Abstract
Diving marine mammals are a diverse group of semi‐ to completely aquatic species. Some species are targets of conservation and rehabilitation efforts; other populations are permanently housed under human care and may contribute to clinical and biomedical investigations. Veterinary medical care for species under human care, at times, may necessitate the use of general anesthesia for diagnostic and surgical indications. However, the unique physiologic and anatomic adaptations of one representative diving marine mammal, the bottlenose dolphin, present several challenges in providing ventilatory and cardiovascular support to maintain adequate organ perfusion under general anesthesia. The goal of this review is to highlight the unique cardiopulmonary adaptations of the completely aquatic bottlenose dolphin (Tursiops truncatus), and to identify knowledge gaps in our understanding of how those adaptations influence their physiology and pose potential challenges for sedation and anesthesia of these mammals.
Keywords: anesthesia, cardiopulmonary, cardiovascular, cetacean, dolphin, perfusion adaptations, physiology, pulmonary, ventilation
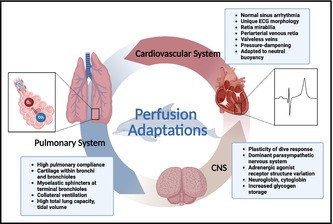
1. INTRODUCTION
Approximately 50–55 million years ago, a terrestrial artiodactyl similar to a small deer made the transition from land to water (Thewissen et al., 2009, 2007). Fossil records suggest that this ancestor of cetaceans became more amphibious over millennia until it became fully aquatic. Cetacea is the mammalian infraorder that includes whales, dolphins and porpoises. The anatomic and physiologic modifications of cetaceans likely provided evolutionary advantages to survival in completely aquatic ecosystems (Dolar et al., 1999; Kooyman & Ponganis, 1997; Piscitelli et al., 2010, 2013). Within the suborder of toothed whales (Odontocetes), a relatively small, shallow‐diving cetacean, the bottlenose dolphin (Tursiops truncatus), is the most extensively studied in its natural environment and while housed under the care of humans. Observational and capture‐release research of wild dolphin populations has provided copious information on dolphin natural history, disease ecology, and diving physiology, as well as historical and current conditions of ocean health (Schwacke et al., 2012; Wells, 2009; Yordy et al., 2010). While housed under human care, bottlenose dolphins often receive comprehensive veterinary medical services and may even contribute to clinical and translational biomedical research (Houser, Finneran, & Ridgway, 2010; Le‐Bert et al., 2018; Meegan, Ardente, et al., 2021; Venn‐Watson et al., 2015, 2022). General anesthesia, however, remains a challenge in dolphins due to a limited number of experienced anesthesiologists and published studies, the significant limitations of current commercially‐available ventilators, and limited anesthetic drug pharmacokinetic studies, including their effects on whole body physiology (Bailey, 2021; Doescher et al., 2018; Dold & Ridgway, 2014; Dover et al., 1999; Higgins & Hendrickson, 2013; Howard et al., 2006; Jones et al., 2023; Le‐Bert et al., 2024; Lee et al., 2019; Lindemann et al., 2023; McCormick & Ridgway, 2018; Medway et al., 1970; Meegan et al., 2015, 2016; Nagel et al., 1964, 1966, 1968; Ridgway, 2002; Ridgway et al., 1975, 1974; Ridgway & McCormick, 1971, 1967; Rosenberg et al., 2017; Russell et al., 2021; Schmitt et al., 2014, 2018; Sommer et al., 1968; Tamura et al., 2017). In this review, we aim to synthesize the current understanding of anesthesia physiology with knowledge of the normal cardiopulmonary physiology and subsequent perfusion adaptations of dolphins and how these adaptations may be modulated during general anesthesia of this completely aquatic marine mammal.
2. HISTORY OF DOLPHIN ANESTHESIA
General anesthesia of dolphins is an infrequently practiced discipline within veterinary medicine. Little technical and practical progress was made between the first dolphin to ever be anesthetized in 1932 and the 1960s (Lilly, 1964; Nagel et al., 1964, 1966). However, during the 1960s and 1970s, Ridgway, Nagel, McCormick and colleagues made significant progress in the successful induction of, and emergence from, anesthesia in dolphins (Medway et al., 1970; Nagel et al., 1964, 1966, 1968; Ridgway et al., 1974; Ridgway & McCormick, 1971, 1967; Sommer et al., 1968). During this period of time, induction was often achieved with intravenous barbiturates (i.e., sodium thiopental, 10–25 mg/kg) and a surgical plane of anesthesia maintained with the volatile gas, halothane, or a nitrous oxide‐oxygen mixture (Table 1). Mechanical ventilation was achieved through adaptation of a Bird Mark 9 large animal ventilator (Bird Respirator Company, Palm Springs, CA) with a custom‐designed apneustic plateau control unit, created to mimic the breath‐holding apneustic breathing pattern of cetaceans. Apneustic plateau ventilation, as coined by Ridgway and McCormick, enabled rapid lung inflation with an inspiratory breath hold at approximately 20–24 mmHg pressure for 15–30 s, followed by airway pressure release, and rapid re‐inflation (Ridgway et al., 1974; Ridgway & McCormick, 1971, 1967). Early ventilation practices using conventional modes of ventilation would result in decreasing trends towards hypoxemia due to hypoventilation (Ridgway et al., 1974). Thus, apneustic plateau ventilation was the standardized approach for mechanical ventilation of dolphins.
TABLE 1.
Summary of general anesthesia performed during anatomic and physiologic studies of dolphins (Tursiops spp.)
| Study objective(s) | No | Age (years) | Weight range (kg) | Pre‐medication agent(s) | Induction agent(s) | Maintenance agent(s) | MAP range (mmHg) | Reversal agent(s) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Effect of halothane anesthesia on hepatic damage during auditory research | 6 (F = 3, M = 3) | N/A | N/A | N/A | Sodium thiopental, 10–15 mg/kg, IV | Halothane | 115 | N/A | Medway et al., 1970; Ridgway & McCormick, 1971 |
| Bispectral index monitor to detect interhemispheric asymmetry | 3 (F = 1, M = 2) | N/A | 212–263 | Diazepam, 0.15 mg/kg, PO (n = 1) | Propofol, 3.03–4.72 mg/kg, IV | N/A | N/A | Howard et al., 2006 | |
| Anesthesia induction and maintenance with thiopental and halothane | 10 | N/A | N/A | N/A | N/A | Halothane | N/A | Ridgway & McCormick, 1967 | |
| 5 | N/A | N/A | N/A | Sodium thiopental, 10 mg/kg, IV | Halothane | N/A | |||
| Surgical approach to the dolphin ear | 4 | N/A | N/A | Atropine, 0.02 mg/kg, IM | Sodium thiopental, 10–15 mg/kg, IV | Halothane, 1–2% | N/A | Ridgway et al., 1974 | |
| Hemodynamic and coronary angiographic studies in the dolphin | 4 | N/A | 80–114 | N/A | Pentobarbital, 10 mg/kg, IP | Nitrous oxide‐oxygen | 122–142 | N/A | Sommer et al., 1968; Nagel et al., 1964; Nagel et al., 1966; Nagel et al., 1968 |
| Return of sound production following anesthetic recovery | 10 (F = 4, M = 6) | 8–46 (mean 32.4) | N/A | Midazolam, 0.08–0.1 mg/kg, IM | Midazolam, 0.02 mg/kg, IV | Sevoflurane | N/A | 1:13 (Midazolam: Flumazenil), IV | Jones et al., 2023 |
| Meperidine, 0.1–0.2 mg/kg, IM | Propofol, 1–4 mg/kg, IV | Naloxone, 0.01 mg/kg, IV | |||||||
| cis‐Atracurium, 0.1 mg/kg, IV | |||||||||
| Apneustic anesthesia ventilation on pulmonary function | 9 (F = 3, M = 6) | 10–42 (mean 32) | 141–292 | Diazepam, 0.08–0.30 mg/kg, PO (n = 2) | Midazolam, 0.02 mg/kg, IV | Sevoflurane, 1.8–2.0% | 80.8+/− 2.9; 86+/−2.6 | Flumazenil, 0.02–0.05 mg/kg, IM/IV | Le‐Bert et al., 2024 |
| Midazolam, 0.08–0.1 mg/kg, IM | Propofol, 2–4 mg/kg, IV | Naloxone, 0.01–0.04 mg/kg, IV (n = 7) | |||||||
| Meperidine, 0.1–0.2 mg/kg, IM | Naltrexone, 0.05–0.20 mg/kg, IV (n = 6) | ||||||||
| Plasma propofol concentrations in dolphins | 6 | 12–27 (mean unk) | N/A | Diazepam, PO | Propofol, 1.97–5.33 mg/kg, IV | Sevoflurane | N/A | N/A | Schmitt et al., 2018 |
| Midazolam, IM | Midazolam, IV | ||||||||
Anesthetic practices in the 60s and 70s evaluated the use of a low solubility anesthetic gas, nitrous oxide, for maintaining a surgical plane of anesthesia. This minimally potent inhalational anesthetic was often combined with a neuromuscular blocking agent (succinylcholine) and a parenteral barbiturate (thiopental) in dolphins. However, a mixed gas anesthetic protocol of 60% nitrous oxide with 40% oxygen did not result in a surgical plane of anesthesia. In one study, an increase to 80% nitrous oxide resulted in lost reflexes and complete unconsciousness following an initial period of hyperexcitability (Ridgway & McCormick, 1971). In a separate study, persistent reflexes and visual tracking at the same concentration of nitrous gas mixture was reported (Ridgway & McCormick, 1967). Further, at 80% nitrous oxide, hypoxemia and cyanosis of the mucus membranes were observed. The combination of continued presence of consciousness and inadequate oxygenation at high inspired nitrous oxide concentrations, led Ridgway and colleagues to cite the nitrous gas mixture as inadequate for major surgery in dolphins, especially as a sole anesthetic agent (Ridgway & McCormick, 1967).
Early hemodynamic studies in these anesthetized dolphins provided insights into cardiovascular function under general anesthesia. Mean arterial pressures in healthy dolphins on halothane gas anesthesia averaged 115 mmHg (normal reported as 120–140 mmHg) (Ridgway & McCormick, 1971). Dolphins on nitrous oxide‐oxygen gas anesthesia ranged between 122 and 142 mmHg (Sommer et al., 1968). Ridgway also observed that the normal, respiratory sinus arrhythmia (RSA) observed in the conscious, non‐anesthetized dolphin transitioned to a normal sinus rhythm, with heart rates between 80 and 160 bpm, after thiopental (15–25 mg/kg) administration (Ridgway et al., 1974; Ridgway & McCormick, 1971, 1967).
While Ridgway published on observational aspects of clinical anesthesia in dolphins, few comprehensive and controlled physiologic studies of anesthetized dolphins have since been conducted (McCormick, 1969; Sommer et al., 1968). Most reports are limited to single case descriptions that document individual dolphins (Tursiops spp.) recovering from surgical or diagnostic procedures, rather than controlled pharmacokinetic or physiologic studies on the effects of ventilation and anesthetics agents (Table 2) (Bailey, 2021; Doescher et al., 2018; Dover et al., 1999; Lee et al., 2019; Lindemann et al., 2023; Meegan et al., 2015, 2016; Meegan, Miller, et al., 2021; Ridgway, 2002; Russell et al., 2021; Schmitt et al., 2014; Tamura et al., 2017). The paucity of comprehensive, controlled anesthetic studies in bottlenose dolphins remain a hurdle in our understanding of the physiology of anesthesia in this species.
TABLE 2.
