Abstract
Background
According to self-reported frequencies, every fifth or sixth dwelling in Germany is affected by dampness and/or mold. This carries a potential risk to health.
Methods
This review is based on pertinent publications retrieved by a selective literature search and inquiry in the GENESIS database, on the AWMF guideline on the medical clinical diagnosis of indoor mold exposure, as updated in 2023, and on the relevant contents of other current guidelines. Based on this research, we present an algorithm for the evaluation of health problems that may be due to mold in indoor environments.
Results
A rational diagnostic work-up begins with history-taking and physical examination, with attention to risk factors—above all, immune compromise and atopy. If there is evidence of atopy, targeted allergy diagnostics should be performed, consisting of a skin prick test and/or measurement of specific IgE antibodies, supplemented whenever indicated by provocative testing and cellular test systems. If the patient’s immune response is compromised, the immediate cessation of mold exposure has absolute priority. Any suspected invasive fungal infection should be evaluated with radiological, microbiological, serological, and immunological testing. Indoor measurements of mold fungi, microbial volatile organic compounds (MVOC), and/or mycotoxins are generally not indicated as part of the medical evaluation; nor are blood or urine tests for particular mold components or metabolites.
Conclusion
Mold in indoor environments should be dealt with by rapid exposure elimination for patients at risk, the rational diagnostic evaluation of any symptoms and signs of disease, and patient education about the possibilities and limitations of diagnostic testing and the generally limited utility of measurements in the affected interior spaces.
CME plus+
This article has been certified by the North Rhine Academy for Continuing Medical Education. The questions on this article can be found at http://daebl.de/RY95.
The submission deadline is 18 April 2025.
Participation is possible at cme.aerztebatt.de
The term “mold” is a collective name for filamentous cell- (hyphae-) and generally also spore-forming microfungi and does not describe a taxonomically defined fungal entity. Molds are a ubiquitous component of our biosphere and, depending on vegetation and anthropogenic sources, are found to varying degrees according to season and region in outdoor air, indoor spaces, and in many workplaces. Anthropogenic sources include, for example, composting plants, waste sorting plants, biogas plants, garden centers, waste management, and agriculture.
Indoor mold infestation is the contamination of surfaces, materials, and/or indoor air by microorganisms and/or biogenic particles and substances that exceeds the general background levels of mold exposure (mold exposure in all indoor spaces)—irrespective of whether the organisms are alive and actively growing there or are dead. Mold species associated with mold infestation primarily include Acremonium spp., Aspergillus penicillioides, Aspergillus restrictus, Aspergillus versicolor, Chaetomium spp., Phialophora spp., Penicillium chrysogenum, Penicillium brevicompactum, Scopulariopsis brevicaulis, Scopulariopsis fusca, Scopulariopsis brumtii, Scopulariopsis chartarum, Stachybotrys chartarum, Tritirachium (Engyodontium) album, and Trichoderma spp. as examples of moisture-related indicators (1), and in rare cases, Aspergillus fumigatus.
In Germany, indoor moisture and mold are a significant health problem with a self-reported frequency of one in every five to six homes.
Mold can only develop and grow if there is sufficient moisture in materials or on surfaces. Therefore, mold growth is usually the result of high humidity, inadequate ventilation, and the cold surfaces of building components (resulting in water condensation) and—in a small number of cases—water damage or rising damp. Molds can grow on a multitude of materials and in a wide range of temperatures (3, 4). Indoor mold growth is always a problem of moisture. In order to permanently prevent the exposure of people who spend time in affected indoor spaces, the cause of moisture needs to be identified and eliminated in a first step.
Particularly for certain risk groups, the growth of indoor mold represents a significant health risk. For example, according to the GENESIS database of the German Federal Statistical Office (Statistisches Bundesamt), 1731 patients with the main diagnosis of aspergillosis (ICD-10 code B44 [including B44.0, B44.1, B44.7, B44.8, B44.9]) were treated as hospital inpatients in 2022. Of these, 117 patients died.
In practice, answering patients’ questions regarding the health risk associated with the detection of indoor mold is primarily a medical task. In order to assess a health risk arising from mold, the health status of the exposed individual (predisposition) needs to be assessed on the one hand, as does the extent of mold infestation and the release of mold spores and/or other components (for example, metabolic products, cell components) (exposure) on the other.
International guidelines on medical clinical diagnosis are available, but these are somewhat older and, as such, can only be applied to a limited extent (4, 5).
