Abstract
Nonalcoholic fatty liver disease (NAFLD) is a group of chronic liver disease which ranges from simple steatosis (NAFL) to non-alcoholic steatohepatitis (NASH) and is characterized by lipid accumulation, inflammation activation, fibrosis, and cell death. To date, a number of preclinical studies or clinical trials associated with therapies targeting fatty acid metabolism, inflammatory factors and liver fibrosis are performed to develop effective drugs for NAFLD/NASH. However, few therapies are cell death signaling-targeted even though the various cell death modes are present throughout the progression of NAFLD/NASH. Here we summarize the four types of cell death including apoptosis, necroptosis, pyroptosis, and ferroptosis in the NAFLD and the underlying molecular mechanisms by which the pathogenic factors such as free fatty acid and LPS induce cell death in the pathogenesis of NAFLD. In addition, we also review the effects of cell death-targeted therapies on NAFLD. In summary, our review provides comprehensive insight into the roles of various cell death modes in the progression of NAFLD, which we hope will open new avenues for therapeutic intervention.
Subject terms: Inflammasome, Metabolic syndrome, Oncogenesis
Highlights
Hepatocyte death is a critical event in the progression of NAFLD.
Different types of hepatocyte death participate in the occurrence and development of NAFLD, including apoptosis, necroptosis, pyroptosis and ferroptosis.
Drugs or compounds targeting the hepatocyte death signaling pathway is a potential direction for the treatment of NAFLD and needs to be further studied.
Facts
The incidence of NAFLD is increasing worldwide and it has become a major chronic liver disease that poses a significant threat to public health.
Several forms of programmed cell death, including apoptosis, necroptosis, pyroptosis, and ferroptosis, play an integral role in the pathogenesis of NAFLD.
A growing body of evidence suggests that various cell death modes may serve as a promising target for the prevention and treatment of NAFLD.
Open questions
Which type of cell death mode plays a dominant role in NAFLD?
Are there other types of cell death modes such as cuproptosis that are also involved in the development of NAFLD?
Given that various cell death modes occur in different types of cells during the pathogenesis of NAFLD, what are the underlying links among them?
Introduction
NAFLD is defined as a clinicopathological syndrome that is not due to alcohol and other well-defined liver-damaging factors. The incidence of NAFLD is increasing worldwide with the rapid economic development and lifestyle changes and it has become a major chronic liver disease that poses a significant threat to public health [1]. Depending on the severity of the disease, NAFLD includes non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), liver fibrosis, and liver cirrhosis. The most widely accepted pathogenesis of NAFLD/NASH is the current “multiple whammy” theory, which encompasses various factors such as genetic susceptibility, epigenetics, intrahepatocellular metabolic and signaling pathways, cellular interactions within the liver, and hepatocyte injury and death, and sheds light on the heterogeneity of NAFLD [2, 3]. The primary causative factors of NAFLD are predominantly attributed to the excessive accumulation of fatty acids in hepatocytes. This leads to lipotoxicity, oxidative stress, organelle dysfunction, and inflammatory responses, which subsequently initiate a cascade of cell death in the liver including apoptosis, necroptosis, pyroptosis, and ferroptosis, and ultimately exacerbate NAFLD progression. Hepatocyte death is a critical event in the progression of NAFLD, therefore a better characterization of hepatocyte death in NAFLD may lead to novel therapeutic.
Since the classification of cell death subroutines was updated by the Nomenclature Committee on Cell Death in 2018, cell death can be divided into accidental cell death (ACD) and regulated cell death (RCD) based on perspectives of morphology, biochemistry, and function. ACD is a process that cells exposed to very harsh environmental conditions (e.g., severe physical, chemical, or mechanical injury) disassemble in a virtually instantaneous and uncontrollable manner. RCD, also known as programmed cell death (PCD), is initiated and propagated by specific molecular mechanisms and is subject to regulation. It can be divided into several subroutines, such as apoptosis, necroptosis, pyroptosis, and ferroptosis [4, 5]. Based on accumulating evidence, RCD is closely related to the initiation and progression of NAFLD [6, 7]. Our review outlines the molecular mechanisms and processes of four major RCD subroutines related to NAFLD. The focus is on the latest advancements in targeting cell death for NAFLD therapy and the mechanisms of the various NAFLD therapies currently available, which mainly depend on different cell death modalities.
Different types of cell death in NALFD
Apoptosis
Overview of apoptosis
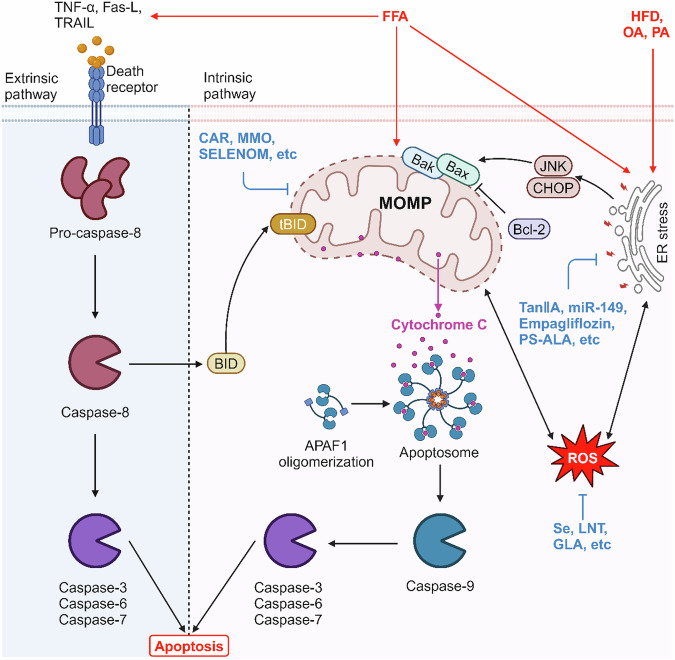
Apoptosis is a genetically determined process to terminates a cell’s life automatically that performs an important role in maintaining organismal homeostasis. It is characterized morphologically by chromatin condensation, cell shrinkage, and the eventual lysis of cells into apoptotic bodies through budding, which is subsequently cleared by phagocytosis. Mechanistically, the apoptotic pathways can be triggered by the exogenous or endogenous pathways [7, 8] (Fig. 1). In detail, the exogenous apoptotic pathway is typically mediated by cell surface death receptors (DRs), such as tumor necrosis factor receptor (TNFR), factor related apoptosis (Fas), TNF-related apoptosis-inducing ligand receptors (TRAIL-R), or toll-like receptors (TLRs) [7]. When bound to natural ligands, these receptors initiate an intracellular cascade reaction that results in the production of a death complex, which then activates caspase-8, followed by caspase-3/6/7 [7]. In the endogenous apoptotic pathway, various cellular stress, such as nuclear DNA damage, cytokine deprivation, endoplasmic reticulum stress (ERs), lysosomal permeabilization, and oxidative stress generate intracellular death signals to promote mitochondrial dysfunction and subsequently mitochondrial outer membrane pore formation (MOMP). Next, cytochrome c is released from the mitochondria into the cytoplasm through the mitochondrial membrane gap and binds to apoptosis protein activator-1 (Apaf-1) in the cytoplasm. And then, Apaf-1 undergoes oligomerization and activates caspase-9 to constitute an activation complex. This complex further activates downstream effector caspase-3/6/7, ultimately leading to the execution of apoptosis [8]. In the context of apoptosis, the mitochondrial pathway plays a crucial role in promoting caspase-8-dependent apoptotic signaling. Specifically, caspase-8 is involved in the cleavage of Bid, a death agonist with a BH3-interacting structural domain. This cleavage generates a truncated form of Bid, named tBid, which translocates to the mitochondria and disrupts the mitochondrial outer membrane. This disruption leads to MOMP, thereby integrating the two pathways into a final common pathway [9]. The regulation of MOMP is governed by the Bcl-2 protein family, comprising anti-apoptotic members (Bcl-2, Bcl-xL) and pro-apoptotic members (Bax, Bak). ERs can induce apoptosis through c-Jun N-terminal kinase (JNK) and C/EBP homologous protein (CHOP), thereby activating the pro-apoptotic protein Bax and disrupting mitochondrial function [10, 11].
Fig. 1. Mechanism of apoptosis in NAFLD.
In NAFLD state, HFD, OA, PA, and FFA lead to hepatocyte apoptosis by inducing increased expression of death receptors, disrupting mitochondrial function, and triggering ER stress.
Apoptosis in NAFLD
Numerous studies have demonstrated that hepatocyte apoptosis plays a crucial role in the progression of NAFLD. Within the population of NAFLD patients, there is a remarkable increase in the number of TUNEL-positive hepatocytes and the level of caspase-3 activity compared to normal individuals. Various animal models of NAFLD have also shown increased levels of caspase-3/8/9 and decreased cytochrome c in mitochondria. Furthermore, the degree of hepatocyte apoptosis is positively correlated with the level of liver inflammation and the stage of fibrosis. Studies have demonstrated pharmacological inhibition or selective knockdown of caspases effectively reduces hepatic apoptosis and inhibits the development of inflammation and fibrosis [7, 12, 13]. All of this indicates that apoptosis is a significant feature for NAFLD [14, 15]. In some clinical studies, the expression of several DRs, including Fas, TRAIL-R1, TRAIL-R2, and TNF receptor 1(TNFR1), was shown to be increased in the livers of NAFLD patients [16–18]. Moreover, the TRAIL knockout mice have been found to avoid diet-induced NASH [19]. In response to free fatty acids (FFA) treatment, cell surface receptors aggregate, leading to caspase-8 recruitment and ultimately resulting in cell death. Notably, compared to the concentrations required for FFA-mediated Fas sensitization or FFA-induced lipid apoptosis, the concentrations required for TRAIL sensitization by FFA are significantly lower. This may be due to the upregulation of DR5 expression through ERs, and then the JNK-dependent mechanisms induced the hepatocyte steatosis sensitivity to TRAIL [20, 21]. In physiological conditions, JNK takes part in cell growth, survival, and death. The FFA induces JNK-dependent hepatocyte apoptosis by activating Bax expression, which triggers the mitochondrial apoptotic pathway [22]. Similarly, oleic acid (OA) treatment upregulated the expression of p-AKT, p-JNK, Bax, and caspase-3 and decreased the Bcl-2 expression in LO2 cells, indicating that OA can induce apoptosis via AKT/JNK signaling in a Bcl-2/ Bax/caspase-dependent manner under stress conditions. This apoptotic mode is associated with mitochondrial dysfunction, as OA treatment causes disruption of mitochondrial membrane potential. Consequently, the impairment of mitochondrial function hinders the process of fatty acid β-oxidation, leading to intracellular fatty acid accumulation which, in turn, stimulates reactive the production of reactive oxygen species (ROS), and excessive ROS further aggravates mitochondrial dysfunction, ultimately triggers apoptosis [23]. In AML-12 cells, the upregulation of Bax, caspase-3, and caspase-9 expressions, as well as the increased apoptosis rate, were observed upon exposure to palmitic acid (PA) [24]. In vivo experiments have demonstrated a significantly increase in caspase-3/9 and Bax expression accompanied by a decrease in the expression of Bcl-2 and cytochrome c in mitochondria within hepatocytes in HFD-fed mice. These findings indicate that fatty acid can indeed promote the development of NAFLD by inducing apoptosis in hepatocytes triggered by mitochondrial dysfunction [15].
Many studies have shown that excessive lipid accumulation in hepatocytes can lead to apoptosis caused by ERs. In HFD-fed mice and OA-treated hepatocytes, the endoplasmic reticulum exhibits significantly swelling and dilation. Additionally, the expression of glucose regulated protein 78 (GRP78) and CHOP, both key hallmarks of ERs, is dramatically enhanced, and the IRE1α/TRAF2/JNK signaling pathway is activated, which in turn increased the expression of Bax and inhibited the expression of Bcl-2, leading to hepatocyte apoptosis [8, 25]. Similarly, PA causes HepG2 cytotoxicity, triggers ERs through the activation of protein kinase R-like ER kinase (PERK)-eukaryotic initiation factor 2α (eIF2α) and activating transcription factor 6 (ATF6) pathways, increases the expression of ERs-associated apoptotic protein CHOP, and ultimately leads to apoptosis [26].
Therefore, in NAFLD, apoptosis appears to be the predominant form of cell death, as there is evidence for the involvement of both pathways of apoptosis. Studying the molecular mechanism of apoptosis can provide a deeper understanding of its association with NAFLD and lead to therapeutic strategies.
Apoptosis-targeted therapies in NAFLD
As a result, an increasing number of drugs have been found to alleviate NAFLD progression by targeting apoptosis (Table 1). An increasing number of studies have shown that increased levels of cellular FFA serve as a catalyst for mitochondrial oxidative damage and apoptosis. Some antioxidants, such as Selenium (Se), prevent apoptosis by improving cellular antioxidant defenses, reducing ROS production, and maintaining cellular redox homeostasis [27]. Similarly to Se, VD supplementation can also reduce oxidative stress-related indexes, which further exerts a therapeutic effect on NAFLD. Besides, HFD-induced mice supplemented with active VD showed decreased levels of P53, P21, and P16, which proved that VD inhibited hepatocyte apoptosis by inhibiting the P53 pathway, but its specific mechanism needs to be investigated [28]. Lentinan (LNT) treatment reduced the ratio of Bax/Bcl-2 protein and caspase-3 activity, ameliorated oxidative stress, and reduced lipid deposition in both PA-induced AML-12 cells and HFD-induced mouse livers. Importantly, these benefits were reversed when peroxisome proliferator-activated receptor (PPARα) was knocked down, suggesting that LNT reduces apoptosis and oxidative stress and delays the progression of NAFLD via the PPARα pathway [29]. As a polyunsaturated fatty acid (PUFA), γ-linolenic acid (GLA) not only reduces intracellular lipid deposition in hepatocytes, but also activates autophagy via the LKB1–AMPK–mTOR pathway, which in turn decreases the expression level of Bax/Bcl-2 and reduces apoptosis [24]. Hydroxycitric acid (HCA), a major component of the natural fruit Garcinia cambogia, exhibits antioxidant effects and activates the nuclear factor erythroid 2-related factor 2 (NRF2)–antioxidant response element (ARE) pathway. This activation inhibits ROS production, regulates the Bcl-2/Bax ratio and PARP cleavage, and reduces apoptosis, in both HFD-induced and FFA-induced HepG2 cells, thereby delaying the process of NAFLD [30]. In the NAFLD model of zebrafish, we observed that swimming exercise can inhibit excessive ROS production, reduce caspase-3 and Bax expression, and increase Bcl-2 levels by activating recombinant Sirtuin 1 (SIRT1)/AMP-activated protein kinase (AMPK) signaling-mediated NRF2, which in turn reduces oxidative stress and ROS-induced apoptosis in zebrafish liver, decreases lipid accumulation in zebrafish NAFLD model liver, and attenuates liver injury [31].
