Abstract
Background:
The objective was to study the effectiveness and diagnostic outcome of frame-based stereotactic brain biopsy (STB) done for contrast non-enhancing lesions using indirect evidence of target selection observed in a plain computed tomography (CT) scan of the head.
Methods:
Data of patients with contrast non-enhancing brain lesions who underwent STB are collected retrospectively from NIMHANS Bangalore, hospital neurosurgery database from January 2021 to March 2023. Those cases subjected to plain CT scans after fixing the stereotactic frame to the head were included in the study. A final histopathological report analysis of these cases was done to assess the diagnostic accuracy.
Results:
A total of 27 such cases were biopsied. The mean age of subjects was 44.04 ± 17.812 years. Most subjects were in the age group 31–40 years (29.6%). About 55.6% were male and 44.4% were female. The most common site of biopsy was the frontal lobe. The most common indirect evidence on CT was perilesional edema at 33.3% and periventricular location at 33.3%, followed by intralesional calcification at 11.1%. Our diagnostic accuracy was 92.59%. The asymptomatic hemorrhage rate was 2%, and an increase in perilesional edema was seen in 2% of cases.
Conclusion:
Indirect targeting is a safe and intuitive method for biopsy of contrast non-enhancing lesions. Due consideration is to be given to various findings visible in non-contrast CT scans of the head as indirect evidence of target selection while performing frame-based STB of contrast non-enhancing lesions. This method will also be helpful in resource-limited centers, especially in low-income countries.
Keywords: Computed tomography scanning, Frameless stereo-tactic brain biopsy, Non-enhancing lesions

INTRODUCTION
Stereotactic brain biopsy (STB) is an invasive but safe diagnostic procedure performed to establish tissue/molecular diagnosis in various spectrums of diseases of the brain. Despite rapid advancement in brain imaging techniques, conventional histopathological, immunohistochemical, and molecular analysis remains the gold standard.[1,5] The procedure aids in obtaining adequate brain tissue representative of the lesion and provides ample diagnostic information for neuro-oncological treatment. At present, frame-based and frame-less STB techniques are in clinical use with non-inferiority of one over another.[8] Traditional teaching is that while subjecting the patient to imaging after fixing the stereotactic frame, the same specific sequence of computed tomography (CT) or magnetic resonance imaging (MRI) scan should be done in which the lesion is better visualized. The enhancing lesion can thus be targeted accurately. The critical step in the frame-based technique is calculating stereotactic coordinates obtained by fusing a preoperative MRI image of the patient with a CT scan taken after fixing the stereotactic frame[5] or from contrast-enhanced T1-weighted MRI/CT.[12]
The problem we are trying to address is selecting a suitable target for a non-enhancing lesion. In such a case, target selection often becomes difficult, such as in the highly eloquent location of low-grade gliomas, resolving/incipient infections, recurrent gliomas, and radiation necrosis, leading to sampling errors.[4] Various adjuncts of imaging, such as positron emission tomography (PET), magnetic resonance perfusion, and magnetic resonance spectroscopy, to help target selection have been published in the literature to address this issue.[5] Using such adjuncts of imaging for mere target selection in case of non-enhancing lesions like low-grade gliomas is time-consuming and increases the cost of the procedure. Thus, we sought a novel targeting method to perform diagnostic STB in non-enhancing lesions without needing higher-order imaging. This method will also be helpful in resource-limited centers, especially in low-income countries. The authors propose a simple method for target selection of such lesions in appropriately selected cases using indirect evidence of target localization during CT-guided frame-based STB.[2,4,7,9,10]
MATERIALS AND METHODS
This study is a retrospective review of clinical, imaging, and histopathological report data of patients with contrast non-enhancing intracranial space-occupying lesions who underwent frame (Leksell system) based stereotactic biopsy in our hospital. As it was a retrospective study of data from histopathological reports and a picture archival communication system, approval from our Institute’s Ethical Committee was not sought. The appropriate case selection for the proposed new method was decided by careful study of preoperative radiological imaging. Imaging prerequisites for case selection are listed below.
