Abstract
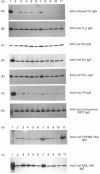
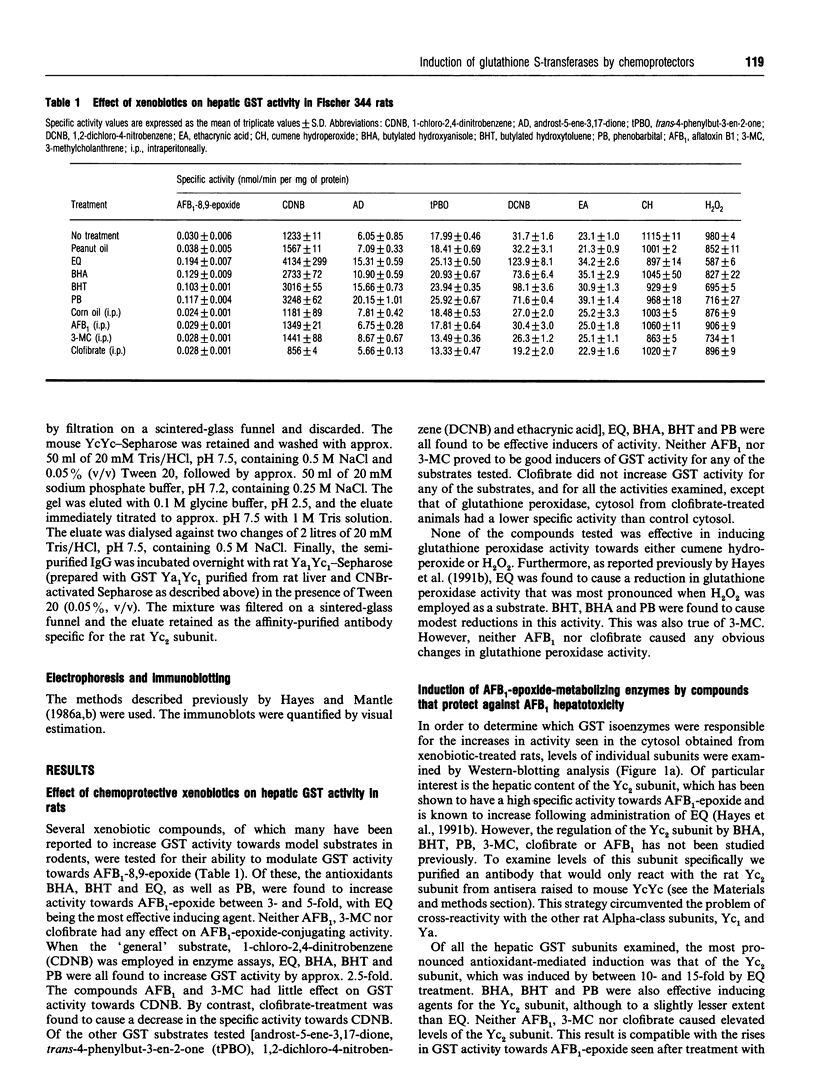
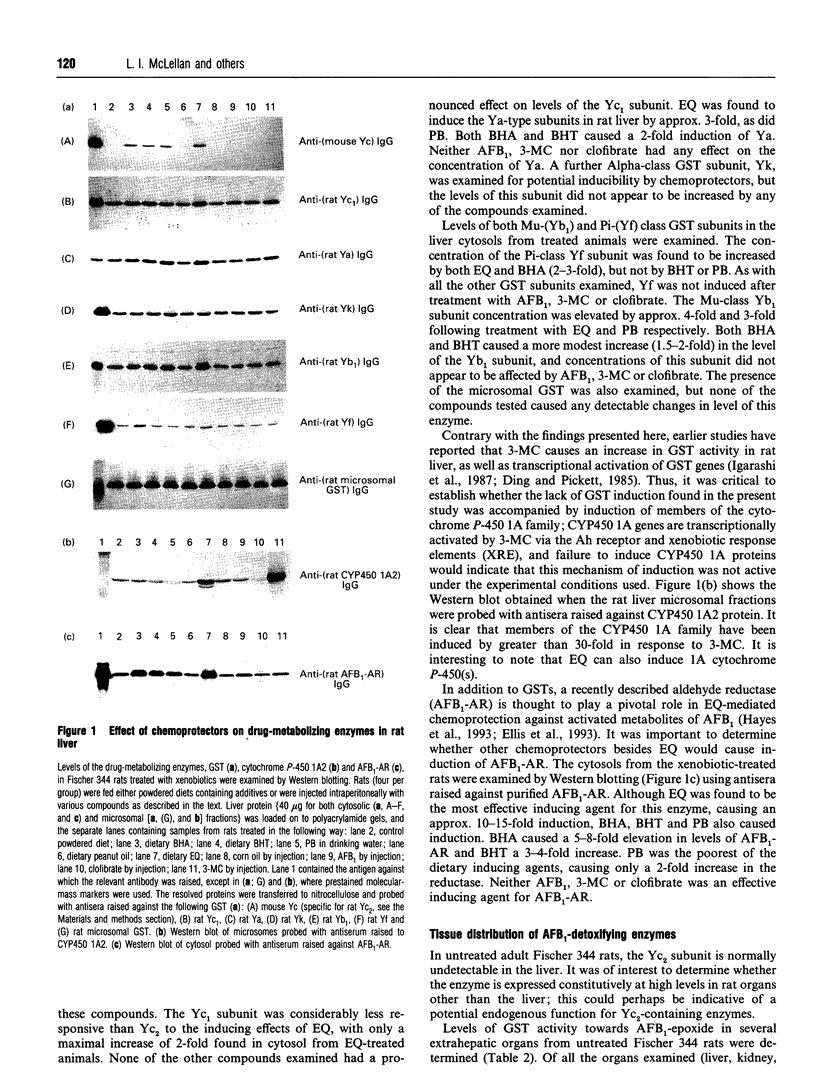
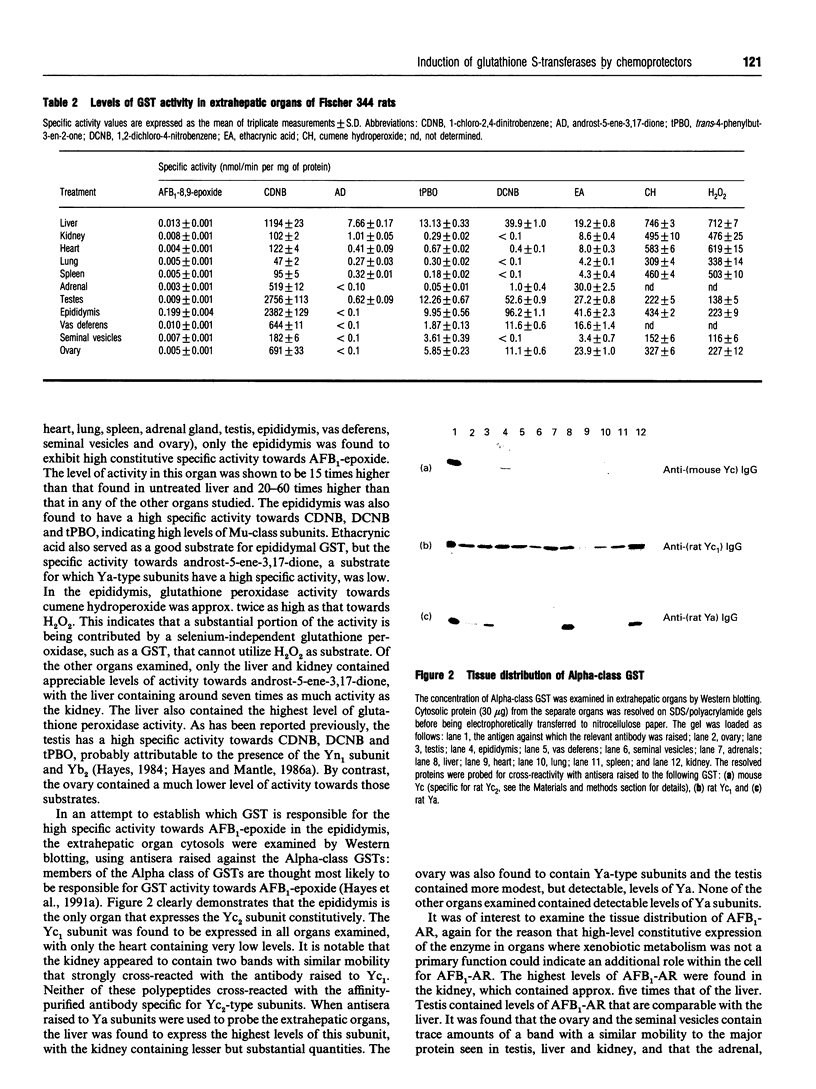
Ingestion of aflatoxin B1 (AFB1) represents a major risk factor in the aetiology of human hepatocellular carcinoma. In the rat, the harmful effects of AFB1 can be prevented by the administration of certain drugs which induce hepatic detoxification enzymes. We have previously shown that treatment of rats with the chemoprotector ethoxyquin (EQ) results in a marked increase in expression of the Alpha-class glutathione S-transferase (GST) Yc2 subunit which has high activity towards AFB1-8,9-epoxide [Hayes, Judah, McLellan, Kerr, Peacock and Neal (1991) Biochem. J. 279, 385-398]. To allow an assessment of whether the increased expression of GST Yc2 represents a general adaptive resistance mechanism to chemical stress, that is invoked by both chemoprotectors and carcinogens, we have examined the effects of EQ, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), phenobarbital (PB), AFB1, 3-methylcholanthrene (3-MC) and clofibrate on the AFB1-glutathione-conjugating activity and the GST subunit levels in rat liver. In addition, the effect of these drugs on the hepatic levels of an aldehyde reductase (AFB1-AR) that metabolizes the cytotoxic dialdehydic form of AFB1 has been studied as this enzyme also appears to be important in chemoprotection. Administration of the antioxidants EQ, BHA or BHT, as well as PB, led to a marked increase in levels of the GST Yc2 subunit in rat liver, and this increase coincided with a substantial rise in the GST activity towards AFB1-8,9-epoxide; neither AFB1, 3-MC nor clofibrate caused induction of Yc2 or any of the GST subunits examined. Among the xenobiotics studied, EQ was found to be the most effective inducing agent for the Yc2 subunit as well as Yc1, Yb1 and Yf. However, PB