Abstract
Human papillomavirus (HPV) is a family of viruses divided into five genera: alpha, beta, gamma, mu, and nu. There is an ongoing discussion about whether beta genus HPVs (β-HPVs) contribute to cutaneous squamous cell carcinoma (cSCC). The data presented here add to this conversation by determining how a β-HPV E6 protein (β-HPV 8E6) alters the cellular response to cytokinesis failure. Specifically, cells were observed after cytokinesis failure was induced by dihydrocytochalasin B (H2CB). β-HPV 8E6 attenuated the immediate toxicity associated with H2CB but did not promote long-term proliferation after H2CB. Immortalization by telomerase reverse transcriptase (TERT) activation also rarely allowed cells to sustain proliferation after H2CB exposure. In contrast, TERT expression combined with β-HPV 8E6 expression allowed cells to proliferate for months following cytokinesis failure. However, this continued proliferation comes with genome destabilizing consequences. Cells that survived H2CB-induced cytokinesis failure suffered from changes in ploidy.
Keywords: Aneuploid, Polyploid, Chromosome, Skin cancer, β-HPV, Telomerase
1. Introduction
Cutaneous squamous cell carcinoma (cSCC) is one of the most common malignancies worldwide (Lomas et al., 2012; Alam and Ratner, 2001). The annual rate of cSCC has risen for thirty straight years (Hollestein et al., 2014). These malignancies represent a tremendous financial burden, especially in fair-skinned populations. As a result, the United States currently spends $3.8 billion annually on treatments (Deady et al., 2014). UV radiation, light skin color, and immunosuppression are the major risk factors implicated in the development of cSCC (Fahradyan et al., 2017). Additionally, it has been hypothesized that cutaneous human papillomavirus of the beta genus (β-HPV) may be another factor in cSCC progression (Howley and Pfister, 2015a; McLaughlin-Drubin, 2015; Tommasino, 2017).
β-HPV types 5 and 8 were first isolated from sun-exposed skin lesions found in individuals with the rare genetic disorder, epidermodysplasia verruciformis (EV) (Orth, 2008). People with EV are prone to β-HPV infections and cSCC (Orth, 2008; Nunes et al., 2018). A similar association has been observed in people taking immunosuppressive drugs after organ transplants (Genders et al., 2015; Boyle et al., 1984; Boxman et al., 1997). Further, animal and epidemiological studies also suggest β-HPV infections are associated with cSCC (Tommasino, 2017; Chahoud et al., 2016; Patel et al., 2008). Yet β-HPV expression in immunocompetent individuals drops significantly as healthy skin progresses to precancerous actinic keratosis (AK), then onto cSCC (Nunes et al., 2018; Winer et al., 2017; Hampras et al., 2017; Weissenborn et al., 2005, 2009; Howley and Pfister, 2015b). In vitro assays suggest that β-HPV proteins, particularly β-HPV E6, alter cell signaling to promote proliferation, impairing genome stability in the process (Wendel and Wallace, 2017; Rollison et al., 2019). These data have led some to hypothesize that β-HPV augments the mutational burden associated with UV, promoting the early stages of malignant conversion. In what has been called the “hit-and-run” model of viral oncogenesis, these mutations result in a tumor that no longer relies on continued viral gene expression (Aldabagh et al., 2013; Hufbauer and Akgül, 2017; de Koning et al., 2007). While this model has merit, other factors seem to dictate the oncogenic potential of β-HPV infections. For example, a recent publication from Strickley et al. helped solidify the growing consensus that immune status is a central determinant of the oncogenic potential associated with β-HPV infections (Strickley et al., 2019). Other factors may also increase or decrease the risk associated with these infections. Given how widespread β-HPV infections are, it remains important to understand the genetic changes that could augment their deleterious characteristics.
The work described here focuses on the maintenance of genome fidelity during cell division. Live cell microscopy and brightfield microscopy demonstrate that failed cytokinesis occurs about 10% of the time that skin cells enter mitosis (Wallace et al., 2014; Dacus et al., 2020). When this occurs, if the cells continue proliferating, they will suffer changes in ploidy (Hayashi and Karlseder, 2013; Alonso-Lecue et al., 2017; Lens and Medema, 2019). Responses to failed cytokinesis are often studied after induction by dihydrocytochalasin B (H2CB). H2CB causes cytokinesis failure by inhibiting actin polymerization. One study used this approach to show that the Hippo pathway kinase LATS was responsible for orchestrating the cellular response to failed cytokinesis, by inducing p53 accumulation and preventing further proliferation (Ganem et al., 2014). β-HPV 8E6 expression inhibits this buildup of p53 by attenuating LATS activation in a p300-dependent manner (Dacus et al., 2020). Despite the impairment of relevant signaling events, β-HPV 8E6 only imparted transient protection from failed cytokinesis. While β-HPV 8E6 expressing cells tolerated the immediate impact of failed cytokinesis, they were not capable of sustained proliferation. Mutations that activate telomerase are common in cSCC and are associated with growth advantages (Cheng et al., 2015; Griewank et al., 2013; Pópulo et al., 2014). Like β-HPV 8E6 expression, TERT expression had a limited ability to promote proliferation after failed cytokinesis. However, expression of β-HPV 8E6 in cells immortalized by telomerase activation promoted short- and long-term proliferation after failed cytokinesis. The survival of H2CB-induced failed cytokinesis was associated with increased aneuploidy.