Summary of single case reports of successful general anesthesia and recovery in dolphins (Tursiops spp.)
| Clinical indication | Age (years) | Sex | Weight (kg) | Pre‐medication agent(s) | Induction agent(s) | Maintenance agent(s) | MAP range (mmHg) | Reversal agent(s) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cerebral spinal fluid sampling | 5 | F | 106 | Diazepam, 0.28 mg/kg, PO | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 93–122 | Flumazenil, 0.01 mg/kg, IM | Russell et al., 2021; Bailey, 2021 |
| Midazolam, 0.05 mg/kg, IM | Propofol, 2 mg/kg, IV | Flumazenil, 0.01 mg/kg, IV | |||||||
| Ventral cervical abscess surgical debridement | 22 | F | 174 | Midazolam, 0.07 mg/kg, IM | Midazolam, 0.04 mg/kg, IV | Sevoflurane | 44–55 | Flumazenil, 0.04 mg/kg, IV | Lee et al., 2019; Meegan et al., 2015; Bailey, 2021 |
| Meperidine, 0.5 mg/kg, IM | Propofol, 2 mg/kg, IV | Naloxone, 0.02 mg/kg, IV | |||||||
| Naltrexone, 0.05 mg/kg, IV | |||||||||
| Renal biopsy, laparoscopy | 27 | F | 150 | Diazepam, 0.27 mg/kg, PO | Propofol, 3.5 mg/kg, IV | Isoflurane | N/A | Flumazenil, 0.001 mg/kg, IM | Dover et al., 1999; Bailey, 2021 |
| Atropine, 0.02 mg/kg, IM | Flumazenil, 0.002 mg/kg, IV | ||||||||
| Electroencephalography | N/A | M | 140 | N/A | Sodium thiopental, IV | Halothane | N/A | Ridgway, 2002 | |
| Lithotripsy | 39 | F | 175 | Midazolam, 0.07 mg/kg, IM | Midazolam, 0.06 mg/kg, IV | Sevoflurane | 57–117 | Flumazenil, 0.024 mg/kg, IV | Bailey, 2021 |
| Propofol, 3.6 mg/kg, IV | |||||||||
| Partial glossectomy | 24 | F | 206 | Diazepam, 0.24 mg/kg, PO | Midazolam, 0.024 mg/kg, IV | Sevoflurane | 63–81 | Flumazenil, 0.017 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.05 mg/kg, IM | Propofol, 4 mg/kg, IV | Flumazenil, 0.01 mg/kg, IM | |||||||
| Corneal scleral mass | 17 | M | 184.5 | Diazepam, 0.22 mg/kg, PO | Midazolam, 0.027 mg/kg, IV | Sevoflurane | N/A | Flumazenil, 0.01 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.054 mg/kg, IM | Propofol, 2.87 mg/kg, IV | Edrophonium, 0.5 mg/kg, IV | |||||||
| Atracurium, 0.1 mg/kg, IV | |||||||||
| Mandibular sequestrum debridement | 18 | M | 220.5 | Diazepam, 0.18 mg/kg, PO | Midazolam, 0.023 mg/kg, IV | Sevoflurane | 25–35 | Flumazenil, 0.026 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.05 mg/kg, IM | Propofol, 1.5 mg/kg, IV | Naloxone, 0.02 mg/kg, IV | |||||||
| Naltrexone, 0.05 mg/kg, IV | |||||||||
| Glossectomy | 44 | M | 215.5 | Diazepam, 0.23 mg/kg, PO | Propofol, 4 mg/kg, IV | Sevoflurane | 44–87 | Flumazenil, 0.019 mg/kg, IV | Doescher et al., 2018; Bailey, 2021 |
| Midazolam, 0.08 mg/kg, IM | |||||||||
| Partial glossectomy | 35 | F | 245 | Diazepam, 0.25 mg/kg, PO | Midazolam, 0.02 mg/kg, IV | Sevoflurane | N/A | Flumazenil, 0.02 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.05 mg/kg, IM | Propofol, 2 mg/kg, IV | ||||||||
| Partial glossectomy | 27 | F | 225 | Diazepam, 0.25 mg/kg, PO | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 98–120 | Flumazenil, 0.022 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.05 mg/kg, IM | Propofol, 1.15 mg/kg, IV | ||||||||
| Atropine, 0.02 mg/kg, IM | |||||||||
| Partial glossectomy | 20 | F | 190 | Diazepam, 0.25 mg/kg, PO | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 33–65 | Flumazenil, 0.025 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.05 mg/kg, IM | Propofol, 2.1 mg/kg, IV | ||||||||
| Lymphadenectomy | 49 | M | 140 | Midazolam, 0.2 mg/kg, IM | Midazolam, 0.035 mg/kg, IV | Sevoflurane | 24–53 | Flumazenil, 0.021 mg/kg, IV | Bailey, 2021 |
| Propofol, 1.5 mg/kg, IV | Naloxone, 0.04 mg/kg, IV | ||||||||
| Gastroscopy for foreign body retrieval | 12 | M | 117 | Midazolam, 0.1 mg/kg, IM | Midazolam, 0.042 mg/kg, IV | Sevoflurane | 76–119 | Flumazenil, 0.016 mg/kg, IV | Bailey, 2021 |
| Propofol, 2.14 mg/kg, IV | Naloxone, 0.017 mg/kg, IV | ||||||||
| Atracurium, 0.2 mg/kg, IV | Naltrexone, 0.2 mg/kg, IV | ||||||||
| Partial glossectomy | 46 | F | 210 | Diazepam, 0.24 mg/kg, PO | Midazolam, 0.02 mg/kg, IV | Sevoflurane | N/A | Flumazenil, 0.03 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.02 mg/kg, IM | Propofol, 2.95 mg/kg, IV | ||||||||
| Oral surgery, ophthalmic examination | 38 | F | 187.5 | Midazolam, 0.08 mg/kg, IM | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 73–96 | Flumazenil, 0.015 mg/kg, IV | Bailey, 2021 |
| Meperidine, 0.1 mg/kg, IM | Propofol, 2.29 mg/kg, IV | Naloxone, 0.02 mg/kg, IV | |||||||
| Ophthalmic surgery | 22 | M | 171 | Diazepam, 0.1 mg/kg, PO | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 60–115 | Flumazenil, 0.018 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.09 mg/kg, IM | Propofol, 3 mg/kg, IV | Naloxone, 0.03 mg/kg, IV | |||||||
| Meperidine, 0.2 mg/kg, IM | |||||||||
| Bronchoscopic dilatation | 8 | M | 196 | Diazepam, 0.2 mg/kg, PO | Midazolam, 0.03 mg/kg, IV | Sevoflurane | 62–84 | Flumazenil, 0.02 mg/kg, IV | Bailey, 2021; Meegan, Ardente, et al., 2021 |
| Midazolam, 0.08 mg/kg, IM | Propofol, 3 mg/kg, IV | Naloxone, 0.02 mg/kg, IV | |||||||
| Meperidine, 0.1 mg/kg, IM | |||||||||
| Partial glossectomy | 16 | M | 181 | Diazepam, 0.25 mg/kg, PO | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 39–42 | Flumazenil, 0.022 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.05 mg/kg, IM | Propofol, 1.65 mg/kg, IV | ||||||||
| Tail abscess surgical debridement | 14 | F1 | 160 | Midazolam, 0.075 mg/kg, IM | Propofol, 1.4 mg/kg, IV | Sevoflurane | Indirect: 36–49 | Flumazenil, 0.015 mg/kg, IV | Tamura et al., 2017 |
| Butorphanol, 0.05 mg/kg, IM | Doxapram, 1 mg/kg, IV | ||||||||
| 14 | F1 | 160 | Midazolam, 0.075 mg/kg, IM | Propofol, 3.7 mg/kg, IV | Sevoflurane | Indirect: 25–95 | Flumazenil, 0.015 mg/kg, IV | Tamura et al., 2017 | |
| Butorphanol, 0.05 mg/kg, IM | Direct: 24 | Doxapram, 1 mg/kg, IV | |||||||
| Superficial keratectomy and cryosurgery of limbal melanoma | 7.5 | F2 | 175 | Diazepam, 0.26 mg/kg, PO | Midazolam, 0.05 mg/kg, IV | Sevoflurane | N/A | Flumazenil, 0.025 mg/kg, IV | Bailey, 2021; Schmitt et al., 2014 |
| Propofol, 5.48 mg/kg, IV | Flumazenil, 0.025 mg/kg, IM | ||||||||
| 10 | F2 | 185 | Butorphanol, 0.11 mg/kg, IM | Midazolam, 0.027 mg/kg, IV | Sevoflurane | N/A | Flumazenil, 0.032 mg/kg, IV | Bailey, 2021 | |
| Midazolam, 0.081 mg/kg, IM | Propofol, 2.4 mg/kg, IV | Edrophonium, 0.5 mg/kg, IV | |||||||
| Atracurium, 0.1 mg/kg, IV | |||||||||
| Dental surgery | 36 | M1 | 263 | Midazolam, 0.08 mg/kg, IM | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 31–67 | Flumazenil, 0.01 mg/kg, IV | Bailey, 2021; Meegan et al., 2016 |
| Propofol, 3.8 mg/kg, IV | |||||||||
| 37 | M1 | 241 | Midazolam, 0.08 mg/kg, IM | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 29–75 | Flumazenil, 0.02 mg/kg, IV | Bailey, 2021 | |
| Propofol, 4.3 mg/kg, IV | Naloxone, 0.01 mg/kg, IV | ||||||||
| Meperidine, 0.4 mg/kg, IM | Naltrexone, 0.05 mg/kg, IV | ||||||||
| 38 | M1 | 234 | Midazolam, 0.08 mg/kg, IM | Midazolam, 0.03 mg/kg, IV | Sevoflurane | 73–84 | Flumazenil, 0.012 mg/kg, IV | Bailey, 2021 | |
| Propofol, 5.5 mg/kg, IV | Naltrexone, 0.05 mg/kg, IV | ||||||||
| Meperidine, 0.25 mg/kg, IM | |||||||||
| Corneal mass excision | 15 | F3 | 180 | Diazepam, 0.25 mg/kg, PO | Propofol, 2.78 mg/kg, IV | Sevoflurane | 67–91 | Flumazenil, 0.014 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.08 mg/kg, IM | Atracurium, 0.1 mg/kg, IV | Edrophonium, 0.5 mg/kg, IV | |||||||
| 16 | F3 | 190 | Diazepam, 0.21 mg/kg, PO | Midazolam, 0.026 mg/kg, IV | Sevoflurane | N/A | Flumazenil, 0.026 mg/kg, IV | Bailey, 2021 | |
| Midazolam, 0.05 mg/kg, IM | Propofol, 2.87 mg/kg, IV | Edrophonium, 0.53 mg/kg, IV | |||||||
| Atracurium, 0.1 mg/kg, IV | |||||||||
| Bronchoscopy | 16 | M2 | 195 | Diazepam, 0.1 mg/kg, PO | Propofol, 4 mg/kg, IV | Sevoflurane | 50–64 | Flumazenil, 0.15 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.08 mg/kg, IM | Meperidine, 0.25 mg/kg, IM | Naloxone, 0.01 mg/kg, IV | |||||||
| Naltrexone, 0.05 mg/kg, IV | |||||||||
| Ophthalmic surgery | 17 | M2 | 202 | Diazepam, 0.2 mg/kg, PO | Midazolam, 0.03 mg/kg, IV | Sevoflurane | N/A | Flumazenil, 0.01 mg/kg, IV | Bailey, 2021 |
| Midazolam, 0.07 mg/kg, IM | Propofol, 2.23 mg/kg, IV | Naloxone, 0.01 mg/kg, IV | |||||||
| Meperidine, 0.25 mg/kg, IM | cis‐Atracurium, 0.1 mg/kg, IV | Naltrexone, 0.05 mg/kg, IV | |||||||
| Partial glossectomy | 37 | F4 | 256 | Diazepam, 0.2 mg/kg, PO | Midazolam, 0.02 mg/kg, IV | Sevoflurane | 66–86 | Flumazenil, 0.03 mg/kg, IV | Bailey, 2021 |
| Tramadol, 1.0 mg/kg, PO | Propofol, 3.05 mg/kg, IV | Naloxone, 0.023 mg/kg, IV | |||||||
| Corneal repair | 38 | F4 | 243 | Diazepam, 0.25 mg/kg, PO | Propofol, 1.0 mg/kg, IV | Sevoflurane | Flumazenil, 0.037 mg/kg, IV | Bailey, 2021 | |
| Midazolam, 0.14 mg/kg, IM | Atracurium, 0.3 mg/kg, IV | ||||||||
| Exploratory laparoscopy | 28 | M | N/A | Ketamine, 1 mg/kg, IM | Midazolam, 0.02 mg/kg, IV | Sevoflurane | N/A | N/A | Lindemann et al., 2023 |
| Midazolam, 0.02 mg/kg, IM | Propofol, 0.6 mg/kg, IV | ||||||||
3. MECHANISMS AND CURRENT APPROACHES TO DOLPHIN ANESTHESIA
Anesthetic and analgesic agents modulate the central nervous system (CNS) via activity on gamma‐aminobutyric acid type A (GABAA), N‐methyl D‐aspartate (NMDA), adrenergic alpha‐2, and opioid receptors. Ion channels, such as the family of neuronal hyperpolarization‐activated cyclic nucleotide‐gated (HCM) and two‐pore domain potassium (K2P) channels, are also known targets for anesthetic agents (Cascella et al., 2020; Pavel et al., 2020). For example, the excitatory glutamate NMDA receptor is associated with neuropathic pain and is antagonized by dissociative anesthetics like ketamine, tiletamine, and phencyclidine. The GABAA receptors are targets for the CNS inhibitory effects of propofol, etomidate, alfaxalone, barbiturates, and benzodiazepines. Alpha‐2 adrenergic agonists, such as dexmedetomidine, tizanidine, and clonidine, produce effects centrally within the locus coeruleus (sedation) and dorsal horn (pain), as well as peripherally to modulate blood pressure, cardiac output, and insulin release from the pancreatic beta cells (Giovannitti Jr et al., 2015). Opioids (morphine, codeine, methadone, tramadol, meperidine, butorphanol, buprenorphine) exert their effects at central and peripheral mu, kappa, and delta opioid receptors and can cause hypotension and sinus bradycardia through depression of sinoatrial node activity. However, the most notable and often critical effects of opioids are seen as centrally‐mediated depression of the respiratory centers, whereby hypoventilation can lead to life‐threatening hypercapnia. Volatile anesthetics, like sevoflurane, isoflurane, and desflurane, depress the response to carbon dioxide in a dose‐dependent fashion and may cause sedation, in part, by inhibiting cholinergic neurotransmission in regions of the brain that regulate arousal (Vacas et al., 2013).
