Abstract
Background:
Intranasal meningoencephaloceles are rarely encountered in pediatric neurosurgery. The symptoms and clinical features may mimic those of nasal polyps or dermoid cysts. Transethmoidal meningoencephalocele is a rare congenital meningoencephalocele of the anterior skull base with diverse clinical presentation. The appropriate surgical intervention is chosen according to the meningoencephalocele type and location. Radiological examinations such as computed tomography and magnetic resonance imaging are helpful for the differential diagnosis of the encephalocele sac and localization of the cranial bone defect.
Case Description:
We are reporting a case of basal meningoencephalocele of the transethmoidal type, which was discovered in a 20-day-old boy presenting with cerebrospinal fluid rhinorrhea, respiratory distress, difficulty in feeding, and meningitis. The preoperative images showed a large herniated intranasal sac with bony discontinuity of the cribriform plate; however, three discrete defects of the cribriform plate with their related discrete herniated sacs were identified intraoperatively. Two staged surgeries were performed in succession: transcranial to separate the sacs from the cranial cavity and seal the anterior fossa floor, followed by transnasal to remove the remnant of the intranasal sacs. Patient symptoms and signs markedly improved after the surgeries.
Conclusion:
We highlight the need for urgent intervention at a very young age if the clinical presentation mandates, and also the importance of meticulous intraoperative identification of all bony and dural defects that might be missed in preoperative images to ensure complete repair and prevent recurrence.
Keywords: Cribriform defect, Meningoencephalocele, Neonate, Transethmoidal

INTRODUCTION
An encephalocele is defined as external herniation of intracranial contents through a skull defect. The term meningocele is used when the herniated sac includes meninges only; however, if the herniated sac contains meninges and part of the brain tissues, the term meningoencephalocele is used instead. Meningoencephalocele occurs with an estimated incidence of one in every 35,000 live births.[5]
Encephalocele can be classified according to the anatomical location of the skull defect. Nearly 75% of encephaloceles are located in the occipital region, whereas 13–15% are located in the frontal ethmoidal region, and 10–12% are located in the basal sphenoidal region.[7] Basal encephaloceles which occur in relation to the cribriform plate and sphenoid sinus may be further classified into transethmoidal (through the cribriform plate), sphenoethmoidal (through the ethmoidal and sphenoidal paranasal sinuses), spheno-orbital (through the superior orbital fissure), and trans-sphenoidal (through the sellar floor).[13] Surgery is the definitive treatment for almost all encephaloceles, with the appropriate surgical approach being selected depending on the location of the defect.[7]
This paper aims to discuss the operative management for a case of a new-born boy who is diagnosed with transethmoidal meningoencephaloceles herniating through three discrete bony defects in the cribriform plate.
CASE PRESENTATION
We report a male baby who was delivered at full term by urgent cesarean section (after the failure of induction of normal labor) due to the development of maternal preeclampsia associated with fetal distress. At birth, the baby was breathing normally, his body weight was 3.1 kg, head circumference was 36 cm, and there were no obvious dysmorphic facial features. Prenatal maternal history was negative for episodes of fever or suspected infections, with all prenatal maternal ultrasonography examinations reported as being normal. Family history was negative for any congenital cranial or facial anomalies.
During the 1st week of life, the mother noticed abnormal noisy nasal breathing and attacks of respiratory distress during bottle feeding. Attempts of nasal cleaning at home yielded an intermittent discharge of watery fluid from the nose. The baby was then seen by an otorhinolaryngologist at the age of 10 days, and imaging was requested.