2. Results
β-HPV 8E6 expressing HFK cannot sustain proliferation after H2CB-induced failed cytokinesis. β-HPV 8E6 hinders the cellular response to genome destabilizing events, including DNA damage and failed cytokinesis (Wendel and Wallace, 2017; Dacus et al., 2020). This study examines the consequences of β-HPV 8E6’s impairment of signaling events stemming from H2CB-induced cytokinesis failure. β-HPV 8E6 reduces H2CB-induced activation of a Hippo tumor suppressor pathway kinase (LATS), p53 stabilization, and the accumulation of apoptotic markers. β-HPV 8E6 also increases the expression of pro-proliferative TEAD-responsive genes. To determine if these alterations allowed cells to survive H2CB-induced failed cytokinesis, we exposed vector control human foreskin keratinocytes (HFK LXSN) and β-HPV 8E6 expressing HFK (HFK β-HPV 8E6) to media containing 4 μM of H2CB for 6 days (Ganem et al., 2014). Cells counted on day 0 are referred to as ‘before’ H2CB. After 6 days of H2CB exposure, cells were counted and are referred to as ‘during’. H2CB was washed out and cells were placed in growth media. Cells were monitored until they reached approximately 90% confluency or stopped proliferating (referred to as ‘after’). At this point, viable cultures were counted, passaged, and considered to have recovered (recovered-HFK LXSN or recovered-HFK β-HPV 8E6) from H2CB exposure. Three independent biological replicates found similar results. β-HPV 8E6 attenuated the immediate consequences of H2CB-associated toxicity (compare the number of HFK LXSN and HFK β-HPV 8E6 after 6 days of H2CB exposure in Fig. 1A). However, neither cell line was capable of sustained proliferation after H2CB (Fig. 1A).
Fig. 1. H2CB-induced failed cytokinesis prevents long-term proliferation.
(A) Three growth curves (biological replicates) comparing HFK LXSN and β-HPV 8E6 cells before, during, and after 6 days of H2CB exposure in 6-well tissue culture plates. HFK LXSN (dashed) and β-HPV 8E6 (solid) data with the same color and number (red, 1; green, 2; and blue, 3) were treated in parallel. (B) Two charts representing GO analysis of common mutations in cSCC. The larger chart on the left represents nodes of similar GO: biological process terms. The smaller chart represents the two GO: biological process terms within the “Proliferation” node. TERT expression allows β-HPV 8E6 HFKs growth after H2CB-induced failed cytokinesis.
β-HPV infections occur in different genetic backgrounds, some of which could act synergistically with β-HPV 8E6 to allow cells to recover from H2CB-induced failed cytokinesis (Martincorena et al., 2015). Given the links between β-HPV and cSCC development, recurrent genetic contributors to cSCC development were examined to identify candidate alterations. Specifically, common mutations from sequencing data of 68 cSCC were ranked by their frequency (Pickering et al., 2014; Li et al., 2015; Gao et al., 2013; Cerami et al., 2012) (Supplemental Data 1). Then, a gene ontology analysis was performed on the top 10% of mutations using the web-based gene ontology software, PANTHER (Mi et al., 2017; The Gene Ontology Resourc, 2019; Ashburner et al., 2000) (Fig. 1B). The biological process “replicative senescence” contained within the “proliferation” node contained commonly mutated genes in cSCC. A complementary gene ontology software also identified “replicative senescence” among the cellular responses enriched within cSCC mutated genes (data not shown). This broad unbiased approach was complimented with a literature-based prioritization of the mutated genes. Among the genes in the “replicative senescence” node, mutations in TERT (the gene encoding telomerase reverse transcriptase, a component of telomerase) were notable. Multiple other studies have identified telomerase activating mutations within TERT promoter region in cSCC (Cheng et al., 2015; Griewank et al., 2013; Pópulo et al., 2014; Scott et al., 2014). Enhanced telomerase activity can promote proliferation despite damage and stress that would normally remove cells from the cell cycle (Urquidi et al., 2000; Victorelli and Passos, 2017; Davoli et al., 2010). It also allows cells immortalized by telomerase activation to continue growing after exposure to cytochalasin B, an unsaturated derivative of H2CB, which shares the ability to inhibit cell division, but unlike H2CB, it affects sugar transport (31, 48–51). These observations suggest that TERT activation is a relevant alteration in cSCC and that it could act on its own or synergize with β-HPV 8E6 to promote growth after cytokinesis failure.