Table 1.
Apoptosis-targeted therapies in NAFLD.
| Treatments | Model | Targets | Signaling pathways or molecular mechanisms | Ref. |
|---|---|---|---|---|
| PinX1 knockdown | HFD-induced mice and primary mice hepatocytes | mTERT | Improves mTERT activity | [45] |
| Se | Primary rat hepatocytes | Oxidative stress | Antioxidant | [27] |
| Vitamin D | HFD-induced mice | Oxidative stress | Antioxidant | [28] |
| Exercise | HFD-induced zebrafish | Oxidative stress | Activates SIRT1/AMPK/NRF2 signaling | [31] |
| HFD-induced rat and HepG2 cells | Srit1 | Activates Sirt1-mediated Drp1 acetylation | [33] | |
| LNT | HFD-induced mice and AML-12 cells | Oxidative stress | Activates PPARα pathway | [29] |
| GLA | AML-12 cells | Oxidative stress | Activates LKB1–AMPK–mTOR pathway | [24] |
| HCA | HFD-induced mice and HepG2 cells | Oxidative stress | Activates NRF2–ARE pathway | [30] |
| PK | HFD-induced rat | Mitochondria | Improves mitochondrial function | [15] |
| A22 | HFD-induced mice and HepG2 cells | Gene promoter i-motif | Upregulates Bcl-2 transcription and translation | [32] |
| BCATc inhibitor 2 | LO2 and HepG2 cells | p-AKT, p-JNK, and mitochondria | Inhibits Bcl-2/Bax/caspase axis and AKT/ERK signaling and improves mitochondrial function | [23] |
| EGCG | HFD-induced mice and LO2 cells | Mitochondria | Inhibits ROS/MAPK pathway | [34] |
| Sulforaphane | HFD-induced mice and HepG2 cells | Ceramide | Inhibits the ceramide | [35] |
| Myriocin | HFD-induced rat | Ceramide | Inhibits the ceramide | [36] |
| Silicon | HFD and HSHCD-induced rat | Mitochondria | Downregulates the mitochondrial (intrinsic) pathway process | [37] |
| CAR | HFD-induced mice and AML-12 cells | PRDX3 | Improves mitochondrial function | [38] |
| MMO | Human Chang liver cells | Mitochondria | Improves mitochondrial function | [39] |
| SELENOM | HFD-induced mice and AML-12 cells | Parkin | Activates AMPKα1–MFN2 pathway and improves mitochondrial function | [40] |
| Tan IIA | HepG2 cells | ER stress | Inhibits PERK/eIF2α and ATF6 signaling pathway | [26] |
| Empagliflozin | HFD-induce mice | ER stress | Inhibits ER stress | [41] |
| MiR-149 | HFD-induced mice and primary mice hepatocytes | ER stress | Downregulates the ATF6 signaling pathway | [42] |
| α-Tocopherol | HCD-induced rabbit | ER stress | Reduces JNK/c-Jun/inflammatory axis and JNK/CHOP/apoptotic signaling | [43] |
| α-Linolenic acid | HFD-induced mice and HepG2 cells | AMPK | Inhibits IRE1α/TRAF2/JNK signaling pathway | [44] |
Emerging evidence indicates that mitochondrial dysfunction plays an important role in the progression of NAFLD, the dysfunctional metabolism associated with NAFLD further exacerbates mitochondrial dysfunction, thereby accelerating the development of NAFLD. However, a large number of drugs have been found to modulate mitochondrial function in a variety of ways, thereby reducing the occurrence of mitochondria-dependent apoptosis in hepatocytes and thus exerting a protective effect against NAFLD. For example, Polygonatum kingianum (PK) has been shown to improve mitochondrial function by enhancing mitochondrial ROS (MtROS) scavenging, and it reverses the upregulation of caspase-3/9 and Bax expression, while restoring the decreased levels of Bcl-2 and cytochrome c in hepatocytes of HFD-induced NAFLD rats [15]. The acridone derivative A22 has demonstrated the ability to upregulate the Bcl-2 expression and block the caspase cascade reaction and mitochondrial apoptotic pathway by selectively binding and stabilizing the Bcl-2 gene promoter i-motif. Consequently, it reduces hepatocyte apoptosis in NAFLD/NASH models and effectively attenuates liver injury [32]. It is well-accepted that aerobic exercise has shown beneficial effects in the prevention and treatment of NAFLD. In a study by Hu et al. on HFD-fed rats and OA-treated HepG2 cells, aerobic exercise regulated the acetylation and activity of dynamically related protein 1 (Drp1) by activating Sirtuins1 (Srit1). This, in turn, reduces ROS generation in hepatocytes, increases mitochondrial membrane potential, mitigates mitochondrial dysfunction, and thus prevents NAFLD [33]. Researchers have found that supplementation of branched-chain amino acids exacerbates OA-induced lipid accumulation and apoptosis. However, BCATc inhibitor 2, a branched-chain amino acid transaminase inhibitor, attenuates the OA-induced activation of JNK and AKT signaling pathways, as well as Bcl-2/Bax/caspase axis. It also reduces MtROS production and prevents potential disruption of mitochondrial membranes, thereby maintaining mitochondrial function and thus exert a protective effect against NAFLD [23]. Epigallocatechin-3-gallate (EGCG) has the potential to attenuate mitochondria-dependent apoptosis in OA-treated LO2 cells and HFD-induced mice hepatocytes by inhibiting the ROS-mediated mitogen-activated protein kinase (MAPK) pathway [34]. Saturated fatty acids (SFA) inhibited apoptosis and downregulated cleaved caspase-3 both in vitro and in vivo, and decreased ceramide and ROS levels. These findings suggest a potential preventive effect against the development of NAFLD. Specifically, ceramide, acting as a second messenger for FFA-induced apoptotic effects, can be targeted to mitigate NAFLD [35]. For instance, the ceramide synthesis inhibitor myriocin reduces the expression of p-JNK, cleaved caspase-3, Bax, and cytochrome c, while increasing the expression of Bcl-2. Additionally, it reduces hepatic lipid accumulation in the hepatocyte of NAFLD rats, leading to a significantly improvement in liver inflammation and apoptosis in NAFLD rats [36]. In a separate study, rats supplemented with silicon in the diet showed a notable reduction in the number of apoptotic cells. The levels of apoptosis-related markers such as cytoplasmic cytochrome c, apoptosis-inducing factor AIF, caspase-3/9, and Bax/Bcl-2 ratio were significantly decreased, suggesting that silicon can reduce hepatocyte apoptosis, attenuate liver injury, and affect NASH development by modulating the mitochondrial intrinsic pathway [37]. In HFD-induced mice hepatocytes and PA-induced AML-12 cells, the researchers found that carnosol (CAR) treatment can maintain mitochondrial membrane potential and inhibit mitochondrial oxidative stress by specifically interacting with and upregulating the expression of the peroxiredoxin 3 (PRDX3), a mitochondrial H2O2 scavenger. This interaction improves mitochondrial function, facilitates ROS clearance, diminishes apoptosis, mitigates liver injury, and decelerates the progression of NAFLD [38]. Huang et al. conducted a study in which they found Meretrix meretrix oligopeptides (MMO), extracted from shellfish, was able to ameliorate mitochondrial dysfunction by decreasing mitochondrial membrane potential and maintaining intracellular ion levels in PA-induced human Chang liver cells, thus exerting an anti-apoptotic effect [39]. Selenoprotein M (SELENOM) acts as an upstream inhibitor of mitochondrial damage, which regulates the pro-mitochondrial autophagy factor Parkin via the AMPKα1–Mitofusin 2 (MFN2) pathway, which in turn regulates mitochondrial membrane potential and mitochondrial autophagy, and reduces mitochondria-associated apoptosis in hepatocytes. Overexpression of SELENOM reversed the upregulation of Bax, Cyt-c, caspase-3/9 and downregulation of Bcl-2 in PA-induced AML-12 cells, attenuating the level of apoptosis. In contrast, SELENOM deficiency increased HFD-induced apoptosis and lipid accumulation in mouse hepatocytes [40].
In addition, ERs-induced apoptosis is particularly important in the progression of NAFLD, as many drugs have been found to reduce the occurrence of apoptosis in hepatocytes by inhibiting ERs. Tanshinone IIA (Tan IIA) significantly inhibited the expression of ERK-related molecules GRP78, ATF6, and CHOP in HepG2 cells induced by PA, suppressed phosphorylation of eIF2α and reduced ERs-induced apoptosis [26]. Following 5 weeks of continuous administration of the hypoglycemic drug empagliflozin in HFD-fed mice, a decrease in NAFLD activity scores (NAS) was detected. The expression of ERs molecules was significantly downregulated, resulting in an increased Bcl-2/Bax ratio, inhibited caspase-8 cleavage, and a significant reduction in hepatocyte apoptosis. It shows that empagliflozin decelerates the progression of NAFLD by reducing ERs and inhibiting hepatocyte apoptosis [41]. MiR-149 can attenuate ERs-induced apoptosis by downregulating the ATF6 signaling pathway, thereby improving NAFLD [42]. Rabbits fed a high-cholesterol diet and supplemented with α-tocopherol exhibited inhibition of cholesterol-induced NASH development. This inhibition was achieved by reducing the JNK/c-Jun/inflammatory axis and JNK/CHOP/apoptotic signaling, which may contribute to resistance against the transformation of NAFLD into NASH [43]. The α-linolenic acid phytosterol ester PS-ALA inhibits ERs by regulating the AMPK signaling pathway, which in turn inhibits the activation of IRE1α/TRAF2/JNK signaling pathway, normalizes the Bcl-2 and Bax expression, and reduces ERs-induced hepatocyte apoptosis to achieve a preventive effect against NAFLD [44].
In addition, some other drugs or molecules inhibit hepatocyte apoptosis in other ways. In HFD-induced mice and PA-treated hepatocytes, knockdown of PIN2/TRF1-interacting telomerase inhibitor 1 (PinX1) could inhibit the expression of cleaved caspase-3 and cleaved poly ADP-ribose polymerase (PARP), one of the cleavage substrates of caspase, by upregulating the expression of telomerase reverse transcriptase (mTERT), exerting an anti-apoptotic effect. While PinX1 is a potential target for the treatment of NAFLD, the specific anti-apoptotic signaling pathway and molecular mechanism remain to be investigated [45]. In a study on NAFLD rat model, long non-coding RNA HULC (lncRNA HULC) could aggravate liver injury associated with NAFLD by activating the MAPK signaling pathway. In addition, the inhibition of HULC can reduce hepatocyte apoptosis and ameliorate liver fibrosis to a certain extent in NAFLD rats, and therefore, it will serve as a potential therapeutic target for NAFLD [46].