Methods
This review is based on pertinent publications retrieved by a selective literature search and inquiry in the GENESIS database, on the AWMF guideline (6) on the medical clinical diagnosis of indoor mold exposure, as updated in 2023, and on the relevant contents of other current guidelines. Based on this research, we present an algorithm for the evaluation of health problems that may be due to mold in indoor environments.
Evaluation of the health risk posed by exposure to indoor mold
Epidemiological studies unanimously show an association between indoor moisture/mold damage and health effects, the most important of which include:
Respiratory symptoms
Eye, nose, and throat irritation
Nasal congestion and wheezing
Dry cough
Sleep disorders
Snoring
The present study confines itself largely to clinical pictures rather than to symptoms. The relevant evidence of associations between moisture/mold damage and its various health effects is summarized in the Box.
Box. Evidence for the link betweenindoor moisture/mold infestation and diseases (in alphabetic order) (6).
Causal link
No evidence
Sufficient evidence for an association*
Allergic airway diseasesAllergic bronchopulmonary aspergillosis (ABPA)
Allergic rhinitis
Allergic rhinoconjunctivitis
Aspergilloma
Aspergillus bronchitis
Asthma (manifestation, progression, exacerbation)
Bronchitis (acute, chronic)
Community acquired Aspergillus pneumonia
Hypersensitivity pneumonitis (HP)
Invasive aspergillosis
Mycosis
Other allergic bronchopulmonary mycoses (ABPM)
Organic dust toxic syndrome (ODTS) (workplace)
Promotion of respiratory infections
Pulmonary aspergillosis (subacute, chronic)
Rhinosinusitis (acute, chronic, invasive or granulomatous, allergic)
Limited or suspected evidence for an association
Atopic eczema/atopic dermatitis/neurodermatitis (manifestation)
Chronic obstructive pulmonary disease (COPD)
Mood disorders
Mucous membrane irritation (MMI)
Odor effects
Sarcoidosis
Inadequate or insufficient evidence for an association
Acute idiopathic pulmonary hemorrhage in infancy
Airborne transmission of mycotoxicosis
Arthritis
Autoimmune diseases
Cancer
Chronic fatigue syndrome (CFS)
Endocrinopathies
Gastrointestinal effects
Multiple chemical sensitivity (MCS)
Multiple sclerosis
Neuropsychological effects
Neurotoxic effects
Renal effects
Reproductive disorders
Rheumatic diseases
Sick building syndrome (SBS)
Sudden infant death syndrome
Teratogenicity
Thyroid diseases
Urticaria
* The diseases listed here can be subsumed under the term building-related illness (BRI), despite the fact that for BRI, the etiology, pathology, pathophysiology, diagnosis, treatment, prevention, and prognosis need to be unequivocally known (9, 10).
It is not possible to prove beyond doubt in individual cases that there is a causal link between exposure to a particular mold and specific health symptoms and clinical pictures. The reasons for this are set out below.
Risk of infection
Molds are ubiquitous. Therefore, even if indoor exposure to one or more mold species that pose a risk of infection according to the definition of the Biological Agents Ordinance has been objectively identified and measured, it is not possible to draw conclusions regarding the cause of a mold infection (11). The mold may have entered the human body in a different environment, for example, outdoor air, a compost heap, the biowaste garbage can, in another indoor space, or at another location. With molds, it is generally not possible to prove that a mold strain in an isolate taken from the patient (for example, mold in bronchoalveolar lavage) and an environmental isolate (for example, mold in indoor air) are the same.
For mold infections to occur in humans, there needs to be a correspondingly high level of immunosuppression. In Germany, this susceptibility (predisposition) to infection can only be assessed by a physician based on the three risk groups of immunosuppression (moderate, severe and very severe immunosuppression/deficiency) set out by the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) (12).
If immune suppression/deficiency of this kind and a source of indoor mold are both present, immediate cessation of exposure may be life-saving. Affected patients must be informed by their physician about the measures they need to take to avoid exposure. In such cases, measuring mold exposure has no benefit in terms of the immediate measures to be taken to protect the immunosuppressed individual, but carries instead the risk of a potentially life-threatening prolongation of exposure (13).
Risk of invasive infections
The risk of invasive mold infections is present almost exclusively in immunosuppressed patients (6). These include in particular individuals:
under immunosuppression according to the classification of the KRINKO at the RKI (12);
with severe influenza;
with severe COVID-19 and respiratory insufficiency.
Patients in these risk groups are often hospitalized. However, spore inhalation may have taken place earlier and thus prior to inpatient admission.