Lesion should be non-contrast enhancing (for example, low-grade gliomas) and
Lesion exerting mass effect/midline shift [Figure 1] distorting surrounding brain normal anatomy such as effacement of adjacent gyri/sulci and obliteration of ventricle [Figure 2]
Lesion location about identifiable subcortical structures such as internal capsule [Figure 3]
Lesion causing radiologically appreciable perilesional edema [Figure 4]
Lesion with calcification [Figure 5].
Figure 1:
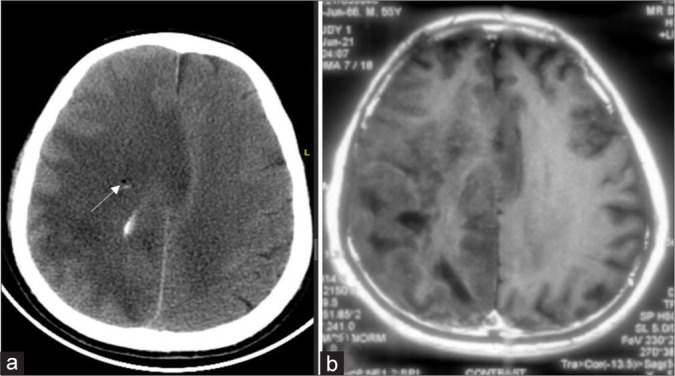
(a) Post procedure plain computed tomography showing midline shift and edema as an indirect evidence at corresponding cuts with a white arrow indicating biopsy site. (b) In an index case of the right diffuse hemispheric glioma preoperative contrast magnetic resonance imaging sequence.
Figure 2:
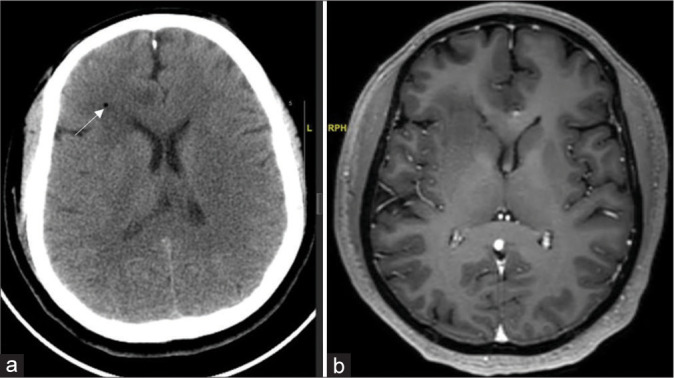
(a) Post procedure plain computed tomography showing right frontal periventricular location in relation to the caudate nucleus as an indirect evidence at corresponding cuts with white arrow indicating biopsy site. (b) In an index case of the right frontal glioma, preoperative contrast magnetic resonance imaging sequence.
Figure 3:
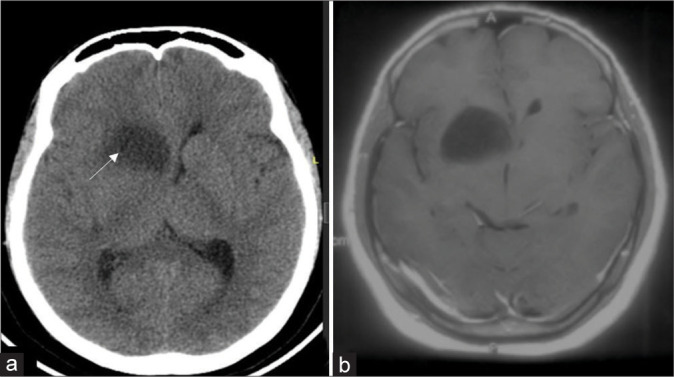
(a) Post procedure plain computed tomography showing lesion location in relation to the anterior limb of right internal capsule as an indirect evidence at corresponding cuts with white arrow indicating biopsy site. (b) In an index case of the right caudate glioma, preoperative contrast magnetic resonance imaging sequence.