With these mechanisms in mind, current approaches to anesthesia of bottlenose dolphins may present several physiologic challenges for the anesthetist. The use of drugs causing and contributing to cardiopulmonary depression, as is also seen in large terrestrial mammals, is an undesired consequence leading to a variety of anesthesia‐associated co‐morbidities (Bukoski et al., 2022; Gozalo‐Marcilla et al., 2014; Menzies et al., 2016; Sage et al., 2018). Currently, no literature exists on the cardiopulmonary impacts of anesthesia protocols on dolphins. Per the experience of the authors, cardiopulmonary derangements, such as hypoventilation, ventilation‐perfusion mismatch, decreased functional residual capacity (FRC), vasodilation, and depression of cardiac contractility are often observed in anesthetized dolphins using commonly accepted anesthetic drugs (e.g., opioids, propofol, benzodiazepines, and inhalation anesthetics) and protocols (e.g., various combinations of ventilation methods and drug selection). These effects can often lead to hypoxemia, hypercapnia, hypotension, and decreased cardiac output (Berry, 2015; Haskins, 2015; Steffey et al., 2015). If not properly mitigated, these effects can impair organ perfusion, reduce oxygen delivery, and predispose the dolphin to organ injury and myopathic conditions (Bailey, 2021; Dold & Ridgway, 2014; Haulena & Schmitt, 2018). For example, decreased work of breathing and subsequent respiratory depression is a characteristic of dolphin sedation (benzodiazepines and opioids) that often demands respiratory support in the form of mechanical ventilation (Dold & Ridgway, 2014; Ridgway & McCormick, 1971, 1967). The use of propofol for induction and inhalation anesthetics for anesthesia maintenance may cause vasodilation and could lead to depressed cardiac contractility (Berry, 2015). Together, the reduced oxygen delivery to muscles could promote rhabdomyolysis, or the breakdown of skeletal muscle fibers, and lead to kidney injury from the breakdown products (e.g., myoglobin) (Bailey et al., 2012). Thus, there is a need to understand the physiologic impacts of anesthesia in dolphins, as well as develop strategies to reduce anesthesia‐associated morbidities.
As noted by Ridgway and colleagues, the out‐of‐water induction of anesthesia abates all spontaneous ventilation in dolphins (Bailey et al., 2022; McCormick & Ridgway, 2018). Mechanical ventilation is, therefore, required to prevent the pathophysiologic consequences of hypoventilation. The most employed mechanical ventilation approach in veterinary species, controlled or conventional mechanical ventilation, mirrors the normal respiratory pattern of terrestrial mammals. Dolphins, however, have an inspiratory breath‐hold respiratory phenotype with significant heart rate variation during each inspiratory‐to‐expiratory cycle (RSA) (Fahlman et al., 2020; Le‐Bert et al., 2024; McCormick, 1969). This cardiopulmonary coupling strategy may improve gas exchange in the conscious diving dolphin; however, it is completely abolished with mechanical ventilation (Fahlman et al., 2020). The uncoupling effect on efficient respiratory gas exchange under anesthesia is unknown and may be of consequence.
While mechanical ventilation is a critical feature of dolphin anesthesia, it can also promote alveolar collapse (atelectasis), leading to ventilation‐perfusion mismatching. While Nagel, Ridgway, and colleagues were able to mechanically mimic dolphin breathing using apneustic plateau ventilation (APV) through the modification of existing large animal ventilators, the availability of this mechanical ventilation strategy to dolphin veterinarians, as well as an understanding of its effect on respiratory gas exchange under anesthesia, are lacking. A ventilation strategy that maintains airway pressure above functional residual capacity (e.g., the point in the breathing cycle where alveoli are more prone to collapse) by decreasing lung volume and pressure from an elevated plateau pressure to an airway pressure at or slightly above functional residual capacity was recently described and tested on pigs, horses, and dolphins (Bratzke et al., 1998; Bukoski et al., 2022, 2024; Le‐Bert et al., 2024). In these studies, the authors compared the cardiopulmonary effects of apneustic anesthesia ventilation (AAV) and conventional mechanical ventilation (CMV) in 12 adult pigs, 10 healthy adult horses, and 10 healthy adult bottlenose dolphins. In the horse and pig studies, the authors found that AAV resulted in significantly higher respiratory system dynamic compliance (change in lung volume over the change in pleural pressure) and lower venous admixture, or physiologic shunt (Bukoski et al., 2022, 2024). In dolphins, AAV resulted in higher arterial oxygen tension and reduced alveolar dead space ventilation (Le‐Bert et al., 2024). Thus, this ventilation strategy demonstrated some physiologic advantages for cardiopulmonary function while mechanically ventilating anesthetized dolphins and warrants further investigation.
Another significant challenge to the physiology of anesthesia in dolphins is the impact of gravity on a species that evolved in a buoyant ocean environment (Le‐Bert et al., 2024). When dolphins are removed from the neutrally buoyant environment, as is often necessary for medical and surgical procedures, the influence of gravity on hemodynamic variables may become an important factor (Figure 1). Resulting pressure gradients across dolphin tissues could contribute to whole body fluid shifts and blood flow redistribution when out of water for anesthetic procedures. Gravity‐induced hemodynamic shifts will be discussed in the next section and should be considered and mitigated in anesthetized dolphins when possible.
FIGURE 1.
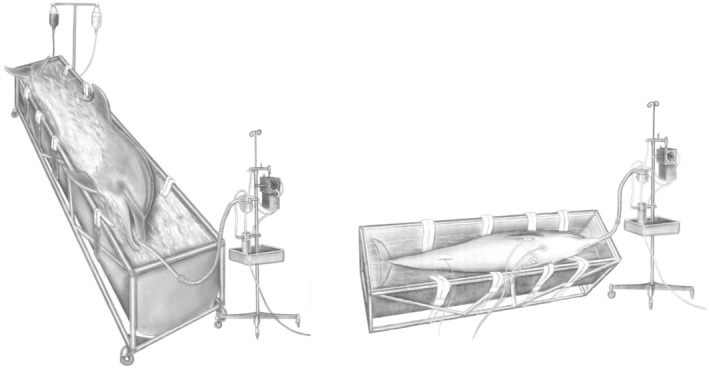
A significant challenge to general anesthesia in dolphins is the impact of gravity on a species that evolved in a buoyant ocean environment. For this reason, Ridgway would perform surgical approaches to the dolphin ear in a partially suspended state—a surgical table‐tank. The water in the surgical table‐tank was also heated to assist with thermoregulation of core body temperature (Image courtesy of the U.S. Navy's Marine Mammal Program).
4. CARDIOPULMONARY ADAPTATIONS RELEVANT TO ANESTHESIA IN BOTTLENOSE DOLPHINS
While cetaceans evolved for life in diverse aquatic habitats, all cetacean species rely on intermittent surfacing to breathe air. Consequently, prolonged intervals of breath‐holding required for locomotion and foraging impact respiratory gas exchange and metabolism (Noren et al., 2012). As such, cetaceans developed specialized anatomic characteristics and physiologic adaptations which must be considered during anesthesia. Here, we expand upon select cardiovascular and pulmonary adaptations to diving and breath‐holding activities and how these adaptations may influence dolphin responses to anesthetic agents.
4.1. Cardiovascular system adaptations
Cetaceans exhibit unique cardiovascular system morphology and physiology to support the circulatory and metabolic requirements of a diving lifestyle. For example, a dorsal‐ventral flattening of the four‐chambered heart limits the impact of chest wall compression on ventricular filling (preload) during a dive (Ochrymowych & Lambertsen, 1984). The cetacean heart is believed to have a Purkinje fiber distribution similar to terrestrial ungulates, also referred to as a Category B, or Type 2, ventricular depolarization pattern (Calloe, 2019; Hamlin, 1970; Hamlin & Smith, 1965; Harms et al., 2013; Kinoshita et al., 2023). These larger Purkinje fibers are believed to increase signal conduction velocity from the atrioventricular node to the ventricular myocardium and may benefit the observed rapid heart rate transitions from a diving bradycardia to a resurfacing tachycardia (Storlund et al., 2021). Conversely, a recent histologic study of the dolphin heart demonstrated the Purkinje fibers actually run just below the endocardium, as seen in humans (Category A ventricular depolarization pattern), and do not extend through the myocardium as is typical of terrestrial ungulates (Kinoshita et al., 2023).
In a meta‐analysis comparing ECG morphology of 50 species of terrestrial mammals and 19 species of marine mammals, marine mammal species exhibited slower atrial (19% longer P‐wave) and ventricular depolarization (24% longer QRS interval), and faster ventricular repolarization (21% shorter QT interval) than terrestrial mammals (Storlund et al., 2021). These electrophysiologic features would suggest an effect of the larger myocardial mass of dolphins influencing the duration of the electrical signal conduction (Storlund et al., 2021). These ECG features are relevant to the physiologic monitoring of both awake and anesthetized dolphins and should, therefore, be considered in the management of perfusion states. More research into the anatomic and physiologic differences contributing to the ventricular activation pattern of dolphins and the potential impact on circulation under general anesthesia is warranted.
The vascular anatomical features of the dolphin are also important when considering the anesthetic effects on dolphin physiology. Cetacean veins and arteries are extremely specialized with respect to circulation, hemodynamics, blood storage, oxygen transport, and thermoregulation. In cetacean appendages (pectoral flippers, tail fluke, dorsal fin), arteries and veins form a complex of vessels known as periarterial venous retia (Meagher et al., 2002). Retia function as counter‐current heat exchangers to support thermoregulation (core body temperature regulation) in the thermally conductive aquatic environment. Highly specialized networks of elaborate vessels, known as retia mirabilia (“wonderful nets”), around the brain and spinal cord (cranial and spinal rete mirabilis), cervicothoracic vertebrae (cervical and thoracospinal retia mirabilia), gonads and eyes (cranial and ophthalmic rete mirabilis), are also key cardiovascular adaptations in cetaceans (Ballarin et al., 2018; Bonato et al., 2019; Costidis, 2012; Cozzi et al., 2017; Lillie et al., 2022; Rommel et al., 1992; Rowlands et al., 2021). These complex vascular structures consist of a single artery with many smaller branching vessels suspended among numerous small veins, giving the appearance of a vascular net or meshwork. They are the major site of blood storage in cetaceans (Bonato et al., 2019; Cozzi et al., 2017). Rete mirabilia may function in maintaining arterial blood pressure and providing adequate cerebral perfusion independent from the peripheral thermoregulatory periarterial venous retia (Lillie et al., 2022; Rowlands et al., 2021). The elaborate morphology of the retial system within the cetacean skull and vertebral canal, and its vascular connections to thoracic and abdominal cavities, likely enables hemodynamic adjustments necessary for diving (Bonato et al., 2019; Nagel et al., 1968; Rowlands et al., 2021). In the natural, neutrally buoyant condition, the lack of pressure gradients across dolphin tissues may necessitate dependence on non‐cardiac pumps to adequately circulate blood throughout the body, for example, via the dorsoventral fluke oscillations of locomotion (Lillie et al., 2022).
Aside from complex vascular retia, true veins are another interesting morphologic feature of the circulatory system in dolphins. Most dolphin veins are valve‐less, which implies the ability for bidirectional blood flow and the reliance on non‐cardiac pumps, such as muscles of locomotion and retia mirabilia, to promote adequate tissue perfusion (Costidis, 2012; Harrison & Tomlinson, 1956). This feature may be particularly important when dolphins are anesthetically immobilized, rendering non‐cardiac pumps temporarily dysfunctional.
In addition to anatomical cardiovascular adaptations advantageous for a diving lifestyle, pelagic (deep‐diving) cetaceans rely on intrinsic oxygen stores via increased hemoglobin (blood), myoglobin (muscle), neuroglobin and cytoglobin (neural), as well as increased blood volumes, to tolerate prolonged dives (Dolar et al., 1999; Noren & Williams, 2000). These features enable continued aerobic metabolism despite prolonged apnea at depth (Ponganis et al., 2011). Myoglobin concentration is 10–30 fold higher in the skeletal muscle of aquatic diving mammals versus terrestrial mammals (Kooyman et al., 1981). Increased myoglobin allows for increased oxygen storage, with subsequent release during breath‐hold underwater exercise. In general, as diving capacity increases across cetacean taxa and ecotypes, skeletal muscle myoglobin concentrations, blood volume, and hemoglobin also increase (Butler & Jones, 1997; Fago et al., 2017; Horvath et al., 1968; Noren & Williams, 2000; Remington et al., 2007; Taboy et al., 2000).
