Abstract
Background:
Collision tumors involving the co-occurrence of two morphologically and genomically distinct neoplasms in the same anatomical site are exceptionally rare in the central nervous system (CNS).
Case Description:
We report a unique case of a CNS collision tumor comprising chronic lymphocytic leukemia and myxopapillary ependymoma in a 77-year-old male with acute neurological decline. Presumed to represent leukemic infiltration, urgent laminectomy was pursued for tissue diagnosis and spinal cord decompression, revealing the unexpected ependymal component.
Conclusion:
This case highlights the diagnostic and therapeutic challenges inherent to managing collision CNS tumors, particularly when one neoplasm is hematological.
Keywords: Collision, Ependymoma, Oncology, Spinal

INTRODUCTION
Collision tumors are defined as the synchronous occurrence of two topographically intermingled but histologically distinct primary neoplasms in the same anatomical location.[8] Such tumors are uncommon occurrences but particularly rare within the central nervous system (CNS).[3] CNS collision tumors pose major diagnostic and therapeutic dilemmas given the need to integrate multimodal treatments tailored toward each morphologically disparate component neoplasm.[1] We describe a unique case of a CNS collision tumor comprised of chronic lymphocytic leukemia (CLL) and myxopapillary ependymoma in a patient presenting with acute neurological decline.
CASE PRESENTATION
A 77-year-old male with a medical history of CLL diagnosed 7 years prior presented with acute onset of severe low back pain radiating bilaterally to the legs associated with urinary retention and overflow incontinence. Medical history was also significant for stroke 3 years earlier with full recovery, hypothyroidism, and chronic low back pain managed conservatively.
His CLL history was notable for recurrent sinopulmonary infections attributed to treatment-related hypogammaglobulinemia necessitating rotating antibiotic prophylaxis. He had undergone chemoimmunotherapy with bendamustine, rituximab, and ibrutinib 2 years prior and was currently on maintenance acalabrutinib.
Three days before the presentation, he suffered a mechanical fall, which exacerbated his chronic back pain and precipitated the new neurological symptoms. On arrival, he exhibited 3/5 strength in the left lower extremity and 4/5 strength on the right with a T12 sensory level. He was unable to ambulate. Magnetic resonance imaging (MRI) of the thoracic spine [Figure 1] revealed a hemorrhagic intradural extramedullary lesion at T12–L1 measuring 2.1 × 1.3 × 1.8 cm, resulting in severe spinal cord compression and edema.
Figure 1:
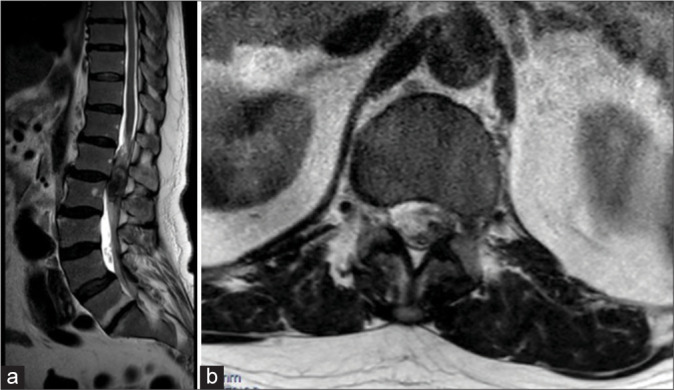
(a) Sagittal and (b) Axial T2 magnetic resonance images show a hemorrhagic intradural extramedullary lesion at T12– L1 measuring 2.1 × 1.3 × 1.8 cm, resulting in severe spinal cord compression.
Given his known history of CLL, the spinal lesion was presumed to represent leukemic infiltration. Hematology was consulted, and acalabrutinib was promptly held, given its propensity to exacerbate bleeding. After extensive discussion of risks and benefits, the decision was made to urgently pursue laminectomy for tissue diagnosis and decompression of the spinal cord.