Figure 4:
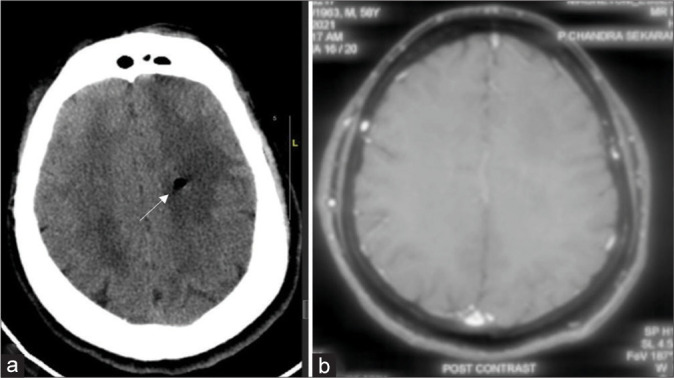
(a) Post procedure plain computed tomography showing mass effect and perilesional edema as an indirect evidence at corresponding cuts with white arrow indicating biopsy site. (b) In an index case of left diffuse hemispheric glioma preoperative contrast magnetic resonance imaging sequence.
Figure 5:
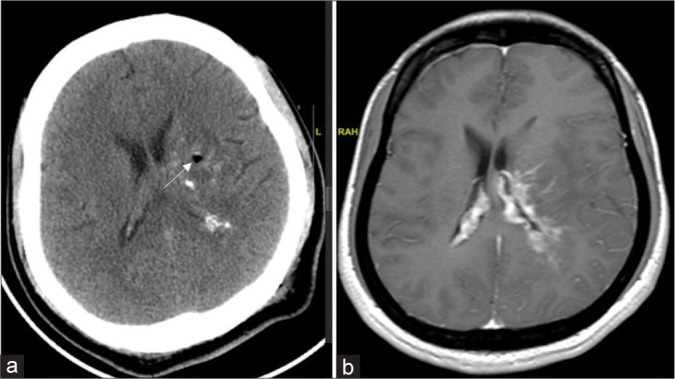
(a) Post procedure plain computed tomography showing intralesional calcification as an indirect evidence at corresponding cuts with a white arrow indicating the biopsy site. (b) In an index case of left thalamocapsular glioma preoperative contrast magnetic resonance imaging sequence.
Using this indirect evidence, most non-enhancing lesions can be precisely localized using non-contrast CT-based planning of target selection and coordinates calculation while performing frame-based STB.
Statistical analysis and software
Data were entered into a Microsoft Excel data sheet and were analyzed using the Statistical Package for the Social Sciences (SPSS) 22 version software. Categorical data were represented in the form of frequencies and proportions. Microsoft Excel and SPSS version 22 (IBM SPSS Statistics, Somers, NY, USA) were used to analyze data.
Description of the procedure
Our institute uses the Leksell system for frame-based STB. All patients underwent contrast MRI preoperatively. On the day of surgery, the radiological images of suitable cases for this new target localization method were studied before starting the procedure. Using aseptic and antiseptic precautions, the Leksell frame was fixed to the patient’s head with two pins in the frontal and two pins in the occipital region under local infiltration of lignocaine at pin sites. All patients underwent a 0.65 mm thin slice plain CT scan with the Leksell stereotactic frame fixed to their heads. We used indirect evidence of non-contrast CT for target localization as illustrated case by case. The target’s stereotactic calculus was planned using MNPS software (Mevis, Sao Paulo SP, Brazil).
The frame center is given the numerical No 100, with the X-axis running horizontally using a grid at the CT scanner console and the Y-axis running vertically. The target coordinates are determined based on this numerical No 100. The fiducial distance recorded in the CT console suggests the Z coordinate of the superoinferior stereotactic plane. We prefer to begin by taking a biopsy sample from the edge of the lesion and then moving to the center to avoid a negative biopsy. All parameters were calculated and then manually applied to the Leksell frame system. The trajectory was planned from the right or left frontal whenever possible to avoid eloquent structures.
