Abstract
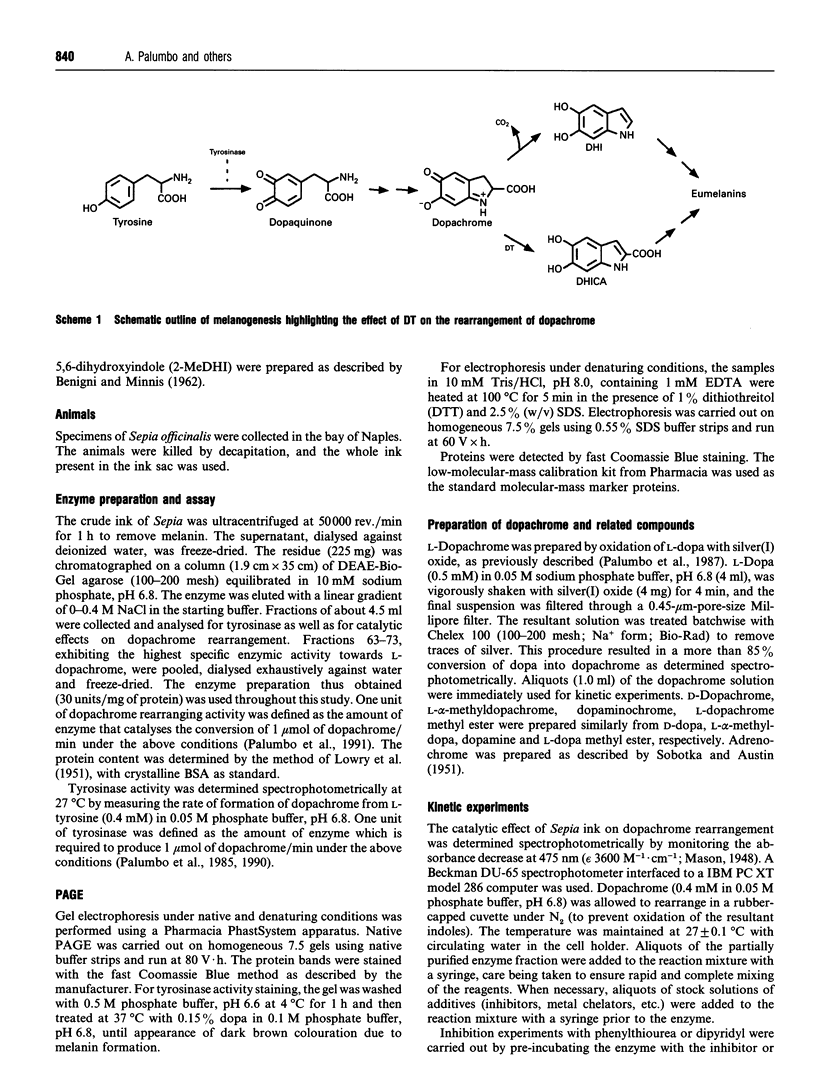
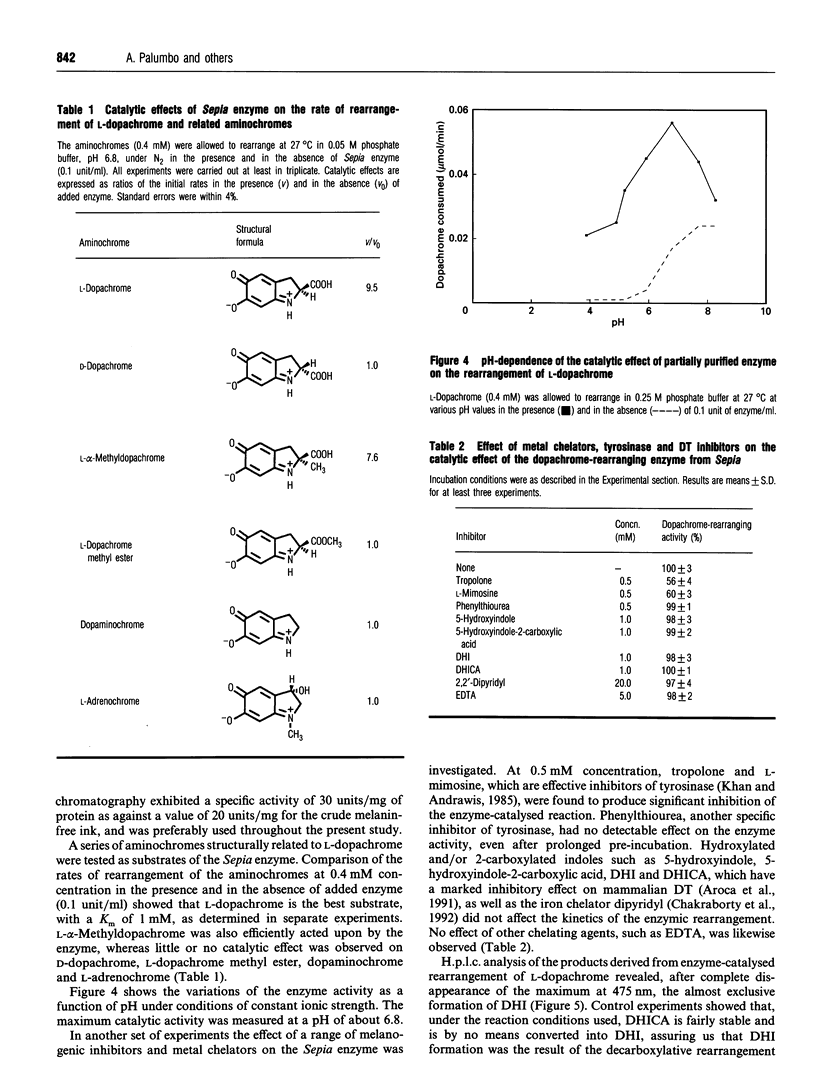
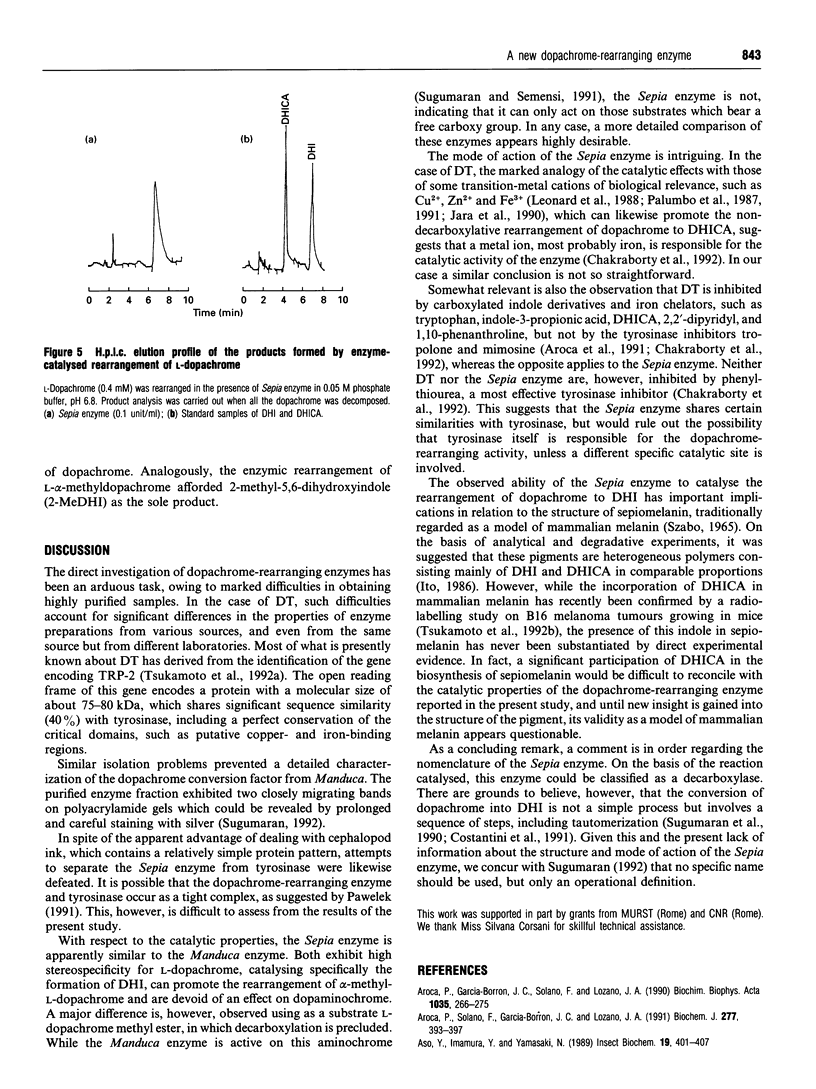
A melanogenic enzyme catalysing the rearrangement of dopachrome has been identified in the ejected ink of the cuttlefish Sepia officinalis. This enzyme occurs as a heat-labile protein which co-migrates with tyrosinase under a variety of chromatographic and electrophoretic conditions. On SDS/PAGE it shows like a single band with an approx. molecular mass of 85 kDa. The enzyme possesses high substrate specificity, acting on L-dopachrome (Km = 1 mM at pH 6.8) and on L-alpha-methyl-dopachrome, but not on D-dopachrome, L-dopachrome methyl ester, dopaminochrome and adrenochrome. Significant inhibition of the catalytic activity was observed with tropolone and L-mimosine. H.p.1.c. analysis of the enzyme-catalysed rearrangement of L-dopachrome revealed the quantitative formation of the decarboxylated product, 5,6-dihydroxyindole. These results point to marked differences between melanogenesis in cephalopod pigment cells and in melanocytes, which may have important implications in relation to the use of sepiomelanin as a model for studies of mammalian melanins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aroca P., Garcia-Borron J. C., Solano F., Lozano J. A. Regulation of mammalian melanogenesis. I: Partial purification and characterization of a dopachrome converting factor: dopachrome tautomerase. Biochim Biophys Acta. 1990 Sep 14;1035(3):266–275. doi: 10.1016/0304-4165(90)90088-e. [DOI] [PubMed] [Google Scholar]
- Aroca P., Solano F., Garcia-Borrón J. C., Lozano J. A. Specificity of dopachrome tautomerase and inhibition by carboxylated indoles. Considerations on the enzyme active site. Biochem J. 1991 Jul 15;277(Pt 2):393–397. doi: 10.1042/bj2770393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J. I., Townsend D., Olds D. P., King R. A. Dopachrome oxidoreductase: a new enzyme in the pigment pathway. J Invest Dermatol. 1984 Aug;83(2):145–149. doi: 10.1111/1523-1747.ep12263381. [DOI] [PubMed] [Google Scholar]
