Abstract
Purpose
In recent times, growing uncertainty has emerged regarding the effectiveness of standard pressure ulcer (PU) risk assessment tools, which are suspected to be no better than clinical judgment, especially in the frail and comorbid elderly population. This study aimed to identify the primary clinical predictive variables for PU development and severity in hospitalized older adults, utilizing a multidimensional frailty assessment, and compare them with the Braden scale.
Patients and methods
The population consisted of 316 patients, admitted to the Geriatric Unit and Transitional Care of San Bartolomeo Hospital in Sarzana (Italy) during the period 21/02/22-01/07/22. The collected information included both anamnestic and laboratory data. A comprehensive geriatric assessment was performed, including also anthropometric and physical performance measurements. Multivariate logistic analysis was used, both in a binary classification test and in the subsequent ordinal classification test of severity levels. The final performance of the model was assessed by ROC curve estimation and AUC comparison with the Braden scale.
Results
Within the population, 152 subjects (48%) developed PU at different levels of severity. The results showed that age, Braden scale (subscales of mobility and friction/shear), Barthel scale, Mini Nutritional Assessment, hemoglobin, and albumin are predictors associated with the development of PU (AUC 85%). The result is an improvement over the use of the Braden scale alone (AUC 75%). Regarding the identification of predictive factors for PU severity, 4AT also emerges as potentially relevant.
Conclusion
Assessing the subject’s nutritional status, physical performance, and functional autonomies enables the effective integration of the Braden scale in identifying patients most susceptible to developing PU. Our findings support the integration of a comprehensive set of methodologically robust frailty determinants into traditional risk assessment tools. This integration reflects the mutual interplay between patients’ frailty, skin frailty, and PU development in very old hospitalized patients.
Keywords: chronic wounds, nutrition, pressure injury, health analytics, precision medicine, chronic diseases
Introduction
The gradual aging of the population has led to a rise in older adults with multimorbidity and frailty, increasing the likelihood of pressure ulcers (PU), particularly during hospitalization. Notably, two-thirds of PU cases occur in patients aged over 70 years,1 who also endure diminished quality of life, disability, higher mortality rates, prolonged hospital stays, and increased complications. Consequently, PU management ranks among the top priorities for healthcare systems.2,3 In line with that, Song et al pointed out that patients with PU had a twofold higher risk of mortality compared to patients without PU during a 3-year follow-up period.4
The prevalence of PU varies between 5% and 15% among in-hospital patients5 and from 3.4% to 32.4% in those admitted to long-term care settings.6
Diminished physiological reserve, coupled with impaired sensory, mobility, and mental status, can predispose older adults to PU development by limiting physiological positional changes. Furthermore, both local risk factors, like immobility leading to local tissue hypoperfusion, and systemic risk factors, such as malnutrition, hypoalbuminemia, and diabetes mellitus, have consistently been linked to PU development, exacerbating clinical outcomes.7 Similarly, growing evidence suggests that poor nutritional status independently increases mortality risk in older adults, even after adjusting for multimorbidity.8
PU are classified according to the International NPUAP/EPUAP (National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel) Pressure Ulcer Classification System;9 the Braden scale10 has proved to be the gold standard for the timely identification of patients at risk of PU formation; its parameters are as follows: sensory perception, moisture, activity, mobility, nutrition, and friction/shear. However, recent evidence has reported degrees of uncertainty about whether standard risk assessment tools may be able to affect PU incidence and severity, as compared to risk assessment through clinical judgment, especially in older and comorbid patients.11
A key element of clinical judgment in the geriatric setting is frailty assessment. To date, in fact, only a paucity of studies have investigated the role of frailty as a key determinant in the development of PU in older adults. Frailty is a common geriatric syndrome characterized by increased vulnerability to environmental stressors, due to diminished physiologic reserve;12 its prevalence rates range from 47.4%13 in in-hospital settings to 52.3% in nursing homes.8 Donini et al, in a retrospective nursing-home-based study, observed that older patients with higher frailty status sustained worsened clinical progression of PU, whereas patients with reduced multimorbidity and higher protein intake at hospital admission were more likely to favorably recover from PU.12,14
Nonetheless, there is still a poor understanding of the interplay between frailty and PU development in older adults, which results in the suboptimal identification of subjects most at risk, offering unequal or inappropriate care processes.