Shaving and disinfection of 2–3 cm scalp over the planned entry point was performed. A small stab incision was placed over the entry point in the scalp after local infiltration of adrenaline and lignocaine. Twist drill trephination is done till the tip crosses the inner table of the skull, with the twist drill tip plunging across the dura. Tissue samples were taken with a Sedan biopsy needle (Micromar, Diadema SP, Brazil), which generates samples of 5 mm. Three samples were collected from the lesion margin before hitting the target. Another three samples were collected from the selected target site. After adequate tissue samples, the biopsy forceps were drawn back, and the skin was closed. The frame was detached from the head. We do postoperative CT scans routinely after 4 hours of the procedure for all the patients in our institute to confirm the biopsy track and to rule out intracerebral bleeding. The patients were kept in the recovery room and discharged home the same day.
RESULTS
The types of cases and indirect evidence can vary. From January 2021 to March 2023, 27 such cases were biopsied. The mean age of subjects was 44.04 ± 17.812 years. Most subjects were in the age group 31–40 years (29.6%), 55.6% were male, and 44.4% were female [Table 1]. The most common biopsy site was the frontal lobe [Table 2]. The most common indirect evidence of CT was perilesional edema in 33.3%, periventricular location in 33.3 %, and intralesional calcification in 11.1 % of the cases [Table 3]. Our diagnostic accuracy was seen in 25 cases (92.59 %), an inconclusive histopathological diagnosis was reported in 2 cases (7.4 %), and none of the cases biopsy was negative [Tables 4-6]. The asymptomatic hemorrhage rate was 2%, and an increase in perilesional edema was seen in 2% of cases. None of the cases needed an open procedure after the biopsy.
Table 1:
Patient characteristics among biopsy cohort (n=27).
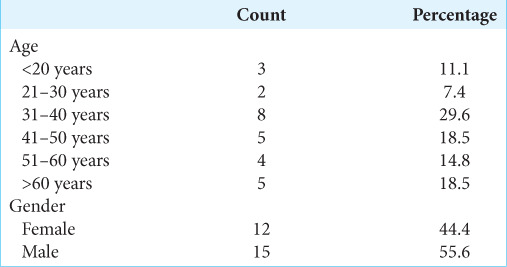
Table 2:
Spectrum of cases that underwent the procedure (n=27).
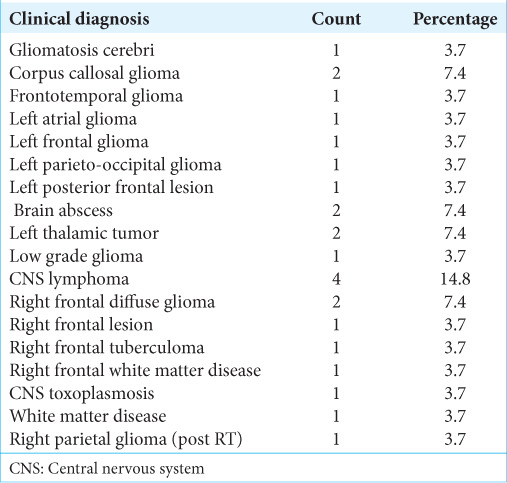
Table 3:
Analysis of indirect evidence in non-contrast CT observed during stereotactic brain biopsies (n=27).
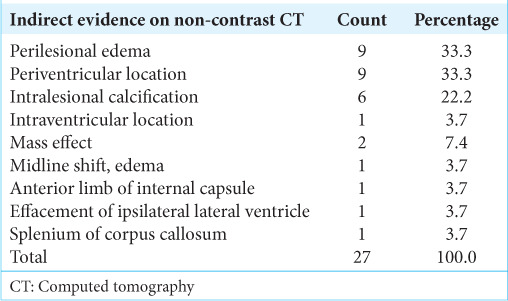
Table 4:
Histopathological diagnosis of the biopsies of 27 patients with non-contrast enhancing brain lesions/pathologies (n=27).