was equally as effective as EQ in increasing levels of the Ya-type subunits, although it was not found to be as potent an inducer of the other GST subunits, including Yc2. In addition to induction of GST, EQ caused a substantial increase in the hepatic content of AFB1-AR. Both BHA and BHT were also able to induce this enzyme but, by contrast, PB was found to be a poor inducer of AFB1-AR. AFB1, 3-MC and clofibrate were unable to serve as inducers of this reductase. The presence of Alpha-class GST, including the Yc2 subunit, was examined in various rat tissues. Constitutive expression of Yc2 was found in the epididymis at levels comparable with that observed in the liver from EQ-treated rats.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branen A. L. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil Chem Soc. 1975 Feb;52(2):59–63. doi: 10.1007/BF02901825. [DOI] [PubMed] [Google Scholar]
- Buetler T. M., Eaton D. L. Complementary DNA cloning, messenger RNA expression, and induction of alpha-class glutathione S-transferases in mouse tissues. Cancer Res. 1992 Jan 15;52(2):314–318. [PubMed] [Google Scholar]
- Buetler T. M., Slone D., Eaton D. L. Comparison of the aflatoxin B1-8,9-epoxide conjugating activities of two bacterially expressed alpha class glutathione S-transferase isozymes from mouse and rat. Biochem Biophys Res Commun. 1992 Oct 30;188(2):597–603. doi: 10.1016/0006-291x(92)91098-b. [DOI] [PubMed] [Google Scholar]
- Ding V. D., Pickett C. B. Transcriptional regulation of rat liver glutathione S-transferase genes by phenobarbital and 3-methylcholanthrene. Arch Biochem Biophys. 1985 Aug 1;240(2):553–559. doi: 10.1016/0003-9861(85)90062-1. [DOI] [PubMed] [Google Scholar]