Starting from this background, the present retrospective study was aimed at identifying the main clinical predictive variables for the development and severity of PU in a hospitalized old-age population based on a multidimensional frailty assessment, as compared with the gold standard Braden scale.
Materials and Methods
This study adheres to the TRIPOD statement, ensuring transparent reporting of key aspects such as study objectives, participant selection, predictor variables, model development, validation methods, performance measures, and potential biases.15 This study was approved by the Clinical Research Ethics Committee of the University of Genoa (No: 2024.54). Written informed consent was obtained from all patients or their families. The study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Three hundred and sixteen patients (F:195–M:121), admitted to the Geriatric Unit and Transitional Care of San Bartolomeo Hospital in Sarzana (La Spezia, Italy) during the period 21/02/22-01/07/22 were consecutively enrolled. Demographic data such as sex, age, smoking habit, and the presence of urinary catheters were obtained. Laboratory data including hemoglobin, leukocyte count, lymphocyte fraction, plasma proteins, albumin, transferrin, creatinine, blood glucose, and CRP were also collected. A Comprehensive Geriatric Assessment (CGA16) was performed within 48 hours after in-hospital admission by an expert geriatrician, including risk of falls (Conley scale17); delirium risk (4AT18); functional status (Barthel19 and IADL20); comorbidity (Cumulative Illness Rating Scale CIRS21); cognitive status (Short Portable Mental Status Questionnaire SPMSQ22); nutritional status (Mini Nutritional Assessment MNA23). The number of medications at hospital admission and discharge and the Anti-Cholinergic Burden (ACB24) were also collected. To assess sarcopenia, Hand Grip (Kuptone dynamometer, model: EH101), MidArm Circumference (MAC), Mid-Thigh Circumference (MTC), and Triceps Skin Fold (TSF) were performed. PU risk was also stratified according to the Braden scale,10 performed by a trained geriatric nurse. Frailty status was classified as the positivity of at least 3 indicators that are part of the CGA.
After imputing the missing data (<10%) through missForest,25,26 we performed an univariate analysis using both p<0.05 in the hypothesis test and AUC increment from a random choice (>10%) as measures of significance for each clinical variable.27 Then, logistic regression analysis and its stepwise form28 were carried out to deal with collinearity. The final performance of the multivariate model was assessed by ROC curve estimation and AUC comparison with the Braden scale; a threshold on the logistic score (0.5) with conventional accuracy estimates (sensitivity and specificity) was used. Moreover, to assess the goodness-of-fit and to evaluate the calibration of the predictive models, the Hosmer–Lemeshow statistical test was employed.29
For the assessment of PU severity, the target clinical variable was defined according to the International NPUAP-EPUAP Pressure Ulcer Classification System (I–IV),9 and a multinomial logistic regression was performed. The low numerosity of class III (11 patients) and IV (15 patients), forced us to merge them, operating on the following classification: absence of PU; presence of grade I PU; presence of advanced grade PU (merged stages II-III-IV).
All analysis processes were cross-validated to avoid the risk of data overfitting and to produce reliable performance estimates of the proposed method. The adopted procedure was the Leave-One-Out Cross-Validation (LOOCV30).
Results
Forty-eight percent of the participants (152 out of 316 patients) developed incident PU with different severity (stage I 58%, stage II 25%, stage III 7%, stage IV 10%) and a prevalent female gender (56% of females, 38% of males). Based on the Braden cut-off point of 19, 79% of patients were at risk of developing PU (248 individuals) but only 59% of them (146 individuals) actually developed PU during in-hospital stay. This estimate showed that the Braden scale had high sensitivity (0.96) but low specificity (0.38) in our frail and old age population.
Patients’ mean age was 85 ± 7 years (ranging from 64 to 101 years) with 56% being oldest old (age 85 and older) with advanced frailty status (82%, 260 out of 316), increased nutritional risk (MNA 13, IQR 9), higher disability (Barthel index 15, IQR 41), higher incident delirium (4AT 5/12, IQR 6) and polypharmacy (average number of drugs: 6, IQR 3) (Table 1).
