Abstract
Introduction:
Ischemia/reperfusion cascade can cause severe tissue damage as documented in myocardial infarction, stroke, and peripheral occlusive vascular; however, Chin et al. first identified unexplained neurological deterioration after decompressive spinal surgery and attributed this to reperfusion injury of the spinal cord. As this appears as hyperintense signal changes in post-operative T2-weighted magnetic resonance image (MRI) sequences, it is termed as “white cord syndrome.”
Case Report:
A 63-year-old man presented with cervical myelopathy due to an ossified posterior longitudinal ligament and ossified ligamentum flavum, having Nurick’s Grade IV posted for surgery. The patient underwent posterior instrumented decompression from 2nd cervical to 5th dorsal spine. Postoperatively, he had neurological deterioration and was investigated and found to have extensive cervical cord edema on MRI. There was no implant malposition.
Conclusion:
WCS has to be ruled out in a case of unexplained neurological deterioration after decompressive spinal surgery, especially in the cervical and dorsal spine. The exact mechanism and treatment of WCS remain unexplained; spine surgeons should warn patients about WCS before surgery to prevent ethical and medicolegal issues.
Keywords: Cervical myelopathy, posterior decompression, ossified posterior longitudinal ligament, white cord syndrome
Learning Point of the Article:
Postoperatively neurological deterioration following cervio-dorsal decompression can be attributed to white cord syndrome that is a radiological diagnosis whose prognosis remains guarded
Introduction
Literature reviews and clinical experience reveal that ischemia/reperfusion cascade can cause severe tissue damage. This injury occurring in myocardial infarction, stroke, and peripheral occlusive vascular disease are well documented, those in the spinal cord compression remained relatively unknown. In 2013, when Chin et al. identified unexplained neurological deterioration after decompressive spinal surgery in the cervical and thoracic spine and described it as white cord syndrome (WCS) [1]. There will be hyperintense signal changes in post-operative T2-weighted magnetic resonance imaging (MRI) sequences. Since then, there are a handful of case reports published but still more or less it remains a puzzle to spine surgeons [2-9]. WCS is a diagnosis of exclusion in cases of acute or subacute worsening of neurology after the surgery after ruling out other causes such as misplaced screws, iatrogenic cord injury, compression due to evolved hematoma, progressive kyphosis, or instability arising due to lack of instrumentation/immobilization [10]. The prognosis of WCS can be devastating if not diagnosed and treated immediately and will remain guarded.
Here, we present a case of ossified posterior longitudinal ligament (OPLL) and hypertrophied ligamentum flavum (HLF) at the cervical and upper dorsal spine, where posterior decompression and instrumentation were done from 2nd cervical to 5th dorsal spine.
Case Report
A 63-year-old man presented with a history of gradual onset weakness of lower limbs and clumsiness of hands for the past 2 years. He was not able to walk, though he could stand with support. For the past 3 months, he felt things were falling off his hand grips. He was not a known diabetic but hypertensive on medication. On examination, bilateral lower limbs had increased tone (grade 3 Ashworth scale), and power could not be assessed. However, he had upward plantar, exaggerated jerks, and sustained clonus of knee and ankle. His upper limbs were normal in tone, and power was 5/5 in most parts except C8 and T1, which had 4/5, and grip strength was 70% on the right side. The jerks were normal, and Hoffmans was negative for him. He had reduced sensation from the C8 dermatome with a diagnosis of cervical myelopathy; the patient was further investigated in the form of an X-ray, MRI, and computed tomography (CT) scan of the cervical spine. MRI revealed a continuous type of OPLL extending from 2nd cervical to 1st dorsal spine and further HLF from dorsal 1st to 4th spine. CT confirmed the MRI findings (Fig. 1a-e). The patient was counseled for surgery, and it was decided to do posterior instrumented decompression from 2nd cervical to 5th dorsal spine. The patient was prone, and the cervical and upper dorsal spine was exposed through a midline incision. The holes for instrumentation were made utilizing bilateral Pars (2nd cervical), lateral mass (3rd to 5th cervical), and pedicle screws (7th cervical and upper dorsal spine up to 4th dorsal). Decompression was done from outside using an ultrasonic scalpel and raising the entire mass with an osteotome, allowing the cord to fall back (Fig. 2a). After this, any attached bone pieces were removed using number 1 Kerrison’s rounger. Screws were placed, rods were connected, and the wound was closed in layers. The patient was shifted to the intensive care unit (ICU), but in post-operative care, we found a reduced power involving the whole of the upper limbs 0/5. An urgent CT was done to see any malposition of the instrumentation but there was found to be none (Fig. 2b and c). He was given high-dose solumedrol and an MRI was ordered. MRI showed diffuse cord edema (T2-weighted hyperintensity) of the whole of the cervical spine, suggestive of “white cord syndrome” (Fig. 2d). The patient completed a high-dose solumedrol dosage and then received dexamethasone on tapering doses for the next 7 days. He was tracheostomized in the ICU and later was weaned off the ventilator. The patient started to show some improvement in regaining shoulder shrugs (C5) and some flickering movement of fingers. The lower limbs started to show increased tonicity. The patient was shifted to the ward and decannulated in the next week. Subsequently, roughly 4 weeks after index surgery, his sutures were removed. He was no longer oxygen dependent, and his power in upper limbs had gained to 3/5. He was discharged home and mobilized in a wheelchair. However, subsequently at 6 months, there was no further recovery.
