Abstract
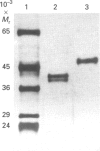
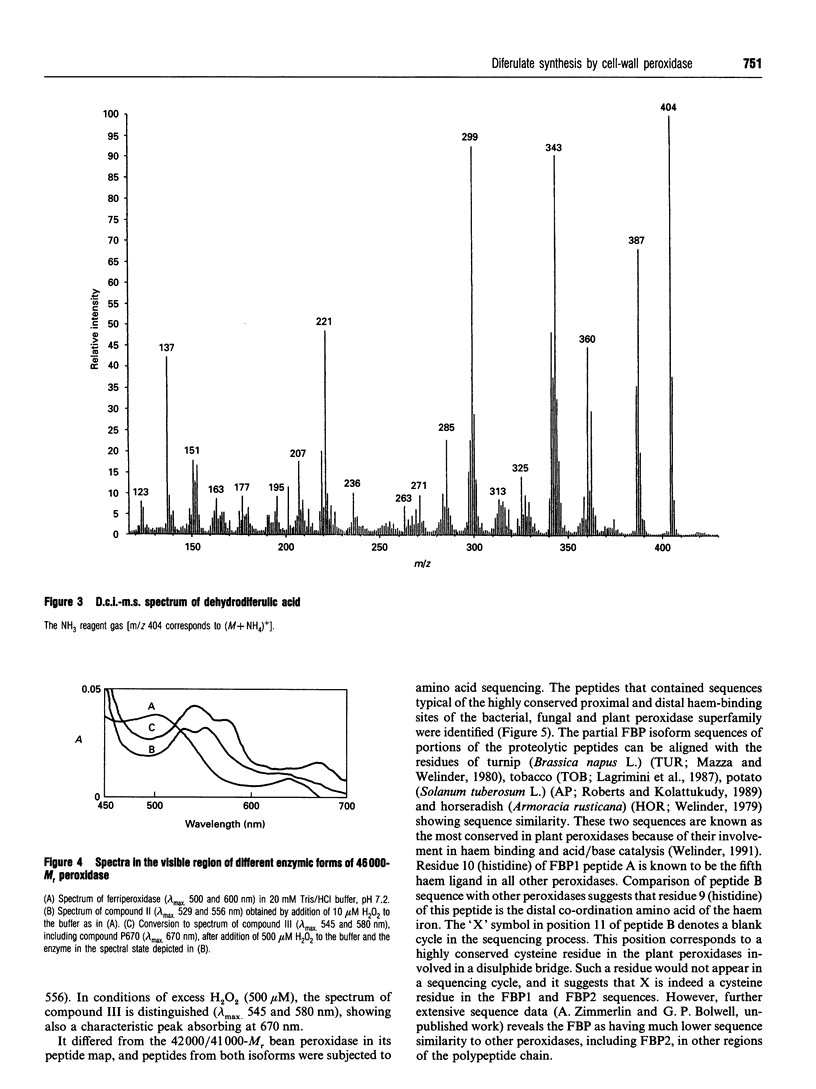
A cationic (pI 8.3) wall-bound peroxidase has been purified to homogeneity from suspension-cultured cells of French bean (Phaseolus vulgaris L.). The enzyme was a glycoprotein and its M(r) was 46,000 as determined by SDS/Page and h.p.l.c. gel filtration. It was localized biochemically to microsomes and the cell wall, and the latter subcellular distribution was confirmed by immunogold techniques. The native enzyme showed absorption maxima at 403, 500 and 640 nm, with an RZ (A405/A280) of 3.3. The peroxidase oxidized guaïacol and natural phenolic acids. By desorption-chemical-ionization mass spectrometry the enzyme was found to oxidize the model compound, ferulic acid, into dehydrodiferulic acid. Kinetics studies indicated an apparent Km of 113.3 +/- 22.9 microM and a Vmax of 144 mumol.min-1.nmol-1 of protein at an H2O2 concentration of 100 microM. In comparison with a second French-bean peroxidase (FBP) and horseradish peroxidase, as a model, it acted with a 6-10-fold higher specificity in this capacity. It is a member of the peroxidase superfamily of bacterial, fungal and plant haem proteins by virtue of its highly conserved amino acid sequence within the proximal and distal haem-binding sites. This is good evidence that this particular FBP may function in constructing covalent cross-linkages in the wall during development and response to pathogens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bao W., O'malley D. M., Whetten R., Sederoff R. R. A laccase associated with lignification in loblolly pine xylem. Science. 1993 Apr 30;260(5108):672–674. doi: 10.1126/science.260.5108.672. [DOI] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989 Nov;91(3):889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992 Oct;13(4):435–448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Mohan R., Bajar M. A., Sherf B. A. Plant peroxidase gene expression and function. Biochem Soc Trans. 1992 May;20(2):333–337. doi: 10.1042/bst0200333. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagrimini L. M., Burkhart W., Moyer M., Rothstein S. Molecular cloning of complementary DNA encoding the lignin-forming peroxidase from tobacco: Molecular analysis and tissue-specific expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7542–7546. doi: 10.1073/pnas.84.21.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N. G., Yamamoto E. Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]
- Macadam J. W., Sharp R. E., Nelson C. J. Peroxidase Activity in the Leaf Elongation Zone of Tall Fescue : II. Spatial Distribution of Apoplastic Peroxidase Activity in Genotypes Differing in Length of the Elongation Zone. Plant Physiol. 1992 Jul;99(3):879–885. doi: 10.1104/pp.99.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza G., Welinder K. G. Covalent structure of turnip peroxidase 7. Cyanogen bromide fragments, complete structure and comparison to horseradish peroxidase C. Eur J Biochem. 1980 Jul;108(2):481–489. doi: 10.1111/j.1432-1033.1980.tb04745.x. [DOI] [PubMed] [Google Scholar]
- Roberts E., Kolattukudy P. E. Molecular cloning, nucleotide sequence, and abscisic acid induction of a suberization-associated highly anionic peroxidase. Mol Gen Genet. 1989 Jun;217(2-3):223–232. doi: 10.1007/BF02464885. [DOI] [PubMed] [Google Scholar]
- Rodgers M. W., Zimmerlin A., Werck-Reichhart D., Bolwell G. P. Microsomally associated heme proteins from French bean: characterization of the cytochrome P450 cinnamate-4-hydroxylase and two peroxidases. Arch Biochem Biophys. 1993 Jul;304(1):74–80. doi: 10.1006/abbi.1993.1323. [DOI] [PubMed] [Google Scholar]
- Welinder K. G. Amino acid sequence studies of horseradish peroxidase. Amino and carboxyl termini, cyanogen bromide and tryptic fragments, the complete sequence, and some structural characteristics of horseradish peroxidase C. Eur J Biochem. 1979 Jun 1;96(3):483–502. doi: 10.1111/j.1432-1033.1979.tb13061.x. [DOI] [PubMed] [Google Scholar]
- Welinder K. G., Mauro J. M., Nørskov-Lauritsen L. Structure of plant and fungal peroxidases. Biochem Soc Trans. 1992 May;20(2):337–340. doi: 10.1042/bst0200337. [DOI] [PubMed] [Google Scholar]