Table 1.
Bivariate Analysis Comparing All Clinical Variables in Patients Who Developed PU and Their Negative Counterparts
| PU-(N=164, 52%) | PU+ (N=152, 48%) | AUC | p | |
|---|---|---|---|---|
| Male Sex, Num (%) | 75 (45) | 46 (30) | 0.098 | 0.005 |
| Age, Median (Range) | 85 (64,101) | 87 (68,101) | 0.093 | 0.004 |
| Smoke Habit, Num (%) | 47 (28) | 28 (18) | 0.068 | 0.035 |
| Triceps Skin Fold | 16 (11) | 16 (10) | 0.039 | 0.232 |
| Mid-Arm Circumference | 26 (5) | 24 (6) | 0.122 | <0.001 |
| Mid-Thigh Circumference | 31 (6) | 29 (5) | 0.122 | <0.001 |
| Hand Grip | 9.3 (7.2) | 5.6 (3.6) | 0.208 | <0.001 |
| Braden-Perception | 3 (1) | 2 (1) | 0.231 | <0.001 |
| Braden-Humidity | 3 (2) | 3 82) | 0.104 | 0.004 |
| Braden-Physical Activity | 3 (3) | 1 (1) | 0.249 | <0.001 |
| Braden-Mobility | 3 (1) | 2 (1) | 0.261 | <0.001 |
| Braden-Nutrition | 3 (1) | 2 (1) | 0.212 | <0.001 |
| Braden-Friction/Shear | 2 (1) | 2 (1) | 0.258 | <0.001 |
| Albumin | 51.6 (7.8) | 48.7 (7.3) | 0.136 | <0.001 |
| Plasma Protein | 6.4 (0.9) | 6.3 (1.1) | 0.076 | 0.024 |
| Hemoglobin | 11.8 (2.9) | 11.6 (3.8) | 0.019 | 0.557 |
| Leukocyte Count | 9.2 (4.6) | 10.0 (7.2) | 0.070 | 0.031 |
| Creatinine | 1.01 (0.59) | 1.08 (0.84) | 0.009 | 0.780 |
| Lymphocytes Fraction | 13 (12) | 10 (12) | 0.042 | 0.197 |
| Plasma Glucose | 121 (37) | 123 (67) | 0.028 | 0.392 |
| CRP | 2.2 (6.7) | 4.7 (8.5) | 0.098 | 0.003 |
| Conley’s Scale | 4 (3) | 5 (3) | 0.154 | <0.001 |
| IADL | 3 (5) | 0 (2) | 0.255 | <0.001 |
| Barthel Index | 55 (55) | 15 (41) | 0.279 | <0.001 |
| CIRS-Comorbidity Index | 5 (3) | 5(3) | 0.051 | 0.082 |
| CIRS-Severity Index | 2.15 (0.6) | 2.23 (0.65) | 0.054 | 0.110 |
| SPMSQ | 4 (4) | 3 (4) | 0.230 | <0.001 |
| MNA | 19 (8) | 13 (9) | 0.237 | <0.001 |
| ACB Score | 1 (2) | 2 (2) | 0.075 | 0.019 |
| N° Medication Pre-Hospitalization | 6 (3) | 6 (3) | 0.005 | 0.859 |
| N° Medication at Discharge | 8 (3) | 8 (3) | 0.033 | 0.297 |
| 4AT | 1 (5) | 5 (6) | 0.195 | <0.001 |
| Urinary Catheter Presence, Num (%) | 83 (50) | 114 (75) | 0.097 | <0.001 |
Notes: When not specified, data refer to each variable’s median (IQR). The bolded values indicate statistically significant results.
Abbreviations: IADL, Instrumental Activities of Daily Living; ACB, Anti-Cholinergic Burden; CFS, Clinical Frailty Scale; CIRS, Cumulative Illness Rating Scale; MNA, Mini Nutritional Assessment; CRP, C-Reactive Protein.