Another semi-invasive entity, chronic pulmonary aspergillosis, can develop in the form of cavitary or fibrosing pulmonary aspergillosis in people with structural lung damage—for example, in chronic obstructive pulmonary disease (COPD), cystic fibrosis, or following tuberculosis.
Risk of sensitizations and allergies
Measurements of mold in indoor air are not able to prove whether exposure to mold leads or has led to sensitization or allergy (14).
However, individuals that are sensitized to mold may develop allergic reactions as a result of indoor moisture/mold damage. Here again, it is more important that exposure ceases than that the mold exposure be objectively measured. This is particularly crucial for patients with allergic asthma or allergic bronchopulmonary aspergillosis (ABPA), since they may experience a worsening of lung function in the case of an allergic reaction to mold exposure.
For most indoor molds, there are no commercially available cutaneous test extracts and only a limited number of specific IgE tests for the detection of sensitization. Therefore, test results to determine a mold allergy, as well as the results of mold measurements taken in rooms used by the respective individuals, cannot be used for a causal risk assessment.
In order to prevent sensitization as well as to protect against allergic reactions in the case of existing sensitization, mold exposure must be ceased (allergen avoidance), that is to say, the mold infestation must be remediated (13).
Risk of toxic reactions
Exposure due to moisture/mold damage is complex and variable. Not only are spores, cell fragments, metabolic products, and mycotoxins from molds present but also bacteria (including actinobacteria), microbial volatile organic compounds (MVOC), β-glucans, mannans, ergosterol, endotoxins, bioaerosol allergens, and mite allergens. Since it is not possible to carry out a quantitative health risk assessment for individual bioaerosol components, it is not necessary to determine individual components with regard to their toxic effect when assessing a mold infestation.
It is known that some molds produce mycotoxins as secondary metabolites in the case of moisture damage. Bound to spores, mycelium, and cell fragments, they contribute as constituents of house dust and the bioaerosol to exposure and likely to inflammatory/irritant mucosal reactions.
The mycotoxin concentrations measured to date in indoor environments (living rooms, kindergartens, schools, and offices) infested with mold are very low, such that acute toxic effects (mycotoxicosis) are not to be expected (15).
The situation is quite different in certain workplaces, for example, in agriculture or at composting plants. Here, levels of airborne mycotoxins can cause considerable health effects (16, 17).
Investigation procedure
In routine practice, the focus is placed on medical history and a clinical assessment of the patient. This involves establishing in a first step whether:
there is evidence of particular exposure to indoor mold, for example, due to moisture/mold damage;
the patient is predisposed to the possible effects of mold;
the symptoms or clinical picture could be caused by mold exposure (6).
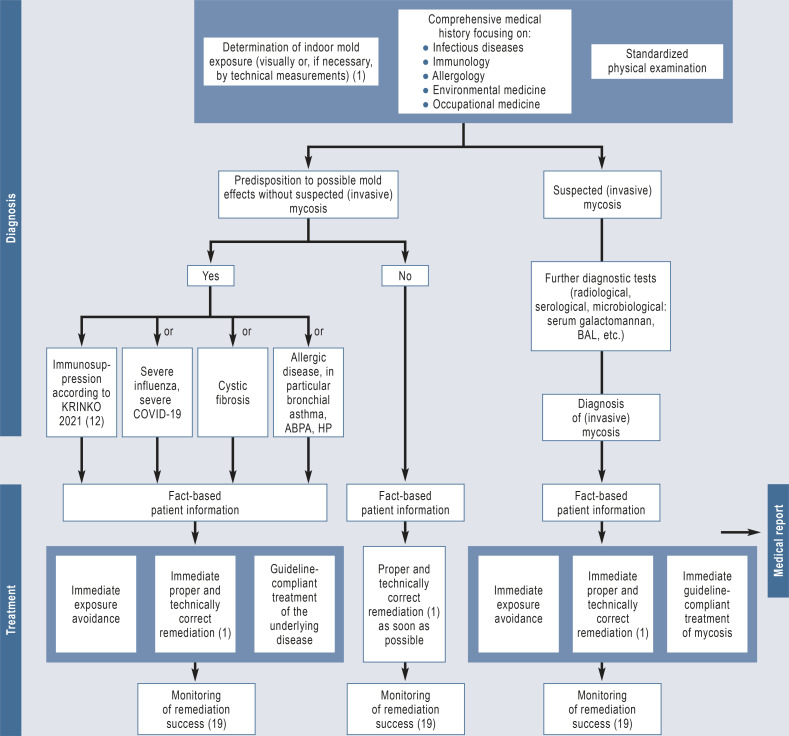
The Figure shows an algorithm for the investigation of suspected indoor mold-related health problems.