Hemoglobin also adds to whole body oxygen stores and is directly proportional to total blood volume (Snyder, 1983). The shallow‐diving bottlenose dolphin, however, does not exhibit increases in red blood cell volume, hemoglobin, or myoglobin, as is measured in the deep‐diving cetaceans (Fahlman et al., 2018). Blood volume in this coastal species is closer to terrestrial mammals at ~7.1% of body mass (Johnson et al., 2009; Ridgway & Johnston, 1966). Early studies found that hemoglobin has a higher affinity for oxygen in the small, shallow‐diving bottlenose dolphin compared to the larger, deep‐diving species (Snyder, 1983). This observation was believed to facilitate oxygen extraction from the lungs during short dives, as well as facilitating oxygen off‐loading to the tissues during deep dives when lungs are collapsed. However, more recent evidence suggests diving mammals have hemoglobin oxygenation properties similar to terrestrial mammals, and that previously observed differences in the oxy‐hemoglobin dissociation curve more likely reflect differences in red blood cell 2,3‐diphosphoglycerate (DPG) concentration (Fago et al., 2017).
Lastly, there is evidence of adaptations in neural mechanisms of cardiovascular control. Specifically, alpha‐adrenergic 2B receptors in another toothed whale, the sperm whale, exhibit protein sequence differences in the polyglutamate acid domain, which likely affect agonist‐induced phosphorylation and receptor activation (Madsen et al., 2002; Small et al., 2001). Relevant to the pharmacology of anesthetic agents, this catecholamine receptor is concentrated in the spinal cord, kidneys and vascular endothelium, and can influence sedation, analgesia, muscle relaxation, bradycardia and systemic vascular resistance. Given these sequence differences of alpha 2B adrenergic receptors, sympathomimetic drugs including alpha 2 adrenergic receptor agonists and antagonists likely exhibit differential binding affinities which may, in turn, impact cardiovascular function in anesthetized dolphins.
4.1.1. Cardiovascular plasticity of the diving dolphin
The mammalian dive response consists of several key events that preserve intrinsic oxygen stores and prevent asphyxia, particularly in vital organs such as the heart and brain (Panneton, 2013). First, activation of facial trigeminal nerve reflexes during submersion induces a parasympathetic response and acetylcholine release (Berk et al., 1991; Ponganis, 2019). Acetylcholine activates heart muscarinic receptors, decreasing heart rate (bradycardia). Bradycardia lowers the chronotropic state of the heart, thereby reducing oxygen consumption, conserving oxygen stores and mitochondrial energy production. Since cardiac output is the product of heart rate and stroke volume (volume of blood ejected per heartbeat), a decrease in heart rate (bradycardia) decreases cardiac output, at least if stroke volume is unchanged. Decreased cardiac output impacts tissue oxygen delivery and thus, perfusion, and limits dive duration.
Bottlenose dolphins decrease heart rate from ~101–111 bpm to ~20–30 bpm within 1 min of water submergence (Houser, Dankiewicz‐Talmadge, et al., 2010; Williams et al., 2015, 1993). Williams and colleagues demonstrated that dive depth and exercise intensity alter the extent of bradycardia in diving dolphins (Williams et al., 2015). In that same study, dolphins at diving depth exhibited ~1.7–3.7 fold increase in heart rate over gliding values during exercise (swimming). Dive depth and duration were important modulators of that exercise response. Thus, Williams and colleagues postulated that the interplay between sympathetic and parasympathetic systems (autonomic conflict) of breath‐hold exercising at depth was responsible for the observed heart rate variability and cardiac anomalies. However, Ponganis and colleagues presented an alternative interpretation of the exercise response in diving marine mammals, de‐emphasizing the concept of autonomic conflict and proposing that instead: (1) sympathetic activation is elevated at dives even without exercise, as evidenced by maximal vasoconstriction, (2) parasympathetic cardiac vagal tone dominates over sympathetic cardiac tone in diving animals (as evidenced by bradycardia), (3) exercise modulation of heart rate during dives primarily involves reduction in parasympathetic tone versus increased sympathetic tone and, finally, (4) benign arrhythmias are common in marine mammals (Ponganis et al., 2017).
Hydrostatic pressure increases with dive depth and, thus, exerts increasing intrathoracic pressures on the dolphin cardiopulmonary system while diving. Changes in hydrostatic pressure while diving are believed to modulate chronotropic and inotropic function of the heart through its influence on pulmonary volumes, blood shunting, baroreceptors, pulmonary stretch receptors, and changes in blood gas tensions (Williams et al., 2015). Mechanical ventilation during anesthesia also increases intrathoracic pressure and can negatively impact cardiac output (Mahmood & Pinsky, 2018). In 1968, Sommer and colleagues measured stroke volume (0.4–0.8 mL/kg) and cardiac output (47–105 mL/min/kg) in four dolphins under anesthesia, noting the likely influence of mechanical ventilation and out‐of‐water experimental conditions on hemodynamic variables (Sommer et al., 1968). More recently, the availability of non‐invasive transthoracic and transesophageal echocardiography has allowed for in‐water cardiovascular evaluation of awake, spontaneously ventilating dolphins (Chetboul et al., 2012; Linnehan et al., 2021; Miedler et al., 2015; Sklansky et al., 2006). In one study, cardiac output was determined by calculating the stroke volume from the integrated blood flow velocity and the aortic cross‐sectional area at the level of the aortic valve using transthoracic echocardiography (Miedler et al., 2015). Dolphins resting at the surface had an average stroke volume of approximately 0.8 mL/kg (136 ± 19 mL) and average cardiac output of 32.2 mL/min/kg (with an average heart rate of 41 bpm). As seen in humans and other species after exercise, heart rate, stroke volume, and, therefore, cardiac output, increased significantly in the surfaced dolphins for up to 4 min following cessation of exercise activity (approximately 104%, 63%, 234%, respectively). However, no studies to date have measured stroke volume in diving dolphins. Therefore, whether exercise modulates either stroke volume or cardiac output in diving dolphins is unknown.
The cardiovascular flexibility noted in dolphins at rest or after exercise may be a critical evolutionary feature to conserve oxygen during diving by regulating pulmonary and systemic perfusion. For example, cardiovascular adjustments likely minimize blood flow to peripheral musculature during dives, while rapidly removing carbon dioxide and replenishing oxygen during the surface interval. Upon ascent, bradycardia is gradually reversed, suggesting that cardiac output and stroke volume increase (Miedler et al., 2015). Thus, a key event during the dive itself is activation of the sympathetic nervous system to elicit peripheral vasoconstriction. Peripheral vasoconstriction ensures that blood flow is shunted away from peripheral tissues and focused on critical central compartments – the brain and heart. Reversal of this vasoconstriction is essential to replenish oxygen and remove carbon dioxide from those peripheral tissues during the surface interval. Blawas and colleagues demonstrated upregulation of the arachidonate 5‐lipoxygenase (ALOX5) gene in dolphins. Since downstream leukotriene metabolites induce vasoconstriction in hypoxic rodent models and humans (Friedman et al., 1984; Ichinose et al., 2001), similar mechanisms may allow marine mammals to tolerate prolonged periods under water (Blawas, Ware, et al., 2021).
Peripheral vasoconstriction while diving increases systemic and target organ vascular resistance, and therefore, perfusion states. Vascular resistance, along with blood flow or cardiac output, determine the blood pressure which is frequently monitored during general anesthesia. In a limited number of reported out‐of‐water, non‐diving, conscious dolphins, the normal mean arterial blood pressure ranged from 120 to 140 mmHg (Ridgway & McCormick, 1971). While no studies exist specifically measuring vascular resistance in resting dolphins, genomic studies of diving marine mammals point to evolutionary pressure on endothelin pathway genes (EDN1, EDN2, EDN3, EDNRA, EDNRB). Further, there is a genetic loss of a renal amino acid transporter of arginine reabsorption (SLC6A18), which would reduce production of the vasodilatory signaling molecule nitric oxide and, thus, be a potential mechanism for promoting vasoconstriction (Hindle, 2020; Huelsmann et al., 2019; Tian et al., 2016). Additional genetic markers contributing to efficient peripheral vasoconstriction in diving dolphins are those of their intrinsic coagulation pathway. Genes encoding coagulation factor XII are absent in dolphins. Specifically, the loss of the kallikrein B1 gene protects against thrombus formation, while key coagulation factors of the extrinsic pathway required for hemostasis of damaged tissue are not lost (Huelsmann et al., 2019; Kokoye et al., 2016; Semba et al., 1998, 2000). Since vasoconstriction‐induced reduction in blood vessel diameter during diving typically increases risk for thrombus formation in other mammals, loss of these genes likely provide an evolutionary advantage to cetaceans (Haulena & Schmitt, 2018; Kokoye et al., 2016).
4.1.2. Respiratory sinus arrhythmia and cardiopulmonary coupling in dolphins
Respiratory sinus arrhythmia (RSA) is the variation of heart rate with the inter‐breath interval and is seen in many species, including humans. RSA is frequently used as an index of cardiac vagal tone and overall fitness and health. Many marine mammal species, including bottlenose dolphins, demonstrate pronounced RSA during surface breathing, resulting in dramatic changes in instantaneous heart rate throughout the inter‐breath interval (Blawas, Nowacek, et al., 2021; Cauture et al., 2019; Fahlman, Miedler, et al., 2019). However, heart rate patterns during prolonged breath‐holds are similar to those resulting from RSA during extended inter‐breath intervals (Blawas, Nowacek, et al., 2021). Cardiorespiratory coupling such as RSA has been proposed in dolphins as a physiologic strategy to optimize gas exchange during the surface interval between prolonged breath‐hold dives (Fahlman et al., 2020; Fahlman, Miedler, et al., 2019; Giardino et al., 2003; Hayano et al., 1996).
4.2. Respiratory system adaptations
Beyond the cardiovascular system, the respiratory system in cetaceans also displays specialized physiologic and anatomic adaptations suitable for a completely aquatic life. For example, the cetacean blowhole is a result of migration of nasal passages to the top of the forehead (Berta et al., 2014). The trachea of cetaceans tends to be short and wide and holds up to 4% of total lung volume (Davenport et al., 2013; Piscitelli et al., 2013). The compliant spiraling rings of the trachea and bronchi are also unique; they retain enough rigidity to remain patent, but enough flexibility to withstand the extreme compression experienced during a dive (Denk et al., 2020; Moore et al., 2013).
Cetacean lungs are unilobular and lie dorsal to the heart while enclosed within a complete mediastinum. The lungs exhibit morphological advantages essential for the explosive, intermittent ventilation observed during surfacing intervals. An abundance of elastic fibers, high pulmonary compliance, collateral ventilation adaptations, and cartilaginous reinforcement of bronchi and bronchioles, allow cetacean alveoli to undergo compression and collapse at extreme hydrostatic pressures (Piscitelli et al., 2013).
In larger airways and the extra‐pulmonary bronchi, smooth muscle replaces elastic layers. The presence of structurally reinforced airways allows for the accumulation of air at high pressures within the dead space when non‐reinforced alveoli would collapse. Alveolar collapse could protect against nitrogen gas absorption or gas emboli when ascending from a dive. Some investigators hypothesize that these attributes, along with evidence of hypoxic pulmonary vasodilation rather than vasoconstriction, and intrapulmonary arteriovenous shunts, enable functional pulmonary shunting at any depth in cetaceans; this ability for pulmonary shunts may not be fully dependent on hydrostatic compression (Garcia Parraga et al., 2018). Thus, even with alveolar collapse, extensive collateral ventilation plus hypoxia‐induced vasodilation may enable continued gas exchange in upper parts of the lung (Gompelmann et al., 2013).
4.2.1. Mechanics of breathing in the dolphin
The inspiratory, breath‐holding breathing pattern of cetaceans is an essential feature required for underwater feeding activities. The defining characteristic of volitional breathing in bottlenose dolphins is a rapid exhalation of large air volumes (~90%–95% of total lung capacity) through the blowhole within approximately 0.26–0.5 s, followed by a slower inspiratory phase ending with blowhole closure. Thus, the respiratory system maintains in an inflated state with the inspired air until the next respiratory cycle (Cotten et al., 2008; Fahlman et al., 2015, 2017; Fahlman, Brodsky, et al., 2019; Piscitelli et al., 2013; Ridgway, 1972).
Existing physiologic measures of lung function and mechanics should be considered when applying mechanical ventilation during general anesthesia of dolphin patients (Table 3). Dolphin airway (alveoli) opening pressures, for example, are 21–25 cm H2O, with maximum lung volume achieved at pressures around 30 cm H2O (Piscitelli et al., 2010). Tidal volumes reported in the literature range from 15 to 22 mL/kg for dolphins resting at the surface (terrestrial mammals are about 7.7 mL/kg), while breathing frequency can be significantly variable, averaging between 0.9 and 3.6 breaths/minute in the resting dolphin (Fahlman et al., 2015; Mortola & Seguin, 2009; Piscitelli et al., 2013; Ridgway, 1972). Total lung capacity in bottlenose dolphins is reported to be between 40 and 138 mL/kg (lung mass ~2.7% of total body mass); however, some of these measurements were acquired from excised lungs, possibly contributing to the wide ranges reported (Fahlman et al., 2015, 2017; Mortola & Seguin, 2009; Piscitelli et al., 2010, 2013).