To determine if TERT activation could promote survival from H2CB, β-HPV 8E6 expression was examined in HFK immortalized by telomerase activation (TERT-HFK). Specifically, the effects of H2CB on long term proliferation were studied in previously characterized HA-tagged β-HPV 8E6 and vector control TERT-HFKs (TERT-HFK β-HPV 8E6 and TERT-HFK LXSN, respectively) (Wang et al., 2016; Dickson et al., 2000). β-HPV 8E6 maintained its previously reported ability to alter the response to H2CB and increase TEAD-responsive gene expression in this genetic background (Supplementary Fig. 1A–C). Further, H2CB exposure was more effective at inducing binucleation and senescence (indicated by senescence-associated β-Galactosidase or SA β-Gal staining) in TERT-HFK cells (Supplementary Fig. 1D,E). These data confirmed that both β-HPV 8E6 and H2CB retained their reported activities in TERT-HFK cells. Next, the impact of H2CB exposure (6 days of 4 μM H2CB) on long-term proliferation was defined for three biological replicates using the growth conditions described in Fig. 1A. β-HPV 8E6 continued to reduce cell death in TERT-HFKs during H2CB exposure (compare cell lines at day 6 in Fig. 2A). However, β-HPV 8E6 was also able to promote recovery from H2CB-induced failed cytokinesis in this genetic background (recovered-TERT-HFK β-HPV 8E6). In each of these long-term growth assays, TERT-HFK β-HPV 8E6 survived for at least 17 days after H2CB exposure (Fig. 2A). Unfortunately, one repeat was contaminated and could not be expanded after survival. In contrast, none of the attempts to grow TERT-HFK LXSN after H2CB exposure were successful (recovered-TERT-HFK LXSN).
Fig. 2. TERT expression promotes recovery from failed cytokinesis.
(A) Three growth curves (biological replicates) comparing TERT-HFK LXSN and β-HPV 8E6 cells before, during, after, and recovered from 6 days of H2CB exposure in 6-well tissue culture plates. LXSN (dashed) and β-HPV 8E6 (solid) data with the same color and number (red, 1; green, 2; and blue, 3) were treated in parallel. ! signifies the premature end of the long-term cultivation due to bacterial contamination. (B) Percent of HFK and TERT-HFK cells capable of long-term growth after 6 days in H2CB.
To obtain recovered-TERT-HFK LXSN to compare to recovered-TERT-HFK β-HPV 8E6 cells, 6 additional replicates were performed in a format with a larger initial population of cells (expansion from a 6-well to 10-cm plate format). In these conditions, only one of the TERT-HFK LXSN cell lines survived (See Fig. 2B). TERT-HFK β-HPV 8E6 cells also survived in each of the experiments conducted in 10-cm plates. Representative growth data for these cells can be found in Supplementary Figure 2. Recovered-TERT-HFK cells were expanded to determine the genomic consequences of surviving H2CB.
3. β-HPV 8E6 exacerbates aneuploidy in TERT-HFKs after recovering from failed cytokinesis
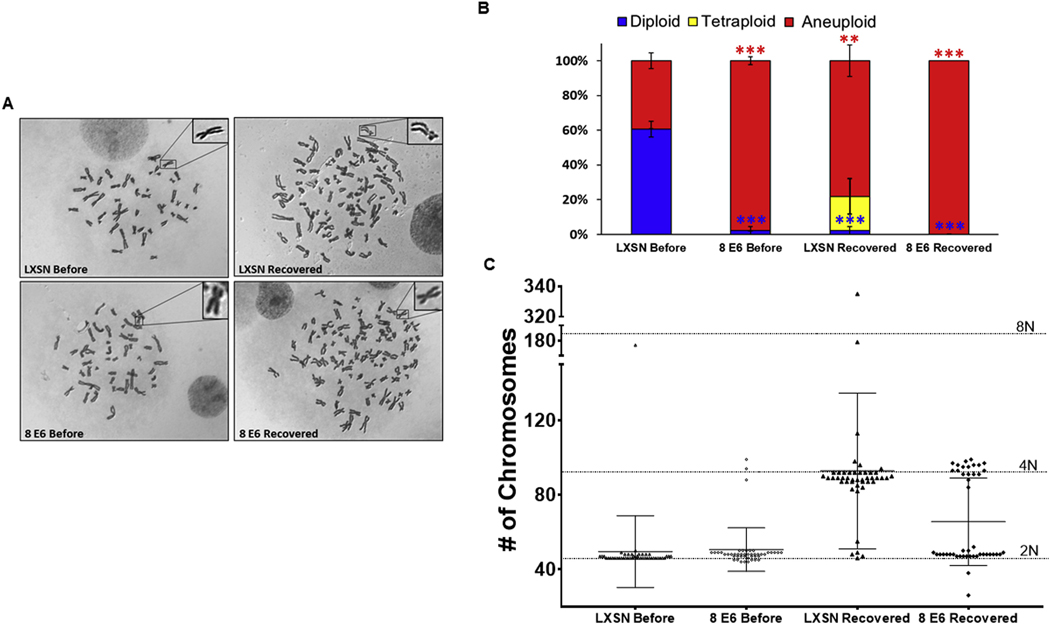
Failed cytokinesis jeopardizes genome integrity, particularly when cells continue to proliferate afterward (Hayashi and Karlseder, 2013; Storchova and Kuffer, 2008; Ganem et al., 2007). To determine if the cells that survived H2CB-induced failed cytokinesis had impaired genomic instability, differential interference contrast microscopy of condensed chromosomes from metaphase spreads was used to compare the ploidy of TERT-HFK LXSN to recovered-TERT-HFK LXSN cells (Fig. 3A). While most TERT-HFK LXSN were diploid before H2CB treatment, many recovered-TERT-HFK LXSN cells had aneuploid