Abstract
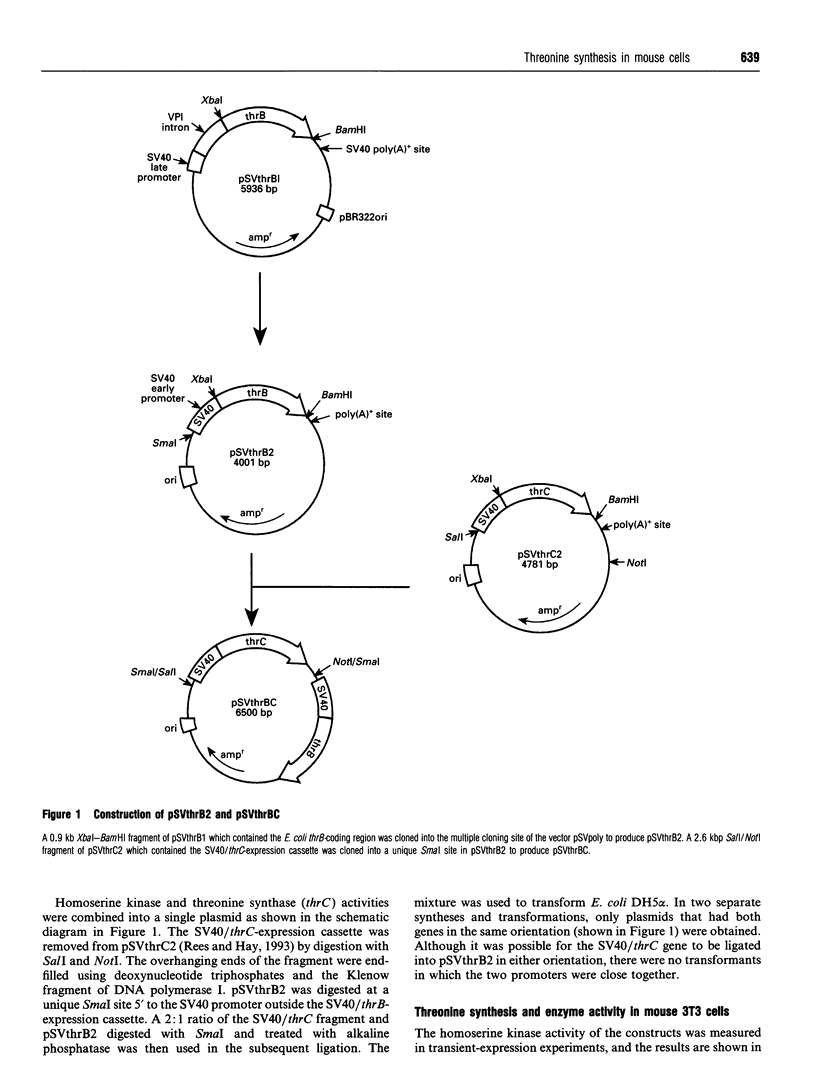
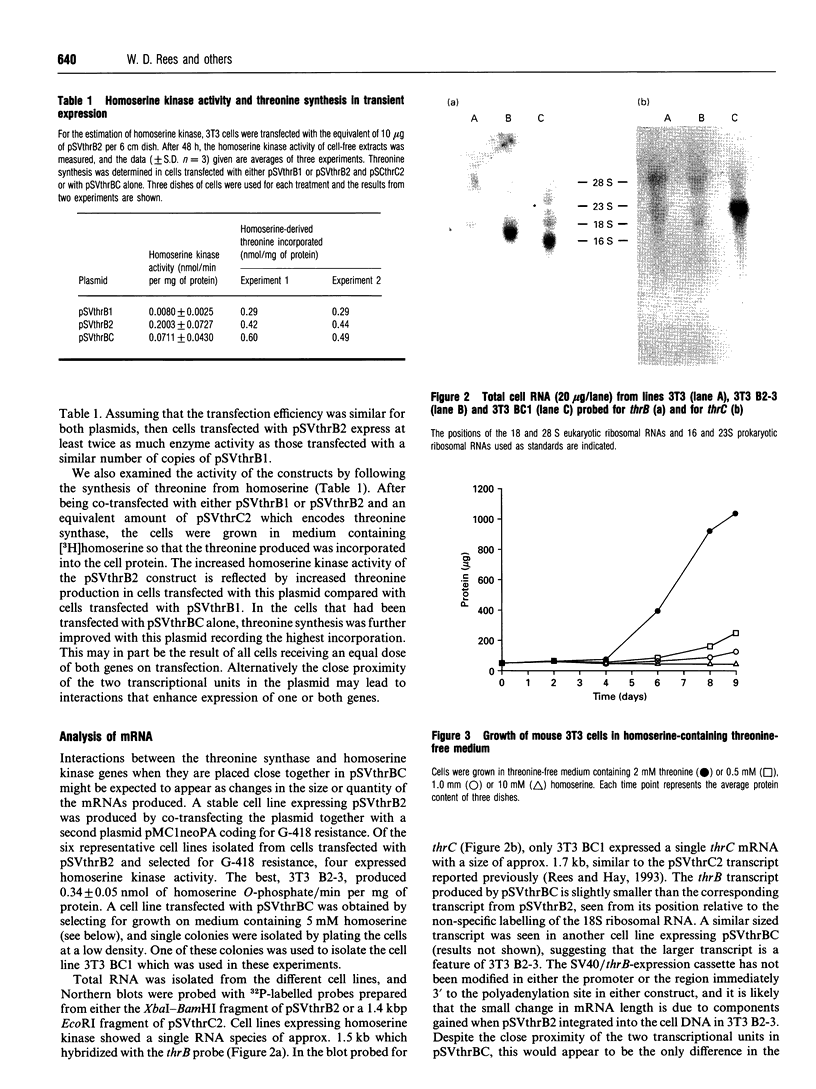
The plasmid pSVthrBC expresses the Escherichia coli thrB (homoserine kinase) and thrC (threonine synthase) genes in mouse cells and enables them to synthesize threonine from homoserine. After transfection with pSVthrBC and culture in medium containing homoserine, only cells that have incorporated pSVthrBC survive. Homoserine at concentrations greater than 1 mM is toxic to mammalian cells. Mouse cells selected from medium containing 5 mM homoserine had incorporated 20-100 copies of the plasmid per cell and had homoserine kinase activities of 0.001-0.012 nmol/min per mg of protein per copy. Cells selected from medium containing 10 mM homoserine had incorporated one or two copies of the plasmid per cell and had homoserine kinase activities of 0.06-0.39 nmol/min per mg of protein per copy. By using high concentrations of homoserine, it is possible to use pSVthrBC to select and isolate cell lines that have one or two copies of the plasmid incorporated into an active region of chromatin. CHO and HeLa cells have also been successfully transfected with pSVthrBC. COS-7 cells are naturally resistant to homoserine as they are able to metabolize homoserine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartier M., Chang M. W., Stanners C. P. Use of the Escherichia coli gene for asparagine synthetase as a selective marker in a shuttle vector capable of dominant transfection and amplification in animal cells. Mol Cell Biol. 1987 May;7(5):1623–1628. doi: 10.1128/mcb.7.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockett M. I., Bebbington C. R., Yarranton G. T. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology (N Y) 1990 Jul;8(7):662–667. doi: 10.1038/nbt0790-662. [DOI] [PubMed] [Google Scholar]