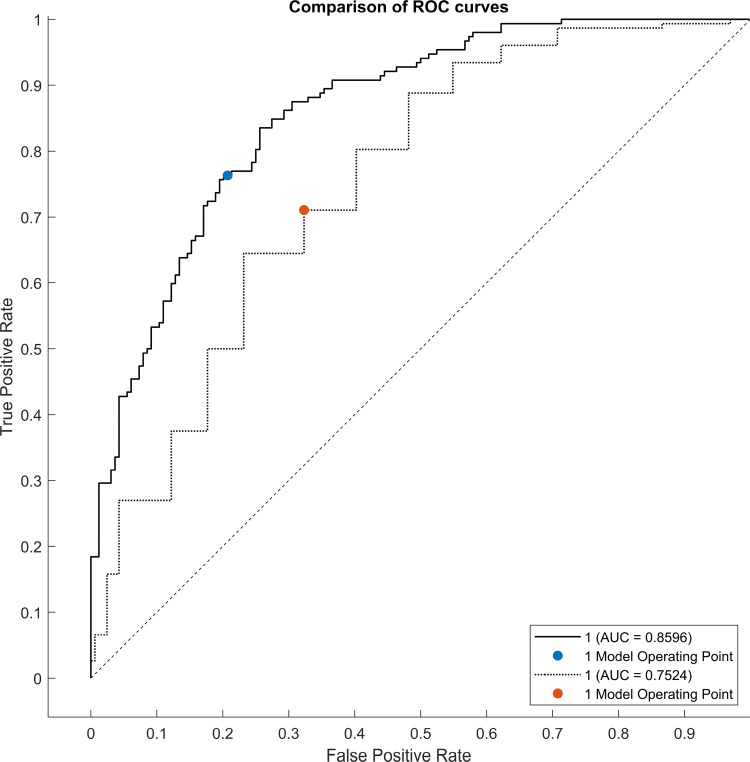
Our results showed that older age (OR 1.53; 95% CI = 1.18–2.08), Braden’s subscale of mobility (OR 0.50; 95% CI=0.32-0-78) and friction/shear (OR 0.52, 95% CI = 0.35–0.78), albumin (OR 0.67, 95% CI = 0.48–0.95), hemoglobin (OR 1.44, 95% CI = 1.04–1.20), Barthel Index (OR 0.65, 95% CI = 0.43–0.96) and MNA (OR 0.49, 95% CI = 0.33–0.72) were the main clinical predictors for the development of PU (AUC of 0.85, 95% CI 0.80–0.89, sensitivity 0.75, specificity 0.79 with cut-off 0.5, as shown in Figure 1), compared to the Braden scale (Table 2).
Figure 1.
Comparison of ROC curves of two classifiers: Braden scale (dotted line) vs our multivariate model (solid line).
Table 2.
Logistic Regression Coefficients and Odds for the Prediction of PU Incidence
| Variables | β | Odds Ratio | 95% CI | p |
|---|---|---|---|---|
| Age | 0.427 | 1.533 | 1.127–2.084 | 0.006 |
| Braden mobility | −0.685 | 0.504 | 0.324–0.784 | 0.002 |
| Braden friction/shear | −0.653 | 0.520 | 0.349–0.776 | 0.001 |
| Albumin | −0.396 | 0.673 | 0.479–0.945 | 0.022 |
| Hemoglobin | 0.367 | 1.444 | 1.044–1.996 | 0.026 |
| Barthel | −0.435 | 0.647 | 0.425–0.985 | 0.046 |
| MNA | −0.714 | 0.490 | 0.332–0.723 | <0.001 |
Note: The bolded values indicate statistically significant results.
Abbreviation: MNA, Mini Nutritional Assessment.
Furthermore, MNA, Barthel index, 4AT screening test, and Braden scale (all sub-scales) were the main clinical predictive variables for PU stage severity (AUC of 0.73, 95% CI 0.66–0.78, sensitivity 0.81, specificity 0.65).