However, at the age of 17 days (before the requested imaging was performed), the baby was admitted to a nearby hospital with the diagnosis of clinical sepsis and suspected meningitis. On admission, he was febrile, tachypneic, in severe respiratory distress, and with continuous yellowish watery nasal discharge. The rest of the physical examination was unremarkable, with no detectable musculoskeletal deformity or neurological deficit. Chest X-ray showed advanced pneumonia. Echocardiogram was normal. Lumbar puncture was done as part of the sepsis work, and analysis of the collected cerebrospinal fluid (CSF) showed a high total leucocytic count with predominant neutrophils. All body cultures, including those from the obtained CSF and the nasal discharge, did not grow any organism. The patient was started on empirical antibiotics (vancomycin and ampicillin) for treatment of neonatal meningitis according to the hospital’s local protocol for a total period of 14 days until repeated CSF analysis and C-reactive protein serum levels were within normal. Computed tomography (CT) followed by magnetic resonance imaging (MRI) of the brain done during the hospital admission showed discontinuity of the cribriform plate on the right side, which seemed to be two medium-sized defects. A soft-tissue mass of low Hounsfield density representing downward intranasal herniation of the intracranial brain tissue was filling the right nasal cavity associated with nasal septal deviation to the left side, causing narrowing of the left nasal cavity. The findings were suggestive of a nasal transethmoidal encephalocele [Figure 1].
Figure 1:
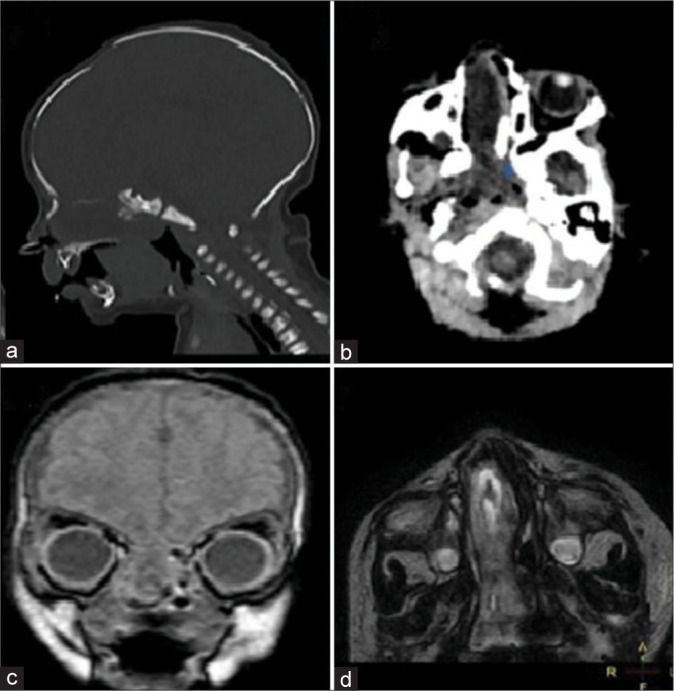
The initial computed tomography (CT) and magnetic resonance imaging (MRI) images used for diagnosis at 21 days of age. (a) CT brain sagittal cuts (bone window) at the level of the right cribriform plate showing two bony defects with a very thin bone plate in between. (b) CT brain axial cuts showing right nasal cavity soft-tissue mass deviating the nasal septum toward the left side. (c) MRI brain coronal images show the continuity of the herniated nasal soft-tissue mass with the intracranial brain tissue through the anterior cranial fossa defect. (d) MRI brain axial images show the herniated soft-tissue mass within the right nasal cavity.
The patient was then referred to another hospital where a pediatric neurosurgeon was available. The surgical intervention was planned to be carried out in two staged surgeries, a transcranial followed by a transnasal one. The transcranial intervention was performed first at the age of 38 days to separate the endonasal mass from its intracranial connection and then seal the defect in the dura and the cribriform lamina. With the patient in a supine position, a bicoronal skin flab was reflected anteriorly with preservation and preparation of a vascularized pericranium flap. A right frontal craniotomy was then performed, followed by extradural subfrontal dissection along the anterior cranial fossa floor to locate the neck of the herniated sac of the encephalocele. Thorough and meticulous dissection and inspection under microscopic magnification identified three discrete encephaloceles herniating through three different and separate cribriform plate bony defects of variable sizes. We assume that the crowdedness of these three herniated sacs in the small neonatal nasal cavity made it difficult to distinguish them separately in the preoperative radiological images. The neck of these three sacs was circumferentially dissected before being transected using bipolar coagulation at their connection with the basal dura of the frontal lobe. No other dural defects or bony could be identified. After delineation of the edges of the three bony defects in the cribriform plate, a four-layer basal dura reconstruction was done using synthetic nonabsorbable dural substitute material augmented with bio glue, with the last most superficial layer being the reflected pericranium flap. The bone flap was then replaced to close the craniotomy defect, and the scalp was closed in the standard fashion after placing a subgaleal vacuum drain, which was kept for 3 days.