genomes. A small subset of recovered-TERT-HFK LXSN cells had tetraploid genomes (Fig. 3B). Chromosome abnormalities were exacerbated by β-HPV 8E6 in the cell line paired with the only recovered-TERT-HFK LXSN cell line. Most TERT-HFK β-HPV 8E6 cells had aneuploid genomes before H2CB exposure and all the recovered-TERT-HFK β-HPV 8E6 were aneuploid (Fig. 3B, C). The length of time in passage is unlikely to explain these data as the cells were analyzed after a similar time in culture. Further, the results were nearly identical when ploidy was determined immediately after recovery or several passages later (data not shown).
Fig. 3. β-HPV 8E6 and cytokinesis failure induce changes in ploidy.
(A) Representative images of metaphase spreads. Insert on the top-right corner shows a magnified chromosome. (B) Relative frequency of diploidy (blue), tetraploidy (yellow), and aneuploidy (red) before H2CB treatment and once cells recovered. Red and blue asterisks denote a significant difference from ‘LXSN Before’ for aneuploidy and diploidy, respectively. (C) Graphical presentation of the distribution of the number of chromosomes among ≥ 45 cells analyzed by metaphase spreads (before or recovered from 6 days of H2CB exposure). Horizontal-dotted lines represent 46 (2 N), 92 (4 N), or 184 (8 N) chromosomes. ** denotes significant difference between indicated samples p ≤ 0.01, *** denotes p ≤ 0.001 (Student’s t-test).
4. Discussion
β-HPVs promote the proliferation of damaged skin cells (Howley and Pfister, 2015a; Tommasino, 2017; Rollison et al., 2019; Wallace et al., 2014; Dacus et al., 2020). β-HPV 8E6 is a critical contributor to this phenotype and acts at least in part by suppressing apoptotic responses (Dacus et al., 2020; Underbrink et al., 2008). As a result, β-HPV infections have been hypothesized to allow the accumulation of potentially tumorigenic mutations. Here, we examine the ability of β-HPV 8E6 to act along with TERT expression to facilitate the survival of cells that do not divide after replicating their genomes. We summarize our observations in Fig. 4. When cytokinesis failure was induced by H2CB, HFK were unable to sustain long-term growth (Fig. 4A). β-HPV 8E6 did not change this outcome (Fig. 4B). Immortalization by telomerase activation rarely allowed cells to recover from.
Fig. 4.
β-HPV 8E6 and telomerase activation affect cell fate after failed cytokinesis. (A) Keratinocytes that experience H2CB-induced cytokinesis failure become binucleated (indicated by two nuclei inside the cell) resulting in cell death and inhibition of long-term proliferation. (B) β-HPV 8E6 (indicated by green nuclei) reduces the death associated with cytokinesis failure but cells remain unable to sustain long-term proliferation. (C) Keratinocytes immortalized by TERT activation (indicated by purple nuclei) experience H2CB-associated binucleation and toxicity, but unlike primary keratinocytes, a small number recover. (D) TERT immortalized keratinocytes that co-express β-HPV 8E6 (indicated by green/purple nuclei) regularly survive H2CB-exposure but have high levels of aneuploidy.
H2CB exposure (Fig. 4C). However, the combination of β-HPV 8E6 expression and TERT expression allowed cells to sustain proliferation for months (presumably indefinitely) after cytokinesis failure and augmented genomic instability (Fig. 4D).
When comparing HFK and TERT-HFK cell lines some caution should be exercised as they were generated from different donors. However, phenotypes are frequently replicated across.