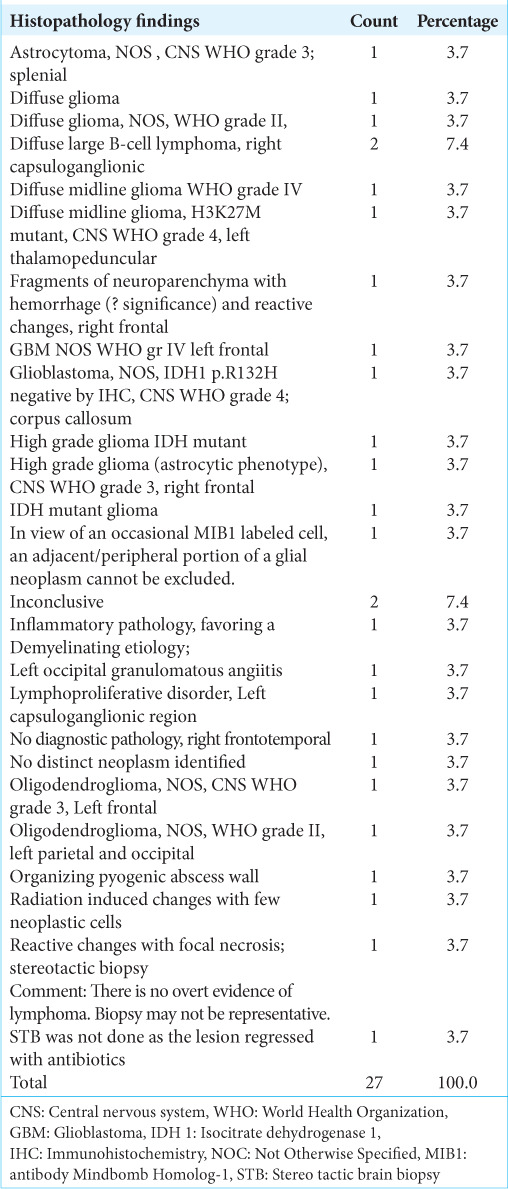
Table 6:
MIB index (n=27).

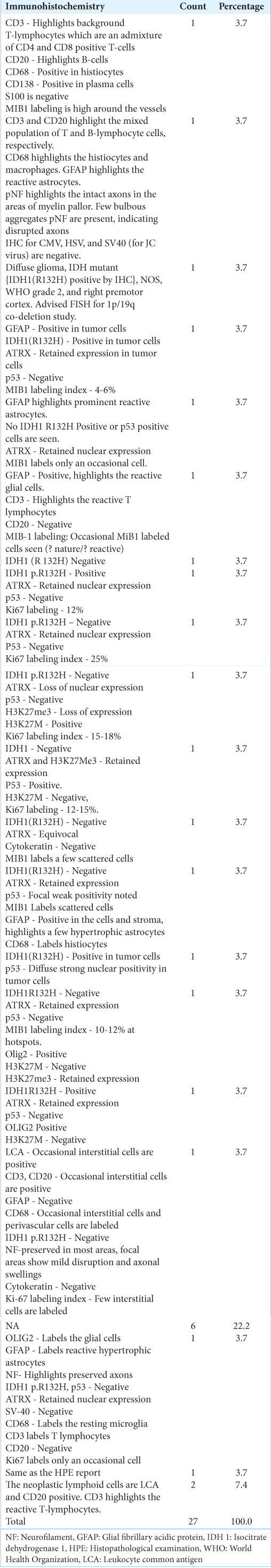
Table 5:
Immunohistochemistry analysis (n=27).