Figure.
Algorithm for the diagnostic work-up of suspected indoor mold-related health disorders
ABPA, allergic bronchopulmonary aspergillosis; BAL, bronchoalveolar lavage; HP, hypersensitivity pneumonitis
From a medical perspective, measurements of indoor mold are rarely indicated (6). However, they serve to document the extent of damage, as well as the causes, and also offer the only possibility to pursue indications of hidden damage (mold damage that is not readily visible, for example, behind a wall unit). Mold measurements are also often required for the planning of remediation works. In the case of visible mold infestation, a quantitative and qualitative determination of the mold species can often be dispensed with (13, 18). What is crucial is that the causes of the infestation be rapidly identified, so that this as well as the primary causes can be eliminated in a correct and proper manner.
In Germany, when measuring indoor mold, the measurement results are evaluated based on the guidelines of the Indoor Air Hygiene Commission of the German Environment Agency (Umweltbundesamt) for the prevention, detection, and remediation of mold infestation in buildings (1). Mold measurements, the tests additionally required for building physics, as well as other tests and the assessment of these measurement results, should be carried out by a publicly appointed and sworn expert (18). Mold differentiation and the accompanying microbiological work should be performed by a laboratory accredited for these methods (1, 18).
Microbiological, immunological, molecular biological, and radiological techniques form core elements of infection diagnostics and, depending on the indication, shall be used to diagnose (invasive) mycosis caused by molds in the abovementioned risk groups (6).
Particularly for individuals in whom indoor mold exposure is at high risk of causing disease, exposure avoidance must begin immediately. These individuals may no longer reside in the rooms/areas affected by moisture/mold damage. Even individuals that are less at risk should keep the amount of time they spend in these rooms/areas to a minimum until the damage has been eliminated and remediated. If there is no other option, not only should rooms be more intensively ventilated but room air purifiers can also be used temporarily, whereby it is essential to ensure that the devices are maintained and cleaned in accordance with the manufacturer’s instructions. The corresponding immediate measures for the short term, the planning and implementation of remediation as well as the monitoring of success should always be supervised by experts in indoor environments.
Likewise, it is essential that the remediation be carried out by a company specialized in these works. Once completed, an inspection of the remediation works is required; this must also be carried out by experts who are independent of the contractors responsible for the remediation. A good instrument for the monitoring of remediation is data sheet 4–12 published by the International Association for Science and Technology of Building Maintenance and Monuments Preservation (WTA) (19), which includes, for example, measurements of total spore count in accordance with DIN ISO 16000–20 (20) following mobilization of existing dust deposits to check the success of cleaning.
However, it is well known that the symptoms of affected individuals can persist for a long time after successful remediation (21, 22).
Recommended medical diagnostic work-up
Core elements of type-1 allergy diagnosis include patient history, skin testing, the determination of specific IgE antibodies (sIgE) as well as—in selected cases—provocation tests. The lack of availability of test extracts is a limitation (23). In the case of ABPA, both sIgE and specific IgG antibodies (sIgG) to Aspergillus fumigatus should be determined (24). In hypersensitivity pneumonitis (HP), skin testing and sIgE play no role; to detect type-III sensitization, sIgG against the antigens in question shall be determined in patients’ sera (25).
The detection of sIgE or a positive reaction in the skin test merely shows that there is a type I sensitization to the respective allergens. A clinically relevant allergy is only present, however, when there are typical symptoms of allergy.
A negative skin- or IgE-test for molds does not reliably rule out sensitization to molds. Reasons for this include the varying composition and quality of test extracts and the lack of availability of relevant allergens.
Invasive mold infections are rare and mostly occur via inhalation. In practice, Aspergillus fumigatus is the most important pathogen of the molds classified in risk groups 2 and 3 according the German TRBA 460 (Technical Rule for Biological Working Materials) (26). Those affected predominantly include individuals with generally severe or very severe immunodeficiency (according to KRINKO grade 2 and grade 3) (12). With regard to the clinical picture and diagnostic criteria, we refer the reader to the study by von Lilienfeld-Toal et al. (27). If the patient has a relevant predisposition, special attention must be paid to this risk and, where necessary, drug or non-drug prophylactic measures must be considered (28). Relevant guidelines on diagnosis and treatment are available (29, 30).