The postoperative course was uneventful, where the patient was admitted to the pediatric intensive care unit for the first few postoperative days for close observation. Postoperative antibiotics were continued for about 2 weeks. Postoperative MRI showed adequate reconstruction of the anterior cranial fossa floor with complete separation of the intracranial and nasal cavities [Figure 2]. In the second stage, a transnasal surgery was carried out successfully by the otorhinolaryngologist service to remove the remaining intranasal soft-tissue mass [Figure 3]. After both surgeries, the patient maintained good oxygen saturation on room air without supplementary oxygen and started bottle-feeding successfully.
Figure 2:
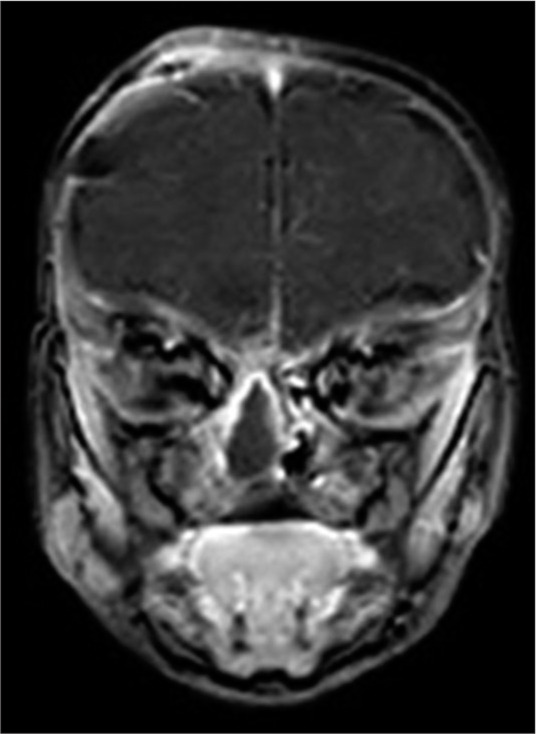
Postoperative magnetic resonance imaging of the brain with coronal images done after the transcranial surgery showing a layered obliteration and separation of the anterior cranial fossa floor from the nasal cavity beneath. The remnant (to be removed later) soft-tissue sac can still be appreciated in the nasal cavity.
Figure 3:
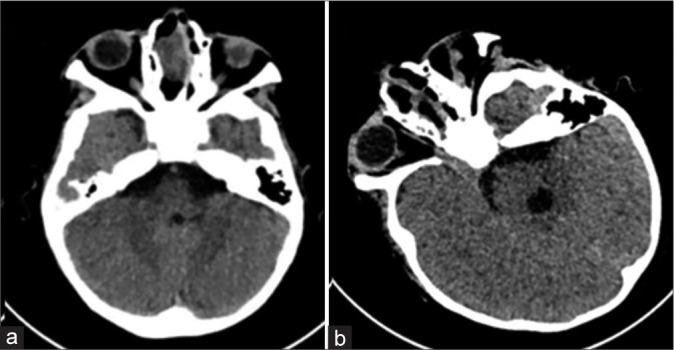
Pre- and post-operative computed tomography of the brain with axial images done before and after the transnasal surgery. (a) The herniated sac remnant can still be visualized occupying the right nasal cavity before surgery. (b) No more brain tissue can be seen within the right nasal cavity after surgery.
DISCUSSION
Manengioencephalocele is a type of open neural tube defect (NTD) characterized by a herniation of brain tissue that is covered by dura matter through a defect in the skull.[1] Basal meningoencephalocele patients, especially transethmoidal ones, usually present with upper airway obstruction;[8] in our case, nasal obstruction and difficulty feeding were the two chief complaints. Diagnosis usually mandates obtaining both CT and MRI images which provide complementary radiological information about skull base bony defects and herniated soft-tissue content.[4] In our case, failure to preoperatively identify the three bony defects may be attributed to the very thin bony lamella in the neonates.