Keratinocytes from separate persons (White et al., 2012; Howie et al., 2011; Meyers et al., 2017). More specific to this study and these cells, the previously reported attenuation of the Hippo pathway kinase LATS activation by β-HPV 8E6 was conserved between both HFK and TERT-HFK cell lines (Supplementary Figure 1 and (Dacus et al., 2020)). These data are consistent with the established idea that telomerase activation promotes carcinogenesis and suggest that β-HPV infections may augment the transformative power of telomerase activation.
Our data also provides other, more specific insights. For instance, we found that β-HPV 8E6 made TERT-HFKs approximately 2.5 times more likely to be aneuploid (Fig. 3C). To our knowledge, this is the first report associating changes in ploidy with β-HPV 8E6. The observation is in line with reports from the Tommasino Lab that describe changes in ploidy in β-HPV 38 E6 and E7 immortalized keratinocytes (Gabet et al., 2008). Unlike our report, they demonstrated that ectopic TERT expression reduced aneuploidy, likely by reducing the chromosomal rearrangements, anaphase bridges, and multipolar mitoses associated with β-HPV 38 E6/E7 immortalization. This could be the result of differences between β-HPV 38 E6 and β-HPV 8E6 or they might be explained by the presence/absence of the β-HPV E7 protein (Tommasino, 2017; Howley and Pfister, 2015b).
In vitro studies on β-HPVs tend to examine the effects of stimuli over a short time interval (hours to days). However, the average β-HPV infection persists for six to eleven months (de Koning et al., 2007; Hampras et al., 2014). TERT-HFK cells provide a system to replicate lengthier conditions and our data demonstrates the utility of such an approach. By removing the restrictive nature of primary cell growth, we were able to describe the changes in ploidy stemming from failed cytokinesis. Based on our data, caution should be exercised when examining these systems as TERT expression can change the cellular response to genome destabilizing events.
Indeed, our data offers proof of principle that phenotypes associated with β-HPV E6 can change based on the genetic context of viral gene expression. There may be genetic environments where cutaneous papillomavirus infections promote cSCC and others where the same infections prevent cSCC. If this were true, it might help explain conflicting reports that describe these infections as oncogenic and oncopreventative (Howley and Pfister, 2015a; Aldabagh et al., 2013; Strickley et al., 2019; Hasche et al., 2018). Moving forward, it will be interesting to determine the ability of β-HPV E6 to synergize with other common mutations and the mechanism by which β-HPV 8E6 increases aneuploidy.
5. Material and methods
5.1. Cell culture
Primary HFK were derived from neonatal human foreskins. HFK and TERT-immortalized-HFK (obtained from Michael Underbrink, University of Texas Medical Branch) were grown in EpiLife medium supplemented with calcium chloride (60 μM), human keratinocyte growth supplement (ThermoFisher Scientific), and penicillin-streptomycin. HPV genes were cloned, transfected, and confirmed as previously described (Wallace et al., 2014). In order not to activate the Hippo pathway via contact inhibition, we carefully monitored the cell density in all experiments. Experiments were aborted if unintended differences in seeding resulted in cell densities that were more than 10% different among cell lines at the beginning of an experiment.
5.2. cBioPortal and gene ontology analysis
Software from (www.cbioportal.org) was used to recognize, analyze, and categorize mutations and transcriptomic data from cutaneous squamous cell carcinomas (Pickering et al., 2014; Li et al., 2015). Analysis of the squamous cell carcinoma samples was done at (http://geneontology.org/) powered by Protein ANalysis THrough Evolutionary Relationships (PANTHER) (The Gene Ontology Resourc, 2019; Ashburner et al., 2000).
5.3. H2CB recovery assay
6-well format: Cells were counted, then either 1.5 × 105 HFK or 5 × 104 TERT-HFK cells were seeded on a 6 well tissue culture plate and grown for 24 h. Cells were then treated with 4 μM H2CB, refreshing the H2CB media every 2 days. After 6 days, the cells were washed with PBS and given fresh EpiLife. Once cells reached 90% confluency, they were counted then moved to new 6 wells. This process was continued until cells were no longer able to be passaged or cells could be moved to a 10 cm plate.
10 cm format: Cells were counted, then 3.0 × 105 cells were seeded on a 10 cm tissue culture plate and grown for 24 h. Cells were then treated with 4 μM H2CB, refreshing the H2CB media every 2 days. After 6 days, the cells were washed with PBS and given fresh EpiLife. Once cells reached 90% confluency they were counted, then 9.0 × 104 cells were reseeded. This process was continued until cells were no longer able to be passaged or for 28 days.