Test methods not recommended outside studies
Environmental monitoring of indoor MVOCs is not indicated in medical diagnostic testing for mold exposure.
Nor is environmental monitoring of mycotoxins in indoor air and house dust indicated in the medical diagnostic work-up.
The determination of specific IgG antibodies as part of the diagnostic work-up for immediate-type mold allergy (type I allergy) is of no diagnostic relevance and shall therefore not be performed. The same applies to the detection of immune complexes, for example, by means of the Ouchterlony assay (6).
Testing for galactomannan in serum (where necessary, also in bronchoalveolar lavage) shall only be carried out for diagnostic purposes in the case of suspected invasive pulmonary aspergillosis in immunosuppressed individuals. In all other cases, it is not indicated for the diagnosis of mold exposure (6).
The determination of serum eosinophil cationic protein (ECP) and β-1,3-D-glucan (BDG) is also not indicated outside the context of studies. As such, it shall not be included in the diagnostic work-up of mold exposure (6).
While the basophil degranulation test, the histamine liberation test (HLT), the basophil activation test using flow cytometry, and the determination of other mediators (sulfidoleukotriene release test, cellular antigen stimulation test [CAST-ELISA]) are used in specialized diagnosis, they should not be performed in the basic diagnostic work-up of allergy patients (6).
Lymphocyte transformation tests (LTT) for molds yield no information on sensitization or allergies and are therefore not indicated as a diagnostic procedure (6, 31).
The whole-blood test (WBT) is not a suitable instrument for the detection of mold sensitization and therefore shall not be performed (6).
Human biomonitoring of mycotoxins is not indicated in the medical diagnosis of indoor mold exposure and therefore shall not be performed (6, 13).
The following diagnostic methods shall not be used in the case of indoor mold exposure since they are not supported by sufficient scientific evidence (list not exhaustive):
Detection of mold in blood
Determination of IgA antibodies against mold
Determination of lymphocyte subpopulations
Determination of cytokines
Determination of oxidative stress
Visual contrast sensitivity (VCS) test
Tear film breakup time (6).
Due to a lack of medical evidence, the following diagnostic methods shall not be performed in the case of indoor mold exposure (list not exhaustive):
Electroacupuncture according to Voll
The bioresonance method
Pendulums
Vega testing
Decoder dermography
Biotonometry
Biotensors
Kirlian photography (plasma printing technique, energetic terminal point diagnosis)
Regulation thermography according to Rost
Auricular diagnosis
Kinesiology
Aurascopy
Iridology
Cytotoxic blood tests
Provocation–neutralization (P-N) testing (6).
Conclusion
When taking the medical history of a patient with indoor-related symptoms and/or the presence of corresponding health problems, physicians should consider the possibility of moisture/mold damage. While mold measurements in the affected indoor spaces are usually not required for the further medical work-up, specialists in the relevant areas (allergology, environmental medicine, pneumology, ENT, occupational medicine, etc.) should be called upon if there is reasonable suspicion of mold-related diseases.
Mold growth indoors must be regarded as a potential health risk from a preventive perspective, even if no quantitative or causal link between the occurrence of individual species and disease symptoms can be considered as confirmed. From a health perspective, moisture damage and/or mold growth indoors always represent a hygiene problem, which—even without health problems—is unacceptable (6). It is far more the case that, in line with the precautionary principle, exposure should be minimized and, if possible, eliminated (1, 32, 33). Therefore, the most important preventive measures in the case of exposure to indoor mold include determining the cause of the moisture/water damage and carrying out proper remediation (1, 34–36).
Questions on the article in issue 8/2025:
Indoor Mold—Important Considerations for Medical Advice to Patients
The submission deadline is 18 April 2025. Only one answer is possible per question.
Please select the answer that is most appropriate.
Question 1
How is the term “mold” defined in the article?
As a taxonomic name for spore-forming microfungi
As a collective term for all inedible fungi
As a collective term for all black fungi
As a collective term for filamentous cell- and spore-forming microfungi
As a taxonomic name for filamentous fungi
Question 2
How often—according to self-reports—are homes in Germany affected by mold?
Every second to third home
Every fifth to sixth home
Every eighth to 10th home
Every 11th to 13th home
Every 15th to 20th home
Question 3
Which sectors are mentioned in the article as examples of workplaces where high mycotoxin exposure may occur?
Agriculture and composting plants
Kindergartens and schools
Offices and administrative bodies
Swimming pools and sports facilities
Joineries and printing companies
Question 4
Which term is used in the article for intoxication due to mold toxins?
