20. doi: 10.1016/0006-291x(92)91518-u. [DOI] [PubMed] [Google Scholar]
- Sadahira Y., Ruan F., Hakomori S., Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9686–9690. doi: 10.1073/pnas.89.20.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., LeKim D., Heyn G. Metabolism of sphingosine bases. XIV. Sphinganine (dihydrosphingosine), an effective donor of the alk-1-enyl chain of plasmalogens. Hoppe Seylers Z Physiol Chem. 1970 Jul;351(7):875–883. doi: 10.1515/bchm2.1970.351.2.875. [DOI] [PubMed] [Google Scholar]
- Stoffel W., LeKim D. Studies on the biosynthesis of plasmalogens. Precursors in the biosynthesis of plasmalogens: on the stereospecificity of the biochemical dehydrogenation of the 1-O-alkyl glyceryl to the 1-O-alk-1'-enyl glyceryl ether bond. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):501–511. doi: 10.1515/bchm2.1971.352.1.501. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Bell R. M. Effect of harvesting methods, growth conditions and growth phase on diacylglycerol levels in cultured human adherent cells. Biochim Biophys Acta. 1988 Mar 25;959(2):185–196. doi: 10.1016/0005-2760(88)90030-6. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Bishop W. R., Bell R. M. Enzymatic quantification of sphingosine in the picomole range in cultured cells. Anal Biochem. 1989 Nov 15;183(1):177–189. doi: 10.1016/0003-2697(89)90186-3. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Huang S., Eyssen H. J., Mannaerts G. P. The deficient degradation of synthetic 2- and 3-methyl-branched fatty acids in fibroblasts from patients with peroxisomal disorders. J Inherit Metab Dis. 1993;16(2):381–391. doi: 10.1007/BF00710285. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Mannaerts G. P. Subcellular localization and membrane topology of sphingosine-1-phosphate lyase in rat liver. J Biol Chem. 1991 Jul 5;266(19):12502–12507. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Matthews T. J., Bolognesi D. P., Bell R. M. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem Biophys Res Commun. 1992 Aug 31;187(1):209–216. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]
- Zhang H., Buckley N. E., Gibson K., Spiegel S. Sphingosine stimulates cellular proliferation via a protein kinase C-independent pathway. J Biol Chem. 1990 Jan 5;265(1):76–81. [PubMed] [Google Scholar]
- Zhang H., Desai N. N., Olivera A., Seki T., Brooker G., Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991 Jul;114(1):155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veldhoven P. P., Mannaerts G. P. Sphingosine-phosphate lyase. Adv Lipid Res. 1993;26:69–98. [PubMed] [Google Scholar]