The calibration adequacy of the predictive models for PU development, the goodness-of-fit between the prediction and the observed incidence of PU in the training group after cross-validation was evaluated. For the Braden scale, the Hosmer–Lemeshow statistic yielded a value of 5.641, with a p-value of 0.1304, indicating suboptimal calibration of the model. Conversely, for our model, the Hosmer–Lemeshow statistic resulted in a value of 2.255, with a p-value of 0.5213, suggesting better model adaptability.
Discussion
Despite being largely preventable, PU remains a significant concern among hospitalized frail older adults. Establishing reliable models for early detection of PU risk is crucial for facilitating the implementation of risk-based prevention strategies and dedicated care pathways aimed at reducing the disease burden.
In our population, the in-hospital incidence of PU was 48%, significantly higher than those reported in previous European studies.31 This elevated incidence may be attributed to our unique demographic profile. In Italy, approximately 22% of the population is estimated to be elderly, with Genoa, located in the Liguria region, having the highest proportion of oldest old individuals (aged 85 years and older).32
It is widely recognized that malnutrition, multimorbidity, and frailty independently increase the risk of PU development in older adults. Additionally, older age33,34 and poor nutritional status,35,36 as assessed by biochemical parameters and mid-arm circumference, are associated with a heightened risk of PU. Similarly, conditions such as diabetes, stroke, and advanced dementia2,37 consistently increase the risk of PU development. Elsorady et al identified associations between the Charlson Comorbidity Index, diabetes mellitus, stroke/transient ischemic attack, dementia, incontinence, chronic kidney disease, and PU development in hospitalized older patients.37 However, the higher prevalence of diabetes among older adults38 may act as a clinical confounder when analyzing the association between age, multimorbidity, and PU.
Currently, neither prevalent chronic diseases in individual patients nor single risk factors are sufficient to fully explain PU development in older adults. This uncertainty may stem from the highly individualized degree of frailty, reflecting patients’ clinical complexity and complicating the establishment of a solid association between old age, multimorbidity, and PU.
So far, the lack of a standardized assessment for frailty hinders the identification of which real-world patients’ clinical phenotypes may be more vulnerable to PU development, with worsened clinical outcomes. In line with that, Capon et al,39 previously underscored a meaningful association between functional decline (ADL score), cognitive decline, low Braden score, and PU development. Similarly, Donini et al12 established an association between the incidence and clinical progression of PU with the degree of frailty, measured through chronological age, cognitive, functional and nutritional status. Although both these findings have the merit of embedding frailty into a wide plethora of PU risk factors, the lack of a methodologically robust frailty assessment limits the generalization of the results.
To the best of our knowledge, our results moved a step forward towards the integration of a set of methodologically robust frailty determinants into traditional risk assessment tools,11 supporting the mutual interplay between patients’ frailty, skin frailty and PU development in very old hospitalized patients. Specifically, older age, Braden scale (sub-scales of mobility and friction/shear), albumin, hemoglobin, Barthel Index, and Mini Nutritional Assessment (MNA) emerged as main predictors for PU development, whilst also 4AT test emerged as a possible predictive variable for PU stage severity.
On one hand, from the patient’s perspective, factors such as malnutrition, low albumin, and hemoglobin levels may lead to reduced skin perfusion and hindered wound healing processes.40 At the same time, the presence of functional decline may accelerate hypomobility, frailty, and disability. On the other hand, from the perspective of skin frailty, impaired Braden’s subscales for friction and mobility may result from factors such as malnutrition, hypomobility, and overall frailty, potentially culminating in overt skin failure.
Notably, delirium is a common geriatric syndrome that in our cohort of hospitalized elders relates to premorbid predisposing conditions such as age and frailty, as well as to a set of precipitant risk factors such as immobility and malnutrition. Although the interaction between an underlying predisposition and a superimposed acute stressor is key to delirium pathophysiology, it could be hypothesized that delirium could trigger the ultimate skin failure, diminishing resilience in response to wound stressors and accelerating both PU progression and frailty. Alternatively, it is widely acknowledged that the presence of delirium is strongly linked to various adverse outcomes, including increased one-year mortality and disability.41 This correlation effectively explains its influence on the severity of PU stages.