Mycocandidosis
Asperticosis
Hyphocytosis
Fumigatosis
Mycotoxicosis
Question 5
What does the abbreviation ABPA stand for in the text?
Allergic bilateral pulmonary aspergillitis
Allergic bronchopulmonary aspergillosis
Advanced biphasic aspergillosis
Advanced bronchopulmonary allergy
Allergic biphasic aspergillosis
Question 6
What information is given in the article regarding guidelines?
There are no German guidelines on the topic of molds.
There is only an outdated AWMF guideline (> 10 years old).
There is a current AWMF guideline on molds.
The first German guideline on the topic of molds is currently in preparation.
The authors call for the first guideline on the topic of molds to be written.
Question 7
According to information in the article, which of the following diagnostic methods form the core elements of the basic diagnostic work-up for type-1 allergy due to indoor mold infestation?
Whole-blood test (WBT)
Cellular antigen stimulation test (CAST-ELISA)
Basophil degranulation tests
Specific IgE antibody determination
Lymphocyte transformation testing for molds
Question 8
Under what circumstances should serum galactomannan be determined in connection with mold exposure?
Only when invasive pulmonary aspergillosis is suspected
Only in patients with cystic fibrosis as an underlying disease
Whenever exposure to indoor mold is suspected
Only if incipient sensitization to molds is suspected
Only in patients with bronchial asthma as an underlying disease
Question 9
For which of the following diseases—according to the article—is there only inadequate or insufficient evidence regarding an association with indoor moisture/mold exposure?
Allergic airway diseases
Asthma
Cancer
Organic dust toxic syndrome (ODTS) in the workplace
Atopic dermatitis
Question 10
According to the article, which of the following diagnostic methods can be used as part of the specialized diagnostic work-up for allergy in the case of indoor mold infestation?
Vega testing
Decoder dermography
Auricular diagnosis
Basophil activation test
Aurascopy
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interest statement
JH, DN, and GAW received honoraria for expert appraisals of case constellations involving mold problems.
MJ received lecture fees from AeDA Online Akademie, BDP, the University Hospital Tübingen, and from the Wisplinghoff Laboratory as well as from Astra Zeneca, Bencard, Boehringer Ingelheim, and HAL Allergy.
JS received lecture fees from AbbVie, Hikma, Pfizer, and Gilead.
JH, DN, BH, JM, JS, and GAW participated in the preparation of the 2023 update of the AWMF guideline on the medical clinical diagnosis of indoor mold exposure (“Medizinisch klinische Diagnostik bei Schimmelpilzexposition in Innenräumen”(AWMF Register No. 161/001).
References
- 1.Innenraumlufthygiene-Kommission des Umweltbundesamtes. Leitfaden zur Vorbeugung, Erfassung und Sanierung von Schimmelbefall in Gebäuden Dessau-Roβlau. Umweltbundesamt. 2017 [Google Scholar]
- 2.Norbäck D, Zock JP, Plana E, et al. Building dampness and mold in European homes in relation to climate, building characteristics and socio-economic status: the European Community Respiratory Health Survey ECRHS II. Indoor Air. 2017;27:921–932. doi: 10.1111/ina.12375. [DOI] [PubMed] [Google Scholar]
- 3.Kommission „Methoden und Qualitätssicherung in der Umweltmedizin“ des Robert Koch-Instituts. Schimmelpilzbelastung in Innenräumen - Befunderhebung, gesundheitliche Bewertung und Maβnahmen. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50:1308–1323. doi: 10.1007/s00103-007-0339-y. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) www.who.int/publications/i/item/9789289041683 (last accessed on 4 December 2023) Kopenhagen: WHO; 2009. Guidelines for indoor air quality: dampness and mould. [PubMed] [Google Scholar]