Transnasal tissue biopsy should be avoided before surgery as it can lead to CSF leak, meningitis, epistaxis, etc.[5] Treatment of meningoencephalocele is almost exclusively surgical.[7] Immediate repair for encephalocele is indicated when there is active CSF leakage, as in our case, due to the risk of developing meningitis.[8] More elective repairs can be carried out in a more delayed fashion at relatively older ages for cases with mild respiratory or feeding difficulties.[10] If left untreated, unrepaired encephaloceles have been attributed to the development of developmental delay, mental and growth retardation, microcephaly, hydrocephalus, ataxia, vision problems, and seizures.[7] In our case, early surgical intervention was necessitated due to the development of CSF leak, meningitis, marked respiratory distress, and alarming feeding difficulties with impending failure to thrive.
The presence of multiple NTD is not a rare diagnosis; 57 cases have been cited in the literature[3], with the three major series (total of 26 cases) reported from India.[2,3,9] Among these 57 cases, in 23 cases, an encephalocele has been reported either in combination with other spinal NTD (15 cases) or as part of multiple encephaloceles (eight cases).[3]
At present, two main hypotheses can explain the embryological development of multiple NTDs. The multisite closure theory[11,12,15] where it is theorized that there are 3–5 sites of initiation of closures, whereas closure below S2 occurs by secondary neurulation, and the interrupted zipper closure theory[3,9] where it is theorized that the neural tube closes starting in the mid-cervical region and progresses toward the caudal and cranial neuropores in a zipper-like fashion, but the process can restarts independently after a point of interruption under the stimulus of the adjacent notochordal segment and the interplay of Sonic hedgehog and its antagonist, Wnt.
Although there are different techniques reported in the literature about approaching basal encephaloceles,[4,14] with the endoscopic approach mostly reserved for children older than 1 year due to the small working transnasal area below this age,[1,6] we opted to follow the commonly implemented surgical approach consisting of a transcranial route through a craniotomy which proved of value to allowing optimal visualization, identification, and surgical handling of the multiple bony defects and herniated sacs.
The prognosis for basal encephalocele surgery is satisfactory in general,[5] with an average low mortality rate of <3%.[14] Long-term neurocognitive development evaluations have been reported to be favorable, especially with anterior encephaloceles,[4] even after excising the herniated brain tissue, which is usually nonfunctional.[13]
CONCLUSION
Transethmoidal encephaloceles management in neonates is faced with the anatomical and physiological limitations of this vulnerable age group; nevertheless, expedient intervention is sometimes mandated when complications such as CSF leak with meningitis or marked respiratory and feeding difficulties develop. Meticulous intraoperative inspection is inevitable in neonates to confirm complete repair of all dural and bony defects, which might not be easily identified in preoperative radiological studies.
Footnotes
How to cite this article: Talahma II, Zawahra AK, Almakhtoob FJ, Shawar FI, Sharabati KM, Dwaik RF, et al. Large transethmoidal meningoencephalocele in a neonate involving three discrete defects in lamina cribriform: A case report. Surg Neurol Int. 2024;15:275. doi: 10.25259/SNI_248_2024
Contributor Information
Imad Ibrahim Talahma, Email: imad.talahma@gmail.com.
Aya Khader Zawahra, Email: ayazawahra88@gmail.com.
Falasteen Jameel Almakhtoob, Email: falasteenalmakhtoob@gmail.com.
Fatima Iyad Shawar, Email: fatimashawer1@gmail.com.
Khulood Marwan Sharabati, Email: khuloodsharabati@gmail.com.
Raghad Faisal Dwaik, Email: raghad.dweik.14@gmail.com.
Marwa K. Abdelshafy, Email: mkabdelshafy@gmail.com.
Ahmed Adel Farag, Email: adelns@outlook.com.
Ahmad M. AbuAyyash, Email: dr.abuayashahmad@gmail.com.