Necroptosis
Overview of necroptosis
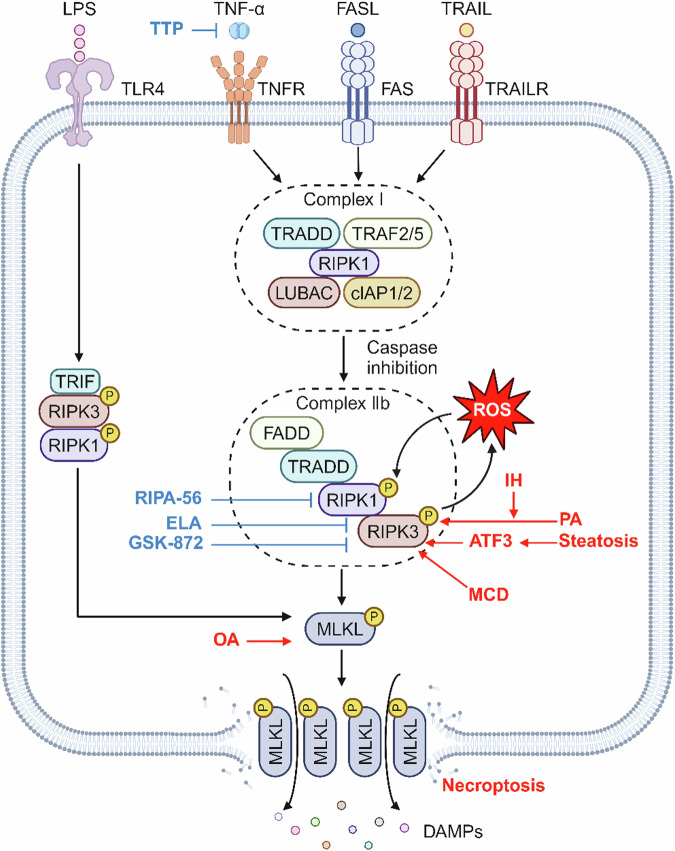
Necroptosis, a type of programmed inflammatory cell death, was first defined in 2005 by Degterev et al. [47] They found that the Fas/TNFR receptor family can activate a common non-apoptotic death pathway even in the absence of intracellular apoptotic signaling and it can be inhibited by specific chemical inhibitors of RIPK1, such as necrostatin-1 (Nec-1) and its modified analog Nec-1 (Fig. 2). As a result, this particular death pathway was named necroptosis. It is morphologically similar to necrosis and is characterized by increased cell volume, organelle swelling, and plasma membrane rupture [48]. The disruption of the plasma membrane leads to a rapid and extensive release of danger-associated molecular patterns (DAMPs), which subsequently trigger inflammation [49]. Although necroptosis is closely associated with apoptosis, it differs in that necroptosis is a specific type of caspase-independent cell death. Necroptosis shares the same death-inducing agents as apoptosis, including TNFα, first apoptotic signaling ligand (FasL), TRAIL, and toll-like receptor 3/4 (TLR3/4). In the context of TNF binding to TNFR1 and subsequent caspase-8 inhibition, the activation of receptor interacting protein 3 (RIPK3) triggers necroptosis [50, 51]. Active RIPK1 is recruited in an oligomeric complex including Fas-associated death domain (FADD) and caspase-8, activated caspase-8 can initiate apoptosis and also prevent necroptosis. Thus, the inhibition of caspase-8 promotes the transition from RIPK1-induced apoptosis (complex IIa) to necroptosis, where RIPK1 recruits and phosphorylates RIPK3, and activated RIPK3 further stimulates the activation of mixed lineage kinase domain-like (MLKL) through the way of phosphorylation. Phosphorylated MLKL translocates to the inner leaflet of the plasma membrane, disrupting cellular integrity and thus leading to necroptosis [52–54]. In addition, MtROS could promote necroptosis. Activated RIPK3 induces phosphorylation of MLKL and ROS production, while RIPK1 is the major target of MtROS, thus forming a positive feedback pathway for necroptosis [55].
Fig. 2. Mechanism of necroptosis in NAFLD.
Various NAFLD triggering factors can lead to the upregulation of RIPK3, triggering RIPK3-dependent necroptosis, and drugs such as ELA and TTP can inhibit necroptosis and protect against NAFLD by acting on different targets of the necroptosis mechanism.
Necroptosis in NAFLD
Necroptosis has been validated to be closely associated with NAFLD and NASH in animal models and human diseases. Expression of necroptosis-associated factors MLKL, RIPK1, and RIPK3 in the liver can be used to diagnose and assess NAFLD progression [56]. Epigenetic silencing of RIPK3 in mouse and human primary hepatocytes prevents necroptosis-mediated liver pathological damage induced by MLKL activation [57]. Both RIPK3 and MLKL expression were increased in liver tissues of NAFLD and NASH patients [58, 59]. Similar to those findings, steatohepatitis was exacerbated after 18 weeks of feeding mice with a high-fat choline-deficient (HFCD) diet, and RIPK3 and MLKL were sequestrated in the insoluble protein fraction of liver lysates from NASH mice, suggesting early events during steatohepatitis progression [58]. MLKL expression in liver tissue of NAFLD patients increases in correlation with the severity of associated pathological changes, such as steatosis, ballooning, and inflammation. Compared to control mice, MLKL-KO mice fed with the HFD exhibited reduced NAS, steatosis score, inflammation, and ballooning degeneration [60]. In the methionine choline-deficient (MCD) diet-fed NASH model, the primary role of caspase-8 is to prevent excessive activation of necroptosis and caspase-8-deficient mice develop large amounts of RIPK3 in hepatocytes, leading to severe liver injury and fibrosis. Conversely, mice lacking RIP3 significantly reduced liver injury, inflammation, and fibrosis induced by the MCD diet compared to wild-type mice, indicating an important role for RIPK3-dependent necroptosis in the progression of NAFLD [61]. In contrast to HFD-fed mice, the heme oxygenase-1 (HO-1) (ROS scavenger) expression and MLKL phosphorylation were increased in sucrose-containing HFD-fed mice, while both indicators were also increased in human NAFLD patients. Excess fructose can induce NAFLD progression by enhancing OA lipotoxicity through ROS production, leading to hepatocyte necroptosis, and its effects are ameliorated by treatment with the reactive oxygen scavenger NAC [62].
RIPK3 was found to be overexpressed in the white adipose tissue (WAT) of HFCD-fed obese mice as well as in visceral WAT of obese humans, and although RIPK3 mediates necroptosis, it primarily inhibits apoptosis, which suppresses inflammation and maintains tissue homeostasis [63]. Interestingly, RIPK3 has been identified as a key mediator of necroptosis that protects mice from HFD-induced liver injury, which contrasts with previous observation in mice on the MCD diet that RIPK3 promotes NAFLD progression. The absence of RIPK3 aggravates HFD-induced liver injury, promotes hepatic inflammation and hepatocyte apoptosis, and is associated with an early fibrotic response. These findings suggest that altered patterns of hepatocyte death can influence NAFLD disease progression, where the complex balance and interplay of different pathways of PCD may be affected by the metabolic state of the liver [61, 64].
Regarding the triggering mechanism of necroptosis in the progression of NAFLD, it is speculated that it may be related to the stimulation of necroptosis-related first activation signals according to the reports. In NASH patients as well as in animal models of NAFLD, the expression of both TNF-α and TNFR1 was found to be upregulated. TNF-α levels positively correlated to the severity of liver disease, and TNFR1 deficiency exerts a protective effect against hepatic steatosis and liver damage [17, 65, 66]. Furthermore, hepatocytes are heavily exposed to TLR4 ligands during NASH [67]. When hepatocytes are exposed to these stimulatory signals, the necroptosis signaling pathway is triggered, which in turn triggers liver injury. Indeed, these necroptosis-associated signaling molecules can also stimulate apoptosis, and both necroptosis and apoptosis may be activated concurrently in liver injury, and RIPK3 can convert apoptosis to necroptosis only when caspase-8 is inhibited [51]. Whereas, with increased hepatic steatosis, the transcription factor ATF3 also increases hepatocyte death by inducing RIPK3 expression and changing the TNFα-dependent cell death pattern from apoptosis to necroptosis [68]. In exploring obstructive sleep apnea hypopnea syndrome (OSAHS)-associated NAFLD, researchers found that intermittent hypoxia (IH) increased RIPK3 upregulation caused by PA treatment of LO2 cells, promoted RIPK3-dependent necroptosis, and increases hepatic oxidative stress, and inflammatory responses. It was also found that IH-exacerbated NAFLD was regulated through the RIPK3-dependent necroptosis NRF2/nuclear factor kappa-B (NF-κB) signaling pathway [69]. Overall, we have to acknowledge the crucial role of necroptosis in the progression of NAFLD.
Necroptosis-targeted therapies in NAFLD
Researchers found that the dual peroxisome proliferator-activated receptor alpha/delta agonist Elafibranor (ELA) exerts a hepatoprotective effect by reducing cleaved RIPK3 and cleaved caspase-3, which in turn reduced hepatocyte necroptosis and apoptosis, inhibited hepatic steatosis, inflammation, and fibrosis, resulting in significantly lowered NASs [70]. RIPA-56, a specific inhibitor of RIPK1, and GSK-872, a RIPK3 inhibitor, have been shown to inhibit the RIPK1/RIPK3/MLKL pathway-mediated necroptosis. In HFD-fed mice, the use of these inhibitors targeting RIPK1/RIPK3 significantly reduces all hepatic features associated with steatohepatitis, including inflammation, liver fibrosis, transaminase activity, and triglyceride levels. Similarly, the same effect was observed by pharmacological inhibition or knockdown of the downstream MLKL of RIPK [69, 71]. In a recent report, researchers found that metformin, a commonly used medication for type 2 diabetes, activates the tristetraprolin (TTP) via the AMPK–SIRT1 pathway in hepatocytes and Kupffer cells (KCs). TTP inhibits TNF-α production in KCs, thereby reducing hepatocyte necroptosis [72]. In addition, AS1842856 attenuates necroptosis in NAFLD mice by specifically inhibiting Forkhead box O (FOXO1), a key downstream factor of the insulin/IGF-1 signaling pathway. RIPK1, RIPK3, and phosphorylated MLKL were significantly downregulated in AML-12 cells treated with AS1842856, thereby mitigating necroptosis marker protein upregulation due to PA treatment. FOXO1 is involved in the regulation of glucose metabolism, however, its role in NAFLD and its relationship with necroptosis and molecular mechanisms remain to be investigated [73].
Pyroptosis
Overview of pyroptosis
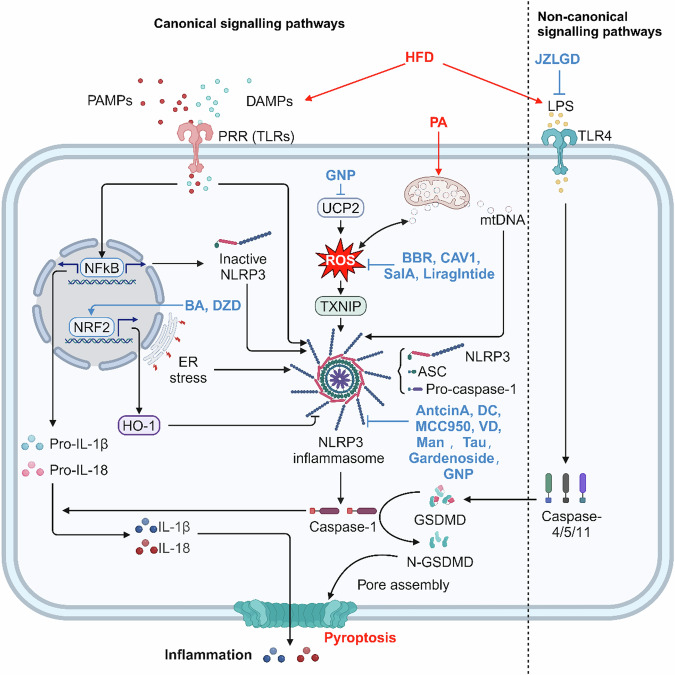
Pyroptosis was initially observed in 1992 by Zychlinsky et al. [74] in Shigella fowleri-infected macrophages and was defined as a caspase-1-dependent mode of pro-inflammatory PCD that is essential for the control of microbial infections. Pyroptosis occurs mainly during intracellular pathogen infection and is characterized by activation of the inflammasome, caspases-dependent formation of membrane pores, rupture of cell membrane swelling, and release of pro-inflammatory cytokines, such as IL-1β, IL-18, and cellular contents. These events collectively trigger subsequent immune responses to infection [75] (Fig. 3).
Fig. 3. Mechanism of pyroptosis in NAFLD.
Various NAFLD triggering factors can activate canonical and non-canonical signaling pathways-induced hepatocyte death through different mechanisms. Antcin A, Dieckol, and others can inhibit pyroptosis and prevent NAFLD by acting on different targets of the pyroptosis mechanism.
Pyroptosis is activated by canonical and non-canonical signaling pathways [76]. Inflammasome activation, known as the canonical signaling pathways, involves two steps. First, the binding of danger associated molecular patterns (DAMPs), pathogen associated molecular pattern (PAMPs), or cytokines to TLRs triggers the activation of NF-κB signaling pathway, resulting in an upregulation in the expression of pro-IL-1β, pro-IL-18, and NOD-like receptor thermal protein domain associated protein 3 (NLRP3). Second, the inflammasome begin to assemble and form. DAMPs caused by environmental factors and cellular damage stimulate inflammasome sensor proteins such as NOD-like receptor proteins (NLRP1, NLRP3, NLRC4, NLRP6), absent in melanoma 2 (AIM2)-like receptors (ALRs), or pyrin families. Typical inflammasome components include the junctional protein named apoptosis-associated speck-like protein containing a CARD (ASC) and the inactive zymogen pro-caspase-1 [77, 78]. NLRP3 inflammasome activates caspase-1 to cleaves pro-IL-1β and pro-IL-18 into the active forms IL-1β and IL-18, meanwhile, cleaves the amino-terminal fragment of the key protein gasdermin D (GSDMD) to form N-GSDMD, which oligomerizes and forms membrane pore formation leading to pyroptosis and subsequent inflammatory response. In the non-canonical activation pathway of NLRP3, the presence of intracellular bacterial lipopolysaccharide (LPS) is detected by caspase-4/5/11, leading to proteolytic activation of GSDMD, which in turn leads to pyroptosis in gram-negative infections [79–81].
Pyroptosis in NAFLD
Previous studies have provided evidence supporting the involvement of pyroptosis, induced by the activation of inflammasomes, in the pathogenic mechanism of NAFLD. Hepatic mRNA levels of NLRP3, pro-caspase-1, IL-1β, and IL-18 were remarkably higher in NAFLD patients than in healthy individuals, and exhibited a strong correlation with lobular inflammation, hepatocyte ballooning, and NASs [82]. The suppression of key signaling molecules involved in the canonical signaling pathways of pyroptosis, (e.g., NLRP3, caspase-1, IL-1β, and GSDMD), was able to attenuate pathological changes such as hepatic steatosis, inflammation, and early fibrogenesis in animal models of NAFLD/NASH. This suggests the crucial role of pyroptosis in the pathogenesis of NAFLD/NASH [83–87]. During the development of NASH, activation of NLRP3 inflammasome mediates caspase-1 activation in bone marrow-derived KCs, which then release pro-inflammatory signals that can activate hepatic stellate cells (HSCs) responsible for collagen deposition and fibrosis in the liver [84]. Additionally, the internalization of NLRP3 inflammasome particles by HSCs induces their activation, resulting in increased secretion of IL-1β and expression of α-smooth muscle actin (α-SMA) [88, 89]. Over time, this process gradually leads to severe liver injury and liver fibrosis.