- 5.French Agency for Food, Environmental and Occupational Health & Safety (ANES) Revised OPINION of the French Agency for Food, Environmental and Occupational Health & Safety on mould in buildings 2016. www.anses.fr/en/system/files/AIR2014SA0016EN.pdf (last accessed on 4 December 2023) [Google Scholar]
- 6.Hurraβ J, Heinzow B, Walser-Reichenbach S, et al. AWMF-Schimmelpilz-Leitlinie „Medizinisch klinische Diagnostik bei Schimmelpilzexposition in Innenräumen“ - Update 2023. www.register.awmf.org/assets/guidelines/161-001l_S2k_Medizinisch-klinische-Diagnostik-bei-Schimmelpilzexposition-in-Innenraeumen_2023-10.pdf (last accessed on 4 December 2023) AWMF-Register-Nr. 161/001. [Google Scholar]
- 7.Rylander R. Microbial cell wall constituents in indoor air and their relation to disease. Indoor Air. 2010;8(Suppl 4):59–68. [Google Scholar]
- 8.Wang J, Janson C, Lindberg E, et al. Dampness and mold at home and at work and onset of insomnia symptoms, snoring and excessive daytime sleepiness. Environ Int. 2020;139 doi: 10.1016/j.envint.2020.105691. 105691. [DOI] [PubMed] [Google Scholar]
- 9.Maroni M, Levy F. Definitions. In: Levy F, Maroni M, editors. NATO/CCMS Pilot study on indoor air quality. 4th plenary meeting. Epidemiology and medical management of building-related complaints and illnesses. Oslo: National Institute of Occupational Health; 1992. pp. 160–161. [Google Scholar]
- 10.Seifert B. Das „sick building“-Syndrom. Öff Gesundh-Wes. 1991;53:376–382. [PubMed] [Google Scholar]
- 11.Hospenthal DR, Kwon-Chung KJ, Bennett JE. Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med Mycol. 1998;36:165–168. [PubMed] [Google Scholar]
- 12.Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI) Anforderungen an die Infektionsprävention bei der medizinischen Versorgung von immunsupprimierten Patienten. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2021;64:232–264. doi: 10.1007/s00103-020-03265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiesmüller GA, Heinzow B, Szewzyk R, Valtanen K, Hurraβ J. Möglichkeiten und Grenzen der gesundheitlichen Bewertung von Schimmelexpositionen im Innenraum. Der Bausachverständige. 2017;3:26–34. [Google Scholar]
- 14.Bush RK, Portnoy JM. The role and abatement of fungal allergens in allergic diseases. J Allergy Clin Immunol. 2001;107(Suppl 3):S430–S440. doi: 10.1067/mai.2001.113669. [DOI] [PubMed] [Google Scholar]
- 15.Kelman BJ, Robbins CA, Swenson LJ, Hardin BD. Risk from inhaled mycotoxins in indoor office and residential environments. Int J Toxicol. 2004;23:3–10. doi: 10.1080/10915810490265423. [DOI] [PubMed] [Google Scholar]
- 16.Degen GH. The challenge to assess workplace related risks from mycotoxin exposure. Mycotoxin Res. 2008;24 doi: 10.1007/BF03032336. i-ii. [DOI] [PubMed] [Google Scholar]
- 17.Kraft S, Buchenauer L, Polte T. Mold, mycotoxins and a dysregulated immune system: a combination of concern? Int J Mol Sci. 2021;22 doi: 10.3390/ijms222212269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrio T, Hurraβ J, Wiesmüller GA, Herr C, Raulf M. Untersuchungsmethoden zur Erfassung einer Schimmelpilzexposition - ein Update. Umweltmedizin - Hygiene - Arbeitsmedizin. 2015;20:115–131. [Google Scholar]
- 19.WTA-Merkblatt 4-12. Berlin: Beuth Verlag; 2021. Ziele und Kontrolle von Schimmelpilzschadensanierungen in Innenräumen. [Google Scholar]
- 20.DIN ISO 16000-20. Berlin: Beuth Verlag; 2014. Innenraumluftverunreinigungen - Teil 20: Nachweis und Zählung von Schimmelpilzen - Bestimmung der Gesamtsporenanzahl (ISO 16000-20:2014-12) [Google Scholar]
- 21.Palomäki E, Reijula K. Evaluating the success of damp building remediation. SJWEH Suppl. 2008;4:35–38. [Google Scholar]
- 22.Rudblad S, Andersson K, Bodin L, Stridh G, Juto JE. Nasal mucosal histamine reactivity among teachers six years after working in a moisture-damaged school. Scand J Work Environ Health. 2005;31:52–58. doi: 10.5271/sjweh.848. [DOI] [PubMed] [Google Scholar]