5.4. RT-qPCR
Cells were lysed, isolated, reverse transcribed, and then RT-qPCR was performed as previously described (Dacus et al., 2020). The following probes (Thermo Scientific) were used: ACTB (Hs01060665_g1), STK4 (Hs00178979_m1), LATS2 (Referred to as LATS in the text) (Hs01059009_m1), YAP1 (Hs00902712_g1), CTGF (Hs00170014_m1), CYR61 (Hs00155479_m1), TEAD1 (Hs00173359_m1), CCND1 (Hs00765553_m1), AXL (Hs01064444_m1), SERPINE1 (Hs00167155_m1).
5.5. Immunoblotting
Cells were washed and lysed, then lysates were run, transferred, probed, and visualized as previously described (Dacus et al., 2020). The following antibodies were used: GAPDH (Santa Cruz Biotechnologies sc-47724), LATS2 (Referred to as LATS in the text, Cell Signaling Technologies D83D6), Phospho-LATS1/2 (Ser909) (Referred to as pLATS in the text) (Cell Signaling Technologies #9157), YAP (Cell Signaling Technologies 4912S), Phospho-YAP (Ser127) (Referred to pYAP in the text) (Cell Signaling Technologies 4911S), AXL (Cell Signaling Technologies 8661S, p300 (Santa Cruz Biotechnologies sc-584).
5.6. Senescence-associated β-galactosidase staining
Cells were seeded onto three 6-well plates and treated with H2CB then stained as previously described (Dacus et al., 2020).
5.7. Chromosome counts via metaphase spread
‘Before’ and after cells recovered from H2CB exposure TERT-immortalized HFK cells were grown to 80% confluency then chromosomes were detected and counted as previously described (Howe et al., 2014).
5.8. Statistical analysis
Unless otherwise noted, statistical significance was determined by a paired Student t-test and was confirmed when appropriate by a two-way analysis of variance (ANOVA) with Turkey’s correction. Only P values less than 0.05 were reported as significant.
Supplementary Material
Acknowledgments:
We thank and acknowledge Jocelyn A. McDonald for assisting with our metaphase spread imaging. Michael Underbrink for providing the TERT-immortalized-HFK. Jazmine A. Snow and Emily Burghardt for constructive criticism of the manuscript.
This work was supported by the Department of Defense CMDRP PRCRP CA160224 (NW) and made possible through generous support from the Les Clow family and the Johnson Cancer Research Center at Kansas State University.
Footnotes
Declarations of interest: none.
CRediT authorship contribution statement
Dalton Dacus: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Elizabeth Riforgiate: Validation, Writing - original draft, Writing - review & editing, Investigation. Nicholas A. Wallace: Conceptualization, Methodology, Validation, Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virol.2020.07.016.
Declaration of competing interest
The authors declare that they have no conflicts of interests related to the work described in our manuscript.
References
- Alam M, Ratner D, 2001. Cutaneous squamous-cell carcinoma. N. Engl. J. Med 344, 975–983. [DOI] [PubMed] [Google Scholar]
- Aldabagh B, Angeles JGC, Cardones AR, Arron ST, 2013. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al 39, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Lecue P, de Pedro I, Coulon V, Molinuevo R, Lorz C, Segrelles C, Ceballos L, López-Aventín D, García-Valtuille A, Bernal JM, Mazorra F, Pujol RM, Paramio J, Ramón Sanz J, Freije A, Toll A, Gandarillas A, 2017. Ineffcient differentiation response to cell cycle stress leads to genomic instability and malignant progression of squamous carcinoma cells. 6. Cell Death Dis. 8, e2901 e2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G, 2000. Gene Ontology: tool for the unification of biology. Nat. Genet 25, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxman ILA, Berkhout RJM, Mulder LHC, Wolkers MC, Bavinck JNB, Vermeer BJ, ter Schegget J, 1997. Detection of human papillomavirus DNA in plucked hairs from renal transplant recipients and healthy volunteers. J. Invest. Dermatol 108, 712–715. [DOI] [PubMed] [Google Scholar]
- Boyle J, Briggs JD, RonaM Mackie, Junor BJR, Aitchison TC, 1984. CANCER, warts, and sunshine IN renal transplant patients: a case-control study. Lancet 323, 702–705. [DOI] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N, 2012. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahoud J, Semaan A, Chen Y, Cao M, Rieber AG, Rady P, Tyring SK, 2016. Association between β-genus human papillomavirus and cutaneous squamous cell carcinoma in immunocompetent individuals—a meta-analysis. JAMA Dermatol 152, 1354–1364. [DOI] [PubMed] [Google Scholar]
- Cheng KA, Kurtis B, Babayeva S, Zhuge J, Tantchou I, Cai D, Lafaro RJ, Fallon JT, Zhong M, 2015. Heterogeneity of TERT promoter mutations status in squamous cell carcinomas of different anatomical sites. Ann. Diagn. Pathol 19, 146–148. [DOI] [PubMed] [Google Scholar]
