Abstract
Background
Isotretinoin is an effective treatment for acne but can cause side effects such as changes in blood lipids and liver enzymes. Laboratory monitoring is essential during treatment, but there is variation in monitoring practices.
Aim
This study aims to investigate the relationship between isotretinoin therapy and its effects on complete blood count in Saudi Arabia to improve patient outcomes.
Methods
The study was a retrospective cohort study conducted at King Khalid University Hospital in Riyadh, Saudi Arabia, between January 2016 and December 2020. Following the inclusion and exclusion criteria, 515 patients were randomly selected for the study. The data was analyzed using SPSS, and descriptive statistics and paired samples t-tests were employed to analyze the data.
Results
In this study, 515 patients were enrolled. Of these participants, 76.7% (n=395) were females and 23.3% (n=120) were males. The mean age of the study participants was 23.98±7.4 years and ranged between 16 and 65 years. The mean dose of Isotretinoin administered was 27.65±9.6 mg/day, with a range of 10–60 mg/day. The mean BMI of the study participants was 24.3±4.1 kg/m2, ranging from 14.3 to 44.8 kg/m2. Regarding the effect of Isotretinoin on laboratory measures, significant statistical differences were found in hemoglobin measurements (t=−3.379, p=0.001), platelets (t=−3.169, p=0.002), neutrophils (%) (t=3.107, p=0.002), total cholesterol (t=−13.017, p=0.000), AST (t=−6.353, p=0.000), ALT (t=−4.352, p=0.000), HDL (t=2.446, p=0.015), and LDL (t=−12.943, p=0.000). However, there were no significant statistical differences in the measurements of WBC, neutrophils (count), or triglycerides. In the Chi-square analysis and Fisher’s Exact test to identify the interaction between BMI, dose, and gender on abnormal lab results, significant interaction was found between participants’ BMI and abnormal HDL measurements (p=0.006). Furthermore, there were significant interactions between Isotretinoin dose (either less than 30 mg/day or 30 mg/day or more) and abnormal neutrophil count (p=0.04), abnormal HDL measurements (p=0.010), and abnormal triglycerides measurements (p=0.020). Moreover, a statistically significant interaction was found between participants’ gender and abnormal hemoglobin measurements (p=0.006), abnormal total cholesterol (p=0.016), abnormal AST measurements (p=0.001), abnormal ALT measurements (p=0.000), abnormal HDL measurements (p=0.000), and abnormal triglycerides measurements (p=0.007).
Conclusion
In conclusion, the study found that isotretinoin therapy has significant effects on several laboratory measures, including hemoglobin, platelets, neutrophils, total cholesterol, AST, ALT, HDL, and LDL. The study also revealed significant interactions between BMI, dose, gender, and abnormal lab results.
Keywords: Isotretinoin, acne treatment, laboratory monitoring, blood lipids, liver enzymes, Saudi Arabia
Introduction
Isotretinoin is an oral vitamin A derivative that is widely prescribed for the treatment of nodulocystic acne lesions, particularly acne vulgaris.1 Its mechanism of action involves reducing sebum production in the skin and altering the size and bacterial micro-population of the sebaceous gland, which leads to a change in skin lipid composition.2 After undergoing hepatic metabolism via oxidation and glucuronidation, it exerts its therapeutic effect on the body. Following metabolism, isotretinoin is excreted in feces and urine.3 Isotretinoin, a retinoid derivative, works by reducing sebaceous gland size and sebum production, altering keratinization. It is metabolized hepatically and excreted via urine and feces, which implicates liver function in its metabolism. These processes can lead to altered lipid metabolism and enzyme activity in the liver, necessitating careful monitoring.