Figure 1.
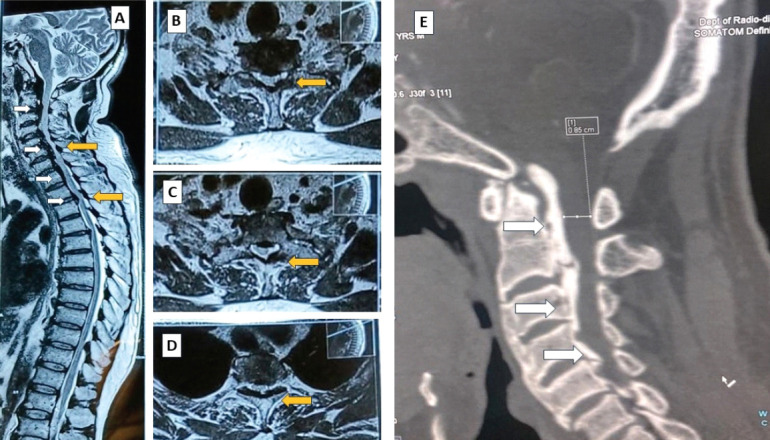
Magnetic resonance image showing multiple level compression of the spinal cord from both anterior and posterior aspects – sagittal section (A), axial section (B-D); computed tomography scan confirming an ossified posterior longitudinal ligament anteriorly (E).
Figure 2.
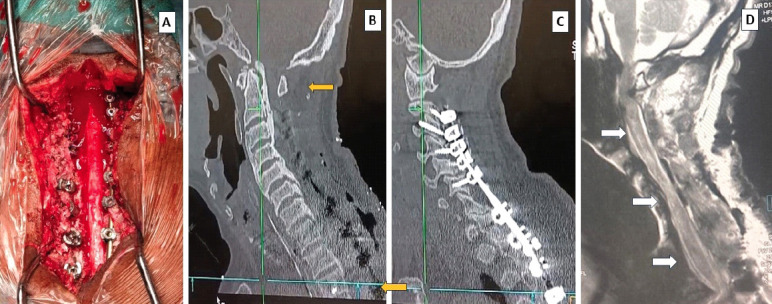
Intraoperative picture showing complete decompression and “fall back” of the cervical cord (A); post-operative computed tomography scan showing adequate decompression as per plan from C1 down (B); the satisfactory position of implants (C); and the magnetic resonance image T2-weight showing the diffuse edema of the whole of the cervical cord demonstrating the “white cord syndrome”(D).
Discussion
WCS is a rare entity characterized by sudden unexplained worsening of neurology after decompressive spinal surgery in cervical and thoracic levels associated with the presence of localized spinal cord edema visualized in post-operative T2-weighted MRI images as hyperintense lesions. The most accepted etiopathology theory of the WCS is a compensatory reperfusion-generated oxygen-free radical injury in a chronically compromised cord after decompression [4]. Even though the cornerstone treatment of any ischemic injury remains immediate reperfusion, there also remains a danger of a paradoxical response. Such response may cause variable levels of tissue damage ranging from transient ischemia to cell death depending upon the susceptibility of the tissue to reperfusion and the duration and severity of hypoperfusion suffered by the tissue before the reperfusion [11]. The spinal cord/central nervous system is poorly capable of self-repair and regeneration following any insult, therefore, the injury is often irreversible and manifested as sensory and motor deficits [12]. The poor regeneration capacity of axonal tissue will be challenged further by the age group where severe degenerative spinal diseases are more prevalent, that is, the elderly, where chances of repair will be even less.