- Chakraborty A. K., Orlow S. J., Pawelek J. M. Evidence that dopachrome tautomerase is a ferrous iron-binding glycoprotein. FEBS Lett. 1992 May 11;302(2):126–128. doi: 10.1016/0014-5793(92)80421-c. [DOI] [PubMed] [Google Scholar]
- Halaban R., Moellmann G. Recent advances in the molecular biology of pigmentation: mouse models. Pigment Cell Res. 1992;Suppl 2:67–78. doi: 10.1111/j.1600-0749.1990.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991 Nov;5(14):2902–2909. [PubMed] [Google Scholar]
- Ito S. Reexamination of the structure of eumelanin. Biochim Biophys Acta. 1986 Aug 6;883(1):155–161. doi: 10.1016/0304-4165(86)90146-7. [DOI] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D. M., Tsukamoto K., Copeland N. G., Gilbert D. J., Jenkins N. A., Hearing V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992 Feb;11(2):527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara J. R., Solano F., Garcia-Borron J. C., Aroca P., Lozano J. A. Regulation of mammalian melanogenesis. II: The role of metal cations. Biochim Biophys Acta. 1990 Sep 14;1035(3):276–285. doi: 10.1016/0304-4165(90)90089-f. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Halaban R., Chintamaneni C. Molecular basis of mouse Himalayan mutation. Biochem Biophys Res Commun. 1989 May 30;161(1):252–260. doi: 10.1016/0006-291x(89)91588-x. [DOI] [PubMed] [Google Scholar]
- Kwon B. S. Pigmentation genes: the tyrosinase gene family and the pmel 17 gene family. J Invest Dermatol. 1993 Feb;100(2 Suppl):134S–140S. doi: 10.1111/1523-1747.ep12465022. [DOI] [PubMed] [Google Scholar]