Despite its efficacy, isotretinoin is known to cause a range of side effects, some of which can be severe.4 The most commonly reported side effects include cheilitis, xerosis, xerostomia, dry nose, epistaxis, pruritus, teratogenicity in women of childbearing age, and alterations in blood lipids and liver enzymes.5 These side effects have been well documented in past and current guidelines, with changes observed in parameters such as hypertriglyceridemia, hypercholesterolemia, elevated liver transaminase, leukopenia, and thrombocytopenia.2 It is believed that the incidence and severity of these side effects may be associated with the dose and/or duration of treatment. Therefore, physicians who prescribe isotretinoin should be updated on the guidelines for monitoring laboratory side effects.1,2
It is hypothesized that isotretinoin may interact with certain essential proteins or enzymes involved in lipid metabolism, although lipid profile abnormalities commonly return to normal after discontinuation of treatment.6 These abnormalities can provoke changes in cell membrane and cellular metabolism, as well as exacerbate oxidative stress in cells, which can disrupt the equilibrium of various lipid parameters. Although isotretinoin, like many retinoids, can increase levels of liver aminotransferases, it has not been clearly implicated in cases of clinically apparent acute liver injury with jaundice.7 Therefore, laboratory monitoring during isotretinoin therapy is essential and typically consists of baseline lipid profile, liver function tests, and complete blood counts.1,2,4,6 While isotretinoin is widely used, monitoring practices vary significantly worldwide. In some regions, frequent laboratory testing is standard, whereas others adopt a more relaxed approach, performing tests only when clinically indicated. Understanding these differences can inform best practices and optimize patient care.
In recent years, several studies have questioned the effectiveness and utility of recurrent laboratory monitoring during isotretinoin therapy, and there remains variation between countries in terms of the frequency and type of laboratory monitoring used in clinical practice. One population study, which included 13,772 patients with acne and assessed laboratory abnormalities during isotretinoin therapy between March 1995 and September 2002, found increased liver transaminase and serum lipid levels, and suggested that these abnormalities were generally transient and reversible.8 Another retrospective cohort study, comprising patients treated with isotretinoin from May 2003 through July 2011, has suggested that, in the absence of known risk factors, complete blood count monitoring should be eliminated, and monitoring of lipid panel and liver function tests should be performed at baseline and after the peak dose is obtained, with no further monitoring necessary if these results are normal.9 According to reported effects, Öktem et al conducted a retrospective study to examine the cost-effectiveness of laboratory monitoring, including complete blood count (CBC), during isotretinoin treatment. Notably, 8.2% of the patients (n = 58) had CBC-related abnormalities, with the median time of occurrence being the second month of treatment. In 3.4%, 3.7%, and 1.6% of the patients, anemia, leukopenia, and thrombocytopenia were observed, respectively.9
Previous studies have demonstrated that isotretinoin can significantly impact lipid profiles and liver enzymes, with variations in the extent and duration of these effects. However, there remains a lack of consensus on the necessity and frequency of monitoring, particularly in specific populations. There is limited knowledge regarding whether monitoring practices have evolved over time in response to studies that challenge frequent laboratory monitoring practices. Reports of systemic complications such as drug-induced hepatotoxicity, pancreatitis, leukopenia, and thrombocytopenia may explain the widespread practice of frequent laboratory monitoring. Lipid profiles, especially triglyceride levels, should be carefully monitored, as they play a crucial role in the development of metabolic syndrome, a condition recognized since the late 1980s.
Due to the widespread use of isotretinoin, the high prevalence of lipid abnormalities and metabolic syndrome in our society, and limited data on the complete blood count, lipid parameters, and liver enzymes during isotretinoin therapy, it is imperative to investigate the relationship between the administration of isotretinoin and its effects on CBC. Currently, there is limited research on this topic in Saudi Arabia. Therefore, this study aims to address this knowledge gap and provide insights for ensuring the safest and most desirable outcomes for patients receiving isotretinoin therapy. The findings from this study could have significant clinical implications, particularly in optimizing monitoring practices for isotretinoin users. Tailoring these practices to the specific needs of populations such as those in Saudi Arabia could improve patient outcomes and reduce the risk of adverse effects.
Methodology
Research Design
A retrospective cohort study was conducted on patients at King Khalid University Hospital (KKUH) in Riyadh, Saudi Arabia. The study utilized data collected between January 2016 and December 2020. The study population comprised individuals who were undergoing isotretinoin treatment during this period.
Research Setting and Sampling
A retrospective cohort study was conducted at King Khalid University Hospital (KKUH) in Riyadh, Saudi Arabia, between January 2016 and December 2020. With the assistance of the Information technology (IT) department, we collected the medical record number (MRN) of all eligible participants. Initially, a total of 2443 patients who were taking isotretinoin during the study period were identified. The healthcare expert-trained investigators reviewed all MRNs to ensure that the patients met the inclusion criteria. After eliminating duplicates and erroneous files, 1547 patient records were retrieved. The inclusion criteria were that the patients must have taken isotretinoin for a minimum of three months, been over 16 years of age, and had baseline and follow-up laboratory data that included complete blood count (CBC) and lipid panel. Using Raosoft sample size calculator with a 5% margin of error and a 97% confidence interval, a sample size of 461 was determined. Following the inclusion and exclusion criteria, 515 patients were randomly selected for the study.