The first step of risk signals for inflammasome activation includes mainly DAMPs and PAMPs, leading to the activation and upregulation of NLRP3, pro-IL-1β, and pro-IL-18. The second step signals are dependent on cellular stress in the process of NAFLD/NASH including MtROS, mtDNA, ERs, and impaired mitochondrial phagocytosis, etc. [89]. Saturated FAs represent an endogenous hazards that upregulate the inflammasome in NASH in the form of a first hit and induce sensitization of hepatocytes to a second hit of LPS, resulting in the release of IL-1β [90]. Interestingly, PA activates NLRP3 inflammasome in mouse KCs by decreasing the mitochondrial membrane potential and inducing the release of mtDNA from mitochondria into the cytoplasm to form mtDNA–NLRP3 inflammasome complexes [91]. Apart from the activation of NLRP3 inflammasome in NAFLD, mitochondrial damage and mtDNA release can also trigger the activation of AIM2 inflammasome [92]. The activation of NLRC4 inflammasome is modulated by TNF-α. Increased TNF-α promotes the activation of NLRC4 inflammasome in the liver, which improves the production of IL-18 and IL-1β, triggers hepatocyte pyroptosis, and accelerates NAFLD progress [93].
Drummer et al. [94] reported that HFD-feeding increased the production of endotoxin LPS by gram-negative bacteria in the intestinal microbiota of mice. The elevated levels of LPS in circulation and intracellularly activates caspase-11 and initiates caspase-11-dependent non-canonical signaling pathways, eventually leading to pyroptosis. Additionally, PA produced by lipolysis in HFD-fed mice also activates caspase-11 and GSDMD cleavage [94]. Furthermore, the involvement of caspase-11 in mediating hepatocyte pyroptosis was verified in an MCD-induced NASH mouse model. The deficiency of caspase-11 significantly reduced liver injury and inflammation in mice, downregulated the activation of GSDMD and IL-1β, and the expression of fibrillation-related factors (TGF-β, α-SMA, and alpha-1 type I collagen) was also reduced, revealing a critical role for caspase-11 in NASH [95].
Pyroptosis-targeted therapies in NAFLD
Recent studies have shown that Exenatide treatment can effectively reduce the expression of NLRP3, caspase-1, and IL-1β in OA/LPS-induced HepG2 cells and MCD-induced db/db mouse livers, which in turn inhibits the pyroptosis signaling pathway to alleviate NASH [96]. Similarly, the herbal formulation Jinlida granule has been found to reduce the expression of NLRP3, caspase-1, IL-1β, and IL-18 in HFD-induced mouse livers as well as FFA-induced HepG2 cells, demonstrating its potential to alleviate liver injury and exert a protective effect against NAFLD by reducing hepatocyte pyroptosis [97]. However, the exact mechanism of their action remains to be studied.
Antcin A, a small triterpene molecule, indirectly inhibits pyroptosis by targeting the assembly and activation of NLRP3 inflammasome [98]. Similar to Antcin A, compounds such as Dieckol (DK), MCC950 (a specific NLRP3 inhibitor), and VD also reduce pyroptosis by targeting NLRP3 inflammasome, thereby attenuating the accumulation of triglycerides and FFA, improving liver injury, and reducing NAFLD scores [99–101]. Mangiferin (Man), on the one hand, downregulated the expression of NLRP3 inflammasome-related proteins to attenuate pyroptosis and inflammatory effects. On the other hand, it upregulates p-AMPKα levels, regulating glucolipid metabolism and ameliorating liver injury, insulin resistance, glucose tolerance, lipid accumulation, and inflammation in NAFLD mice [102]. In an As2O3-induced NASH model, researchers found that taurine (Tau) inhibits pyroptosis and attenuates hepatic inflammation through inhibition of the cathepsin B (CTSB)–NLRP3 inflammatory vesicle pathway [103]. Gardenoside, a natural botanical ingredient, ameliorates lipid accumulation and liver fibrosis by reducing the expression of pyroptosis-associated protein. The knockdown of CCCTC binding factor (CTCF) or dipeptidyl peptidase-4 (DPP4) in HFD-fed mice and PA + LPS-treated AML-12 cells yields similar effects to Gardenoside treatment, while CTCF overexpression counteracted this alteration. These findings suggest that Gardenoside inhibits via the CTCF/DPP4 signaling pathway [104]. Genipin (GNP), acting as an inhibitor of uncoupling protein-2 (UCP2), effectively reversed HFD-induced liver injury and inhibited NLRP3 inflammasome activation. Notably, its inhibitory effect on inflammasome was verified to be related to UCP2–ROS signaling [105]. Besides, lncRNA growth-arrest specific transcript 5 (GAS5) also inhibits NLRP3 inflammasome-mediated hepatocyte pyroptosis via binding miR-28a-5p in NAFLD [106].
Caveolin-1 (CAV1) may inhibit NLRP3-mediated pyroptosis through the ROS/thioredoxin-interacting protein (TXNIP) pathway. In detail, CAV1 scavenges ROS, preventing the separation of TXNIP from thioredoxin and its binding to NLRP3, thus inhibiting the assembly and activation of the NLRP3 inflammasome and attenuating liver injury [107]. Apart from CAV1, additional chemical medicines, such as Berberine (BBR) and Salvianolic acid A (SalA), have been found to inhibit pyroptosis during the NAFLD process by modulating TXNIP-induced NLRP3 inflammasome activation [108, 109]. Additionally, Liraglutide has shown promise in human and experimental studies for the prevention of NASH by inhibiting NLRP3 inflammasome activation and pyroptosis in PA + LPS-treated hepatocytes. This is achieved through the reduction of lipid accumulation, maintenance of mitochondrial function, and decrease in ROS production [110].
It was well known that, under physiological conditions, the NRF2/HO-1 axis is essential for resistance to oxidative stress, and upregulation of this axis significantly suppresses the expression of NLRP3, caspase-1, and ASC, inhibits inflammasome formation, blocks GSDMD activation, and prevents the release of IL-1β and IL-18. In exploring the mechanisms underlying the lipid-lowering and anti-inflammatory effects of Baicalin (BA), it was discovered that BA reduced FFA-induced HepG2 cells and HFD-induced mice hepatocyte pyroptosis via the NRF2/HO-1/NLRP3 pathway. However, the regulation of NRF2 by BA needs to be further investigated [111, 112]. Indeed, Danshen zexie decoction, like BA, protects against HFD-induced inflammation and liver injury by upregulating of this axis [113].
In studies of non-canonical signaling pathways, it has been found that Jiangzhi Ligan Decoction (JZLGD) ameliorated steatosis, inflammation, and pyroptosis in HFD-fed rats not only by inhibiting the NLRP3/caspase-1/GSDMD-mediated typical pyroptosis pathway but also by decreasing the levels of LPS in the hepatic portal vein of NAFLD rats, which prevented caspase-11 and GSDMD activation, inhibited the LPS/caspase-11/GSDMD-mediated non-canonical signaling pathways, and alleviated NALFD [114].
Ferroptosis
Overview of ferroptosis
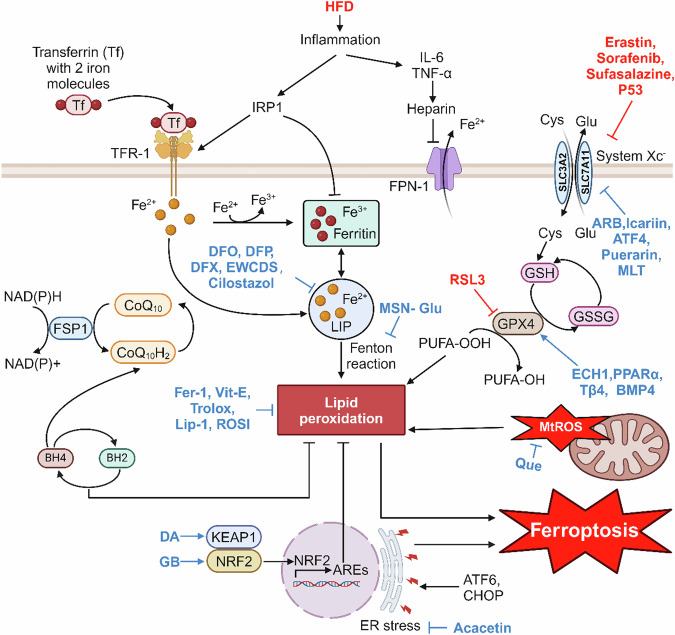
Ferroptosis is a novel mode of PCD that is iron-dependent and distinct from apoptosis, necroptosis, and pyroptosis. Early in 2012, it was first described by Dixon et al. [115] and the central events of ferroptosis involve intracellular iron overload, excessive accumulation of lipid ROS, and lipid peroxidation. It differs morphologically, physiologically, and genetically from apoptosis, necroptosis, and pyroptosis, making it a unique form of cell death (Fig. 4). Morphologically, it is characterized by cell membrane rupture and effacement, a decline or even vanishment of mitochondrial cristae, mitochondrial atrophy, and normal nuclear morphology but it lacks of chromatin condensation [115].
Fig. 4. Mechanism of ferroptosis in NAFLD.
Iron metabolism imbalance in the liver during NAFLD development triggers ferroptosis, leading to lipid peroxidation and accelerated NAFLD progression, and drugs such as EWCDS and BMP4 can inhibit ferroptosis and protect against NAFLD by acting on different targets of the ferroptosis mechanism.
Ferroptosis is intertwined in three aspects: amino acid and lipid metabolism, regulation of ROS, and iron regulation. As we know, the key components of the cell membrane are phospholipids (PLs), and when PUFAs are part of the membrane PLs, they become substrates for the peroxidation of cellular ferroptosis. The ferroptosis activator Erastin can act directly on systemic Xc−, the reverse transporter of cystine/glutamate in the cell membrane, to inhibit amino acid uptake, which in turn affects glutathione (GSH) synthesis, causing a decrease in glutathione peroxidase 4 (GPX4) activity [115, 116]. Under basal conditions, GPX4 converts GSH to oxidized glutathione (GSSG) and reduces cytotoxic lipid peroxides (L-OOH) to the corresponding alcohols (L-OH). In response to GPX4 inhibition, cellular antioxidant capacity is rapidly reduced, which in turn leads to the accumulation of intracellular lipid ROS, oxidative damage, and ultimately ferroptosis. Unlike the canonical ferroptosis pathway due to GPX4 inactivation, intracellular Fe2+ accumulation, which can react with peroxides in the Fenton reaction, generates excess hydroxyl radicals, induces lipid peroxidation, and drives cellular ferroptosis [117, 118]. Systemic Xc− inhibitors (e.g., Sorafenib, Sulfasalazine, and Erastin) are considered as class I ferroptosis inducers, whereas directly GPX4 inhibitors such as RAS selective lethality protein 3 (RSL3), are referred to as class II inducers [119–121]. Some transcription factors regulate ferroptosis-related genes in response to oxidative stress, for example, p53 decreases cystine uptake by inhibiting the expression of Solute carrier family 7 member 11 (SLC7A11), one of the components of the Xc− system, thereby increasing cellular susceptibility to ferroptosis [122]. And ferroptosis induced due to GPX4 deficiency can be inhibited by ferroptosis suppressor protein 1 (FSP1) through the NAD(P)H–FSP1–CoQ10 axis. This is a signaling pathway parallel to the Cyst(e)ine–GSH–GPX4 axis, which plays an important role in the inhibition of lipid peroxidation and ferroptosis [123]. In addition, there exists another pathway that inhibits ferroptosis, the GCH1–BH4–DHFR axis. Tetrahydrobiopterin (BH4), a downstream factor of GTP cyclohydrolase-1(GCH1), on the one hand, inhibits ferroptosis by promoting the synthesis of CoQ10 and, on the other hand, inhibits lipid peroxidation as an antioxidant, which, in turn, inhibits ferroptosis [124, 125].
Ferroptosis in NAFLD
Numerous studies have demonstrated the presence of iron overload in the liver tissue of NAFLD patients, that excess free iron can generate hydroxyl radicals and ROS via the Fenton reaction, promoting lipid peroxidation, and that redox imbalance is a central pathogenic mechanism of NAFLD and its progression [118, 126]. Maintaining a balance of iron metabolism in the body prevents obesity and increases insulin sensitivity, both of which are typical of NAFLD patients [127, 128]. It is estimated that one-third of patients with NAFLD present with methemoglobinemia, mainly with mild hepatic iron deposition (50–150 μmol/g) [128]. Kowdley et al. examined the connection between increased human serum ferritin (SF) and NAFLD severity, and they found that SF > 1.5 × upper limit of normal (ULN) independently predicted the development of advanced liver fibrosis in patients with NAFLD and that elevated SF was independently linked with elevated NAS even in patients without hepatic iron deposition correlated [129]. Indeed, iron metabolism disorders and ferroptosis occur in the early stages of the NAFLD process. On the one hand, in the liver of HFD-fed rats, the activity of iron regulatory protein 1 (IRP1) is increased, and it affects iron uptake and storage in cells by transcriptionally regulating the expression of ferritin, transferrin receptor-1 (TfR1) and iron export protein ferroportin (FPN-1). On the other hand, HFD induced in a time-dependent manner the expression of hepatic pro-inflammatory cytokine IL-6 and transcription factor C/EBPα, both of which promoted the expression of ferroportin, and, in turn, the downregulation of FPN-1, ultimately leading to an increase in iron content (HIC) in hepatocytes. These findings suggest a strong link between an imbalance of iron metabolism, inflammatory damage, and disease progression [130].