The patient underwent bilateral T12 and L1 laminectomies. Opening of the dura revealed diffuse hemorrhage and a vascular mass within the subarachnoid space tracking from the filum terminale. Frozen section analysis of the specimen revealed two distinct neoplasms. Final pathology [Figures 2-4] confirmed the diagnosis of a collision tumor comprised of CLL infiltrating myxopapillary ependymoma World Health Organization (WHO) grade 2.
Figure 2:
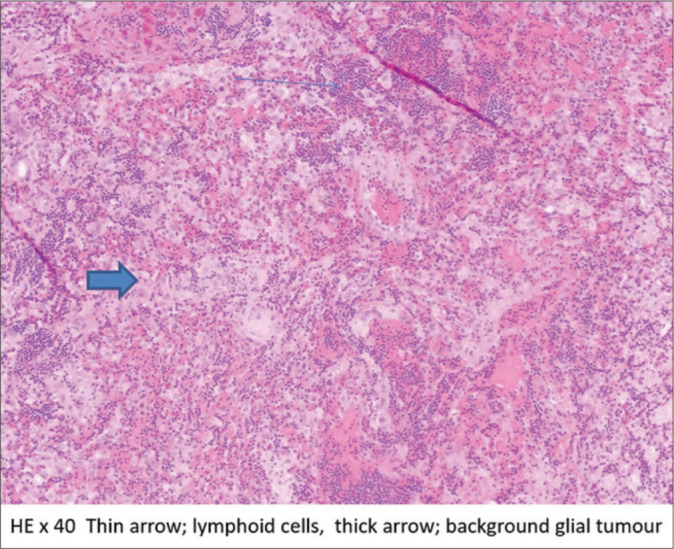
Hematoxylin and eosin (HE) ×40 histopathology image showing the collision of the tumors.
Figure 4:
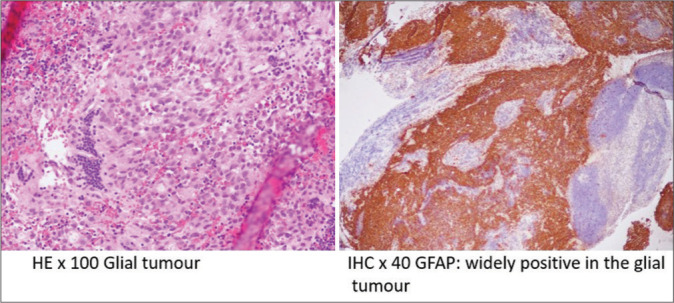
Hematoxylin and eosin (HE) ×100 and immunohistochemistry (IHC) ×40 GFAP histopathology images of the myxopapillary ependymoma. GFAP: Glial fibrillary acidic protein.
Figure 3:
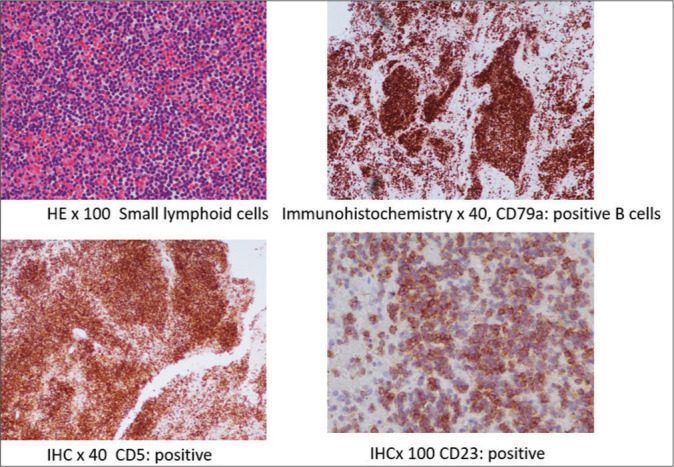
Hematoxylin and eosin (HE) ×100, immunohistochemistry (IHC) ×40, CD79a, IHC ×40 CD5, and IHC ×100 CD23 histopathology images of the chronic lymphocytic leukaemia.