Data Collection Procedure
A data collection spreadsheet was utilized for the study, and demographic information such as gender, age, body mass index (BMI), and isotretinoin dosage were recorded for each patient at the initiation of treatment. The complete blood count (CBC) values were collected, including white blood cells (WBC) [4.3 to 11 x 109/mm3], hemoglobin (Hgb) [130 to 180 g/L in males, 120 to 160 g/L in females], platelets, hematocrit (Hct) [45% to 62% in males, 37% to 48% in females], and neutrophil auto count [1.8 to 7.8 x 103/mm^3] and percentage. The lipid panel and liver function test (LFT) values were also recorded, including alanine transaminase (ALT) [≤40 U/L], aspartate aminotransferase (AST) [≤40 U/L], total cholesterol (≤5.20 mmol/L), triglyceride (<2.30 mmol/L), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). Baseline and follow-up laboratory values were both recorded.
In addition to recording baseline and follow-up laboratory values, the mean duration of isotretinoin treatment was calculated for the study population. The mean duration of treatment was 5.2 months, with a range from 3.3 to 7.8 months. This data was derived from patient records and helps in understanding the correlation between treatment length and the observed laboratory outcomes.
To ensure the accuracy and reliability of the laboratory data, all equipment used for testing was routinely calibrated and maintained according to the manufacturer’s specifications. Calibration procedures were performed daily before testing commenced, and equipment was standardized using control samples with known values to verify performance. Regular audits and quality checks were conducted to monitor and confirm the precision of measurements.
This study was rigorously reviewed and approved by the Institutional Review Board (IRB) at the College of Medicine, King Saud University, Riyadh, Saudi Arabia (Research Project No. E-21-6093). Given the retrospective nature of the study, informed consent was not obtained directly from each patient. This requirement was waived by the IRB due to the study’s reliance on anonymized retrospective data, which posed minimal risk to the participants. The waiver was granted on the basis that the study would significantly contribute to the understanding of isotretinoin’s effects on laboratory values without compromising patient safety or confidentiality. All patient data were de-identified and handled in accordance with the ethical standards of the Helsinki Declaration.
Data Analysis
The data collected in this study was analyzed using the Statistical Package of Social Sciences (SPSS) (v.26, IBM Corporation, Chicago, IL, U.SA). Descriptive statistics such as frequencies, percentages, means, and standard deviations were employed to describe the participants’ socio-demographic characteristics and the repeated measurements of laboratory results. Paired samples t-tests were performed to detect statistically significant differences between the baseline and follow-up laboratory measurements. Furthermore, Chi-square analysis was conducted to investigate the relationship between participants’ BMI, isotretinoin dose, gender, and abnormal laboratory measurements identified in the study. A statistical significance threshold of (α≤.05) was applied to determine statistically significant findings.
Results
A total of 515 patients enrolled in this study. The results shown in Table 1 represent the socio-demographic characteristics of the study participants. About 76.7% (n=395) of the study participants were females, whereas 23.3% (n=120) were males. The mean age of the study participants was (23.98±7.4) and ranging between 16 and 65 years. In addition, it was found that the minimum and maximum Isotretinoin dose were 10 and 60 mg/day, respectively, with a mean dose of (27.65±9.6). Moreover, the Body Mass Index (BMI) of the study participants ranged between 14.3 and 44.8 kg/m2 and the mean of the participants BMI was found to be (24.3±4.1).
Table 1.