In addition to iron overload, lipid peroxidation is an essential process for the development of ferroptosis [131]. Loguercio et al. [132] observed that more than 90% of patients with NAFLD exhibited elevated levels of lipid peroxidation markers (malondialdehyde [MDA] and 4-hydroxynonenal [4-HNE]), and in particular, patients with NASH had significantly higher MDA and 4-HNE than patients with steatosis [132]. Qi et al. [133] demonstrated that in the MCD-induced NASH model, treatment with the GPX4 activator sodium selenite, the iron chelator desferrioxamine (DFO), and the ferroptosis inhibitor Liproxstatin-1(Lip-1) inhibited the ferroptosis, suppressed inflammatory response, and alleviated NASH, while conversely RSL3 (a ferroptosis inducer) downregulates GPX4 levels and exacerbates the severity of NASH [133]. Likewise, the presence of ferroptosis was confirmed in an arsenic-induced NASH model by detecting the expression of ferroptosis-related proteins as well as mitochondrial morphology [134]. Tsurusaki et al. [135] found that necroptosis precedes apoptosis in the process of NAFLD through a choline-deficient, ethionine-supplemented (CDE) diet model. However, after administration of necroptosis and ferroptosis inhibitors on top of the CDE diet, it was found that ferroptosis inhibitors almost completely reversed hepatocyte death, whereas necroptosis inhibitors failed to block the onset of cell death, suggesting that hepatocyte ferroptosis may be one of the initial modes of cell death at the onset of steatohepatitis and may be a trigger for the initial inflammation of steatohepatitis [135]. In summary, these studies prove that ferroptosis is involved in NAFLD and accelerates its developmental process.
Ferroptosis-targeted therapies in NAFLD
Therefore, inhibition of ferroptosis is a promising therapeutic strategy for NAFLD, and an increasing number of drugs targeting various pathways involved in the occurrence of ferroptosis have been identified, which can effectively target different ferroptosis-related factors. Consequently, these drugs inhibit ferroptosis, reduce lipid peroxidation and oxidative stress in cells, and slow down the progression of NAFLD. Notably, some ferroptosis inhibitors such as iron chelators, Fer-1, Lip-1, ROSI (ACSL4 inhibitor), and Trolox (antioxidant vitamin E analog) have been demonstrated to significantly inhibit ferroptosis in NAFLD animal models induced by arsenic, CDE-fed, MCD-fed, HFD-fed, and so on, and attenuate liver inflammation reactions, lipid accumulation, and liver injury [134–138].
In addition to some traditional iron chelators including DFO, deferiprone (DFP), and deferasirox (DFX), a novel iron nano-chelator fluorescent protein-based carbon dots (EWCDs) can effectively reduce ROS production, inhibit apoptosis, and alleviate inflammation, making EWCDs a potential treatment for zebrafish iron overload-induced NAFLD. What’s more, EWCDs can inhibit hepatocyte apoptosis and reduce inflammation by attenuating ERs [139]. Similarly, Insulin-like growth factor binding protein 7 (IGFBP7) deletion in the zebrafish NAFLD model significantly increased the expression of the nuclear receptor coactivator 4 (NCOA4), increased NCOA4-mediated ferritin autophagy (ferritinophagy), maintained intracellular iron homeostasis, and reduced the occurrence of hepatocyte ferroptosis [140]. Cilostazol inhibits ectopic erythrocyte phagocytosis by hepatic inhibition of increased platelet and erythrocyte accumulation, which in turn attenuates hepatic iron overload and prevents ferroptosis [141]. The above drugs reduce ferroptosis by directly or indirectly reducing the accumulation of Fe2+ in cells.
Accordingly, there are many other drugs that ultimately target Systemic Xc− in various ways, thereby reducing cystine uptake and inhibiting ferroptosis. The natural antioxidant arbutin (ARB) is able to target demethylase fat mass and obesity-related protein to promote methylation of the SLC7A11 gene, one of the components of Xc−, improving HFD-induced NAFLD in vivo and in vitro [142]. Icariin, a flavonoid abundant in the herb Epimedium, ameliorated MCD diet-induced lipid peroxidation, restored mitochondrial structure in the livers of NASH mice, and exerted hepatoprotective effects through the activation of Nrf2, which in turn significantly elevated the protein levels of GPX4 and a cystine/glutamate transporter (xCT) [143]. Activating transcription factor 4 (ATF4) was also able to inhibit ferroptosis and improve liver function by increasing the expression of SLC7A11 [144]. Similarly, in HFD combined with streptozotocin (STZ) injected mice and PA-treated AML-12 cells, researchers found that Puerarin treatment was able to effectively inhibit ferroptosis and reduce lipid peroxidation by activating the SIRT1/NRF2 pathway and increasing the expression of HO-1, SLC7A11, and GPX4 proteins [145]. HO-1 is the major and rate-limiting enzyme of heme metabolism, which catalyzes the catabolism of heme to biliverdin, free iron, and carbon monoxide, and HO-1 deficiency exacerbates ROS accumulation, lipid peroxidation, and iron overload in hepatocytes [146]. In FFAs-treated HepG2 cells and HFD-induced NAFLD mice, researchers found that Melatonin treatment increased NRF2 and HO-1 levels to protect cells from oxidative environment, and also upregulated the expression of GPX4 and SLC7A11 to inhibit ferroptosis [147].
In addition, in the antioxidant signaling pathway Keap1/NRF2/ARE, Gao et al. found that a substance called dehydroabietic acid binds to Keap1, activates NRF2-ARE, induces downstream gene expression, which in turn upregulates GSH, GPX4, inhibits ROS accumulation and lipid peroxidation, suppresses ferroptosis and NAFLD [148]. Yang et al. [149] found that Ginkgolide B activated the NRF2 signaling pathway by promoting NRF2 translocation to the nucleus and upregulated GPX4, which in turn inhibited ferroptosis in PA/OA-induced HepG2 cells and HFD-induced mice hepatocytes. In addition, there are a number of genes and small molecule compounds that have been shown to upregulate GPX4 through other pathways. For instance, enoyl coenzyme A hydratase 1 (ECH1) can reduce ferroptosis by upregulating GPX4 on the one hand, and reduce apoptosis by inhibiting ERK on the other hand, thus improving NAFLD [150]. In addition, PPARα inhibits ferroptosis by promoting GPX4 expression and inhibiting plasma transferrin (TRF) expression. The family of PPARs regulates energy metabolism, of which PPARα is mainly expressed in the liver and can directly induce GPX4 expression [151]. Shu et al. [152] found that time-restricted feeding suppressed the expression of the circadian gene Per2, and that specific knockdown of Per2 in hepatocytes promoted the expression of PPARα, thereby reducing lipid peroxidation levels, inhibiting the ferroptosis-related genes expression, improving mitochondrial morphology, and ultimately effectively mitigating NASH. Another novel strategy and treatment target for NAFLD identified by Zhu et al. [153] is thymosin beta 4 (Tβ4), which can exert a protective effect against NAFLD by inhibiting ferroptosis in PA-induced LO2 cells and HFD-induced rat hepatocytes through upregulation of GPX4 expression [153]. Similarly the protective effect of bone morphogenetic protein 4 (BMP4) on NAFLD is mediated by regulating GPX4 expression through a non-transcriptional mechanism. The overexpression of BMP4 in LO2 cells and HepG2 cells can upregulate the expression of GPX4, reduce ROS and MDA levels, mitigate ferroptosis, and reduce liver injury [154].
There are also drugs that inhibit ferroptosis through other pathways that also exert a protective effect against NAFLD. Quercetin can attenuate lipid droplet accumulation and lipid peroxidation, ameliorate liver injury, and alleviate NAFLD by inhibiting MtROS-mediated ferroptosis [155]. Jiang et al. [156] demonstrated that ERs are an upstream signal for ferroptosis in NAFLD, and proposed a drug, the natural flavonoid Acacetin, which inhibits ferroptosis and reduces hepatic lipid accumulation by inhibiting ERs. Liraglutide, an agonist of GLP-1R, can increase acetyl-CoA carboxylase (ACC) phosphorylation through activation of AMPK, which in turn attenuates Erastin- or RSL3-induced cellular ferroptosis in the presence of high glucose, suggesting that Liraglutide ameliorates T2DM-associated NAFLD by inhibiting ferroptosis through activation of AMPK/ACC signaling [157].
In addition to these traditional iron death drugs, Zhao et al. developed for the first time a novel nanodrug: the N-(3-triethoxysilylpropyl)gluconamide (Glu) modified magnesium silicide (Mg2Si) nanosheets (MSN-Glu), which antagonizes the free heme-induced Fenton reaction by targeting H2 delivery to hepatocytes, reduces oxidative stress, lipid peroxidation, and subsequent ferroptosis, and significantly improves hepatic metabolic function in NAFLD mice [158].
Cell death in different stages of NAFLD
NAFLD refers to a spectrum of liver damage ranging from NAFL to NASH and advanced liver fibrosis/cirrhosis. Chronic lipid accumulation in the liver causes inflammation, converts NAFL to NASH, and promotes hepatic fibrosis, with advanced progression to liver cirrhosis and hepatocellular carcinoma.
In recent years, a large number of studies have demonstrated that different types of cell death (including apoptosis, pyroptosis, necroptosis, and ferroptosis) occur concurrently in the progression of NAFL to NASH and that their occurrence correlates with the severity of the disease. Compared with patients with steatosis alone (NAFL), patients with NASH have a significantly increased level of apoptosis [12], as well as pyroptosis with elevated expression of caspase-1 [159] and necroptosis with increased expression of RIP3 [58]. In addition, the activation of HSC have been wildly reported to function in the progression of NASH to cirrhosis [160]. Notably, all the four types of cell deaths were reported to accelerate NASH by promoting HSC activation. Gaul et al. demonstrate that primary mouse and human hepatocytes can undergo pyroptosis with subsequent release of NLRP3 inflammasome proteins, which activates HSCs with increased secretion of IL-1β and α-SMA expression [159]. Human HSCs incubated with conditioned medium from steatotic hepatocytes show fibrogenic activation and enhanced resistance to apoptosis, suggesting that apoptotic hepatocytes could stimulate HSCs in patients with NASH [161]. Moreover, loss of MLKL, the terminal effector in necroptosis pathway, ameliorates liver fibrosis by inhibiting hepatocyte necroptosis and HSC activation [162]. As to ferroptosis, it plays as a “double-edged sword” in liver fibrosis. On the one hand, hepatocyte ferroptosis can aggravate liver fibrogenesis by activating HSCs [163], while on the other hand, treatment with Erastin in mice or Sorafenib monotherapy in advanced fibrotic patients with hepatocellular carcinoma could alleviate liver fibrosis by inducing HSC ferroptosis [164, 165]. Taken together, in the stage from NAFL to NASH, levels of cell death could be used as indicators of the pathogenesis with detection of the death-related markers, whereas in the stage from NASH to cirrhosis, cell death pathways are effective therapeutic targets in blockage of HSC activation.
The cross-talk among different cell deaths in NAFLD
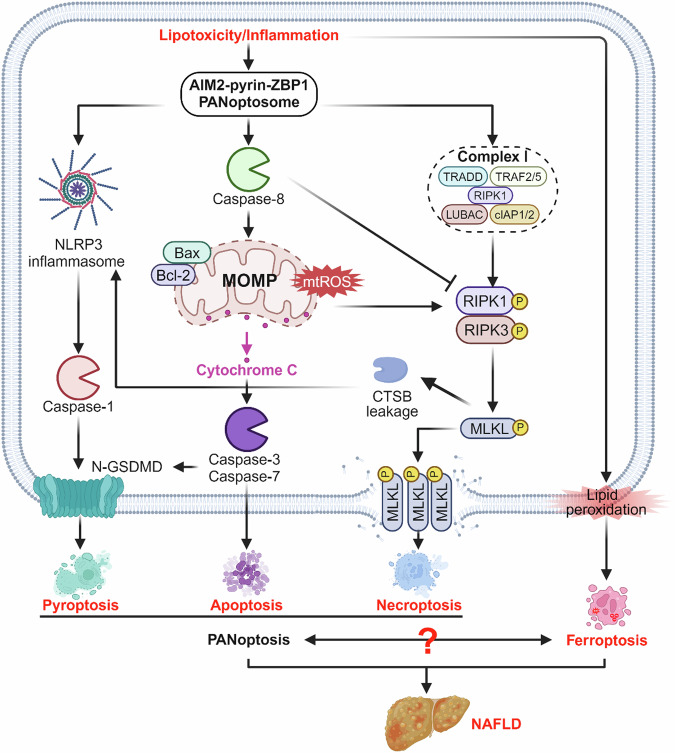
Given that the different types of PCD, including apoptosis, necroptosis, pyroptosis, and ferroptosis, can coexist during the pathogenesis of NAFLD, there might be extensive interactions between the different modes of cell death. PANoptosis, including apoptosis, pyroptosis, and necroptosis, is newly proposed as a coordinated cell death pathway in recent years, which has been reported to play critical roles in the pathogenesis of NAFLD [166, 167]. During infections, AIM2 regulated the innate immune sensors Pyrin and ZBP1 to drive PANoptosis and provide host protection [168]. Si-Wu-Tang, a traditional Chinese herbal prescription, was reported to protect against hepatitis-mediated hepatocyte PANoptosis through inhibiting mtDNA release-mediated ZBP1 activation [167]. In monocytes and macrophages, caspase-1 activates caspases-3/7 and induces apoptosis in the absence of GSDMD while caspases-3/7 specifically block pyroptosis by cleaving GSDMD during apoptosis, which is called “bidirectional crosstalk” [169]. Whereas in the progression of NAFL to NASH, ghrelin shows beneficial effects via inhibition of TNF-α-induced hepatocyte apoptosis and pyroptosis [170]. Sodium sulfite, a biological derivative of the air pollutant sulfur dioxide, could trigger hepatic apoptosis, necroptosis, and pyroptosis in mice and hepatocye cell line [171]. Interestingly, in a 3-week non-alcoholic steatohepatitis mouse model, Elafibranor showed strong benefits on NASH with improvement in hepatic inflammation, necroptosis, and apoptosis, but did not alter pyroptosis [172]. Tβ4, a multifunctional polypeptide, improved PA-induced LO2 damage through inhibition of apoptosis and ferroptosis. Furthermore, GPX4 knockdown attenuated therapeutic effect of Tβ4 on rat liver and LO2 cells with upregulation of apoptosis-related Bcl-2, Bax, and caspase-3 [153]. Besides, in addition to blocking the ferroptosis markers ACSL4 and ALOX15, ferroptosis inhibitor liproxstatin-1 also inhibited hepatic apoptosis, pyroptosis, and necroptosis by inhibiting cleavages of PANoptosis-related caspase-8 and caspase-6 in MAFLD mouse liver [173]. Taken together, more and more evidence regarding the interactions among apoptosis, pyroptosis and necroptosis and the relationship between panoptosis and ferroptosis is provided in pathogenesis of NAFLD interactions. However, although the ferroptosis and panoptosis are common targets of drugs, the underlying molecular mechanism is yet unknown (Fig. 5).