DISCUSSION
The introduction of STB has proved critical in arriving at tissue diagnosis for various brain pathologies, including neoplasm. Studies showed no statistically significant differences in the accuracy and retrieval of diagnostic tissue between frame-based and frameless methods. Preoperative planning is based on careful selection of the particular imaging sequence from CT or MRI, in which the target is better visualized, mainly in contrast sequences. Sampling errors are of particular concern in the case of contrast non-enhancing targets/lesions. Advanced imaging modalities such as perfusion sequences, PET, and magnetic resonance spectroscopy are used as adjuncts for better visualization of such targets/lesions reported high diagnostic yield in small studies (n = 12–32) by few authors.[12]
There are several factors to discuss when considering a new method for targeting. One important aspect is the diagnostic yield, which is influenced by the patient’s age, volume of the biopsied lesion, and histopathology.[11] In a recent meta-analysis, among the 15 studies included, 10 of them accounted for the size of the lesion. Over 90% of the biopsied lesions were observed to be larger than 1 cm in diameter. Therefore, the results of the meta-analysis may not apply to lesions smaller than 1 cm. In such cases, many neurosurgeons prefer using frame-based biopsy techniques. However, the impact of the number of biopsy specimens on the diagnostic yield cannot be adequately analyzed due to limited data, as only four studies have provided information on this aspect.
Another factor is morbidity and mortality; it is known that the risk is influenced by the location of the lesion, with higher risks associated with lesions situated in deep gray matter, brainstem, and eloquent regions. Unfortunately, most identified studies did not provide information on lesion location. Some surgeons opt for frame-based approaches when dealing with lesions in the pineal region, brainstem, basal ganglia, thalamus, posterior fossa, and deep perivascular regions, as frame-based systems offer more precise stereotactic guidance.[3] Furthermore, it is generally believed that the number of biopsy specimens taken can affect the risk of complications. However, more data are needed to quantify the contribution of the number of biopsy samples to the observed morbidity and mortality rates in this study. Finally, certain intracranial lesions have an increased risk of hemorrhage. Patients with cancer and intracranial lesions who have recently undergone chemotherapy or have a hematologic malignancy may have thrombocytopenia or other forms of coagulopathy, which further increases the risk. Fortunately, our targeting system does not add to any of these complications while providing a 92.59% diagnostic accuracy, comparable to previously reported studies.[6]
In terms of procedure duration, our study supported the notion that indirect evidence-targeted biopsy offers time savings compared to the large amount of time needed for higher-order imaging after frame fixation in other methods. However, the time saved may vary depending on institutional workflow, including travel time to and from imaging units and surgeon preference. It is worth noting that although time savings are essential, transporting patients under general anesthesia has its risks. Therefore, it is crucial to consider these factors when interpreting our results.
Limitations
We acknowledge certain limitations to our research. First, some of the other parameters (age, size of the lesion, lesion location, etc.) may be predictive of the location of the lesion, which we have not considered. Further research focused on the other parameters may demonstrate differences not shown in our study. Second, indirect targeting may only be applicable in some cases due to the small numbers. A prospective evaluation may reveal the advantages and disadvantages of our suggested target selection technique.
CONCLUSION
Indirect targeting is a safe and intuitive method for the biopsy of non-enhancing lesions. This eliminates the need for higher-order imaging and, thus, saves time and money spent while not compromising diagnostic accuracy and safety. Due consideration is to be given to the findings observed in non-contrast CT scans of the head as indirect evidence of target selection while performing frame-based STB of contrast non-enhancing lesions. A more extensive series with a comparison arm of direct targeting comparing diagnostic accuracy and safety will be the next step in our series.
Footnotes
How to cite this article: Lingaraju TS, Prabhuraj AR, Nandeesh BN, Saini J, Pruthi N. Computed tomography-guided frame-based stereotactic brain biopsy of non-enhancing lesions using indirect evidence of target selection, technical consideration, and early clinical experience. Surg Neurol Int. 2024;15:286. doi: 10.25259/SNI_187_2024
Contributor Information
TS Lingaraju, Email: drlingarajuts@gmail.com.
AR Prabhuraj, Email: drprabhuraj@yahoo.co.in.
BN Nandeesh, Email: nandeeshbn@gmail.com.