Socio-Demographic Characteristics of the Enrolled Patients
| Variable | M ±SD | F (%) | Min-Max |
|---|---|---|---|
| Gender 1. Female 2. Male |
-------- |
395 (76.7) 120 (23.3) |
|
| Age (Years) | 23.98±7.4 | 16–65 | |
| Isotretinoin Dose (mg/day) | 27.65±9.6 | -------- | 10–60 |
| Body Mass Index (BMI) (kg/m2) | 24.3±4.1 | 14.3–44.8 |
The results shown in Table 2 represent the effect of Isotretinoin administration on repeated measures of Complete Blood Count (CBC), Liver Enzymes, Total Cholesterol, and Triglycerides. The results showed that there were significant statistical differences in hemoglobin measurements (t=−3.379, p=0.001), platelets (t=−3.169, p=0.002), neutrophils (%) (t=3.107, p=0.002), total cholesterol (t=−13.017, p=0.000), AST (t=−6.353, p=0.000), ALT (t=−4.352, p=0.000), HDL (t=2.446, p=0.015), and LDL (t=−12.943, p=0.000). However, there were no significant statistical differences in the measurements of WBC, Neutrophils (Count), or Triglycerides.
Table 2.
Effect of Oral Isotretinoin Administration on Repeated Measures of Complete Blood Count (CBC), Liver Enzymes, Total Cholesterol, and Triglycerides
| Parameter at Baseline and During Treatment | Mean Difference | N | Paired-Samples t-Test | P-value |
|---|---|---|---|---|
| Hemoglobin | −0.1289 | 515 | −3.379 | 0.001* |
| Platelets | −6.0223 | 515 | −3.169 | 0.002* |
| WBC | 0.0868 | 515 | 1.208 | 0.228 |
| Hct | −0.1897 | 515 | −1.770 | 0.077 |
| Neutrophils (Count) | 0.14716 | 515 | 1.759 | 0.079 |
| Neutrophils (%) | 2.291 | 515 | 3.107 | 0.002* |
| Total Cholesterol | −0.3966 | 515 | −13.017 | 0.000* |
| AST | −4.242 | 515 | −6.353 | 0.000* |
| ALT | −2.930 | 515 | −4.352 | 0.000* |
| HDL | 0.0596 | 515 | 2.446 | 0.015* |
| LDL | −0.3671 | 515 | −12.943 | 0.000* |
| Triglycerides | −0.0750 | 515 | −0.491 | 0.623 |
Note: *Significant at significance level (α≤0.05).
The results presented in Table 3 shows the Chi-square analysis and Fisher’s Exact test to identify the interaction between BMI, dose, and gender on one hand and abnormal lab results on Isotretinoin therapy. The results showed that there was a significant interaction between participants’ BMI and abnormal HDL measurements (p=0.006). In addition, it was found that there were significant interaction between Isotretinoin dose (either less than 30 mg/day or 30 mg/day or more) and abnormal neutrophils count (p=0.04), abnormal HDL measurements (p=0.010) and abnormal triglycerides measurements (p=0.020).
Table 3.
Risk Factors for Abnormal Lab Results on Isotretinoin Therapy
| Parameter | BMI | Dose | Gender | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal (n=377) | Abnormal (n=138) | P-value | ˂30 (n=362) | ≥30 (n=153) | P-value | Male (n=120) | Female (n=395) | P-value | |
| Hemoglobin | |||||||||
| Normal | 363 (96.3) | 131 (94.9) | 0.489 | 349 (96.4) | 145 (94.7) | 0.391 | 120 (100) | 374 (94.7) | 0.006* |