The results of the calibration analysis showed that our model demonstrates better goodness-of-fit than the use of the Braden scale alone, although the performance of the latter was better than reported in previous works.42
The study’s limitations include the relatively small sample size, single in-hospital setting, and retrospective design, which led to relatively incomplete PU prevalence rates before hospitalization. Moreover, the CGA was conducted as a single-point assessment within 48 hours of hospitalization, limiting the ability to demonstrate whether delirium presence or its duration/persistence could influence PU severity modulation and the individual’s frailty trajectory during the hospital stay.
The small sample size did not enable us to test more complex and powerful machine learning methods like ANN,43–46 requiring typically much more data than multivariate logistic regression to be trained effectively. Although non-linear methods, including neural networks and boosting algorithms, hold tremendous promise for advancing our understanding of PU and have already been widely used in the adult population.47,48 Future research on PU should aim to refine the application of such modern algorithms, incorporating essential variables derived from CGA. This approach will enhance the accuracy and effectiveness of predicting PU in this population, requiring further exploration and validation.
Conclusions
PU risk assessment in very old individuals presents low-certainty evidence regarding the efficacy of available ulcer risk assessment tools in reducing both the incidence and severity when compared to risk assessment based on clinical judgment.
Furthermore, it remains uncertain whether aligning information obtained from clinical judgment and standardized assessment tools positively influences PU development in frail older individuals. Additionally, it could be argued that timely PU assessment serves as a preliminary step toward developing an effective care plan to mitigate the impact of identified risk factors. Despite these uncertainties, the present findings endorse the strong predictive capability of a structured clinical judgment based on a methodologically robust frailty stratification, incorporating Braden subitems, within a hospitalized population of very old individuals.
The impact of interventions provided to these patients, such as devices, documented pressure care plans, referrals to skin specialists or dieticians, and the multi-component model’s ability to predict one-year mortality, are ongoing processes aimed at investigating patient outcomes and providing a foundation for future care implementation.
Further studies in other settings are warranted, as well as randomized trials in real-world old age populations; hopefully, the latest frontier of machine learning methods will help fill this gap of knowledge39 in the future, establishing whether undertaken risk assessment makes any difference to PU incidence even after adjusting for patients’ frailty.
Acknowledgments
The authors have no acknowledgments to disclose.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zaidi SRH, Sharma S. Pressure Ulcer. StatPearls. StatPearls Publishing; 2022. Available from. http://www.ncbi.nlm.nih.gov/books/NBK553107/. Accessed August 28, 2022. [Google Scholar]
- 2.Jaul E, Barron J, Rosenzweig JP, Menczel J. An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr. 2018;18(1):305. doi: 10.1186/s12877-018-0997-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyder CH, Wang Y, Metersky M, et al. Hospital-acquired pressure ulcers: results from the national medicare patient safety monitoring system study. J Am Geriatr Soc. 2012;60(9):1603–1608. doi: 10.1111/j.1532-5415.2012.04106.x [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Shen H, Cai J, Zha M, Chen H. The relationship between pressure injury complication and mortality risk of older patients in follow up: a systematic review and meta analysis. Int Wound J. 2019;16(6):1533–1544. doi: 10.1111/iwj.13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81(4):881–890. doi: 10.1016/j.jaad.2018.12.069 [DOI] [PubMed] [Google Scholar]
- 6.Anthony D, Alosoumi D, Safari R. Prevalence of pressure ulcers in long-term care: a global review. J Wound Care. 2019;28(11):702–709. doi: 10.12968/jowc.2019.28.11.702 [DOI] [PubMed] [Google Scholar]