Fig. 5. Cross-talk among different cell deaths.
The three modes of death, including apoptosis, pyroptosis, and necroptosis, which coexist in NAFLD and interact with each other, are called PANoptosis. However, the interaction between PANoptosis and ferroptosis is not yet known.
Limitations
There exist several limitations, as different cell death subroutines display distinct feature, while sharing many similar characteristics with considerable overlap and cross-talk. Though it is recognized that various types of cell death may collectively contribute to the onset and progression of NAFLD, the specific dominant role of each type and the potential synergy resulting from the simultaneous activation of multiple pathways in hepatocytes remain elusive. To date, certain cell death modes, such as cuproptosis [174], remain poorly understood, and no literature has been found on the direct association with NAFLD.
Conclusions and perspectives
This article reviews the molecular mechanisms of four different forms of cell death, demonstrates the existence of multiple forms of hepatocyte death in NAFLD and the acceleration of NAFLD progression by these forms of cell death in in vitro and in vivo experiments, and summarizes the therapeutic agents or potential therapeutic targets for the different forms of cell death in the existing studies. However, our understanding of the association between different forms of cell death and NAFLD is incomplete, and the pathophysiologic mechanisms are not yet clearly elucidated. Current studies have not yet been able to answer the question of the interrelationships and influences of the various forms of cell death in the disease, as well as the sequence and predominance of the various forms of cell death at different stages of the disease progression. Future research needs to focus on understanding the linkages between different forms of cell death pathways and their possible roles in NAFLD to help us better understand the complex mechanisms of the disease and to find more effective therapeutic agents that target cell death.
Acknowledgements
The figures were created with BioRender software (BioRender.com).
Author contributions
H-lX: conceptualization, writing—original draft. S-rW: investigation, writing—original draft. Y-A: visualization, investigation. Q-W: visualization, investigation. Y-hX: investigation, validation. C-hD: investigation, validation. P-pZ: investigation, validation. Y-L: investigation, validation. B-tX: conceptualization, writing—review & editing. Z-zJ: conceptualization, writing—review & editing.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82270358), Sichuan Science and Technology Program (Nos. 2024NSFSC0588; 2022YFS0617; 2023ZYD0095), and Luzhou Science and Technology Program (2023JYJ027).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hui-li Xu, Sheng-rong Wan.
Contributor Information
Bu-tuo Xu, Email: xu651044138@163.com.
Zong-zhe Jiang, Email: jiangzongzhe555@swmu.edu.cn.
References
- 1.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24. 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64. 10.1016/j.cell.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22. 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. 10.1038/s41422-019-0164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautheron J, Gores GJ, Rodrigues CMP. Lytic cell death in metabolic liver disease. J Hepatol. 2020;73:394–408. 10.1016/j.jhep.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shojaie L, Iorga A, Dara L. Cell death in liver diseases: a review. Int J Mol Sci. 2020;21:9682. 10.3390/ijms21249682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. 10.1038/nrm1496 [DOI] [PubMed] [Google Scholar]
- 9.Li S, Zhao Y, He X, Kim T, Kuharsky DK, Rabinowich H, et al. Relief of extrinsic pathway inhibition by the bid-dependent mitochondrial release of smac in Fas-mediated hepatocyte apoptosis. J Biol Chem. 2002;277:26912–20. 10.1074/jbc.M200726200 [DOI] [PubMed] [Google Scholar]
- 10.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. 10.1038/cdd.2017.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–80. 10.1038/sj.cdd.4401378 [DOI] [PubMed] [Google Scholar]
- 12.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–43. 10.1016/S0016-5085(03)00907-7 [DOI] [PubMed] [Google Scholar]
- 13.Hatting M, Zhao G, Schumacher F, Sellge G, Al MM, Gaβler N, et al. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology. 2013;57:2189–201. 10.1002/hep.26271 [DOI] [PubMed] [Google Scholar]
- 14.Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, et al. The pan‐caspase inhibitor Emricasan (IDN‐6556) decreases liver injury and fibrosis in a murine model of non‐alcoholic steatohepatitis. Liver Int. 2014;35:953–66. 10.1111/liv.12570 [DOI] [PubMed] [Google Scholar]
- 15.Yang XX, Wang X, Shi TT, Dong JC, Li FJ, Zeng LX, et al. Mitochondrial dysfunction in high-fat diet-induced nonalcoholic fatty liver disease: the alleviating effect and its mechanism of Polygonatum kingianum. Biomed Pharmacother. 2019;117:109083. 10.1016/j.biopha.2019.109083 [DOI] [PubMed] [Google Scholar]
- 16.Alkhouri N, Alisi A, Okwu V, Matloob A, Ferrari F, Crudele A, et al. Circulating soluble Fas and Fas ligand levels are elevated in children with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:2353–9. 10.1007/s10620-015-3614-z [DOI] [PubMed] [Google Scholar]
- 17.Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–63. 10.1053/jhep.2001.29628 [DOI] [PubMed] [Google Scholar]
- 18.Afonso MB, Castro RE, Rodrigues CMP. Processes exacerbating apoptosis in non-alcoholic steatohepatitis. Clin Sci. 2019;133:2245–64. 10.1042/CS20190068 [DOI] [PubMed] [Google Scholar]
- 19.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150:956–67. 10.1053/j.gastro.2015.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–31. 10.1136/gut.2006.118059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazanave SC, Mott JL, Bronk SF, Werneburg NW, Fingas CD, Meng XW, et al. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J Biol Chem. 2011;286:39336–48. 10.1074/jbc.M111.280420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhi H, Bronk SF, Werneburg W, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–101. 10.1074/jbc.M510660200 [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Sun GF, Pan XA, Qu XH, Yang P, Chen ZP, et al. BCATc inhibitor 2 ameliorated mitochondrial dysfunction and apoptosis in oleic acid-induced non-alcoholic fatty liver disease model. Front Pharmacol. 2022;13:1025551. 10.3389/fphar.2022.1025551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang Y, Zhang Z, Tu J, Wang Z, Gao X, Deng K, et al. γ-Linolenic acid prevents lipid metabolism disorder in palmitic acid-treated alpha mouse liver-12 cells by balancing autophagy and apoptosis via the LKB1-AMPK-mTOR pathway. J Agric Food Chem. 2021;69:8257–67. 10.1021/acs.jafc.1c02596 [DOI] [PubMed] [Google Scholar]
- 25.Sozen E, Ozer NK. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated mini-review. Redox Biol. 2017;12:456–61. 10.1016/j.redox.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Hu R, Yin C, Xiao Y. Tanshinone IIA reduces palmitate‐induced apoptosis via inhibition of endoplasmic reticulum stress in HepG2 liver cells. Fundam Clin Pharmacol. 2020;34:249–62. 10.1111/fcp.12510 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Li S, Jiang H, Liu B, Lv Z, Guo C, et al. Effects of selenium on apoptosis and abnormal amino acid metabolism induced by excess fatty acid in isolated rat hepatocytes. Mol Nutr Food Res. 2017;61:1700016. [DOI] [PubMed]
- 28.Liu Y, Wang M, Xu W, Zhang H, Qian W, Li X, et al. Active vitamin D supplementation alleviates initiation and progression of nonalcoholic fatty liver disease by repressing the p53 pathway. Life Sci. 2020;241:117086. 10.1016/j.lfs.2019.117086 [DOI] [PubMed] [Google Scholar]
- 29.Du T, Fang Q, Zhang Z, Zhu C, Xu R, Chen G, et al. Lentinan protects against nonalcoholic fatty liver disease by reducing oxidative stress and apoptosis via the PPARα pathway. Metabolites. 2022;12:55. 10.3390/metabo12010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, Park M, Myung C. Garcinia cambogia ameliorates non-alcoholic fatty liver disease by inhibiting oxidative stress-mediated steatosis and apoptosis through NRF2-ARE activation. Antioxidants. 2021;10:1226. 10.3390/antiox10081226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou Y, Chen Z, Sun C, Yang D, Zhou Z, Peng X, et al. Exercise intervention mitigates pathological liver changes in NAFLD zebrafish by activating SIRT1/AMPK/NRF2 signaling. Int J Mol Sci. 2021;22:10940. 10.3390/ijms222010940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Wang J, Gong X, Zhang M, Kang S, Shu B, et al. Upregulation of BCL-2 by acridone derivative through gene promoter i-motif for alleviating liver damage of NAFLD/NASH. Nucleic Acids Res. 2020;48:8255–68. 10.1093/nar/gkaa615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Z, Zhang H, Wang Y, Li B, Liu K, Ran J, et al. Exercise activates Sirt1-mediated Drp1 acetylation and inhibits hepatocyte apoptosis to improve nonalcoholic fatty liver disease. Lipids Health Dis. 2023;22:33. 10.1186/s12944-023-01798-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D, Liu Z, Wang Y, Zhang Q, Li J, Zhong P, et al. Epigallocatechin-3-gallate alleviates high-fat diet-induced nonalcoholic fatty liver disease via inhibition of apoptosis and promotion of autophagy through the ROS/MAPK signaling pathway. Oxid Med Cell Longev. 2021;2021:5599997. 10.1155/2021/5599997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Xie S, Teng W. Sulforaphane attenuates nonalcoholic fatty liver disease by inhibiting hepatic steatosis and apoptosis. Nutrients. 2021;14:76. 10.3390/nu14010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang M, Li C, Liu Q, Wang A, Lei M. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front Endocrinol. 2019;10:665. 10.3389/fendo.2019.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcimartín A, López-Oliva ME, Sántos-López JA, García-Fernández RA, Macho-González A, Bastida S, et al. Silicon alleviates nonalcoholic steatohepatitis by reducing apoptosis in aged Wistar rats fed a high–saturated fat, high-cholesterol diet. J Nutr. 2017;147:1104–12. 10.3945/jn.116.243204 [DOI] [PubMed] [Google Scholar]
- 38.Geng Y, Wang Y, Sun R, Kang X, Zhao H, Zhu M, et al. Carnosol alleviates nonalcoholic fatty liver disease by inhibiting mitochondrial dysfunction and apoptosis through targeting of PRDX3. Toxicol Appl Pharmacol. 2021;432:115758. 10.1016/j.taap.2021.115758 [DOI] [PubMed] [Google Scholar]
- 39.Huang F, Zhao S, Yu F, Yang Z, Ding G. Protective effects and mechanism of meretrix meretrix oligopeptides against nonalcoholic fatty liver disease. Mar Drugs. 2017;15:31. 10.3390/md15020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai J, Huang J, Yang J, Chen X, Zhang H, Zhu Y, et al. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: the role of the AMPKα1–MFN2 pathway and Parkin mitophagy. Cell Mol Life Sci. 2022;79:354. 10.1007/s00018-022-04385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, et al. Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in high fat diet fed ApoE(−/−) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci. 2021;22:818. 10.3390/ijms22020818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, Liu Y, Yang L, Liu P, Zhang Y, Wang X. MiR-149 attenuates endoplasmic reticulum stress-induced inflammation and apoptosis in nonalcoholic fatty liver disease by negatively targeting ATF6 pathway. Immunol Lett. 2020;222:40–8. 10.1016/j.imlet.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 43.Demirel-Yalciner T, Sozen E, Ozaltin E, Sahin A, Ozer NK. alpha‐Tocopherol supplementation reduces inflammation and apoptosis in high cholesterol mediated nonalcoholic steatohepatitis. BioFactors. 2021;47:403–13. 10.1002/biof.1700 [DOI] [PubMed] [Google Scholar]
- 44.Han H, Xue T, Li Jie, Guo Y, Li X, Wang L, et al. Plant sterol ester of α-linolenic acid improved non-alcoholic fatty liver disease by attenuating endoplasmic reticulum stress-triggered apoptosis via activation of the AMPK. J Nutr Biochem. 2022;107:109072. 10.1016/j.jnutbio.2022.109072 [DOI] [PubMed] [Google Scholar]
- 45.Huang E, Xu K, Gu X, Zhu Q. PinX1 depletion improves liver injury in a mouse model of nonalcoholic fatty liver disease via increasing telomerase activity and inhibiting apoptosis. Cytogenet Genome Res. 2021;161:449–62. 10.1159/000518284 [DOI] [PubMed] [Google Scholar]
- 46.Shen X, Guo H, Xu J, Wang J. Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J Cell Physiol. 2019;234:18169–79. 10.1002/jcp.28450 [DOI] [PubMed] [Google Scholar]
- 47.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. 10.1038/nchembio711 [DOI] [PubMed] [Google Scholar]
- 48.Zong W, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. 10.1101/gad.1376506 [DOI] [PubMed] [Google Scholar]
- 49.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–23. 10.1016/j.immuni.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 50.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18:127–36. 10.1038/nrm.2016.149 [DOI] [PubMed] [Google Scholar]