| Abnormal | 14 (3.7) | 7 (5.1) | 13 (3.6) | 8 (5.3) | 0 (0) | 21 (5.3) | |||
| Platelets | |||||||||
| Normal | 369 (97.9) | 136 (98.6) | 0.624 | 357 (98.6) | 148 (96.7) | 0.156 | 118 (98.3) | 387 (98) | 0.803 |
| Abnormal | 8 (2.1) | 2 (1.4) | 5 (1.4) | 5 (3.3) | 2 (1.7) | 8 (2) | |||
| WBC | |||||||||
| Normal | 364 (96.6) | 133 (96.4) | 0.923 | 350 (96.7) | 147 (96.1) | 0.731 | 119 (99.2) | 378 (95.7) | 0.069 |
| Abnormal | 13 (3.4) | 5 (3.6) | 12 (3.3) | 6 (3.9) | 1 (0.8) | 17 (4.3) | |||
| Hct | |||||||||
| Normal | 367 (97.3) | 138 (100) | 0.069 | 354 (97.8) | 151 (98.7) | 0.497 | 120 (100) | 385 (97.5) | 0.127 |
| Abnormal | 10 (2.7) | 0 (0) | 8 (2.2) | 2 (1.3) | 0 (0) | 10 (2.5) | |||
| Neutrophils (Count) | |||||||||
| Normal | 373 (98.9) | 136 (98.6) | 0.716 | 360 (99.4) | 149 (97.4) | 0.04* | 119 (99.2) | 390 (98.7) | 0.699 |
| Abnormal | 4 (1.1) | 2 (1.4) | 2 (0.6) | 4 (2.6) | 1 (0.8) | 5 (1.3) | |||
| Neutrophils (%) | |||||||||
| Normal | 331 (87.8) | 115 (83.3) | 0.187 | 319 (88.1) | 127 (83) | 0.119 | 109 (90.8) | 337 (85.3) | 0.120 |
| Abnormal | 46 (12.2) | 23 (16.7) | 43 (11.9) | 26 (17) | 11 (9.2) | 58 (14.7) | |||
| Total Cholesterol | |||||||||
| Normal | 116 (30.8) | 46 (33.3) | 0.283 | 116 (32) | 46 (30.1) | 0.659 | 27 (22.5) | 135 (34.2) | 0.016* |
| Abnormal | 261 (69.2) | 92 (66.7) | 246 (68) | 107 (69.9) | 93 (77.5) | 260 (65.8) | |||
| AST | |||||||||
| Normal | 364 (96.6) | 135 (97.8) | 0.460 | 348 (96.1) | 151 (99) | 0.126 | 111 (92.5) | 388 (98.2) | 0.001* |
| Abnormal | 13 (3.4) | 3 (2.2) | 14 (3.9) | 2 (1) | 9 (7.5) | 7 (1.8) | |||
| ALT | |||||||||
| Normal | 343 (91) | 120 (87) | 0.179 | 330 (91.2) | 137 (89.5) | 0.564 | 90 (75) | 377 (95.4) | 0.000* |
| Abnormal | 34 (9) | 18 (13) | 32 (8.8) | 16 (10.5) | 30 (25) | 18 (4.6) | |||
| HDL | |||||||||
| Normal | 355 (94.2) | 120 (87) | 0.006* | 341 (94.2) | 134 (87.6) | 0.010* | 101 (84.2) | 374 (94.7) | 0.000* |
| Abnormal | 22 (5.8) | 18 (13) | 21 (5.8) | 19 (12.4) | 19 (15.8) | 21 (5.3) | |||
| LDL | |||||||||
| Normal | 296 (78.5) | 111 (80.4) | 0.635 | 282 (77.9) | 125 (81.7) | 0.333 | 96 (80) | 311 (78.7) | 0.765 |
| Abnormal | 81 (21.5) | 27 (19.6) | 80 (22.1) | 28 (18.3) | 24 (20) | 84 (21.3) | |||
| Triglycerides | |||||||||
| Normal | 360 (95.5) | 134 (97.1) | 0.413 | 352 (97.2) | 142 (92.8) | 0.020* | 110 (91.7) | 384 (97.2) | 0.007* |
| Abnormal | 17 (4.5) | 4 (2.9) | 10 (2.8) | 11 (7.2) | 10 (8.3) | 11 (2.8) | |||
Note: *Significant at significance level (α≤0.05).
Furthermore, it was found that there was a statistically significant interaction between participants’ gender and abnormal hemoglobin measurements (p=0.006), abnormal total cholesterol (p=0.016), abnormal AST measurements (p=0.001), abnormal ALT measurements (p=0.000), abnormal HDL measurements (p=0.000), and abnormal triglycerides measurements (p=0.007).