Waeel O. Hamouda, Email: wohamouda@outlook.com.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Abdel-Aziz M, El-Bosraty H, Qotb M, El-Hamamsy M, El-Sonbaty M, Abdel-Badie H, et al. Nasal encephalocele: Endoscopic excision with anesthetic consideration. Int J Pediatr Otorhinolaryngol. 2010;74:869–73. doi: 10.1016/j.ijporl.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad FU, Dwarakanath S, Sharma BS, Mahapatra AK. Multiple neural tube defects: A clinical series of seven cases and their embryological basis. Pediatr Neurosurg. 2008;44:280–7. doi: 10.1159/000131676. [DOI] [PubMed] [Google Scholar]
- 3.Deora H, Srinivas D, Shukla D, Devi BI, Mishra A, Beniwal M, et al. Multiple-site neural tube defects: Embryogenesis with complete review of existing literature. Neurosurg Focus. 2019;47:E18. doi: 10.3171/2019.8.FOCUS19437. [DOI] [PubMed] [Google Scholar]
- 4.Franco D, Alonso N, Ruas R, da Silva Freitas R, Franco T. Transsphenoidal meningoencephalocele associated with cleft lip and palate: Challenges for diagnosis and surgical treatment. Childs Nerv Syst. 2009;25:1455–8. doi: 10.1007/s00381-009-0918-3. [DOI] [PubMed] [Google Scholar]
- 5.Jabre A, Tabaddor R, Samaraweera R. Transsphenoidal meningoencephalocele in adults. Surg Neurol. 2000;54:183–8. doi: 10.1016/s0090-3019(00)00270-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee JA, Byun YJ, Nguyen SA, Schlosser RJ, Gudis DA. Endonasal endoscopic surgery for pediatric anterior cranial fossa encephaloceles: A systematic review. Int J Pediatr Otorhinolaryngol. 2020;132:109919. doi: 10.1016/j.ijporl.2020.109919. [DOI] [PubMed] [Google Scholar]
- 7.Macfarlane R, Rutka JT, Armstrong D, Phillips J, Posnick J, Forte V, et al. Encephaloceles of the anterior cranial fossa. Pediatr Neurosurg. 1995;23:148–58. doi: 10.1159/000120952. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan C, Rath GP, Dash HH, Bithal PK. Perioperative management of children with encephalocele: An institutional experience. J Neurosurg Anesthesiol. 2011;23:352–6. doi: 10.1097/ANA.0b013e31821f93dc. [DOI] [PubMed] [Google Scholar]
- 9.Mahalik SK, Vaze D, Kanojia RP, Narasimhan KL, Rao KL. Multiple neural tube defects may not be very rare. Childs Nerv Syst. 2013;29:609–19. doi: 10.1007/s00381-012-1976-5. [DOI] [PubMed] [Google Scholar]
- 10.Naidich TP, Altman NR, Braffman BH, McLone DG, Zimmerman RA. Cephaloceles and related malformations. AJNR Am J Neuroradiol. 1992;13:655–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Nakatsu T, Uwabe C, Shiota K. Neural tube closure in humans initiates at multiple sites: Evidence from human embryos and implications for the pathogenesis of neural tube defects. Anat Embryol (Berl) 2000;201:455–66. doi: 10.1007/s004290050332. [DOI] [PubMed] [Google Scholar]
- 12.O’Rahilly R, Müller F. The two sites of fusion of the neural folds and the two neuropores in the human embryo. Teratology. 2002;65:162–70. doi: 10.1002/tera.10007. [DOI] [PubMed] [Google Scholar]
- 13.Tirumandas M, Sharma A, Gbenimacho I, Shoja MM, Tubbs RS, Oakes WJ, et al. Nasal encephaloceles: A review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739–44. doi: 10.1007/s00381-012-1998-z. [DOI] [PubMed] [Google Scholar]
- 14.Turgut M, Özcan OE, Benli K, Özgen T, Gürçay Ö, Sağlam S, et al. Congenital nasal encephalocele: A review of 35 cases. J Cranio Maxillofacial Surg. 1995;23:1–5. doi: 10.1016/s1010-5182(05)80246-x. [DOI] [PubMed] [Google Scholar]
- 15.Van Allen MI, Kalousek DK, Chernoff GF, Juriloff D, Harris M, McGillivray BC, et al. Evidence for multi-site closure of the neural tube in humans. Am J Med Genet. 1993;47:723–43. doi: 10.1002/ajmg.1320470528. [DOI] [PubMed] [Google Scholar]