Surgical decompression itself is a traumatic event that may trigger a proinflammatory cascade along with the oxygen free radicals released as a result of reperfusion, resulting in the formation of inflammatory mediators such as interleukin-1β, tumor necrosis factor-α, or 8-oxodeoxyguanosine DNA [13, 14]. These inflammatory mediators will cause a breach in the blood-spinal cord barrier and propagate the tissue damage. Thus, the pathophysiology of WCS is complex and involves either or a combination of ischemia, endothelial cell impairment, neuroinflammation, changes in vascular architecture, oligodendrocyte and neuronal apoptosis, and disruption of the blood–spinal cord barrier [15].
Even though the risk factors that are responsible for WCS are not extensively identified, it is hypothesized that the duration of spinal cord compression before decompression surgery, chronic hypertension, and advanced age can cause higher levels of tissue damage due to greater oxidative stress. From the literature review, the average age of patients reported to have WCS is 60.3 years [16], and our patient’s age is 63 years. Liao et al. have described a diagnostic criterion for this syndrome which includes paralysis occurring within 3 h of surgery due to decompression in a severely compressed after excluding all possible causative factors, including post-operative hematoma; and response to high-dose methylprednisolone in the form of partial/complete recovery [10].
The management of WCS is not very clear. Remote ischemic pre-conditioning (RIPC) is considered to reduce the risk of WCS in patients undergoing decompressive spinal surgery; a prospective study that investigated the efficacy of RIPC found that RIPC had significantly reduced neuron-specific enolase and S-100B levels in decompressive spinal surgery. If the surgery is done with neuromonitoring, then a drop-in motor evoked potential (MEP) and somatosensory evoked potential (SSEP) may be noted intraoperatively in acute WCS. However, in subacute WCS. This will not be a reliable tool for early suspicion. SSEP and MEP drops are useful for quick detection and early decisions and have helped patients [2, 5, 6, 7]. Regulation of mean arterial pressure (MAP) has been suggested to reduce the risk of WCS. However, there is level 1 evidence regarding the maintenance of MAP in spinal cord injury. Busack and Eagleton stated that patients with spinal cord injury need a MAP higher than 85 mmHg for stable cerebral perfusion. These patients need a higher MAP than normal for the functioning of the autoregulatory zone for cerebral perfusion [3]. However, if WCS is caused due to reperfusion injury, then increasing the blood flow can turn detrimental. Gallagher et al. have stated that dropping the MAP will be beneficial, as it has been discussed in the case of acute spinal cord trauma [17]. However, Turner et al. have stated that reducing MAP will reduce the SSEP and MEP [18]. Xie et al. found the administration of propofol to be useful. Propofol reduces the permeability of the blood–spinal cord barrier by downregulating the levels of nuclear factor-kB, and it also reduces histological damage to the spinal cord [19]. Yamazaki et al. proposed the administration of high-dose methylprednisolone within 8 h of injury is proposed as a treatment for WCS. Steroids reduce oxidative stress by upregulating anti-inflammatory markers. It also reduces lipid peroxidation and prevents intracellular potassium depletion; additional decompression is also usually done once WCS is diagnosed [20].
Conclusion
WCS has to be ruled out in a case of unexplained neurological deterioration after decompressive spinal surgery, especially in the cervical and dorsal spine. A handful of case reports have been published in the last decade regarding WCS. However, the exact mechanism and treatment of WCS remain unexplained; further studies are needed to predict the risk of occurrence and management of WCS. Spine surgeons should warn the patients about WCS before surgery to prevent ethical and medicolegal issues.
Clinical Message.
WCS is a radiological diagnosis in patients with unexplained neurological deterioration after decompressive spinal surgery, especially in the cervical and dorsal spine. Spine surgeons should warn the patients about WCS before surgery to prevent ethical and medicolegal issues.