- 23.Steiβ JO, Lindemann H, Brosig B, Zimmer KP. Wichtige Aspekte bei der Betreuung chronisch kranker Kinder und Jugendlicher am Beispiel des Asthma bronchiale. Dtsch Med Wochenschr. 2013;138:2613–2618. doi: 10.1055/s-0033-1349640. [DOI] [PubMed] [Google Scholar]
- 24.Saxena P, Choudhary H, Muthu V, et al. Which are the optimal criteria for the diagnosis of ABPA? A latent class analysis. J Allergy Clin Immunol Pract. 2021;9:328–335. doi: 10.1016/j.jaip.2020.08.043. [DOI] [PubMed] [Google Scholar]
- 25.Quirce S, Vandenplas O, Campo P, et al. Berufsbedingte exogen-allergische Alveolitis: ein EAACI-Positionspapier. Allergologie. 2018;41:449–469. [Google Scholar]
- 26.TRBA 460. Einstufung von Pilzen in Risikogruppen. GMBl 2016, Nr. 29/30 vom 22.07.2016, S. 562, 4. Änderung: GMBl. Nr. 45 vom 10.11.2020, 1009. www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRBA/TRBA-460.html (last accessed on 4 December 2023) [Google Scholar]
- 27.von Lilienfeld-Toal M, Wagener J, Einsele H, Cornely OA, Kurzai O. Invasive fungal infection—new treatments to meet new challenges. Dtsch Arztebl Int. 2019;116:271–278. doi: 10.3238/arztebl.2019.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stemler J, Mellinghoff SC, Khodamoradi Y, et al. Primary prophylaxis of invasive fungal diseases in patients with haematological malignancies: 2022 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO) J Antimicrob Chemother. 2023;78:1813–1826. doi: 10.1093/jac/dkad143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruhnke M, Behre G, Buchheidt D, et al. Diagnosis of invasive fungal diseases in haematology and oncology: 2018 update of the recommendations of the infectious diseases working party of the German Society for Hematology and Medical Oncology (AGIHO) Mycoses. 2018;61:796–813. doi: 10.1111/myc.12838. [DOI] [PubMed] [Google Scholar]
- 30.Ruhnke M, Cornely OA, Schmidt-Hieber M, et al. Treatment of invasive fungal diseases in cancer patients—revised 2019. Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Mycoses. 2020;63:653–682. doi: 10.1111/myc.13082. [DOI] [PubMed] [Google Scholar]
- 31.Kommission „Methoden und Qualitätssicherung in der Umweltmedizin“ des Robert Koch-Instituts. „Qualitätssicherung beim Lymphozytentransformationstest“ - Addendum zum LTT-Papier der RKI-Kommission „Methoden und Qualitätssicherung in der Umweltmedizin“. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2008;51:1070–1076. doi: 10.1007/s00103-008-0641-3. [DOI] [PubMed] [Google Scholar]
- 32.Landesgesundheitsamt Baden-Württemberg, Arbeitskreis „Qualitätssicherung - Schimmelpilze in Innenräumen“. Stuttgart: Landesgesundheitsamt Baden-Württemberg; 2004. Schimmelpilze in Innenräumen - Nachweis, Bewertung, Qualitätsmanagement. Abgestimmtes Arbeitsergebnis des Arbeitskreises „Qualitätssicherung - Schimmelpilze in Innenräumen“ am Landesgesundheitsamt Baden-Württemberg, 14.12.2001 (überarbeitet Dezember 2004) [Google Scholar]
- 33.Mücke W, Lemmen C. Landsberg/Lech: ecomed Verlagsgesellschaft; 2004. Schimmelpilze. Vorkommen - Gesundheitsgefahren - Schutzmaβnahmen. [Google Scholar]
- 34.Deutsche Gesetzliche Unfallversicherung (DGUV) Information 201-028. Handlungsanleitung Gesundheitsgefährdungen durch Biostoffe bei der Schimmelpilzsanierung. www.publikationen.dguv.de/widgets/pdf/download/article/644 (last accessed on 4 December 2023) 2022 [Google Scholar]
- 35.Landesgesundheitsamt Baden-Württemberg. www.gesundheitsamt-bw.de/fileadmin/LGA/_DocumentLibraries/SiteCollectionDocuments/03_Fachinformationen/FachpublikationenInfo_Materialien/Schimmelpilzsanierung_Handlungsempfehlung.pdf (last accessed on 4 December 2023) Stuttgart: Landesgesundheitsamt Baden-Württemberg; 2011. Handlungsempfehlung für die Sanierung von mit Schimmelpilzen befallenen Innenräumen. [Google Scholar]
- 36.Oswald R. Angemessene Antworten auf das komplexe Problem der Schimmelursachen? Stellungnahme zum DIN-Fachbericht 4108-8 Vermeidung von Schimmelwachstum in Wohngebäuden. Der Bausachverständige. 2011;7:32–37. [Google Scholar]