Abstract
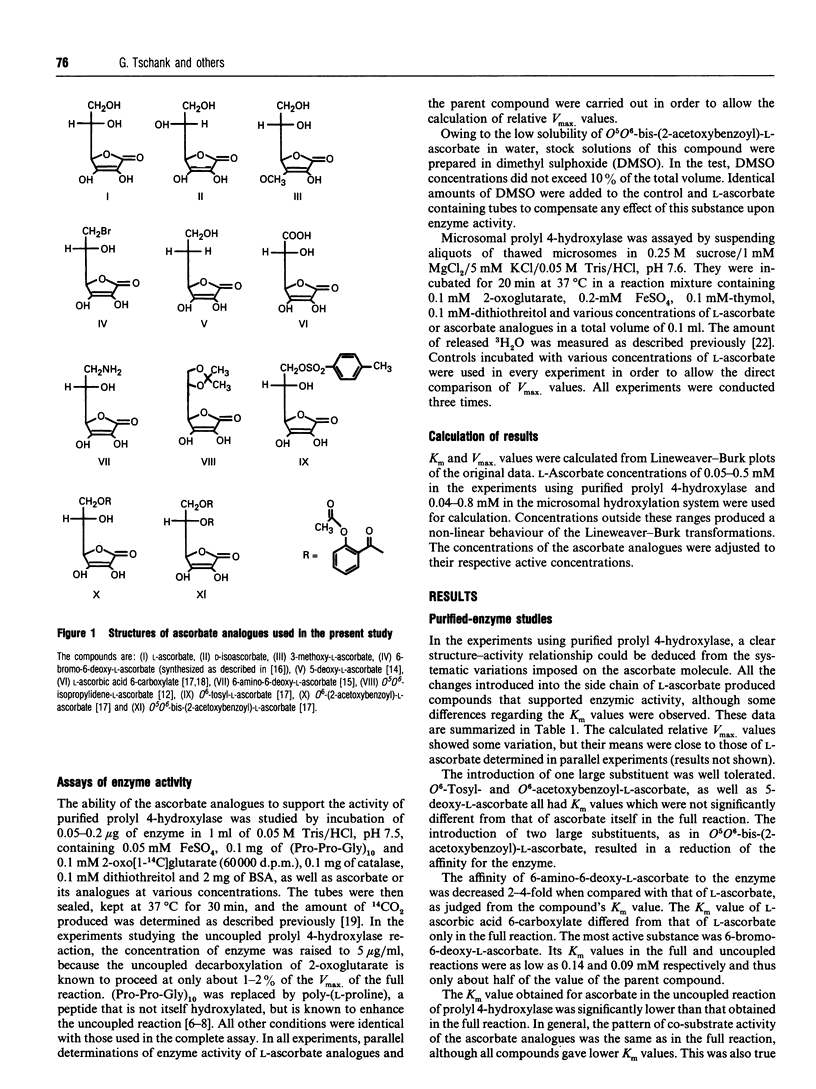
The ability of structural analogues of ascorbate to serve as substitutes for this reducing agent in the prolyl 4-hydroxylase reaction was studied. In experiments using the purified enzyme, variations of the compounds' side chain were compatible with co-substrate activity. The presence of very large hydrophobic substituents or a positively charged group caused an increase in the observed Km values. A negative charge and smaller modifications did not change the affinity to the enzyme when compared with L-ascorbate. 6-Bromo-6-deoxy-L-ascorbate had a lower Km than the physiological reductant. Substitution at the -OH group in ring position 3 prevented binding to the enzyme. The same pattern of activity was observed when the full and uncoupled prolyl 4-hydroxylase reactions were studied. The Vmax. values with all compounds were similar. The reaction of microsomal prolyl 4-hydroxylase was supported by D-isoascorbate, O6-tosyl-L-ascorbate and 5-deoxy-L-ascorbate, giving the same dose-response behaviour as L-ascorbate itself. Again, 6-bromo-6-deoxy-L-ascorbate gave a lower Km and a similar Vmax. value. L-Ascorbic acid 6-carboxylate produced substrate inhibition at concentrations above 0.3 mM. The Km and Vmax. values calculated from concentrations up to 0.2 mM were similar to those of L-ascorbate. The enzyme activity observed with 6-amino-6-deoxy-L-ascorbate was very low in the microsomal hydroxylation system. The calculated Vmax. value was lower than that of L-ascorbate, suggesting a restriction of the access of this compound to the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Counts D. F., Cardinale G. J., Udenfriend S. Prolyl hydroxylase half reaction: peptidyl prolyl-independent decarboxylation of alpha-ketoglutarate. Proc Natl Acad Sci U S A. 1978 May;75(5):2145–2149. doi: 10.1073/pnas.75.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong L., Kemp A. Stoicheiometry and kinetics of the prolyl 4-hydroxylase partial reaction. Biochim Biophys Acta. 1984 May 31;787(1):105–111. doi: 10.1016/0167-4838(84)90113-4. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel H. M., Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: applicability to classification and design of inhibitors. J Theor Biol. 1982 Jan 21;94(2):421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Berg R. A. An improved method for the purification of vertebrate prolyl hydroxylase by affinity chromatography. Coll Relat Res. 1981 Jul;1(4):345–353. doi: 10.1016/s0174-173x(81)80011-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Helaakoski T., Tasanen K., Vuori K., Myllylä R., Parkkonen T., Pihlajaniemi T. Molecular biology of prolyl 4-hydroxylase. Ann N Y Acad Sci. 1990;580:132–142. doi: 10.1111/j.1749-6632.1990.tb17925.x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Hydroxylation of proline in synthetic polypeptides with purified protocollagen hydroxylase. J Biol Chem. 1967 Sep 25;242(18):4007–4012. [PubMed] [Google Scholar]
- Majamaa K., Günzler V., Hanauske-Abel H. M., Myllylä R., Kivirikko K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986 Jun 15;261(17):7819–7823. [PubMed] [Google Scholar]
- Myllylä R., Kuutti-Savolainen E. R., Kivirikko K. I. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978 Jul 28;83(2):441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Majamaa K., Günzler V., Hanauske-Abel H. M., Kivirikko K. I. Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl 4-hydroxylase and lysyl hydroxylase. J Biol Chem. 1984 May 10;259(9):5403–5405. [PubMed] [Google Scholar]
- Peterkofsky B., Tschank G., Luedke C. Iron-dependent uptake of ascorbate into isolated microsomes. Arch Biochem Biophys. 1987 Apr;254(1):282–289. doi: 10.1016/0003-9861(87)90104-4. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. V., Adams E. Partial reaction of prolyl hydroxylase. (Gly-PRO-Ala)n stimulates alpha-ketoglutarate decarboxylation without prolyl hydroxylation. J Biol Chem. 1978 Sep 25;253(18):6327–6330. [PubMed] [Google Scholar]
- Tschank G., Hanauske-Abel H. M., Peterkofsky B. The effectiveness of inhibitors of soluble prolyl hydroxylase against the enzyme in the cisternae of isolated bone microsomes. Arch Biochem Biophys. 1988 Mar;261(2):312–323. doi: 10.1016/0003-9861(88)90346-3. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- de Jong L., Albracht S. P., Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron. The role of ascorbate in peptidyl proline hydroxylation. Biochim Biophys Acta. 1982 Jun 4;704(2):326–332. doi: 10.1016/0167-4838(82)90162-5. [DOI] [PubMed] [Google Scholar]