- Körner A. M., Pawelek J. Dopachrome conversion: a possible control point in melanin biosynthesis. J Invest Dermatol. 1980 Aug;75(2):192–195. doi: 10.1111/1523-1747.ep12522650. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamoreux M. L., Woolley C., Pendergast P. Genetic controls over activities of tyrosinase and dopachrome conversion factor in murine melanocytes. Genetics. 1986 Aug;113(4):967–984. doi: 10.1093/genetics/113.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard L. J., Townsend D., King R. A. Function of dopachrome oxidoreductase and metal ions in dopachrome conversion in the eumelanin pathway. Biochemistry. 1988 Aug 9;27(16):6156–6159. doi: 10.1021/bi00416a049. [DOI] [PubMed] [Google Scholar]
- Palumbo A., Misuraca G., D'Ischia M., Prota G. Effect of metal ions on the kinetics of tyrosine oxidation catalysed by tyrosinase. Biochem J. 1985 Jun 15;228(3):647–651. doi: 10.1042/bj2280647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A., Solano F., Misuraca G., Aroca P., Garcia Borron J. C., Lozano J. A., Prota G. Comparative action of dopachrome tautomerase and metal ions on the rearrangement of dopachrome. Biochim Biophys Acta. 1991 Nov 14;1115(1):1–5. doi: 10.1016/0304-4165(91)90003-y. [DOI] [PubMed] [Google Scholar]
- Palumbo A., d'Ischia M., Misuraca G., Carratú L., Prota G. Activation of mammalian tyrosinase by ferrous ions. Biochim Biophys Acta. 1990 Mar 26;1033(3):256–260. doi: 10.1016/0304-4165(90)90129-k. [DOI] [PubMed] [Google Scholar]
- Palumbo A., d'Ischia M., Misuraca G., Prota G. Effect of metal ions on the rearrangement of dopachrome. Biochim Biophys Acta. 1987 Aug 13;925(2):203–209. doi: 10.1016/0304-4165(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M. After dopachrome? Pigment Cell Res. 1991 Mar;4(2):53–62. doi: 10.1111/j.1600-0749.1991.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Prota G. Regulatory mechanisms of melanogenesis: beyond the tyrosinase concept. J Invest Dermatol. 1993 Feb;100(2 Suppl):156S–161S. [PubMed] [Google Scholar]