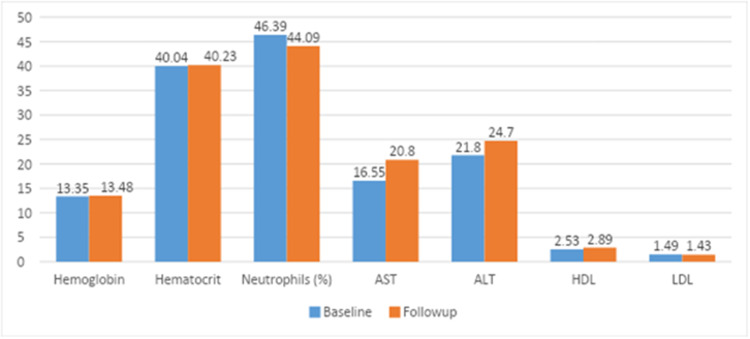
The results presented in Figure 1 show the mean differences between the laboratory measures at baseline and follow-up. The results showed that hemoglobin mean score at baseline measurement increased from 13.35 to 13.48 at follow-up, hematocrit mean score increased from 40.04 at baseline to 40.23 at follow-up, neutrophils percentage mean score decreased from 46.39 at baseline to 44.09 at follow-up, AST mean score increased from 16.55 at baseline to 20.8 at follow-up, ALT mean score increased from 21.8 at baseline to 24.7 at follow-up, HDL mean score increased from 2.53 at baseline to 2.89 at follow-up, and LDL mean score decreased from 1.49 at baseline to 1.43 at follow-up.
Figure 1.
Baseline and Follow-up mean scores of the significantly differed laboratory measures (Hemoglobin, Hct: Hematocrit, Neutrophils percentage).
Abbreviations: AST, Aspartate Transaminase; ALT, Alanine Aminotransferase; HDL, High-Density Lipoprotein; LDL, Low-Density Lipoprotein.
Discussion
The study found that most of the participants were females (76.7%), and the mean age was 23.98 years, ranging between 16 and 65 years. The mean dose of Isotretinoin was 27.65 mg/day, with a range of 10–60 mg/day, and the mean BMI was 24.3 kg/m2, ranging between 14.3 and 44.8 kg/m2. These findings provide information about the characteristics of patients who are typically given Isotretinoin, which can be used to guide clinical decision-making.
The study also found that Isotretinoin administration had a significant effect on various measures of CBC, liver enzymes, and lipids. The decrease in total cholesterol and LDL and the increase in HDL are consistent with the known effects of Isotretinoin on lipid metabolism. The decrease in hemoglobin measurements, platelets, and neutrophils and the increase in AST and ALT may be related to the adverse effects of Isotretinoin on the liver and blood cells.10,11
The findings of this study are consistent with previous research that has shown that Isotretinoin can affect various measures of CBC, liver enzymes, and lipids. Different studies confirmed that Isotretinoin administration resulted in significant changes in lipid profile and liver function tests.11,12 Another study found that Isotretinoin administration was associated with a decrease in hemoglobin levels, platelet counts, and white blood cell counts.13
The results of this study provide important insights into the effect of Isotretinoin on various blood parameters and lipids. The findings suggest that Isotretinoin administration has a significant effect on hemoglobin measurements, platelets, neutrophils, total cholesterol, AST, ALT, HDL, and LDL. These results are consistent with previous studies that have shown that Isotretinoin can affect various blood parameters and lipids.
The decrease in hemoglobin measurements, platelets, and neutrophils observed in this study may be related to the known adverse effects of Isotretinoin on blood cells. Isotretinoin is known to cause a decrease in the number of white blood cells, which can lead to a decrease in neutrophil counts. Isotretinoin can also cause thrombocytopenia, which can result in a decrease in platelet counts. The decrease in hemoglobin measurements may be related to the effect of Isotretinoin on iron metabolism. Iron deficiency can lead to a decrease in hemoglobin levels.14
The increase in AST and ALT observed in this study may be related to the known adverse effects of Isotretinoin on liver function. Isotretinoin can cause hepatotoxicity, which can result in an increase in AST and ALT levels.15,16 The decrease in total cholesterol and LDL observed in this study may be related to the effect of Isotretinoin on lipid metabolism. Isotretinoin is known to cause a decrease in the production of sebum, which is rich in lipids. The increase in HDL observed in this study may be related to the effect of Isotretinoin on lipid metabolism. Isotretinoin is known to cause an increase in the clearance of LDL, which can result in an increase in HDL levels.17
It is important to note that there were no significant statistical differences in the measurements of WBC, Neutrophils (Count), or Triglycerides observed in this study. This may be due to the relatively sample size considerations and the short duration of Isotretinoin administration in this study. Further studies with larger sample sizes and longer durations of Isotretinoin administration are needed to confirm these findings.
The results of the Chi-square analysis and Fisher’s Exact test showed that there was a significant interaction between participants’ BMI and abnormal HDL measurements (p=0.006). This suggests that BMI may play a role in the development of abnormal HDL measurements in patients on Isotretinoin therapy.