TABLE 3.
Summary of cardiopulmonary variables relevant to perfusion adaptations of awake and anesthetized bottlenose dolphins (Tursiops truncatus).
| Reported values | References | |
|---|---|---|
| Cardiovascular variables | ||
| Heart rate (beats/min) | 20–140 a | Williams et al., 2015; Williams et al., 1993; Ponganis et al., 2017 |
| Mean arterial pressure (mmHg) | 120–142 b | Sommer et al., 1968; Ridgway & McCormick, 1971 |
| Cardiac index (mL/min/kg) | 47–105 c | Sommer et al., 1968 |
| 18.2–49.5 d | Miedler et al., 2015 | |
| Stroke volume index (mL/beat/kg) | 0.4–0.8 c | Sommer et al., 1968 |
| 0.5–0.9 d | Miedler et al., 2015 | |
| Blood volume (mL/kg) | 65–83 (mean 71) | Johnson et al., 2009; Ridgway & Johnston, 1966 |
| Hemoglobin (g/dL) | 13.2–15.3 (mean 14.4) | Ridgway & Johnston, 1966 |
| Oxygen‐carrying capacity, blood (mL O2/dL) e | 17.7–20.5 (mean 19.3) | Ridgway & Johnston, 1966 |
| Myoglobin (g 100/g muscle) | 2.5–3.5 | Kooyman & Ponganis, 1997; Dolar et al., 1999; Ridgway & Johnston, 1966 |
| Oxygen‐carrying capacity, muscle | ||
| Muscle mass (mL O2/kg muscle) e | 33.5–46.9 | Kooyman & Ponganis, 1997 |
| Body mass (mL O2/kg body mass) | 13.3 | Pabst D, et al., 1999 |
| Total oxygen (mL O2/kg) | 29–36 | Pabst D, et al., 1999; Kooyman & Ponganis, 1997; Noren & Williams, 2000 |
| Pulmonary variables | ||
| Breathing frequency (breaths/min) | 0.9–3.6 | Piscitelli et al., 2010; Piscitelli et al., 2013; Fahlman et al., 2015; Fahlman et al., 2017 |
| Inspiratory phase time (ms) | 430–660 | Fahlman et al., 2015; Fahlman et al., 2017; Fahlman, Brodsky, et al., 2019 |
| Expiratory phase time (ms) | 260–500 | Piscitelli et al., 2013; Fahlman et al., 2015; Fahlman et al., 2017; Fahlman, Brodsky, et al., 2019 |
| Inspiratory flow rate (L/s) | 9.8–20.2 | Fahlman et al., 2015 |
| Expiratory flow rate (L/s) | 16.5–37.5 | Fahlman et al., 2015 |
| Tidal volume (mL/kg) | 15–22 | Fahlman et al., 2015; Fahlman et al., 2017; Fahlman, Miedler, et al., 2019; Mortola & Seguin, 2009 |
| Total lung capacity (mL/kg) | 40–138 | Piscitelli et al., 2010; Piscitelli et al., 2013; Fahlman et al., 2015; Fahlman et al., 2017; Mortola & Seguin, 2009 |
| Airway (alveolar) opening pressure (cm H2O) | 21–25 | Piscitelli et al., 2010; Piscitelli et al., 2013 |
| Peak airway pressure (cm H2O) | 30 | Piscitelli et al., 2010; Piscitelli et al., 2013 |
| Dynamic compliance (L/cm H2O) | 0.37 ± 0.04 f | Fahlman et al., 2015 |
Normal respiratory sinus arrhythmia accounts for heart rate variability in adult bottlenose dolphins at the surface and during diving conditions.
Mean arterial pressures obtained in awake as well as anesthetized out‐of‐water dolphins. No significant differences were found between both conditions, therefore, data was pooled.
Variable data obtained in anesthetized out‐of‐water dolphins via indocyanine green dye‐dilution curves (n = 4).
Variable data obtained in awake in‐water dolphins via echocardiogram measurements (n = 14).
Calculated value, that is, oxygen capacity is calculated from measured [Mb] or [Hb] assuming a conversion factor of 1.34 mL O2.
Estimated as tidal volume divided by tidal change in transpulmonary pressure measured using an esophageal pressure catheter.
4.2.2. Control of breathing in dolphins
Breathing in mammals is a complex physiologic process involving respiratory neurons of the brainstem. Brainstem respiratory neurons generate the breathing rhythm, and then modulate that rhythm through complex feedback from chemoreceptors (both central and peripheral) and mechanoreceptors. The fundamental breathing signal is then exposed to breath pattern formation, crafting the detailed spatiotemporal distribution to the different respiratory muscles that generate a breath, as well as major inputs to breathing from cortical areas governing the volitional control of breathing (Ashhad et al., 2022).
The central and peripheral chemoreceptors sense carbon dioxide in the blood and neural tissue, while only the peripheral chemoreceptors sense changes in oxygen in the blood, triggering chemoreflexes that manifest as increased breathing (Guyenet & Bayliss, 2015; McCulloch et al., 1997). These chemoreceptors normally operate in the manner of a negative feedback loop to preserve oxygen and carbon dioxide homeostasis.
In 1969, McCormick evaluated the effects of mixed inspired gases on peripheral and central chemoreceptor responses of conscious dolphins (McCormick, 1969). In this study, increasing the inspired carbon dioxide to 5% (with normal oxygen levels) induced a ventilatory response characterized by an increase in respiratory rate. This ventilatory response persisted, even when inspired oxygen concentrations were artificially increased to 40%. Conversely, when inspired oxygen was reduced to 10%, respiratory rate again increased, consistent with a hypoxic chemoreflex elicited by peripheral (carotid body) chemoreceptors. Both responses in dolphins are consistent with normal mammalian peripheral and central chemoreflexes in response to increased inspired carbon dioxide or decreased inspired oxygen concentrations at sea level.
During diving behavior, these responses appear to be reversibly blunted. Pelagic marine mammals appear to tolerate arterial oxygen tensions (paO2) as low as 12 mmHg and venous oxygen tensions (pvO2) as low as 3 mmHg during free diving conditions (Meir et al., 2009; Ponganis et al., 2011). Dolphin venous oxygen tensions (pvO2) during voluntary breath holds at the surface were measured as low as 18–20 mmHg (Williams et al., 1999). It was proposed by Stephenson that dis‐facilitation, rather than overt inhibition of ventilation, during diving occurs due to profound hypocapnia, decreasing the drive to breathe below the CO2 apneic threshold during long dives (Stephenson, 2005). In Stephenson's model, the most important factor leading to dis‐facilitation of respiratory drive occurs during the surface interval between dives. Stephenson speculated that surface breathing drastically decreases arterial carbon dioxide (<30 mmHg) and increases arterial oxygen (~120 mmHg), minimizing all chemoreflex drive to breathing (i.e., dis‐facilitation). Similar to terrestrial mammals, the drive to breathe in pelagic marine mammals is likely influenced more by central chemoreceptor responses to carbon dioxide versus peripheral chemoreceptor response to decreased oxygen levels (Stephenson, 2005). This may be an important consideration when allowing for permissive hypercapnia during emergence from general anesthesia.
A summary of the relevant, published cardiopulmonary measurements, obtained in both unanesthetized and anesthetized bottlenose dolphins is provided in Table 3 for reference.
5. CONCLUDING REMARKS
The ability to promote an anesthetic state in dolphins with minimal cardiopulmonary derangements is critical to reduce anesthesia‐associated morbidities and improve clinical outcomes. Current practices in dolphin anesthesia involve use of agents that cause respiratory depression and undermine cardiac function. While many of these effects can be mitigated with controlled mechanical ventilation and chronotropic, inotropic and vasopressive agents, these interventions are often complex, requiring specialized equipment and drug delivery methods. As such, limited peer‐reviewed publications exist on the pharmacokinetics of anesthetic drugs in bottlenose dolphins. Therefore, extrapolation of drug dosages from terrestrial species and human medicine is often performed. As evidenced from the distinct features of dolphin cardiopulmonary physiology, as well as genomic and receptor differences that could impact binding and metabolism of anesthetic agents as outlined above, the mechanisms of anesthesia may not translate from comparative species. Consideration of the specialized structure and function of the cardiopulmonary system of dolphins should, therefore, guide anesthetic practices to minimize its effects on cardiopulmonary depression and promote hemodynamic stability in the anesthetized dolphin. For example, investigations into partial and total intravenous anesthetic techniques that would limit reliance on inhalation anesthetics may provide cardio‐pulmonary sparing effects and improve perfusion states. Additionally, evaluation of mixed gas ventilation approaches, such as lower inspired oxygen concentrations, may reduce morbidities associated with absorption atelectasis. And finally, limiting the gravitational effects on dolphin circulation by investigating buoyant materials may improve hemodynamics and thus, perfusion, under general anesthesia.
Though we recognize the need to improve anesthesia protocols in dolphins, a significant hurdle in the advancement of dolphin anesthesiology is the relative lack of dolphins under human care around the world. The low availability of study subjects and resource expertise makes controlled basic physiologic and pharmacologic studies difficult to complete. Bottlenose dolphins considered ‘charismatic megafauna,’ are not routinely subjected to risky and complex biomedical studies. A greater understanding of dolphin physiology will continue to contribute to improvements in anesthesia protocols and medical management and may even inspire advances in human biomedical research and health care. However, in the West, dolphins are not managed as laboratory animals and thus, opportunities for controlled biomedical studies will remain limited. Often these opportunities present by observations incidental to emergent or urgent clinical interventions and dependent on gradually evolving approaches to medical management of healthy dolphins among larger holders of this species. For these reasons, it is of utmost importance these and smaller holding institutions maintain a network of scientists and collaborators with expertise in cetacean medicine, physiology, and anesthesiology. Only with these collaborations can the practice and discipline of dolphin anesthesiology eliminate the hurdle of inexperience and advance anesthetic practices for the improvement of cetacean care around the world.
FUNDING INFORMATION
Funding for this manuscript was provided to C. Le‐Bert through the Naval Information Warfare Center Pacific, Naval Innovative Science and Engineering (NISE) Program, P‐NISE‐AR‐23‐230,205;76,725.
Le‐Bert, C. R. , Mitchell, G. S. , & Reznikov, L. R. (2024). Cardiopulmonary adaptations of a diving marine mammal, the bottlenose dolphin: Physiology during anesthesia. Physiological Reports, 12, e16183. 10.14814/phy2.16183
REFERENCES
- Ashhad, S. , Kam, K. , Del Negro, C. A. , & Feldman, J. L. (2022). Breathing rhythm and pattern and their influence on emotion. Annual Review of Neuroscience, 45, 223–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, J. , Dold, C. , & Ridgway, S. (2022). Cetacean Anesthesia. In: Zoo Animal and Wildlife Immobilization and Anesthesia.