- FLAVIN M., SLAUGHTER C. Threonine synthetase mechanism: studies with isotopic hydrogen. J Biol Chem. 1960 Apr;235:1112–1118. [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Franchi-Gazzola R., White M. F. The cluster-tray method for rapid measurement of solute fluxes in adherent cultured cells. Anal Biochem. 1981 Aug;115(2):368–374. doi: 10.1016/0003-2697(81)90019-1. [DOI] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël N., Chenciner N., Streeck R. E. Amplifiable expression vectors for mammalian cell lines. Gene. 1987;51(2-3):197–204. doi: 10.1016/0378-1119(87)90308-8. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Kaufman R. J., Latt S. A., Weinberg R. A. Construction and use of a dominant, selectable marker: a Harvey sarcoma virus-dihydrofolate reductase chimera. Mol Cell Biol. 1983 Jan;3(1):32–43. doi: 10.1128/mcb.3.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Wolman S., Vogel A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature. 1970 Dec 5;228(5275):938–passim. doi: 10.1038/228938a0. [DOI] [PubMed] [Google Scholar]
- Rees W. D., Hay S. M., Flint H. J. Expression of Escherichia coli homoserine kinase in mouse 3T3 cells. Biochem J. 1992 Feb 1;281(Pt 3):865–870. doi: 10.1042/bj2810865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. D., Hay S. M. The expression of Escherichia coli threonine synthase and the production of threonine from homoserine in mouse 3T3 cells. Biochem J. 1993 Apr 1;291(Pt 1):315–322. doi: 10.1042/bj2910315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Wahl G. M. Escherichia coli aspartate transcarbamylase: a novel marker for studies of gene amplification and expression in mammalian cells. Mol Cell Biol. 1986 Sep;6(9):3050–3058. doi: 10.1128/mcb.6.9.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Schnieke A. SVpoly: a versatile mammalian expression vector. Nucleic Acids Res. 1990 May 11;18(9):2829–2829. doi: 10.1093/nar/18.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Delbrück S., Eckhart W., Meinkoth J., Vitto L., Wahl G. The cloning and reintroduction into animal cells of a functional CAD gene, a dominant amplifiable genetic marker. Cell. 1981 Dec;27(2 Pt 1):267–277. doi: 10.1016/0092-8674(81)90410-4. [DOI] [PubMed] [Google Scholar]