Moreover, the results also revealed significant interactions between Isotretinoin dose and abnormal neutrophil count, abnormal HDL measurements, and abnormal triglyceride measurements. Specifically, patients receiving a dose of less than 30 mg/day or 30 mg/day or more were more likely to have abnormal neutrophil count, abnormal HDL measurements, and abnormal triglyceride measurements. This indicates that the dose of Isotretinoin may affect the development of abnormalities in these laboratory parameters.
These findings are in line with previous studies that have reported the association between Isotretinoin therapy and changes in laboratory parameters, such as lipid profile and liver enzymes. However, the present study also highlights the importance of considering factors such as BMI, dose, and gender when evaluating the effects of Isotretinoin therapy on laboratory parameters.
Moreover, the results showed that there was a significant interaction between gender and abnormal hemoglobin measurements, abnormal total cholesterol, abnormal AST measurements, abnormal ALT measurements, abnormal HDL measurements, and abnormal triglycerides measurements. This indicates that gender may be an important factor to consider when evaluating the effects of Isotretinoin therapy on laboratory parameters.
Despite that previous studies have also reported the association between Isotretinoin therapy and changes in laboratory parameters, such as lipid profile, liver enzymes, and blood cell counts, the present study suggests that gender may also play a role in the development of abnormalities in these laboratory parameters.15,16,18
It is important to note that the mechanisms underlying these interactions between gender and laboratory parameters are not yet fully understood. However, it is possible that hormonal differences between males and females may contribute to these differences. For example, estrogen has been shown to have a protective effect on lipid metabolism and may explain why females are less likely to have abnormal lipid profiles compared to males.19
The results of the present study showed that the mean differences between the laboratory measures at baseline and follow-up in patients receiving isotretinoin therapy. The findings indicate that there were changes in the laboratory parameters over time. The hemoglobin and hematocrit levels showed a slight increase, which could be due to the improvement in the patients’ nutritional status, as isotretinoin therapy can cause anorexia, leading to malnutrition. Moreover, the decrease in neutrophils percentage mean score could be due to the drug’s immunomodulatory effects. The increase in AST and ALT mean scores could be attributed to the hepatotoxicity of isotretinoin. This finding is consistent with the known side effects of the drug on liver function, as isotretinoin therapy can cause liver damage in some patients. The increase in HDL mean score and the decrease in LDL mean score could be attributed to the effect of isotretinoin on lipid metabolism. Isotretinoin has been shown to decrease serum triglyceride and LDL levels while increasing HDL levels. However, these changes may not be significant clinically.
Limitations
This study has several limitations that must be taken into account when interpreting the results. Firstly, the study was retrospective in design, which means that it is prone to selection bias and confounding variables. Secondly, the study only included patients from a single hospital, which may limit the generalizability of the findings to other populations. Thirdly, the study did not include a control group, which makes it difficult to determine whether the observed changes in laboratory values were a result of the isotretinoin treatment or other factors. Fourthly, the study relied on the accuracy of medical record data, which may contain errors or omissions. Lastly, the study did not follow up with patients after treatment completion to assess the long-term effects of isotretinoin on laboratory values. Despite these limitations, the study provides valuable information about the effects of isotretinoin on laboratory values in a Saudi Arabian population.