- Bailey, J. E. (2021, May 23–26). Cetacean anesthesia: a review of 34 general anesthesia events, lessons learned, and future plans [Conference presentation abstract]. Fifty‐first annual conference of the International Association for Aquatic Animal Medicine, Virtual Conference. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Bailey, J. E. , Flanagan, C. , Meegan, J. , Le‐Bert, C. , Johnson, S. , Gomez, F. , Lutmerding, B. , Smith, C. , Jensen, E. , Silva, N. , Silva, J. , Colitz, C. , Latimer, F. G. , Nunes, A. , Silveira, M. , Neto, M. , & Palma, L. (2012, May 12–16). Cogent evidence of rhabdomyolysis in a California sea lion (Zalophus californianus) and a South African fur seal (Arctocephalus pusillus pusillus) during anesthesia [Conference presentation abstract]. Forty‐third annual conference of the International Association for Aquatic Animal Medicine, Atlanta, GA, USA. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Ballarin, C. , Bagnoli, P. , Peruffo, A. , & Cozzi, B. (2018). Vascularization of the trachea in the bottlenose dolphin: Comparison with bovine and evidence for evolutionary adaptations to diving. Royal Society Open Science, 5, 171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk, W. A. , Shea, M. J. , & Crevey, B. J. (1991). Bradycardic responses to vagally mediated bedside maneuvers in healthy volunteers. The American Journal of Medicine, 90, 725–729. [PubMed] [Google Scholar]
- Berry, S. H. (2015). Injectable anesthetics. In Grimm K. A., Lamont L. A., Tranquilli W. J., Greene S. A., & Robertson S. A. (Eds.), Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones (pp. 277–297). Wiley; . [Google Scholar]
- Berta, A. , Ekdale, E. G. , & Cranford, T. W. (2014). Review of the cetacean nose: Form, function, and evolution. Anatomical Record (Hoboken), 297, 2205–2215. [DOI] [PubMed] [Google Scholar]
- Blawas, A. M. , Nowacek, D. P. , Allen, A. S. , Rocho‐Levine, J. , & Fahlman, A. (2021). Respiratory sinus arrhythmia and submersion bradycardia in bottlenose dolphins (Tursiops truncatus). The Journal of Experimental Biology, 224,jeb234096. [DOI] [PubMed] [Google Scholar]
- Blawas, A. M. , Ware, K. E. , Schmaltz, E. , Zheng, L. , Spruance, J. , Allen, A. S. , West, N. , Devos, N. , Corcoran, D. L. , Nowacek, D. P. , Eward, W. C. , Fahlman, A. , & Somarelli, J. A. (2021). An integrated comparative physiology and molecular approach pinpoints mediators of breath‐hold capacity in dolphins. Evolution, Medicine, and Public Health, 9, 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato, M. , Bagnoli, P. , Centelleghe, C. , Maric, M. , Brocca, G. , Mazzariol, S. , & Cozzi, B. (2019). Dynamics of blood circulation during diving in the bottlenose dolphin (Tursiops truncatus): The role of the retia mirabilia. The Journal of Experimental Biology, 222, jeb198457. [DOI] [PubMed] [Google Scholar]
- Bratzke, E. , Downs, J. B. , & Smith, R. A. (1998). Intermittent CPAP: A new mode of ventilation during general anesthesia. Anesthesiology, 89, 334–340. [DOI] [PubMed] [Google Scholar]
- Bukoski, A. , Downs, J. , Hodgson, D. S. , Le‐Bert, C. R. , Thomen, R. , Flors, L. , Thombs, L. , & Bailey, J. (2024). Cardiopulmonary effects of apneustic anesthesia ventilation in anesthetized pigs: A new mode of ventilation for anesthetized veterinary species. Frontiers in Veterinary Science, 11, 1378617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukoski, A. , Hodgson, D. , Downs, J. , LeBert, C. , Thombs, L. , & Bailey, J. (2022). An implementation of apneustic anesthesia ventilation in the horse: Comparison with conventional mechanical ventilation. Veterinary Anaesthesia and Analgesia, 49(4), 372–381. [DOI] [PubMed] [Google Scholar]
- Butler, P. J. , & Jones, D. R. (1997). Physiology of diving of birds and mammals. Physiological Reviews, 77, 837–899. [DOI] [PubMed] [Google Scholar]
- Calloe, K. (2019). The transient outward potassium current in healthy and diseased hearts. Doctoral Dissertation. January 2019. Volume 225. Supplement 717. 10.1111/apha.13225 [DOI] [PubMed]
- Cascella, M. , Bimonte, S. , & Di Napoli, R. (2020). Delayed emergence from anesthesia: What we know and how we act. Local and Regional Anesthesia, 13, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauture, F. , Sterba‐Boatwright, B. , Rocho‐Levine, J. , Harms, C. , Miedler, S. , & Fahlman, A. (2019). Using respiratory sinus arrhythmia to estimate inspired tidal volume in the bottlenose dolphin (Tursiops truncatus). Frontiers in Physiology, 10, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetboul, V. , Lichtenberger, J. , Mellin, M. , Mercera, B. , Hoffmann, A. C. , Chaix, G. , Trehiou‐Sechi, E. , Misbach, C. , Petit, A. , Lefebvre, H. P. , Gaide, N. , Tissier, R. , & Delfour, F. (2012). Within‐day and between‐day variability of transthoracic anatomic M‐mode echocardiography in the awake bottlenose dolphin (Tursiops truncatus). Journal of Veterinary Cardiology, 14, 511–518. [DOI] [PubMed] [Google Scholar]
- Costidis, A. (2012). The Morphology of the Venous System in the Head and Neck of the Bottlenose Dolphin (Tursiops truncatus) and Florida Manatee (Trichechus manatus latirostris). In: Veterinary Medical Sciences University of Florida. p. 354.
- Cotten, P. B. , Piscitelli, M. A. , McLellan, W. A. , Rommel, S. A. , Dearolf, J. L. , & Pabst, D. A. (2008). The gross morphology and histochemistry of respiratory muscles in bottlenose dolphins, Tursiops Truncatus . Journal of Morphology, 269, 1520–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi, B. , Huggenberger, S. , & Oelschlaeger, H. (2017). Anatomy of Dolphins: Insights Into Body Structure and Function. Elsevier. [Google Scholar]
- Davenport, J. , Cotter, L. , Rogan, E. , Kelliher, D. , & Murphy, C. (2013). Structure, material characteristics and function of the upper respiratory tract of the pygmy sperm whale. The Journal of Experimental Biology, 216, 4639–4646. [DOI] [PubMed] [Google Scholar]
- Denk, M. , Fahlman, A. , Dennison‐Gibby, S. , Song, Z. , & Moore, M. (2020). Hyperbaric tracheobronchial compression in cetaceans and pinnipeds. The Journal of Experimental Biology, 223, jeb217885. [DOI] [PubMed] [Google Scholar]
- Doescher, B. M. , Pawloski, J. , & Bailey, J. E. (2018, May 19–23). General anesthesia to facilitate the surgical resection of an oral squamous cell carcinoma in a geriatric bottlenose dolphin (Tursiops truncatus) [Conference presentation abstract]. Forty‐ninth annual conference of the International Association for Aquatic Animal Medicine, Long Beach, CA, USA. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Dolar, M. L. L. , Suarez, P. , Ponganis, P. J. , & Kooyman, G. L. (1999). Myoglobin in pelagic small cetaceans. The Journal of Experimental Biology, 202, 227–236. [DOI] [PubMed] [Google Scholar]
- Dold, C. , & Ridgway, S. (2014). Cetaceans. In Zoo animal and wildlife immobilization and anesthesia (2nd ed., pp. 679–691). John Wiley & Sons, Inc. [Google Scholar]
- Dover, S. R. , Beusse, D. , Walsh, M. T. , McBain, J. F. , & Ridgway, S. (1999). Laparoscopic techniques for the bottlenose dolphin (Tursiops truncatus) [Conference presentation abstract]. Thirtieth annual conference of the International Association for Aquatic Animal Medicine, Boston, MA, USA. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Fago, A. , Parraga, D. G. , Petersen, E. E. , Kristensen, N. , Giouri, L. , & Jensen, F. B. (2017). A comparison of blood nitric oxide metabolites and hemoglobin functional properties among diving mammals. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 205, 35–40. [DOI] [PubMed] [Google Scholar]
- Fahlman, A. , Brodsky, M. , Miedler, S. , Dennison, S. , Ivancic, M. , Levine, G. , Rocho‐Levine, J. , Manley, M. , Rocabert, J. , & Borque‐Espinosa, A. (2019). Ventilation and gas exchange before and after voluntary static surface breath‐holds in clinically healthy bottlenose dolphins, Tursiops truncatus . The Journal of Experimental Biology, 222, jeb192211. 10.1242/jeb.192211 [DOI] [PubMed] [Google Scholar]
- Fahlman, A. , Jensen, F. H. , Tyack, P. L. , & Wells, R. S. (2018). Modeling tissue and blood gas kinetics in coastal and offshore common bottlenose dolphins, Tursiops truncatus . Frontiers in Physiology, 9, 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlman, A. , Loring, S. H. , Levine, G. , Rocho‐Levine, J. , Austin, T. , & Brodsky, M. (2015). Lung mechanics and pulmonary function testing in cetaceans. The Journal of Experimental Biology, 218, 2030–2038. [DOI] [PubMed] [Google Scholar]
- Fahlman, A. , Miedler, S. , Marti‐Bonmati, L. , Ferrero Fernandez, D. , Muñoz Caballero, P. , Arenarez, J. , Rocho‐Levine, J. , Robeck, T. , & Blawas, A. (2020). Cardiorespiratory coupling in cetaceans; a physiological strategy to improve gas exchange? The Journal of Experimental Biology, 223, jeb226365. [DOI] [PubMed] [Google Scholar]
- Fahlman, A. , Miedler, S. , Rocho‐Levine, J. , Jabois, A. , Arenarez, J. , Marti‐Bonmati, L. , Garcia‐Parraga, D. , & Cauture, F. (2019). Re‐evaluating the significance of the dive response during voluntary surface apneas in the bottlenose dolphin, Tursiops Truncatus . Scientific Reports, 9, 8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlman, A. , Moore, M. J. , & Garcia‐Parraga, D. (2017). Respiratory function and mechanics in pinnipeds and cetaceans. The Journal of Experimental Biology, 220, 1761–1773. [DOI] [PubMed] [Google Scholar]
- Friedman, Z. , Lunyong, V. E. , Courtney, J. , Smith, H. , Berkowitz, P. , & Sun, F. (1984). Prostaglandin formation in the isolated human ductus arteriosus, aorta, pulmonary and umbilical arteries. Prostaglandins, Leukotrienes, and Medicine, 14, 279–286. [DOI] [PubMed] [Google Scholar]
- Garcia Parraga, D. , Moore, M. , & Fahlman, A. (2018). Pulmonary ventilation‐perfusion mismatch: A novel hypothesis for how diving vertebrates may avoid the bends. Proceedings of the Biological Sciences, 285, 20180482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino, N. D. , Glenny, R. W. , Borson, S. , & Chan, L. (2003). Respiratory sinus arrhythmia is associated with efficiency of pulmonary gas exchange in healthy humans. American Journal of Physiology. Heart and Circulatory Physiology, 284, H1585–H1591. [DOI] [PubMed] [Google Scholar]
- Giovannitti, J. A., Jr. , Thoms, S. M. , & Crawford, J. J. (2015). Alpha‐2 adrenergic receptor agonists: A review of current clinical applications. Anesthesia Progress, 62, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompelmann, D. , Eberhardt, R. , & Herth, F. J. (2013). Collateral ventilation. Respiration, 85, 515–520. [DOI] [PubMed] [Google Scholar]
- Gozalo‐Marcilla, M. , Gasthuys, F. , & Schauvliege, S. (2014). Partial intravenous anaesthesia in the horse: A review of intravenous agents used to supplement equine inhalation anaesthesia. Part 1: Lidocaine and ketamine. Veterinary Anaesthesia and Analgesia, 41, 335–345. [DOI] [PubMed] [Google Scholar]
- Guyenet, P. G. , & Bayliss, D. A. (2015). Neural control of breathing and CO2 homeostasis. Neuron, 87, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin, R. L. (1970). Electrocardiogram of bottle‐nosed dolphin (Tursiops truncatus). American Journal of Veterinary Research, 31, 501–505. [PubMed] [Google Scholar]
- Hamlin, R. L. , & Smith, C. R. (1965). Categorization of common domestic mammals based upon their ventricular activation process. Annals of the New York Academy of Sciences, 127, 195–203. [DOI] [PubMed] [Google Scholar]
- Harms, C. A. , Jensen, E. D. , Townsend, F. I. , Hansen, L. J. , Schwacke, L. H. , & Rowles, T. K. (2013). Electrocardiograms of bottlenose dolphins (Tursiops truncatus) out of water: Habituated collection versus wild postcapture animals. Journal of Zoo and Wildlife Medicine, 44, 972–981. [DOI] [PubMed] [Google Scholar]
- Harrison, R. , & Tomlinson, J. (1956). Observations on the venous system in certain Pinnipedia and cetacean. Proceedings of the Zoological Society of London, 126, 205–233. [Google Scholar]
- Haskins, S. C. (2015). Monitoring anesthetized patients. In Grimm K. A., Lamont L. A., Tranquilli W. J., Greene S. A., & Robertson S. A. (Eds.), Veterinary anesthesia and analgesia: The fifth edition of Lumb and Jones (pp. 86–113). Wiley. [Google Scholar]