- 7.Dube A, Sidambe V, Verdon A, et al. Risk factors associated with heel pressure ulcer development in adult population: a systematic literature review. J Tissue Viability. 2022;31(1):84–103. doi: 10.1016/j.jtv.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 8.Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940–945. doi: 10.1016/j.jamda.2015.06.025 [DOI] [PubMed] [Google Scholar]
- 9.Kottner J, Cuddigan J, Carville K, et al. Prevention and treatment of pressure ulcers/injuries: the protocol for the second update of the international clinical practice guideline 2019. J Tissue Viability. 2019;28(2):51–58. doi: 10.1016/j.jtv.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nurs Res. 1987;36(4):205–210. doi: 10.1097/00006199-198707000-00002 [DOI] [PubMed] [Google Scholar]
- 11.Moore ZE, Patton D. Risk assessment tools for the prevention of pressure ulcers. Cochrane wounds group, ed. Cochrane Database Syst Rev. 2019;2019(1). doi: 10.1002/14651858.CD006471.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donini LM, De Felice MR, Tagliaccica A, De Bernardini L, Cannella C. Comorbidity, frailty, and evolution of pressure ulcers in geriatrics. Med Sci Monit Int Med J Exp Clin Res. 2005;11(7):CR326–336. [PubMed] [Google Scholar]
- 13.Doody P, Asamane EA, Aunger JA, et al. The prevalence of frailty and pre-frailty among geriatric hospital inpatients and its association with economic prosperity and healthcare expenditure: a systematic review and meta-analysis of 467,779 geriatric hospital inpatients. Ageing Res Rev. 2022;80:101666. doi: 10.1016/j.arr.2022.101666 [DOI] [PubMed] [Google Scholar]
- 14.Donini LM, De Felice MR, Tagliaccica A, De Bernardini L, Cannella C. Nutritional status and evolution of pressure sores in geriatric patients. J Nutr Health Aging. 2005;9(6):446–454. [PubMed] [Google Scholar]
- 15.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. Eur J Clin Invest. 2015;45(2):204–214. doi: 10.1111/eci.12376 [DOI] [PubMed] [Google Scholar]
- 16.Parker SG, McCue P, Phelps K, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing. 2018;47(1):149–155. doi: 10.1093/ageing/afx166 [DOI] [PubMed] [Google Scholar]
- 17.Conley D, Schultz AA, Selvin R. The challenge of predicting patients at risk for falling: development of the Conley Scale. Medsurg Nurs off J Acad Med-Surg Nurses. 1999;8(6):348–354. [PubMed] [Google Scholar]
- 18.Bellelli G, Morandi A, Davis DHJ, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496–502. doi: 10.1093/ageing/afu021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 21.Conwell Y, Forbes NT, Cox C, Caine ED. Validation of a measure of physical illness burden at autopsy: the cumulative illness rating scale. J Am Geriatr Soc. 1993;41(1):38–41. doi: 10.1111/j.1532-5415.1993.tb05945.x [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients†. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 23.Guigoz Y, Vellas B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. In: Vellas B, Garry P, Guigoz Y, editors. Nestle Nutrition Workshop Series: Clinical & Performance Program. Vol 1. KARGER; 1999:3–12. doi: 10.1159/000062967 [DOI] [PubMed] [Google Scholar]
- 24.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–320. doi: 10.2217/1745509X.4.3.311 [DOI] [Google Scholar]
- 25.Stekhoven DJ, Buhlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–118. doi: 10.1093/bioinformatics/btr597 [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Lynn HS. Accuracy of random-forest-based imputation of missing data in the presence of non-normality, non-linearity, and interaction. BMC Med Res Methodol. 2020;20(1):199. doi: 10.1186/s12874-020-01080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L, Wang J, Wei J. AVC: selecting discriminative features on basis of AUC by maximizing variable complementarity. BMC Bioinf. 2017;18(S3):50. doi: 10.1186/s12859-017-1468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(1):17. doi: 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. Third edition ed. New Jersey: Wiley; 2013. [Google Scholar]
- 30.Cheng H, Garrick DJ, Fernando RL. Efficient strategies for leave-one-out cross validation for genomic best linear unbiased prediction. J Anim Sci Biotechnol. 2017;8(1):38. doi: 10.1186/s40104-017-0164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore Z, Avsar P, Conaty L, Moore DH, Patton D, O’Connor T. The prevalence of pressure ulcers in Europe, what does the European data tell us: a systematic review. J Wound Care. 2019;28(11):710–719. doi: 10.12968/jowc.2019.28.11.710 [DOI] [PubMed] [Google Scholar]
- 32.Istituto Nazionale di Statistica. Indicatori demografici;2023.