- 51.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–52. 10.1038/s41575-018-0065-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–65. 10.1056/NEJMra1310050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–46. 10.1016/j.molcel.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 54.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–81. 10.1016/j.celrep.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Su SS, Zhao S, Yang Z, Zhong C, Chen X, et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat Commun. 2017;8:14329. 10.1038/ncomms14329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun HJ, Jiao B, Wang Y, Zhang YH, Chen G, Wang ZX, et al. Necroptosis contributes to non-alcoholic fatty liver disease pathoetiology with promising diagnostic and therapeutic functions. World J Gastroenterol. 2024;30:1968–81. 10.3748/wjg.v30.i14.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preston SP, Stutz MD, Allison CC, Nachbur U, Gouil Q, Tran BM, et al. Epigenetic silencing of RIPK3 in hepatocytes prevents MLKL-mediated necroptosis from contributing to liver pathologies. Gastroenterology. 2022;163:1643–57. 10.1053/j.gastro.2022.08.040 [DOI] [PubMed] [Google Scholar]
- 58.Afonso MB, Rodrigues PM, Carvalho T, Caridade M, Borralho P, Cortez-Pinto H, et al. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin Sci. 2015;129:721–39. 10.1042/CS20140732 [DOI] [PubMed] [Google Scholar]
- 59.Gautheron J, Vucur M, Luedde T. Necroptosis in nonalcoholic steatohepatitis. Cell Mol Gastroenterol Hepatol. 2015;1:264–5. 10.1016/j.jcmgh.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saeed WK, Jun DW, Jang K, Oh JH, Chae YJ, Lee JS, et al. Decrease in fat de novo synthesis and chemokine ligand expression in non‐alcoholic fatty liver disease caused by inhibition of mixed lineage kinase domain‐like pseudokinase. J Gastroenterol Hepatol. 2019;34:2206–18. 10.1111/jgh.14740 [DOI] [PubMed] [Google Scholar]
- 61.Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, et al. A positive feedback loop between RIP3 and JNK controls non‐alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062–74. 10.15252/emmm.201403856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanazawa J, Kakisaka K, Suzuki Y, Yonezawa T, Abe H, Wang T, et al. Excess fructose enhances oleatic cytotoxicity via reactive oxygen species production and causes necroptosis in hepatocytes. J Nutr Biochem. 2022;107:109052. 10.1016/j.jnutbio.2022.109052 [DOI] [PubMed] [Google Scholar]
- 63.Gautheron J, Vucur M, Schneider AT, Severi I, Roderburg C, Roy S, et al. The necroptosis-inducing kinase RIPK3 dampens adipose tissue inflammation and glucose intolerance. Nat Commun. 2016;7:11869. 10.1038/ncomms11869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roychowdhury S, McCullough RL, Sanz-Garcia C, Saikia P, Alkhouri N, Matloob A, et al. Receptor interacting protein 3 protects mice from high‐fat diet‐induced liver injury. Hepatology. 2016;64:1518–33. 10.1002/hep.28676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, et al. Tumour necrosis factor signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–24. 10.1136/gut.2005.071118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-κB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–17. 10.1111/j.1572-0241.2004.40009.x [DOI] [PubMed] [Google Scholar]
- 67.Roh YS, Seki E. Toll‐like receptors in alcoholic liver disease, non‐alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28:38–42. 10.1111/jgh.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inaba Y, Hashiuchi E, Watanabe H, Kimura K, Oshima Y, Tsuchiya K, et al. The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice. Nat Commun. 2023;14:167. 10.1038/s41467-023-35804-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Zhou L, Zhou Y, Wang L, Jiang W, Liu L, et al. Intermittent hypoxia aggravates non-alcoholic fatty liver disease via RIPK3-dependent necroptosis-modulated Nrf2/NFκB signaling pathway. Life Sci. 2021;285:119963. 10.1016/j.lfs.2021.119963 [DOI] [PubMed] [Google Scholar]
- 70.Briand F, Heymes C, Bonada L, Angles T, Charpentier J, Branchereau M, et al. A 3‐week nonalcoholic steatohepatitis mouse model shows elafibranor benefits on hepatic inflammation and cell death. Clin Transl Sci. 2020;13:529–38. 10.1111/cts.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majdi A, Aoudjehane L, Ratziu V, Islam T, Afonso MB, Conti F, et al. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J Hepatol. 2020;72:627–35. 10.1016/j.jhep.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 72.Park J, Rah SY, An HS, Lee JY, Roh GS, Ryter SW, et al. Metformin-induced TTP mediates communication between Kupffer cells and hepatocytes to alleviate hepatic steatosis by regulating lipophagy and necroptosis. Metabolism. 2023;141:155516. 10.1016/j.metabol.2023.155516 [DOI] [PubMed] [Google Scholar]
- 73.Ding HR, Tang ZT, Tang N, Zhu ZY, Liu HY, Pan CY, et al. Protective properties of FOXO1 inhibition in a murine model of non-alcoholic fatty liver disease are associated with attenuation of ER stress and necroptosis. Front Physiol. 2020;11:177. 10.3389/fphys.2020.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9. 10.1038/358167a0 [DOI] [PubMed] [Google Scholar]
- 75.de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 2018;26:146–61. 10.1038/s41418-018-0106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–22. 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 77.Schroder K, Tschopp J. The Inflammasomes. Cell. 2010;140:821–32. 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 78.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20. 10.1038/nri.2016.58 [DOI] [PubMed] [Google Scholar]
- 79.Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567–95. 10.1146/annurev-immunol-073119-095439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–57. 10.1038/s41577-019-0228-2 [DOI] [PubMed] [Google Scholar]
- 81.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 82.Mitsuyoshi H, Yasui K, Hara T, Taketani H, Ishiba H, Okajima A, et al. Hepatic nucleotide binding oligomerization domain‐like receptors pyrin domain‐containing 3 inflammasomes are associated with the histologic severity of non‐alcoholic fatty liver disease. Hepatol Res. 2017;47:1459–68. 10.1111/hepr.12883 [DOI] [PubMed] [Google Scholar]
- 83.Dixon LJ, Flask CA, Papouchado BG, Feldstein AE, Nagy LE. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS ONE. 2013;8:e56100. 10.1371/journal.pone.0056100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dixon LJ, Berk M, Thapaliya S, Papouchado BG, Feldstein AE. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab Investig. 2012;92:713–23. 10.1038/labinvest.2012.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mirea AM, Stienstra R, Kanneganti TD, Tack CJ, Chavakis T, Toonen EJM, et al. Mice deficient in the IL-1β activation genes Prtn3, Elane, and Casp1 are protected against the development of obesity-induced NAFLD. Inflammation. 2020;43:1054–64. 10.1007/s10753-020-01190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68:773–82. 10.1016/j.jhep.2017.11.040 [DOI] [PubMed] [Google Scholar]
- 87.Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med. 2014;92:1069–82. 10.1007/s00109-014-1170-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. 10.1002/hep.26592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu L, Hong W, Lu S, Li Y, Guan Y, Weng X, et al. The NLRP3 inflammasome in non-alcoholic fatty liver disease and steatohepatitis: therapeutic targets and treatment. Front Pharmacol. 2022;13:780496. 10.3389/fphar.2022.780496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–44. 10.1002/hep.24341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan J, Ou Z, Cai C, Li P, Gong J, Ruan XZ, et al. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 2018;332:111–20. 10.1016/j.cellimm.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 92.Xu L, Zhou J, Che J, Wang H, Yang W, Zhou W, et al. Mitochondrial DNA enables AIM2 inflammasome activation and hepatocyte pyroptosis in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2021;320:G1034–G1044. 10.1152/ajpgi.00431.2020 [DOI] [PubMed] [Google Scholar]
- 93.Chen Y, Ma K. NLRC4 inflammasome activation regulated by TNF-α promotes inflammatory responses in nonalcoholic fatty liver disease. Biochem Biophys Res Commun. 2019;511:524–30. 10.1016/j.bbrc.2019.02.099 [DOI] [PubMed] [Google Scholar]
- 94.Drummer C 4th, Saaoud F, Jhala NC, Cueto R, Sun Y, et al. Caspase-11 promotes high-fat diet-induced NAFLD by increasing glycolysis, OXPHOS, and pyroptosis in macrophages. Front Immunol. 2023;14:1113883. 10.3389/fimmu.2023.1113883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Y, Zhao H, Lu J, Lin K, Ni J, Wu G, et al. Caspase-11–mediated hepatocytic pyroptosis promotes the progression of nonalcoholic steatohepatitis. Cell Mol Gastroenterol Hepatol. 2021;12:653–64. 10.1016/j.jcmgh.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y, Wang DW, Wang D, Duan BH, Kuang HY. Exenatide attenuates non-alcoholic steatohepatitis by inhibiting the pyroptosis signaling pathway. Front Endocrinol. 2021;12:663039. 10.3389/fendo.2021.663039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hao YY, Cui WW, Gao HL, Wang MY, Liu Y, Li CR, et al. Jinlida granules ameliorate the high-fat-diet induced liver injury in mice by antagonising hepatocytes pyroptosis. Pharm Biol. 2022;60:274–81. 10.1080/13880209.2022.2029501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruan S, Han C, Sheng Y, Wang J, Zhou X, Guan Q, et al. Antcin A alleviates pyroptosis and inflammatory response in Kupffer cells of non-alcoholic fatty liver disease by targeting NLRP3. Int Immunopharmacol. 2021;100:108126. 10.1016/j.intimp.2021.108126 [DOI] [PubMed] [Google Scholar]
- 99.Oh S, Son M, Byun KA, Jang JT, Choi CH, Son KH, et al. Attenuating effects of dieckol on high-fat diet-induced nonalcoholic fatty liver disease by decreasing the NLRP3 inflammasome and pyroptosis. Mar Drugs. 2021;19:318. 10.3390/md19060318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037–46. 10.1016/j.jhep.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, Shang X, Jin S, Ma Z, Wang H, Ao N, et al. Vitamin D ameliorates high-fat-diet-induced hepatic injury via inhibiting pyroptosis and alters gut microbiota in rats. Arch Biochem Biophys. 2021;705:108894. 10.1016/j.abb.2021.108894 [DOI] [PubMed] [Google Scholar]
- 102.Yong Z, Ruiqi W, Hongji Y, Ning M, Chenzuo J, Yu Z, et al. Mangiferin ameliorates HFD-induced NAFLD through regulation of the AMPK and NLRP3 inflammasome signal pathways. J Immunol Res. 2021;2021:4084566. 10.1155/2021/4084566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018;9:946. 10.1038/s41419-018-1004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shen T, Lei T, Chen L, Zhu BB, Xu BL, Zhang CP, et al. Gardenoside hinders caspase-1-mediated hepatocyte pyroptosis through the CTCF/DPP4 signaling pathway. Front Physiol. 2021;12:669202. 10.3389/fphys.2021.669202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhong H, Liu M, Ji Y, Ma M, Chen K, Liang T, et al. Genipin reverses HFD-induced liver damage and inhibits UCP2-mediated pyroptosis in mice. Cell Physiol Biochem. 2018;49:1885–97. 10.1159/000493651 [DOI] [PubMed] [Google Scholar]
- 106.Chen T, Meng Y, Zhou Z, Li H, Wan L, Kang A, et al. GAS5 protects against nonalcoholic fatty liver disease via miR-28a-5p/MARCH7/NLRP3 axis-mediated pyroptosis. Cell Death Differ. 2023;30:1829–48. 10.1038/s41418-023-01183-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang X, Li Y, Fu D, You T, Wu S, Xin J, et al. Caveolin-1 ameliorates acetaminophen-aggravated inflammatory damage and lipid deposition in non-alcoholic fatty liver disease via the ROS/TXNIP/NLRP3 pathway. Int Immunopharmacol. 2023;114:109558. 10.1016/j.intimp.2022.109558 [DOI] [PubMed] [Google Scholar]
- 108.Mai W, Xu Y, Xu J, Zhao D, Ye L, Yu G, et al. Berberine inhibits nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP axis. Front Pharmacol. 2020;11:185. 10.3389/fphar.2020.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ding C, Zhao Y, Shi X, Zhang N, Zu G, Li Z, et al. New insights into salvianolic acid A action: regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci Rep. 2016;6:28734. 10.1038/srep28734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu X, Hao M, Liu Y, Ma X, Lin W, Xu Q, et al. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur J Pharmacol. 2019;864:172715. 10.1016/j.ejphar.2019.172715 [DOI] [PubMed] [Google Scholar]
- 111.Shi H, Zhang Y, Xing J, Liu L, Qiao F, Li J, et al. Baicalin attenuates hepatic injury in non-alcoholic steatohepatitis cell model by suppressing inflammasome-dependent GSDMD-mediated cell pyroptosis. Int Immunopharmacol. 2020;81:106195. 10.1016/j.intimp.2020.106195 [DOI] [PubMed] [Google Scholar]
- 112.Shi H, Qiao F, Lu W, Huang K, Wen Y, Ye L, et al. Baicalin improved hepatic injury of NASH by regulating NRF2/HO-1/NRLP3 pathway. Eur J Pharmacol. 2022;934:175270. 10.1016/j.ejphar.2022.175270 [DOI] [PubMed] [Google Scholar]