Postoperatively, lower extremity strength improved to antigravity function bilaterally. Given the known chemosensitivity profile of each component, he was restarted on acalabrutinib and began temozolomide. Surveillance MRIs [Figure 5] have shown no evidence of recurrence.
Figure 5:
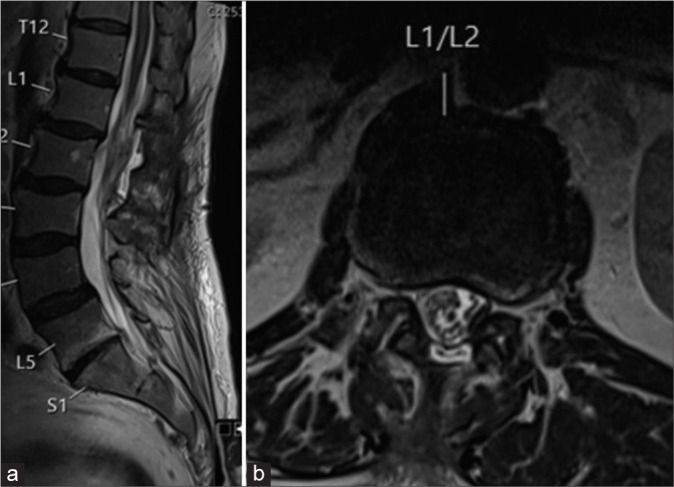
(a) Sagittal and (b) Axial T2 magnetic resonance images show the complete resection of the solid tumor.
DISCUSSION
Ependymomas are glial tumors that arise from Radial galial cells and account for 2–9% of all neuroepithelial neoplasms.[4] Myxopapillary ependymomas arise exclusively from the filum terminale and conus medullaris, representing 13% of all ependymomas. Despite being classified as WHO Grade 2, they are prone to recurrence, with 5- and 10-year progression-free survival rates of 76% and 42%.[5] Maximal safe resection is the treatment of choice for myxopapillary ependymomas due to their tendency to recur locally. However, the risks of postoperative deficits must be balanced against the extent of removal. Adjuvant radiation is often administered, especially in cases of subtotal resection, although its role following gross total resection remains controversial. Systemic therapy is usually reserved for disease progression.[7]
CLL is the most common adult leukemia in the Western world, with a propensity for CNS involvement.[2] While rare, extramedullary CLL manifesting as an intracranial mass lesion has been reported.[6]
Collision CNS tumors are exceptionally rare. A meta-analysis found the most common combinations to be meningioma with glioblastoma or metastasis.[8] Collision between two primary CNS tumors was less frequent than with systemic cancer.[3] To the best of our knowledge, this is the first reported case of CLL colliding with myxopapillary ependymoma.
Managing collision CNS tumors requires understanding the dynamics of each component to integrate surgery, radiation, and systemic therapy. Resection, in this case, was appropriate given the ependymoma’s tendency to recur.[9] Adjuvant radiation was deferred due to infection risk with the patient’s hypogammaglobulinemia. Systemic agents active against both CLL and ependymoma were selected. Interdisciplinary collaboration was integral to optimizing outcomes.
CLL is the most common adult leukemia in the Western world, with a propensity for CNS involvement.[2] While rare, extramedullary CLL manifesting as an intracranial mass lesion has been reported.[6]
Collision CNS tumors are exceptionally rare. A meta-analysis found the most common combinations to be meningioma with glioblastoma or metastasis.[8] Collision between two primary CNS tumors was less frequent than with systemic cancer.[3] The co-occurrence of CLL and myxopapillary ependymoma, in this case, is exceptionally rare. While collision tumors involving meningiomas or metastases[3] are occasionally reported, the combination of two primary CNS neoplasms is uncommon. This appears to be the first documented instance of concurrent CLL and myxopapillary ependymoma. To the best of our knowledge, this is the first reported case of CLL colliding with myxopapillary ependymoma. The pathogenesis underlying collision tumors remains poorly understood. Hence, we are not sure immunosuppressive effects of CLL that may have promoted the development or progression of the ependymoma or CLL-associated immune defects, which could reduce tumor surveillance and create a more permissive environment for oncogenesis. Future studies analyzing genetic alterations in each component may provide insight into potential shared mechanistic links.