- Haulena, M. , & Schmitt, T. (2018). Anesthesia. In Gulland F. M. D., Dierauf L. A., & Whitman K. L. (Eds.), CRC handbook of marine mammal medicine (pp. 567–606). CRC Press. [Google Scholar]
- Hayano, J. , Yasuma, F. , Okada, A. , Mukai, S. , & Fujinami, T. (1996). Respiratory sinus arrhythmia: A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation, 94, 842–847. [DOI] [PubMed] [Google Scholar]
- Higgins, J. L. , & Hendrickson, D. A. (2013). Surgical procedures in pinniped and cetacean species. Journal of Zoo and Wildlife Medicine, 44, 817–836. [DOI] [PubMed] [Google Scholar]
- Hindle, A. G. (2020). Diving deep: Understanding the genetic components of hypoxia tolerance in marine mammals. Journal of Applied Physiology (Bethesda, MD: 1985), 128, 1439–1446. [DOI] [PubMed] [Google Scholar]
- Horvath, S. M. , Chiodi, H. , Ridgway, S. H. , & Azar, S. (1968). Respiratory and electrophoretic characteristics of hemoglobin of porpoises and sea lion. Comparative Biochemistry and Physiology, 24, 1027–1033. [DOI] [PubMed] [Google Scholar]
- Houser, D. S. , Dankiewicz‐Talmadge, L. A. , Stockard, T. K. , & Ponganis, P. J. (2010). Investigation of the potential for vascular bubble formation in a repetitively diving dolphin. The Journal of Experimental Biology, 213, 52–62. [DOI] [PubMed] [Google Scholar]
- Houser, D. S. , Finneran, J. J. , & Ridgway, S. H. (2010). Research with navy marine mammals benefits animal care, conservation and biology. International Journal of Comparative Psychology, 23, 249–268. [Google Scholar]
- Howard, R. S. , Finneran, J. J. , & Ridgway, S. H. (2006). Bispectral index monitoring of unihemispheric effects in dolphins. Anesthesia and Analgesia, 103, 626–632. [DOI] [PubMed] [Google Scholar]
- Huelsmann, M. , Hecker, N. , Springer, M. S. , Gatesy, J. , Sharma, V. , & Hiller, M. (2019). Genes lost during the transition from land to water in cetaceans highlight genomic changes associated with aquatic adaptations. Science Advances, 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose, F. , Zapol, W. M. , Sapirstein, A. , Ullrich, R. , Tager, A. M. , Coggins, K. , Jones, R. , & Bloch, K. D. (2001). Attenuation of hypoxic pulmonary vasoconstriction by endotoxemia requires 5‐lipoxygenase in mice. Circulation Research, 88, 832–838. [DOI] [PubMed] [Google Scholar]
- Johnson, S. , Venn‐Watson, S. , Cassle, S. , Smith, C. , Jensen, E. , & Ridgway, S. (2009). Use of phlebotomy treatment in Atlantic bottlenose dolphins with iron overload. Journal of the American Veterinary Medical Association, 235, 194–200. [DOI] [PubMed] [Google Scholar]
- Jones, B. L. , McClain, A. M. , Sportelli, J. J. , & Le‐Bert, C. R. (2023). Return of sound production as a biomarker of bottlenose dolphin emergence from anesthesia. Animals, 13. 10.3390/ani13152531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, R. , Ebisawa, K. , Okabayashi, K. , Narita, T. , Nakayama, S. , & Koie, H. (2023). Aquatic environments change the cardiac morphology of dolphins. The Journal of Veterinary Medical Science, 85, 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoye, Y. , Ivanov, I. , Cheng, Q. , Matafonov, A. , Dickeson, S. K. , Mason, S. , Sexton, D. J. , Renne, T. , McCrae, K. , Feener, E. P. , & Gailani, D. (2016). A comparison of the effects of factor XII deficiency and prekallikrein deficiency on thrombus formation. Thrombosis Research, 140, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyman, G. L. , Castellini, M. A. , & Davis, R. W. (1981). Physiology of diving in marine mammals. Annual Review of Physiology, 43, 343–356. [DOI] [PubMed] [Google Scholar]
- Kooyman, G. L. , & Ponganis, P. J. (1997). The challenges of diving of depth: The deepest sea divers have unique ways of budgeting their oxygen supply and responding to pressure. American Scientist, 85, 530–539. [Google Scholar]
- Le‐Bert, C. R. , Bukoski, A. , Downs, J. , Hodgson, D. S. , Thombs, L. , Ridgway, S. H. , & Bailey, J. (2024). Apneustic anesthesia ventilation improves pulmonary function in anesthetized bottlenose dolphins (Tursiops truncatus). Frontiers in Veterinary Science, 11. 10.3389/fvets.2024.1287478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le‐Bert, C. R. , Smith, C. R. , Poindexter, J. , Ardente, A. , Meegan, J. , Wells, R. S. , Venn‐Watson, S. , Jensen, E. D. , & Sakhaee, K. (2018). Comparison of potential dietary and urinary risk factors for ammonium urate nephrolithiasis in two bottlenose dolphin (Tursiops truncatus) populations. American Journal of Physiology. Renal Physiology, 315, F231–F237. [DOI] [PubMed] [Google Scholar]
- Lee, C. , Jensen, E. D. , Meegan, J. , Ivancic, M. , Bailey, J. , Hendrickson, D. , Weiss, J. , Grindley, J. , Costidis, A. M. , & Wisbach, G. (2019). Surgical Management of a Chronic Neck Abscess in a U.S. navy bottlenose dolphin. Military Medicine, 184, e360–e364. [DOI] [PubMed] [Google Scholar]
- Lillie, M. A. , Vogl, A. W. , Gerard, S. G. , Raverty, S. , & Shadwick, R. E. (2022). Retia mirabilia: Protecting the cetacean brain from locomotion‐generated blood pressure pulses. Science, 377, 1452–1456. [DOI] [PubMed] [Google Scholar]
- Lilly, J. C. (1964). Animals in aquatic environments: Adaptations of mammals to the ocean. In Dill D. B. (Ed.), Handbook of physiology: Environment (pp. 741–747). American Physiology Society. [Google Scholar]
- Lindemann, D. M. , Hendrickson, D. A. , Bailey, J. , Le‐Bert, C. R. , Erlacher‐Reid, C. , Staggs, L. , DiRocco, S. , Montano, G. , Weisbrod, T. C. , Dennison, S. , Stedman, N. , & Robeck, T. R. (2023, May 21–24). General anesthesia, exploratory laparoscopy, and mass removal in a bottlenose dolphin (Tursiops truncatus) [Conference presentation abstract]. Fifty‐third annual conference of the International Association for Aquatic Animal Medicine, Salt Lake City, UT, USA. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Linnehan, B. K. , Gomez, F. M. , Huston, S. M. , Hsu, A. , Takeshita, R. , Colegrove, K. M. , Harms, C. A. , Barratclough, A. , Deming, A. C. , Rowles, T. K. , Musser, W. B. , Zolman, E. S. , Wells, R. S. , Jensen, E. D. , Schwacke, L. H. , & Smith, C. R. (2021). Cardiac assessments of bottlenose dolphins (Tursiops truncatus) in the northern Gulf of Mexico following exposure to deepwater horizon oil. PLoS One, 16, e0261112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen, O. , Willemsen, D. , Ursing, B. M. , Arnason, U. , & de Jong, W. W. (2002). Molecular evolution of the mammalian alpha 2B adrenergic receptor. Molecular Biology and Evolution, 19, 2150–2160. [DOI] [PubMed] [Google Scholar]
- Mahmood, S. S. , & Pinsky, M. R. (2018). Heart‐lung interactions during mechanical ventilation: The basics. Annals of Translational Medicine, 6, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, J. G. (1969). Relationship of sleep, respiration, and anesthesia in the porpoise: A preliminary report. Proceedings of the National Academy of Sciences of the United States of America, 62, 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, J. G. , & Ridgway, S. H. (2018). History of the development of anesthesia for the dolphin: A quest to study a brain as large as Man's. Anesthesiology, 129, 11–21. [DOI] [PubMed] [Google Scholar]
- McCulloch, P. F. , Ollenberger, G. P. , Bekar, L. K. , & West, N. H. (1997). Trigeminal and chemoreceptor contributions to bradycardia during voluntary dives in rats. The American Journal of Physiology, 273, R814–R822. [DOI] [PubMed] [Google Scholar]
- Meagher, E. M. , McLellan, W. A. , Westgate, A. J. , Wells, R. S. , Frierson, J. D. , & Pabst, D. A. (2002). The relationship between heat flow and vasculature in the dorsal fin of wild bottlenose dolphins Tursiops truncatus . The Journal of Experimental Biology, 205, 3475–3486. [DOI] [PubMed] [Google Scholar]
- Medway, W. , McCormick, J. G. , Ridgway, S. H. , & Crump, J. F. (1970). Effects of prolonged halothane anesthesia on some cetaceans. Journal of the American Veterinary Medical Association, 157, 576–582. [PubMed] [Google Scholar]
- Meegan, J. M. , Ardente, A. J. , Poindexter, J. R. , Baird, M. , Novick, B. , Parry, C. , Jensen, E. D. , Venn‐Watson, S. , Sakhaee, K. , & Smith, C. R. (2021). Dietary effects on urinary physicochemistry in navy bottlenose dolphins (Tursiops truncatus) for the prevention of ammonium urate kidney stones. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 321, R723–R731. [DOI] [PubMed] [Google Scholar]
- Meegan, J. M. , Cotte, L. S. , Ivancic, M. , Thornton, J. J. , Deming, A. C. , Bailey, J. E. , Emory‐Gomez, F. M. , Costidis, A. M. , Wisbach, G. G. , Hendrickson, D. , Weiss, J. S. , Gallus, D. P. , Smith, C. R. , & Jensen, E. D. (2015, April 6–10). Medical and surgical management of a chronic cervical abscess in a bottlenose dolphin (Tursiops truncatus) [Conference presentation abstract]. Forty‐sixth annual conference of the International Association for Aquatic Animal Medicine, Chicago, IL, USA. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Meegan, J. M. , Miller, R. , Ross, K. , Gildea, T. , McClain, A. , Bailey, J. , Cendejas, V. , Linnehan, B. , Gomez, F. , Le‐Bert, C. , & Jensen, E. D. (2021, May 23–26). Multidisciplinary management of complex airway stenosis in a bottlenose dolphin (Tursiops truncatus) [Conference presentation abstract]. Fifty‐first annual conference of the International Association for Aquatic Animal Medicine, Virtual Conference. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Meegan, J. M. , Woody, A. D. , Bailey, J. E. , Jensen, E. D. , Gomez, F. M. , Garman, L. S. , Lutmerding, B. A. , Smith, C. R. , Nau, M. , Ivancic, M. , & Ridgway, S. H. (2016, May 21–26). Surgical management of osteomyelitis and dental disease in a bottlenose dolphin (Tursiops truncatus) using general anesthesia [Conference presentation abstract]. Forty‐seventh annual conference of the International Association for Aquatic Animal Medicine, Virginia Beach, VA, USA. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Meir, J. U. , Champagne, C. D. , Costa, D. P. , Williams, C. L. , & Ponganis, P. J. (2009). Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 297, R927–R939. [DOI] [PubMed] [Google Scholar]
- Menzies, M. P. , Ringer, S. K. , Conrot, A. , Theurillat, R. , Kluge, K. , Kutter, A. P. , Jackson, M. , Thormann, W. , & Bettschart‐Wolfensberger, R. (2016). Cardiopulmonary effects and anaesthesia recovery quality in horses anaesthetized with isoflurane and low‐dose S‐ketamine or medetomidine infusions. Veterinary Anaesthesia and Analgesia, 43, 623–634. [DOI] [PubMed] [Google Scholar]
- Miedler, S. , Fahlman, A. , Valls Torres, M. , Alvaro Alvarez, T. , & Garcia‐Parraga, D. (2015). Evaluating cardiac physiology through echocardiography in bottlenose dolphins: Using stroke volume and cardiac output to estimate systolic left ventricular function during rest and following exercise. The Journal of Experimental Biology, 218, 3604–3610. [DOI] [PubMed] [Google Scholar]
- Moore, C. , Moore, M. , Trumble, S. , Niemeyer, M. , Lentell, B. , McLellan, W. , Costidis, A. , & Fahlman, A. (2013). A comparative analysis of marine mammal tracheas. Journal of Experimental Biology, 217, 1154–1166. [DOI] [PubMed] [Google Scholar]
- Mortola, J. P. , & Seguin, J. (2009). End‐tidal CO2 in some aquatic mammals of large size. Zoology (Jena, Germany), 112, 77–85. [DOI] [PubMed] [Google Scholar]
- Nagel, E. L. , Morgane, P. J. , & McFarland, W. L. (1964). Anesthesia for the bottlenose dolphin, Tursiops truncatus . Science, 146, 1591–1593. [DOI] [PubMed] [Google Scholar]
- Nagel, E. L. , Morgane, P. J. , & McFarland, W. L. (1966). Anesthesia for the bottlenose dolphin. Veterinary Medicine, Small Animal Clinician, 61, 229–233. [Google Scholar]
- Nagel, E. L. , Morgane, P. J. , McFarland, W. L. , & Galliano, R. E. (1968). Rete mirabile of dolphin: Its pressure‐dampening effect on cerebral circulation. Science, 161, 898–900. [DOI] [PubMed] [Google Scholar]
- Noren, S. R. , & Williams, T. M. (2000). Body size and skeletal muscle myoglobin of cetaceans: Adaptations for maximizing dive duration. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 126, 181–191. [DOI] [PubMed] [Google Scholar]
- Noren, S. R. , Williams, T. M. , Ramirez, K. , Boehm, J. , Glenn, M. , & Cornell, L. (2012). Changes in partial pressures of respiratory gases during submerged voluntary breath hold across odontocetes: Is body mass important? Journal of Comparative Physiology B Biochemical, Systemic, and Environmental Physiology, 182, 299–309. [DOI] [PubMed] [Google Scholar]