- 113.Biao Y, Chen J, Liu C, Wang R, Han X, Li L, et al. Protective effect of Danshen zexie decoction against non-alcoholic fatty liver disease through inhibition of ROS/NLRP3/IL-1β pathway by Nrf2 signaling activation. Front Pharmacol. 2022;13:877924. 10.3389/fphar.2022.877924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yin K, Zhou X, Jiang W, Wang L, Dai Z, Tang B. Jiangzhi Ligan Decoction inhibits GSDMD-mediated canonical/noncanonical pyroptosis pathways and alleviates high-fat diet-induced nonalcoholic fatty liver disease. Dis Markers. 2021;2021:9963534. 10.1155/2021/9963534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lo M, Wang YZ, Gout PW. The xc− cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. 10.1002/jcp.21366 [DOI] [PubMed] [Google Scholar]
- 117.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol. 2019;15:1137–47. 10.1038/s41589-019-0408-1 [DOI] [PubMed] [Google Scholar]
- 119.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62‐Keap1‐NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84. 10.1002/hep.28251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–40. 10.1038/sj.leu.2402238 [DOI] [PubMed] [Google Scholar]
- 121.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. 10.1038/nature14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. 10.1038/s41586-019-1707-0 [DOI] [PubMed] [Google Scholar]
- 124.Wang S, Liu Z, Geng J, Li L, Feng X. An overview of ferroptosis in non-alcoholic fatty liver disease. Biomed Pharmacother. 2022;153:113374. 10.1016/j.biopha.2022.113374 [DOI] [PubMed] [Google Scholar]
- 125.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. 10.1021/acscentsci.9b01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Videla LA, Valenzuela R. Perspectives in liver redox imbalance: toxicological and pharmacological aspects underlying iron overloading, nonalcoholic fatty liver disease, and thyroid hormone action. BioFactors. 2021;48:400–15. 10.1002/biof.1797 [DOI] [PubMed] [Google Scholar]
- 127.Folgueras AR, Freitas-Rodríguez S, Ramsay AJ, Garabaya C, Rodríguez F, Velasco G, et al. Matriptase-2 deficiency protects from obesity by modulating iron homeostasis. Nat Commun. 2018;9:1350. 10.1038/s41467-018-03853-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rametta R, Fracanzani AL, Fargion S, Dongiovanni P. Dysmetabolic hyperferritinemia and dysmetabolic iron overload syndrome (DIOS): two related conditions or different entities? Curr Pharm Des. 2020;26:1025–35. 10.2174/1381612826666200131103018 [DOI] [PubMed] [Google Scholar]
- 129.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. 10.1002/hep.24706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meli R, Mattace Raso G, Irace C, Simeoli R, Di Pascale A, Paciello O, et al. High fat diet induces liver steatosis and early dysregulation of iron metabolism in rats. PLoS ONE. 2013;8:e66570. 10.1371/journal.pone.0066570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29:467–80. 10.1038/s41418-022-00941-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, et al. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J Hepatol. 2001;35:568–74. 10.1016/S0168-8278(01)00192-1 [DOI] [PubMed] [Google Scholar]
- 133.Qi J, Kim JW, Zhou Z, Lim CW, Kim B. Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation–mediated cell death in mice. Am J Pathol. 2020;190:68–81. 10.1016/j.ajpath.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 134.Wei S, Qiu T, Wang N, Yao X, Jiang L, Jia X, et al. Ferroptosis mediated by the interaction between Mfn2 and IREα promotes arsenic-induced nonalcoholic steatohepatitis. Environ Res. 2020;188:109824. 10.1016/j.envres.2020.109824 [DOI] [PubMed] [Google Scholar]
- 135.Tsurusaki S, Tsuchiya Y, Koumura T, Nakasone M, Sakamoto T, Matsuoka M, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10:449. 10.1038/s41419-019-1678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–79. 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, et al. Targeting ferroptosis alleviates methionine‐choline deficient (MCD)‐diet induced NASH by suppressing liver lipotoxicity. Liver Int. 2020;40:1378–94. 10.1111/liv.14428 [DOI] [PubMed] [Google Scholar]
- 138.Zhang J, Xie H, Yao J, Jin W, Pan H, Pan Z, et al. TRIM59 promotes steatosis and ferroptosis in non-alcoholic fatty liver disease via enhancing GPX4 ubiquitination. Hum Cell. 2023;36:209–22. 10.1007/s13577-022-00820-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu L, He M, Liu S, Dou X, Li L, Gu N, et al. Fluorescent egg white-based carbon dots as a high-sensitivity iron chelator for the therapy of nonalcoholic fatty liver disease by iron overload in zebrafish. ACS Appl Mater Interfaces. 2021;13:54677–89. 10.1021/acsami.1c14674 [DOI] [PubMed] [Google Scholar]
- 140.Wang Y, Bo J, Zhao Z, Han Y, Zhang Q, Liu L. Depletion of Igfbp7 alleviates zebrafish NAFLD progression through inhibiting hepatic ferroptosis. Life Sci. 2023;332:122086. 10.1016/j.lfs.2023.122086 [DOI] [PubMed] [Google Scholar]
- 141.Park JB, Ko K, Baek YH, Kwon WY, Suh S, Han SH, et al. Pharmacological prevention of ectopic erythrophagocytosis by cilostazol mitigates ferroptosis in NASH. Int J Mol Sci. 2023;24:12862. 10.3390/ijms241612862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiang T, Xiao Y, Zhou J, Luo Z, Yu L, Liao Q, et al. Arbutin alleviates fatty liver by inhibiting ferroptosis via FTO/SLC7A11 pathway. Redox Biol. 2023;68:102963. 10.1016/j.redox.2023.102963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi J, Choi H, Chung J. Icariin supplementation suppresses the markers of ferroptosis and attenuates the progression of nonalcoholic steatohepatitis in mice fed a methionine choline-deficient diet. Int J Mol Sci. 2023;24:12510. 10.3390/ijms241512510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He F, Zhang P, Liu J, Wang R, Kaufman RJ, Yaden BC, et al. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J Hepatol. 2023;79:362–77. 10.1016/j.jhep.2023.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang M, Xia L, Song J, Hu H, Zang N, Yang J, et al. Puerarin ameliorates metabolic dysfunction-associated fatty liver disease by inhibiting ferroptosis and inflammation. Lipids Health Dis. 2023;22:202. 10.1186/s12944-023-01969-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yuan X, Li L, Zhang Y, Ai R, Li D, Dou Y, et al. Heme oxygenase 1 alleviates nonalcoholic steatohepatitis by suppressing hepatic ferroptosis. Lipids Health Dis. 2023;22:99. 10.1186/s12944-023-01855-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saha M, Das S, Manna K, Saha KD. Melatonin targets ferroptosis through bimodal alteration of redox environment and cellular pathways in NAFLD model. Biosci Rep. 2023;43:BSR20230128. 10.1042/BSR20230128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao G, Xie Z, Li EW, Yuan Y, Fu Y, Wang P, et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med. 2021;75:540–52. 10.1007/s11418-021-01491-4 [DOI] [PubMed] [Google Scholar]
- 149.Yang Y, Chen J, Gao Q, Shan X, Wang J, Lv Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology. 2020;445:152599. 10.1016/j.tox.2020.152599 [DOI] [PubMed] [Google Scholar]
- 150.Liu B, Yi W, Mao X, Yang L, Rao C. Enoyl coenzyme A hydratase 1 alleviates nonalcoholic steatohepatitis in mice by suppressing hepatic ferroptosis. Am J Physiol Endocrinol Metab. 2021;320:E925–E937. 10.1152/ajpendo.00614.2020 [DOI] [PubMed] [Google Scholar]
- 151.Xing G, Meng L, Cao S, Liu S, Wu J, Li Q, et al. PPARα alleviates iron overload‐induced ferroptosis in mouse liver. EMBO Rep. 2022;23:e52280. 10.15252/embr.202052280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shu YY, Gao WK, Chu HK, Yang L, Pan XL, Ye J. Attenuation by time-restricted feeding of high-fat and high-fructose diet-induced NASH in mice is related to Per2 and ferroptosis. Oxid Med Cell Longev. 2022;2022:8063897. 10.1155/2022/8063897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu Z, Zhang Y, Huang X, Can L, Zhao X, Wang Y, et al. Thymosin beta 4 alleviates non-alcoholic fatty liver by inhibiting ferroptosis via up-regulation of GPX4. Eur J Pharmacol. 2021;908:174351. 10.1016/j.ejphar.2021.174351 [DOI] [PubMed] [Google Scholar]
- 154.Wang X, Ma B, Wen X, You H, Sheng C, Bu L, et al. Bone morphogenetic protein 4 alleviates nonalcoholic steatohepatitis by inhibiting hepatic ferroptosis. Cell Death Discov. 2022;8:234. 10.1038/s41420-022-01011-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiang JJ, Zhang GF, Zheng JY, Sun JH, Ding SB. Targeting Mitochondrial ROS-Mediated Ferroptosis by Quercetin Alleviates High-Fat Diet-Induced Hepatic Lipotoxicity. Front Pharmacol. 2022;13:876550. 10.3389/fphar.2022.876550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang Z, Sun H, Miao J, Sheng Q, Xu J, Gao Z, et al. The natural flavone acacetin protects against high-fat diet-induced lipid accumulation in the liver via the endoplasmic reticulum stress/ferroptosis pathway. Biochem Biophys Res Commun. 2023;640:183–91. 10.1016/j.bbrc.2022.12.014 [DOI] [PubMed] [Google Scholar]
- 157.Guo T, Yan W, Cui X, Liu N, Wei X, Sun Y, et al. Liraglutide attenuates type 2 diabetes mellitus-associated non-alcoholic fatty liver disease by activating AMPK/ACC signaling and inhibiting ferroptosis. Mol Med. 2023;29:132. 10.1186/s10020-023-00721-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao M, Jin Z, Xia C, Chen S, Zeng L, Qin S, et al. Inhibition of free heme-catalyzed Fenton-like reaction prevents non-alcoholic fatty liver disease by hepatocyte-targeted hydrogen delivery. Biomaterials. 2023;301:122230. 10.1016/j.biomaterials.2023.122230 [DOI] [PubMed] [Google Scholar]
- 159.Gaul S, Leszczynska A, Alegre F, Kaufmann B, Johnson CD, Adams LA, et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J Hepatol. 2021;74:156–67. 10.1016/j.jhep.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wiering L, Subramanian P, Hammerich L. Hepatic stellate cells: dictating outcome in nonalcoholic fatty liver disease. Cell Mol Gastroenterol Hepatol. 2023;15:1277–92. 10.1016/j.jcmgh.2023.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kanda T, Matsuoka S, Yamazaki M, Shibata T, Nirei K, Takahashi H, et al. Apoptosis and non-alcoholic fatty liver diseases. World J Gastroenterol. 2018;24:2661–72. 10.3748/wjg.v24.i25.2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo R, Jia X, Ding Z, Wang G, Jiang M, Li B, et al. Loss of MLKL ameliorates liver fibrosis by inhibiting hepatocyte necroptosis and hepatic stellate cell activation. Theranostics. 2022;12:5220–36. 10.7150/thno.71400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cho SS, Yang JH, Lee JH, Baek JS, Ku SK, Cho IJ, et al. Ferroptosis contribute to hepatic stellate cell activation and liver fibrogenesis. Free Radic Biol Med. 2022;193:620–37. 10.1016/j.freeradbiomed.2022.11.011 [DOI] [PubMed] [Google Scholar]
- 164.Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16:1482–505. 10.1080/15548627.2019.1687985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A, et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083–103. 10.1080/15548627.2018.1503146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ni K, Meng L. Mechanism of PANoptosis in metabolic dysfunction-associated steatotic liver disease. Clin Res Hepatol Gastroenterol. 2024;48:102381. 10.1016/j.clinre.2024.102381 [DOI] [PubMed] [Google Scholar]
- 167.Ma Z, Xie K, Xue X, Li J, Yang Y, Wu J, et al. Si-Wu-Tang attenuates hepatocyte PANoptosis and M1 polarization of macrophages in non-alcoholic fatty liver disease by influencing the intercellular transfer of mtDNA. J Ethnopharmacol. 2024;328:118057. 10.1016/j.jep.2024.118057 [DOI] [PubMed] [Google Scholar]
- 168.Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti TD. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 2021;597:415–9. 10.1038/s41586-021-03875-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–14.e4. 10.1016/j.chembiol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, et al. Ghrelin reduces TNF-α-induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metab. 2019;104:21–37. [DOI] [PubMed] [Google Scholar]
- 171.Liu M, Lu J, Hu J, Chen Y, Deng X, Wang J, et al. Sodium sulfite triggered hepatic apoptosis, necroptosis, and pyroptosis by inducing mitochondrial damage in mice and AML-12 cells. J Hazard Mater. 2024;467:133719. 10.1016/j.jhazmat.2024.133719 [DOI] [PubMed] [Google Scholar]
- 172.Briand F, Heymes C, Bonada L, Angles T, Charpentier J, Branchereau M, et al. A 3-week nonalcoholic steatohepatitis mouse model shows elafibranor benefits on hepatic inflammation and cell death. Clin Transl Sci. 2020;13:529–38. 10.1111/cts.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tong J, Lan XT, Zhang Z, Liu Y, Sun DY, Wang XJ, et al. Ferroptosis inhibitor liproxstatin-1 alleviates metabolic dysfunction-associated fatty liver disease in mice: potential involvement of PANoptosis. Acta Pharmacol Sin. 2023;44:1014–28. 10.1038/s41401-022-01010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tian Z, Jiang S, Zhou J, Zhang W. Copper homeostasis and cuproptosis in mitochondria. Life Sci. 2023;334:122223. 10.1016/j.lfs.2023.122223 [DOI] [PubMed] [Google Scholar]