- Dacus D, Cotton C, McCallister TX, Wallace NA, 2020. Beta Human Papillomavirus 8E6 Attenuates LATS Phosphorylation after Failed Cytokinesis. Journal of Virology 94. 10.1128/JVI.02184-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T, Denchi EL, de Lange T, 2010. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell 141, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning MNC, Struijk L, Bavinck JNB, Kleter B, ter Schegget J, Quint WGV, Feltkamp MCW, 2007. Betapapillomaviruses frequently persist in the skin of healthy individuals. J. Gen. Virol 88, 1489–1495. [DOI] [PubMed] [Google Scholar]
- Deady S, Sharp L, Comber H, 2014. Increasing skin cancer incidence in young, affluent, urban populations: a challenge for prevention. Br. J. Dermatol 171, 324–331. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG, 2000. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell Biol 20, 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahradyan A, Howell AC, Wolfswinkel EM, Tsuha M, Sheth P, Wong AK, 2017. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabet A-S, Accardi R, Bellopede A, Popp S, Boukamp P, Sylla BS, Londoño-Vallejo JA, Tommasino M, 2008. Impairment of the telomere/telomerase system and genomic instability are associated with keratinocyte immortalization induced by the skin human papillomavirus type 38. FASEB J 22, 622–632. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Storchova Z, Pellman D, 2007. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev 17, 157–162. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Cornils H, Chiu S-Y, O’Rourke KP, Arnaud J, Yimlamai D, Théry M, Camargo FD, Pellman D, 2014. Cytokinesis failure triggers Hippo tumor suppressor pathway activation. Cell 158, 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N, 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genders RE, Mazlom H, Michel A, Plasmeijer EI, Quint KD, Pawlita M, van der Meijden E, Waterboer T, de Fijter H, Claas FH, Wolterbeek R, Feltkamp MCW, Bouwes Bavinck JN, 2015. The presence of betapapillomavirus antibodies around transplantation predicts the development of keratinocyte carcinoma in organ transplant recipients: a cohort study. J. Invest. Dermatol 135, 1275–1282. [DOI] [PubMed] [Google Scholar]
- Griewank KG, Murali R, Schilling B, Schimming T, Möller I, Moll I, Schwamborn M, Sucker A, Zimmer L, Schadendorf D, Hillen U, 2013. TERT promoter mutations are frequent in cutaneous basal cell carcinoma and squamous cell carcinoma. PLoS ONE 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampras SS, Giuliano AR, Lin H-Y, Fisher KJ, Abrahamsen ME, Sirak BA, Iannacone MR, Gheit T, Tommasino M, Rollison DE, 2014. Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PLOS ONE 9, e104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampras SS, Rollison DE, Giuliano AR, McKay-Chopin S, Minoni L, Sereday K, Gheit T, Tommasino M, 2017. Prevalence and concordance of cutaneous beta human papillomavirus infection at mucosal and cutaneous sites. J. Infect. Dis 216, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasche D, Vinzón SE, Rösl F, 2018. Cutaneous papillomaviruses and non-melanoma skin cancer: causal agents or innocent bystanders? Front. Microbiol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MT, Karlseder J, 2013. DNA damage associated with mitosis and cytokinesis failure. Oncogene 32, 4593–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollestein LM, de Vries E, Aarts MJ, Schroten C, Nijsten TEC, 2014. Burden of disease caused by keratinocyte cancer has increased in The Netherlands since 1989. J. Am. Acad. Dermatol 71, 896–903. [DOI] [PubMed] [Google Scholar]
- Howe B, Umrigar A, Tsien F, 2014. Chromosome preparation from cultured cells. J Vis Exp. 10.3791/50203. e50203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie HL, Koop JI, Weese J, Robinson K, Wipf G, Kim L, Galloway DA, 2011. Beta-HPV 5 and 8 E6 promote p300 degradation by blocking AKT/p300 association. PLoS Pathog. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley PM, Pfister HJ, 2015a. Beta genus papillomaviruses and skin cancer. Virology 290–296 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley PM, Pfister HJ, 2015b. Beta genus papillomaviruses and skin cancer. Virology 290–296 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufbauer M, Akgül B, 2017. Molecular mechanisms of human papillomavirus induced skin carcinogenesis. Viruses 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens SMA, Medema RH, 2019. Cytokinesis defects and cancer. Nat Rev Cancer 19, 32–45. [DOI] [PubMed] [Google Scholar]
- Li YY, Hanna GJ, Laga AC, Haddad RI, Lorch JH, Hammerman PS, 2015. Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res 21, 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas A, Leonardi-Bee J, Bath-Hextall F, 2012. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol 166, 1069–1080. [DOI] [PubMed] [Google Scholar]