Jitender Saini, Email: jsaini76@gmail.com.
Nupur Pruthi, Email: pruthi_nupur@yahoo.co.in.
Ethical approval
The Institutional Review Board approval is not required as the study was retrospective in nature.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Akshulakov SK, Kerimbayev TT, Biryuchkov MY, Urunbayev YA, Farhadi DS, Byvaltsev VA. Current trends for improving safety of stereotactic brain biopsies: Advanced optical methods for vessel avoidance and tumor detection. Front Oncol. 2019;9:947. doi: 10.3389/fonc.2019.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernov MF, Muragaki Y, Ochiai T, Taira T, Ono Y, Usukura M, et al. Spectroscopy-supported frame-based image-guided stereotactic biopsy of parenchymal brain lesions: Comparative evaluation of diagnostic yield and diagnostic accuracy. Clin Neurol Neurosurg. 2009;111:527–35. doi: 10.1016/j.clineuro.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Dhawan S, He Y, Bartek J, Jr, Alattar AA, Chen CC. Comparison of frame-based versus frameless intracranial stereotactic biopsy: Systematic review and meta-analysis. World Neurosurg. 2019;127:607–16.e4. doi: 10.1016/j.wneu.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Eigenbrod S, Trabold R, Brucker D, Eros C, Egensperger R, La Fougere C, et al. Molecular stereotactic biopsy technique improves diagnostic accuracy and enables personalized treatment strategies in glioma patients. Acta Neurochir (Wien) 2014;156:1427–40. doi: 10.1007/s00701-014-2073-1. [DOI] [PubMed] [Google Scholar]
- 5.Farahmand D, Keil F, Gohring M, Dinc N, Seifert V, Marquardt G, et al. Prognostic risk factors for postoperative hemorrhage in stereotactic biopsies of lesions in the basal ganglia. Clin Neurol Neurosurg. 2018;174:180–4. doi: 10.1016/j.clineuro.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 6.He Z, Zhu CX, Chan DT, Cheung TC, Ng HK, Mok VC, et al. Diagnostic accuracy and field for improvement of frameless stereotactic brain biopsy: A focus on nondiagnostic cases. J Neurol Surg A Cent Eur Neurosurg. 2024;85:48–61. doi: 10.1055/a-1994-8033. [DOI] [PubMed] [Google Scholar]
- 7.Hemm S, Rigau V, Chevalier J, Picot MC, Bauchet L, El Fertit H, et al. Stereotactic coregistration of 201Tl SPECT and MRI applied to brain tumor biopsies. J Nucl Med. 2005;46:1151–7. [PubMed] [Google Scholar]
- 8.Kesserwan MA, Shakil H, Lannon M, McGinn R, Banfield L, Nath S, et al. Frame-based versus frameless stereotactic brain biopsies: A systematic review and meta-analysis. Surg Neurol Int. 2021;12:52. doi: 10.25259/SNI_824_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirotte B, Goldman S, Massager N, David P, Wikler D, Vandesteene A, et al. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med. 2004;45:1293–8. [PubMed] [Google Scholar]
- 10.Pirotte BJ, Lubansu A, Massager N, Wikler D, Goldman S, Levivier M. Results of positron emission tomography guidance and reassessment of the utility of and indications for stereotactic biopsy in children with infiltrative brainstem tumors. J Neurosurg. 2007;107(5 Suppl):392–9. doi: 10.3171/PED-07/11/392. [DOI] [PubMed] [Google Scholar]
- 11.Tsermoulas G, Mukerji N, Borah AJ, Mitchell P, Ross N. Factors affecting diagnostic yield in needle biopsy for brain lesions. Br J Neurosurg. 2013;27:207–11. doi: 10.3109/02688697.2012.722239. [DOI] [PubMed] [Google Scholar]
- 12.Winn HR. Vol. 4. Netherlands: Elsevier Health Sciences; 2022. Youmans and Winn neurological surgery e-book. [Google Scholar]