Managing collision CNS tumors requires understanding the dynamics of each component to integrate surgery, radiation, and systemic therapy. Resection, in this case, was appropriate given the ependymoma’s tendency to recur.[9] Adjuvant radiation was deferred due to infection risk with the patient’s hypogammaglobulinemia. Systemic agents active against both CLL and ependymoma were selected. Overall, this case highlights the importance of an individualized, multidisciplinary approach to complex collision tumors. As novel systemic agents emerge, the integration of surgery, radiation, and drug therapy will be key to improving outcomes. Molecular profiling may also shed light on potential therapeutic targets and biological links between synchronous primary malignancies.
CONCLUSION
We present an exceptional case of a CNS collision tumor comprised of synchronous infiltration by CLL and myxopapillary ependymoma. Collision CNS tumors create major diagnostic and therapeutic dilemmas necessitating broad clinicopathologic correlation and an individualized, cross-disciplinary management strategy.
Footnotes
How to cite this article: Aly A, Nagaraju S, Price R. Collision tumor comprised of chronic lymphocytic leukemia and myxopapillary ependymoma. Surg Neurol Int. 2024;15:282. doi: 10.25259/SNI_658_2023
Contributor Information
Ahmed Aly, Email: ahmedhma88@gmail.com.
Santhosh Nagaraju, Email: ahmedhma88@gmail.com.
Rupert Price, Email: ahmedhma88@gmail.com.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Bougaci N, Litrico S, Burel-Vandenbos F, Paquis P. Unusual cauda equina syndrome due to multifocal ependymoma infiltrated by lymphoma. J Spine Surg. 2017;3:697–701. doi: 10.21037/jss.2017.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kikushige Y. Pathogenesis of chronic lymphocytic leukemia and the development of noveltherapeutic strategies. J Clin Exp Hematop. 2020;60:146–58. doi: 10.3960/jslrt.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merrill SA, Sharma A, Carlin RE, McCullough AE, Porter AB, Bendok BR, et al. A rare intracranial collision tumor of meningioma and metastatic uterine adenocarcinoma: Case report and literature review. World Neurosurg. 2021;145:340–7. doi: 10.1016/j.wneu.2020.09.108. [DOI] [PubMed] [Google Scholar]
- 4.Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22:IV1–96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghunathan A, Wani K, Armstrong TS, Vera-Bolanos E, Fouladi M, Gilbertson R, et al. Histological predictors of outcome in ependymoma are dependent on anatomic site within the central nervous system. Brain Pathol. 2013;23:584–94. doi: 10.1111/bpa.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratterman M, Kruczek K, Sulo S, Shanafelt TD, Kay NE, Nabhan C. Extramedullary chronic lymphocytic leukemia: Systematic analysis of cases reported between 1975 and 2012. Leuk Res. 2014;38:299–303. doi: 10.1016/j.leukres.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Rudà R, Bruno F, Pellerino A, Soffietti R. Ependymoma: Evaluation and management updates. Curr Oncol Rep. 2022;24:985–93. doi: 10.1007/s11912-022-01260-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed S, Karambizi DI, Baker A, Groh DM, Toms SA. A comparative report on intracranial tumor-to-tumor metastasis and collision tumors. World Neurosurg. 2018;116:454–63.e2. doi: 10.1016/j.wneu.2018.04.109. [DOI] [PubMed] [Google Scholar]
- 9.Weber DC, Wang Y, Miller R, Villà S, Zaucha R, Pica A, et al. Long-term outcome of patients with spinal myxopapillary ependymoma: Treatment results from the MD Anderson Cancer Center and institutions from the Rare Cancer Network. Neuro Oncol. 2015;17:588–95. doi: 10.1093/neuonc/nou293. [DOI] [PMC free article] [PubMed] [Google Scholar]