- Shibahara S. Functional analysis of the tyrosinase gene and brown-locus protein gene promoters. J Invest Dermatol. 1993 Feb;100(2 Suppl):146S–149S. [PubMed] [Google Scholar]
- Sugumaran M. Naming of dopachrome conversion factor (DCF) Pigment Cell Res. 1992 Nov;5(5 Pt 1):203–204. doi: 10.1111/j.1600-0749.1992.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Sugumaran M., Semensi V. Quinone methide as a new intermediate in eumelanin biosynthesis. J Biol Chem. 1991 Apr 5;266(10):6073–6078. [PubMed] [Google Scholar]
- Takeuchi T., Tanaka S., Tanaka M. Expression of tyrosinase gene in transgenic mice: programmed versus non-programmed expression. J Invest Dermatol. 1993 Feb;100(2 Suppl):141S–145S. [PubMed] [Google Scholar]
- Townsend D., Oetting W. S., Polman T., King R. A. Purification and characterization of dopachrome tautomerase (DT). Pigment Cell Res. 1992;Suppl 2:32–35. doi: 10.1111/j.1600-0749.1990.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K., Jackson I. J., Urabe K., Montague P. M., Hearing V. J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992 Feb;11(2):519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto K., Palumbo A., D'Ischia M., Hearing V. J., Prota G. 5,6-Dihydroxyindole-2-carboxylic acid is incorporated in mammalian melanin. Biochem J. 1992 Sep 1;286(Pt 2):491–495. doi: 10.1042/bj2860491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder A. J., Wittbjer A., Rosengren E., Rorsman H. The mouse brown (b) locus protein has dopachrome tautomerase activity and is located in lysosomes in transfected fibroblasts. J Cell Sci. 1993 Sep;106(Pt 1):153–166. doi: 10.1242/jcs.106.1.153. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Takeuchi S., Kudo T., Sato C., Takeuchi T. Melanin production in cultured albino melanocytes transfected with mouse tyrosinase cDNA. Jpn J Genet. 1989 Apr;64(2):121–135. doi: 10.1266/jjg.64.121. [DOI] [PubMed] [Google Scholar]