- 33.Blanc G, Meier MJ, Stocco JGD, Roehrs H, Crozeta K, Barbosa DA. Effectiveness of enteral nutritional therapy in the healing process of pressure ulcers: a systematic review. Rev Esc Enferm USP. 2015;49(1):152–161. doi: 10.1590/S0080-623420150000100020 [DOI] [PubMed] [Google Scholar]
- 34.Lima Serrano M, González Méndez MI, Carrasco Cebollero FM, Lima Rodríguez JS. Risk factors for pressure ulcer development in Intensive Care Units: a systematic review. Med Intensiva Engl Ed. 2017;41(6):339–346. doi: 10.1016/j.medine.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 35.Montalcini T, Moraca M, Ferro Y, et al. Nutritional parameters predicting pressure ulcers and short-term mortality in patients with minimal conscious state as a result of traumatic and non-traumatic acquired brain injury. J Transl Med. 2015;13(1):305. doi: 10.1186/s12967-015-0660-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bluestein D, Javaheri A. Pressure ulcers: prevention, evaluation, and management. Am Fam Physician. 2008;78(10):1186–1194. [PubMed] [Google Scholar]
- 37.Elsorady KE, Nouh AH. Biomarkers and clinical features associated with pressure injury among geriatric patients. Electron J Gen Med. 2023;20(1):em431. doi: 10.29333/ejgm/12636 [DOI] [Google Scholar]
- 38.Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65–99-year-old adults: findings from the international diabetes federation diabetes atlas. Diabet Res Clin Pract. 2020;162:108078. doi: 10.1016/j.diabres.2020.108078 [DOI] [PubMed] [Google Scholar]
- 39.Capon A, Pavoni N, Mastromattei A, Di Lallo D. Pressure ulcer risk in long-term units: prevalence and associated factors. J Adv Nurs. 2007;58(3):263–272. doi: 10.1111/j.1365-2648.2007.04232.x [DOI] [PubMed] [Google Scholar]
- 40.Ferris AE, Harding KG. Are chronic wounds a feature of frailty? Br J Gen Pract. 2020;70(694):256–257. doi: 10.3399/bjgp20X709829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han JH, Shintani A, Eden S, et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56(3):244–252.e1. doi: 10.1016/j.annemergmed.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen HL, Cao YJ, Wang J, Huai BS. Calibration power of the Braden scale in predicting pressure ulcer development. J Wound Care. 2016;25(11):655–659. doi: 10.12968/jowc.2016.25.11.655 [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro F, Fidalgo F, Silva A, Metrôlho J, Santos O, Dionisio R. Literature review of machine-learning algorithms for pressure ulcer prevention: challenges and opportunities. Informatics. 2021;8(4):76. doi: 10.3390/informatics8040076 [DOI] [Google Scholar]
- 44.Levy JJ, Lima JF, Miller MW, Freed GL, O’Malley AJ, Emeny RT Investigating the potential for machine learning prediction of patient outcomes: a retrospective study of hospital acquired pressure injuries. Health Inform. 2020; 2020–2023. doi: 10.1101/2020.03.29.20047084 [DOI] [Google Scholar]
- 45.Walther F, Heinrich L, Schmitt J, Eberlein-Gonska M, Roessler M. Prediction of inpatient pressure ulcers based on routine healthcare data using machine learning methodology. Sci Rep. 2022;12(1):5044. doi: 10.1038/s41598-022-09050-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen HL, Yu SJ, Xu Y, et al. Artificial neural network: a method for prediction of surgery-related pressure injury in cardiovascular surgical patients. J Wound Ostomy Continence Nurs. 2018;45(1):26–30. doi: 10.1097/WON.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 47.Cai JY, Zha ML, Song YP, Chen HL. Predicting the development of surgery-related pressure injury using a machine learning algorithm model. J Nurs Res. 2021;29(1):e135. doi: 10.1097/JNR.0000000000000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu C, Chen H, Shen W, Feng L. A new nomogram score for predicting surgery‐related pressure ulcers in cardiovascular surgical patients. Int Wound J. 2017;14(1):226–232. doi: 10.1111/iwj.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]