- Ochrymowych, C. , & Lambertsen, R. H. (1984). Anatomy and vasculature of a minke whale heart. The American Journal of Anatomy, 169, 165–175. [DOI] [PubMed] [Google Scholar]
- Pabst, D. A. , Rommel, S. A. , & McLellan, W. A. (1999). Functional morphology of marine mammals. In Reynolds J. E. & Rommel S. A. (Eds.), Biology of marine mammals (pp. 15–72). Smithsonian Press. [Google Scholar]
- Panneton, W. M. (2013). The mammalian diving response: An enigmatic reflex to preserve life? Physiology (Bethesda), 28, 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel, M. A. , Petersen, E. N. , Wang, H. , Lerner, R. A. , & Hansen, S. B. (2020). Studies on the mechanism of general anesthesia. Proceedings of the National Academy of Sciences of the United States of America, 117, 13757–13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli, M. A. , McLellan, W. A. , Rommel, S. A. , Blum, J. E. , Barco, S. G. , & Pabst, D. A. (2010). Lung size and thoracic morphology in shallow‐ and deep‐diving cetaceans. Journal of Morphology, 271, 654–673. [DOI] [PubMed] [Google Scholar]
- Piscitelli, M. A. , Raverty, S. A. , Lillie, M. A. , & Shadwick, R. E. (2013). A review of cetacean lung morphology and mechanics. Journal of Morphology, 274, 1425–1440. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J. (2019). State of the art review: From the seaside to the bedside: Insights from comparative diving physiology into respiratory, sleep and critical care. Thorax, 74, 512–518. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J. , McDonald, B. I. , Tift, M. S. , & Williams, C. L. (2017). Heart rate regulation in diving sea lions: The vagus nerve rules. The Journal of Experimental Biology, 220, 1372–1381. [DOI] [PubMed] [Google Scholar]
- Ponganis, P. J. , Meir, J. U. , & Williams, C. L. (2011). In pursuit of Irving and Scholander: A review of oxygen store management in seals and penguins. The Journal of Experimental Biology, 214, 3325–3339. [DOI] [PubMed] [Google Scholar]
- Remington, N. , Stevens, R. D. , Wells, R. S. , Hohn, A. , Dhungana, S. , Taboy, C. H. , Crumbliss, A. L. , Henkens, R. , & Bonaventura, C. (2007). Genetic diversity of coastal bottlenose dolphins revealed by structurally and functionally diverse hemoglobins. Gene, 398, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway, S. H. (1972). Homeostasis in the aquatic environment. In Ridgway S. H. (Ed.), Mammals of the Sea: Biology and Medicine (pp. 590–747). Charles C. Thomas. [Google Scholar]
- Ridgway, S. H. (2002). Asymmetry and symmetry in brain waves from dolphin left and right hemispheres: Some observations after anesthesia, during quiescent hanging behavior, and during visual obstruction. Brain, Behavior and Evolution, 60, 265–274. [DOI] [PubMed] [Google Scholar]
- Ridgway, S. H. , Green, R. F. , & Sweeney, J. C. (1975). Mandibular anesthesia and tooth extraction in the bottlenosed dolphin. Journal of Wildlife Diseases, 11, 415–418. [DOI] [PubMed] [Google Scholar]
- Ridgway, S. H. , & Johnston, D. G. (1966). Blood oxygen and ecology of porpoises of three genera. Science, 151, 456–458. [DOI] [PubMed] [Google Scholar]
- Ridgway, S. H. , & McCormick, J. G. (1967). Anesthetization of porpoises for major surgery. Science, 158, 510–512. [DOI] [PubMed] [Google Scholar]
- Ridgway, S. H. , & McCormick, J. G. (1971). Anesthesia of the porpoise. In Textbook of Veterinary Anesthesia (pp. 394–403). Williams and Wilkins Co. . [Google Scholar]
- Ridgway, S. H. , McCormick, J. G. , & Wever, E. G. (1974). Surgical approach to the dolphin's ear. The Journal of Experimental Zoology, 188, 265–276. [DOI] [PubMed] [Google Scholar]
- Rommel, S. A. , Pabst, D. A. , McLellan, W. A. , Mead, J. G. , & Potter, C. W. (1992). Anatomical evidence for a countercurrent heat exchanger associated with dolphin testes. The Anatomical Record, 232, 150–156. [DOI] [PubMed] [Google Scholar]
- Rosenberg, J. F. , Haulena, M. , Bailey, J. E. , Hendrickson, D. A. , Ivancic, M. , & Raverty, S. A. (2017). Emergency anesthesia and exploratory laparotomy in a compromised Pacific white‐sided dolphin (Lagenorhynchus obliquidens). Journal of Zoo and Wildlife Medicine, 48, 581–585. [DOI] [PubMed] [Google Scholar]
- Rowlands, C. E. , McLellan, W. A. , Rommel, S. A. , Costidis, A. M. , Yopak, K. E. , Koopman, H. N. , Glandon, H. L. , & Ann, P. D. (2021). Comparative morphology of the spinal cord and associated vasculature in shallow versus deep diving cetaceans. Journal of Morphology, 282, 1415–1431. [DOI] [PubMed] [Google Scholar]
- Russell, J. , Jeffery, N. , Bailey, J. , Osborn, S. , Le‐Bert, C. , Whitehead, H. , & Nollens, H. (2021). Cerebrospinal fluid sampling in a bottlenose dolphin (Tursiops truncatus) under general anesthesia. Journal of Zoo and Wildlife Medicine, 51, 1056–1061. [DOI] [PubMed] [Google Scholar]
- Sage, A. M. , Keating, S. C. , Lascola, K. M. , Schaeffer, D. J. , & Clark‐Price, S. C. (2018). Cardiopulmonary effects and recovery characteristics of horses anesthetized with xylazine‐ketamine with midazolam or propofol. Veterinary Anaesthesia and Analgesia, 45, 772–781. [DOI] [PubMed] [Google Scholar]
- Schmitt, T. L. , Knych, H. K. , Mama, K. R. , Nollens, H. H. , Croft, L. , DiRocco, S. , Erlacher‐Reid, C. , & Bailey, J. E. (2018, May 19–23). Plasma concentrations and disposition of propofol in bottlenose dolphins (Tursiops truncatus) during general anesthesia [Conference presentation abstract]. Forty‐ninth annual conference of the International Association for Aquatic Animal Medicine, Long Beach, CA, USA. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Schmitt, T. L. , Nollens, H. H. , Esson, D. W. , St. Leger, J. , & Bailey, J. E. (2014, May 17–22). Superficial keratectomy and cryosurgery of a limbal melanoma under general anesthesia in a bottlenose dolphin (Tursiops truncatus gillii) [Conference presentation abstract]. Forty‐fifth annual conference of the International Association for Aquatic Animal Medicine, Gold Coast, QLD, Australia. https://www.vin.com/proceedings/proceedings.aspx?orgid=76&said=‐1
- Schwacke, L. H. , Zolman, E. S. , Balmer, B. C. , De Guise, S. , George, R. C. , Hoguet, J. , Hohn, A. A. , Kucklick, J. R. , Lamb, S. , Levin, M. , Litz, J. A. , McFee, W. E. , Place, N. J. , Townsend, F. I. , Wells, R. S. , & Rowles, T. K. (2012). Anaemia, hypothyroidism and immune suppression associated with polychlorinated biphenyl exposure in bottlenose dolphins (Tursiops truncatus). Proceedings of the Biological Sciences, 279, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba, U. , Shibuya, Y. , Okabe, H. , Hayashi, I. , & Yamamoto, T. (2000). Whale high‐molecular‐weight and low‐molecular‐weight kininogens. Thrombosis Research, 97, 481–490. [DOI] [PubMed] [Google Scholar]
- Semba, U. , Shibuya, Y. , Okabe, H. , & Yamamoto, T. (1998). Whale Hageman factor (factor XII): Prevented production due to pseudogene conversion. Thrombosis Research, 90, 31–37. [DOI] [PubMed] [Google Scholar]
- Sklansky, M. , Levine, G. , Havlis, D. , West, N. , Renner, M. , Rimmerman, C. , & Stone, R. (2006). Echocardiographic evaluation of the bottlenose dolphin (Tursiops truncatus). Journal of Zoo and Wildlife Medicine, 37, 454–463. [DOI] [PubMed] [Google Scholar]
- Small, K. M. , Brown, K. M. , Forbes, S. L. , & Liggett, S. B. (2001). Polymorphic deletion of three intracellular acidic residues of the alpha 2B‐adrenergic receptor decreases G protein‐coupled receptor kinase‐mediated phosphorylation and desensitization. The Journal of Biological Chemistry, 276, 4917–4922. [DOI] [PubMed] [Google Scholar]
- Snyder, G. K. (1983). Respiratory adaptations in diving mammals. Respiration Physiology, 54, 269–294. [DOI] [PubMed] [Google Scholar]
- Sommer, L. , McFarland, W. , Galliano, R. , Nagel, E. , & Morgane, P. (1968). Hemodynamic and coronary angiographic studies in the bottlenose dolphin (Tursiops truncatus). American Journal of Physiology‐Legacy Content, 215, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Steffey, E. P. , Mama, K. R. , & Brosnan, R. J. (2015). Inhalation Anesthestics. In Grimm K. A., Lamont L. A., Tranquilli W. J., Greene S. A., & Robertson S. A. (Eds.), Veterinary anesthesia and analgesia: The fifth edition of Lumb and Jones (pp. 297–331). John Wiley & Sons, Inc. [Google Scholar]
- Stephenson, R. (2005). Physiological control of diving behaviour in the Weddell seal Leptonychotes weddelli: A model based on cardiorespiratory control theory. The Journal of Experimental Biology, 208, 1971–1991. [DOI] [PubMed] [Google Scholar]
- Storlund, R. L. , Rosen, D. A. S. , & Trites, A. W. (2021). Electrocardiographic scaling reveals differences in electrocardiogram interval durations between marine and terrestrial mammals. Frontiers in Physiology, 12, 690029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboy, C. H. , Faulkner, K. M. , Kraiter, D. , Bonaventura, C. , & Crumbliss, A. L. (2000). Concentration‐dependent effects of anions on the anaerobic oxidation of hemoglobin and myoglobin. The Journal of Biological Chemistry, 275, 39048–39054. [DOI] [PubMed] [Google Scholar]
- Tamura, J. , Yanagisawa, M. , Endo, Y. , Ueda, K. , Koga, H. , Izumisawa, Y. , & Yamashita, K. (2017). Anesthetic Management of an Indo‐Pacific Bottlenose Dolphin (Tursiops aduncus) requiring surgical debridement of a tail abscess. Journal of Zoo and Wildlife Medicine, 48, 200–203. [DOI] [PubMed] [Google Scholar]
- Thewissen, J. G. , Cooper, L. N. , Clementz, M. T. , Bajpai, S. , & Tiwari, B. N. (2007). Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature, 450, 1190–1194. [DOI] [PubMed] [Google Scholar]
- Thewissen, J. G. M. , Cooper, L. N. , George, J. C. , & Bajpai, S. (2009). From land to water: The origin of whales, dolphins, and porpoises. Evolution: Education and Outreach, 2, 272–288. [Google Scholar]
- Tian, R. , Wang, Z. , Niu, X. , Zhou, K. , Xu, S. , & Yang, G. (2016). Evolutionary genetics of hypoxia tolerance in cetaceans during diving. Genome Biology and Evolution, 8, 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacas, S. , Kurien, P. , & Maze, M. (2013). Sleep and anesthesia–common mechanisms of action. Sleep Medicine Clinics, 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn‐Watson, S. , Reiner, J. , & Jensen, E. D. (2022). Pentadecanoylcarnitine is a newly discovered endocannabinoid with pleiotropic activities relevant to supporting physical and mental health. Scientific Reports, 12, 13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn‐Watson, S. K. , Parry, C. , Baird, M. , Stevenson, S. , Carlin, K. , Daniels, R. , Smith, C. R. , Jones, R. , Wells, R. S. , Ridgway, S. , & Jensen, E. D. (2015). Increased dietary intake of saturated fatty acid Heptadecanoic acid (C17:0) associated with decreasing ferritin and alleviated metabolic syndrome in dolphins. PLoS One, 10, e0132117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, R. S. (2009). Learning from nature: Bottlenose dolphin care and husbandry. Zoo Biology, 28, 635–651. [DOI] [PubMed] [Google Scholar]
- Williams, T. , Friedl, W. , & Haun, J. (1993). The physiology of bottlenose dolphins (Tursiops truncatus): Heart rate, metabolic rate and plasma lactate concentration during exercise. The Journal of Experimental Biology, 179, 31–46. [DOI] [PubMed] [Google Scholar]
- Williams, T. M. , Fuiman, L. A. , Kendall, T. , Berry, P. , Richter, B. , Noren, S. R. , Thometz, N. , Shattock, M. J. , Farrell, E. , Stamper, A. M. , & Davis, R. W. (2015). Exercise at depth alters bradycardia and incidence of cardiac anomalies in deep‐diving marine mammals. Nature Communications, 6, 6055. [DOI] [PubMed] [Google Scholar]
- Williams, T. M. , Haun, J. E. , & Friedl, W. A. (1999). The diving physiology of bottlenose dolphins (Tursiops truncatus): I. Balancing the demands of exercise for energy conservation at depth. The Journal of Experimental Biology, 202, 2739–2748. [DOI] [PubMed] [Google Scholar]
- Yordy, J. E. , Wells, R. S. , Balmer, B. C. , Schwacke, L. H. , Rowles, T. K. , & Kucklick, J. R. (2010). Partioning of persistent organic pollutants between blubber and blood of wild bottlenose dolphins: Implications for biomonitoring and health. Environmental Science & Technology, 44, 4789–4795. [DOI] [PubMed] [Google Scholar]