- Martincorena I, Roshan A, Gerstung M, Ellis P, Loo PV, McLaren S, Wedge DC, Fullam A, Alexandrov LB, Tubio JM, Stebbings L, Menzies A, Widaa S, Stratton MR, Jones PH, Campbell PJ, 2015. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, 2015. Human papillomaviruses and non-melanoma skin cancer. Semin. Oncol 42, 284–290. [DOI] [PubMed] [Google Scholar]
- Meyers JM, Uberoi A, Grace M, Lambert PF, Munger K, 2017. Cutaneous HPV8 and MmuPV1 E6 proteins target the NOTCH and TGF-β tumor suppressors to inhibit differentiation and sustain keratinocyte proliferation. PLoS Pathog. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD, 2017. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 45, D183–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EM, Talpe-Nunes V, Sichero L, 2018. Epidemiology and biology of cutaneous human papillomavirus. Clinics 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G, 2008. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr. Top. Microbiol. Immunol 321, 59–83. [DOI] [PubMed] [Google Scholar]
- Patel AS, Karagas MR, Perry AE, Nelson HH, 2008. Exposure profiles and human papillomavirus infection in skin cancer: an analysis of 25 genus β-types in a population-based study. J. Invest. Dermatol 128, 2888–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, Tsai KY, Curry JL, Tetzlaff MT, Lai SY, Yu J, Muzny DM, Doddapaneni H, Shinbrot E, Covington KR, Zhang J, Seth S, Caulin C, Clayman GL, El-Naggar AK, Gibbs RA, Weber RS, Myers JN, Wheeler DA, Frederick MJ, 2014. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res 20, 6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pópulo H, Boaventura P, Vinagre J, Batista R, Mendes A, Caldas R, Pardal J, Azevedo F, Honavar M, Guimarães I, Manuel Lopes J, Sobrinho-Simões M, Soares P, 2014. TERT promoter mutations in skin cancer: the effects of sun exposure and X-irradiation. J. Invest. Dermatol 134, 2251–2257. [DOI] [PubMed] [Google Scholar]
- Rollison DE, Viarisio D, Amorrortu RP, Gheit T, Tommasino M, 2019. An emerging issue in oncogenic virology: the role of beta human papillomavirus types in the development of cutaneous squamous cell carcinoma. J. Virol 93, e01003–e01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GA, Laughlin TS, Rothberg PG, 2014. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol 27, 516–523. [DOI] [PubMed] [Google Scholar]
- Storchova Z, Kuffer C, 2008. The consequences of tetraploidy and aneuploidy. J. Cell Sci 121, 3859–3866. [DOI] [PubMed] [Google Scholar]
- Strickley JD, Messerschmidt JL, Awad ME, Li T, Hasegawa T, Ha DT, Nabeta HW, Bevins PA, Ngo KH, Asgari MM, Nazarian RM, Neel VA, Jenson AB, Joh J, Demehri S, 2019. Immunity to commensal papillomaviruses protects against skin cancer. Nature 575, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The gene ontology resource: 20 years and still GOing strong. Nucleic. Acids Res 47, D330–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasino M, 2017. The biology of beta human papillomaviruses. Virus Res. 231, 128–138. [DOI] [PubMed] [Google Scholar]
- Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA, 2008. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J. Virol 82, 10408–10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquidi V, Tarin D, Goodison S, 2000. Role of telomerase in cell senescence and oncogenesis. Annu. Rev. Med 51, 65–79. [DOI] [PubMed] [Google Scholar]
- Victorelli S, Passos JF, 2017. Telomeres and cell senescence - size matters not. EBioMedicine 21, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace NA, Robinson K, Galloway DA, 2014. Beta human papillomavirus E6 expression inhibits stabilization of p53 and increases tolerance of genomic instability. J. Virol 88, 6112–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dupuis C, Tyring SK, Underbrink MP, 2016. Sterile α motif domain containing 9 is a novel cellular interacting partner to low-risk type human papillomavirus E6 proteins. PLoS ONE 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, Majewski S, Pfister H, Wieland U, 2005. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J. Invest. Dermatol 125, 93–97. [DOI] [PubMed] [Google Scholar]
- Weissenborn SJ, De Koning MNC, Wieland U, Quint WGV, Pfister HJ, 2009. Intrafamilial transmission and family-specific spectra of cutaneous betapapilloma-viruses. J. Virol 83, 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel SO, Wallace NA, 2017. Loss of genome fidelity: beta HPVs and the DNA damage response. Front. Microbiol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EA, Kramer RE, Tan MJA, Hayes SD, Harper JW, Howley PM, 2012. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J. Virol 86, 13174–13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer RL, Gheit T, Cherne S, Lin J, Stern JE, Poljak M, Feng Q, Tommasino M, 2017. Prevalence and correlates of beta human papillomavirus detection in fingernail samples from mid-adult women. Papillomavirus Res 5, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.