Conclusion
In conclusion, this study sheds light on the effect of Isotretinoin administration on various blood parameters and lipids. The findings suggest that Isotretinoin has a significant effect on hemoglobin measurements, platelets, neutrophils, total cholesterol, AST, ALT, HDL, and LDL. These results are consistent with previous studies that have shown that Isotretinoin can affect various blood parameters and lipids. It is important to monitor blood parameters and lipids in patients who are taking Isotretinoin for the treatment of acne. The study also highlights the importance of considering factors such as BMI, dose, and gender when evaluating the effects of Isotretinoin therapy on laboratory parameters. Further studies with larger sample sizes and longer durations of Isotretinoin administration are needed to confirm these findings and to determine the long-term effects of Isotretinoin on blood parameters and lipids.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Khalil NY, Darwish IA, Al-Qahtani AA. Isotretinoin. In Profiles Drug Subst Excipients Relat Methodol. 2020;45:pp. 119–157. [DOI] [PubMed] [Google Scholar]
- 2.Mahrle G, Bauermeister-Jasso K, Enderer K. Roaccutan in acne and rosacea. Z Hautkr. 1985;60(1–2):120–125 [PubMed] [Google Scholar]
- 3.Almuhanna SA, Alamri Y, Alanazi AM, Almuhanna SA, Pinjabi L, Alsnaidi NA. Prevalence and associated risk factors of acne relapse among Saudi acne vulgaris patients using isotretinoin. Saudi Pharm J. 2020;28(3):374–379. doi: 10.1016/j.jsps.2020.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry MD, McEvoy GK. Isotretinoin: new therapy for severe acne. Clin Pharm. 1983;2(1):12–19. [PubMed] [Google Scholar]
- 5.Ward A, Brogden RN, Heel RC, Speight TM, Avery GS. Isotretinoin: a review of its pharmacological properties and therapeutic efficacy in acne and other skin disorders. Drugs. 1984;28(1):6–37. doi: 10.2165/00003495-198428010-00002 [DOI] [PubMed] [Google Scholar]
- 6.Villani A, Nastro F, Di Vico F, Fabbrocini G, Annunziata MC, Genco L. Oral isotretinoin for acne: a complete overview. Expert Opin Drug Saf. 2022;21(8):1027–1037. doi: 10.1080/14740338.2022.2102605 [DOI] [PubMed] [Google Scholar]
- 7.Strauss JS, Krowchuk DP, Leyden JJ, et al. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56(4):651–663. doi: 10.1016/j.jaad.2006.08.048 [DOI] [PubMed] [Google Scholar]
- 8.Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8). doi: 10.1001/archderm.142.8.1016 [DOI] [PubMed] [Google Scholar]
- 9.Öktem A, Hayran Y, Arı E, Yalçın B. Minimize the regular laboratory monitoring during the systemic isotretinoin treatment: data of 704 patients with acne vulgaris. J Dermatolog Treat. 2019;30(8):813–817. doi: 10.1080/09546634.2019.1591578 [DOI] [PubMed] [Google Scholar]
- 10.Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87(3):382–387. doi: 10.1590/S0365-05962012000300005 [DOI] [PubMed] [Google Scholar]
- 11.Kizilyel O, Metin MS, Elmas ÖF, Çayir Y, Aktas A. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94(5):234–238. [PubMed] [Google Scholar]
- 12.Alajaji A, Alrawaf FA, Alosayli SI, Alqifari HN, Alhabdan BM, Alnasser MA. Laboratory abnormalities in acne patients treated with oral isotretinoin: a retrospective epidemiological study. Cureus. 2021;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutlu Ö. Effect of isotretinoin treatment on the inflammatory markers in patients with acne vulgaris: can monocyte/HDL be a new indicator for inflammatory activity of isotretinoin treatment? Cutan Ocul Toxicol. 2020;39(1):67–70. doi: 10.1080/15569527.2019.1701485 [DOI] [PubMed] [Google Scholar]
- 14.Alyasi A, Al Hawsawi K, Malebari BA, Mandili R, Alqasim D. Isotretinoin-induced thrombocytosis in a patient with acne vulgaris: a case report. Cureus. 2021. doi: 10.7759/cureus.16716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazarian RS, Zheng E, Halverstam C, Cohen SR, Wolkoff AW. Prolonged serum alanine aminotransferase elevation associated with isotretinoin administration. Case Reports Hepatol. 2019;2019:1–3. doi: 10.1155/2019/9270827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varol F, Selimoglu M, Karadag N, Gungor S. Isotretinoin hepatotoxicity or isotretinoin induced autoimmune hepatitis? Indian J Paediatr Dermatol. 2021;22(1):70. doi: 10.4103/ijpd.IJPD_87_20 [DOI] [Google Scholar]
- 17.Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Fam Med Prim Care. 2018;7(1):171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapała J, Lewandowska J, Placek W, Owczarczyk-Saczonek A. adverse events in isotretinoin therapy: a single-arm meta-analysis. Int J Environ Res Public Health. 2022;19(11):6463. doi: 10.3390/ijerph19116463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. J Biomed Biotechnol. 2011;Vol. 2011:187103. [DOI] [PMC free article] [PubMed] [Google Scholar]