Biography






Footnotes
Conflict of Interest: Nil
Source of Support: Nil
Consent: The authors confirm that informed consent was obtained from the patient for publication of this case report
References
- 1.Chin KR, Seale J, Cumming V. “White cord syndrome”of acute tetraplegia after anterior cervical decompression and fusion for chronic spinal cord compression:A case report. Case Rep Orthop. 2013;2013:697918. doi: 10.1155/2013/697918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antwi P, Grant R, Kuzmik G, Abbed K. “White cord syndrome”of acute hemiparesis after posterior cervical decompression and fusion for chronic cervical stenosis. World Neurosurg. 2018;113:33–6. doi: 10.1016/j.wneu.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Busack CD, Eagleton BE. White cord syndrome causing transient tetraplegia after posterior decompression and fusion. Ochsner J. 2020;20:334–8. doi: 10.31486/toj.19.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giammalva GR, Maugeri R, Graziano F, Gulì C, Giugno A, Basile L, et al. White cord syndrome after non-contiguous double-level anterior cervical decompression and fusion (ACDF):A “no reflow phenomenon”? Interdiscip Neurosurg. 2017;7:47–9. [Google Scholar]
- 5.Kalidindi KK, Sath S. “White cord syndrome”of acute tetraplegia after posterior cervical decompression and resulting hypoxic brain injury. Asian J Neurosurg. 2020;15:756–8. doi: 10.4103/ajns.AJNS_240_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathkour M, Werner C, Riffle J, Scullen T, Dallapiazza RF, Dumont A, et al. Reperfusion “white cord''syndrome in cervical spondylotic myelopathy:Does mean arterial pressure goal make a difference?Additional case and literature review. World Neurosurg. 2020;137:194–9. doi: 10.1016/j.wneu.2020.01.062. [DOI] [PubMed] [Google Scholar]
- 7.Papaioannou I, Repantis T, Baikousis A, Korovessis P. Late-onset ”white cord syndrome“in an elderly patient after posterior cervical decompression and fusion:A case report. Spinal Cord Ser Cases. 2019;5:28. doi: 10.1038/s41394-019-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal DN, Lunati MP, Kukowski NR, Michael KW. White cord syndrome and acute tetraplegia after posterior cervical decompression. JBJS Case Connect. 2021;11(2) doi: 10.2106/JBJS.CC.20.00281. doi:10.2106/JBJS. CC.20.00281. [DOI] [PubMed] [Google Scholar]
- 9.Sepulveda F, Carballo L, Carnevale M, Yañez P. White cord syndrome in a pediatric patient:A case report and review. Radiol Case Rep. 2020;15:2343–7. doi: 10.1016/j.radcr.2020.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao YX, He SS, He ZM. 'White cord syndrome', a rare but disastrous complication of transient paralysis after posterior cervical decompression for severe cervical spondylotic myelopathy and spinal stenosis:A case report. Exp Ther Med. 2020;20:90. doi: 10.3892/etm.2020.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, et al. Oxidative stress in ischemic brain damage:Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–17. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang T, Wu L, Wang H, Fang J, Yao N, Xu Y. Inflammation level after decompression surgery for a rat model of chronic severe spinal cord compression and effects on ischemia-reperfusion injury. Neurol Med Chir (Tokyo) 2015;55:578–86. doi: 10.2176/nmc.oa.2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, et al. Acute spinal cord injury, part I:Pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–64. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Fehlings MG, Tetreault L, Hsieh PC, Traynelis V, Wang MY. Introduction:Degenerative cervical myelopathy:Diagnostic, assessment, and management strategies, surgical complications, and outcome prediction. Neurosurg Focus. 2016;40:E1. doi: 10.3171/2016.3.FOCUS16111. [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Dong HL, Li YZ, Luo ZJ, Sun L, Yang QZ, et al. Effects of remote ischemic preconditioning on biochemical markers and neurologic outcomes in patients undergoing elective cervical decompression surgery:A prospective randomized controlled trial. J Neurosurg Anesthesiol. 2010;22:46–52. doi: 10.1097/ANA.0b013e3181c572bd. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher MJ, Hogg FR, Zoumprouli A, Papadopoulos MC, Saadoun S. Spinal cord blood flow in patients with acute spinal cord injuries. J Neurotrauma. 2019;36:919–29. doi: 10.1089/neu.2018.5961. [DOI] [PubMed] [Google Scholar]
- 18.Turner JD, Eastlack RK, Mirzadeh Z, Nguyen S, Pawelek J, Mundis GM., Jr Fluctuations in spinal cord perfusion during adult spinal deformity correction identify neurologic changes:Proof of concept. World Neurosurg. 2016;85:365.e1–6. doi: 10.1016/j.wneu.2015.08.067. [DOI] [PubMed] [Google Scholar]
- 19.Xie LJ, Huang JX, Yang J, Yuan F, Zhang SS, Yu QJ, et al. Propofol protects against blood-spinal cord barrier disruption induced by ischemia/reperfusion injury. Neural Regen Res. 2017;12:125–32. doi: 10.4103/1673-5374.199004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki M, Koda M, Okawa A, Aiba A. Transient paraparesis after laminectomy for thoracic ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum. Spinal Cord. 2006;44:130–4. doi: 10.1038/sj.sc.3101807. [DOI] [PubMed] [Google Scholar]