- Ellis E. M., Judah D. J., Neal G. E., Hayes J. D. An ethoxyquin-inducible aldehyde reductase from rat liver that metabolizes aflatoxin B1 defines a subfamily of aldo-keto reductases. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10350–10354. doi: 10.1073/pnas.90.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foliot A., Touchard D., Mallet L. Inhibition of liver glutathione S-transferase activity in rats by hypolipidemic drugs related or unrelated to clofibrate. Biochem Pharmacol. 1986 May 15;35(10):1685–1690. doi: 10.1016/0006-2952(86)90324-2. [DOI] [PubMed] [Google Scholar]
- Friling R. S., Bensimon A., Tichauer Y., Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Numoto S., Furuya K., Furukawa N. T., Williams G. M. Effects of the hepatocarcinogen nafenopin, a peroxisome proliferator, on the activities of rat liver glutathione-requiring enzymes and catalase in comparison to the action of phenobarbital. Cancer Res. 1985 Oct;45(10):5011–5019. [PubMed] [Google Scholar]
- Groopman J. D., Cain L. G., Kensler T. W. Aflatoxin exposure in human populations: measurements and relationship to cancer. Crit Rev Toxicol. 1988;19(2):113–145. doi: 10.3109/10408448809014902. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., McLellan L. I., Kerr L. A., Peacock S. D., Neal G. E. Ethoxyquin-induced resistance to aflatoxin B1 in the rat is associated with the expression of a novel alpha-class glutathione S-transferase subunit, Yc2, which possesses high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem J. 1991 Oct 15;279(Pt 2):385–398. doi: 10.1042/bj2790385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., McLellan L. I., Neal G. E. Contribution of the glutathione S-transferases to the mechanisms of resistance to aflatoxin B1. Pharmacol Ther. 1991;50(3):443–472. doi: 10.1016/0163-7258(91)90053-o. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., Neal G. E., Nguyen T. Molecular cloning and heterologous expression of a cDNA encoding a mouse glutathione S-transferase Yc subunit possessing high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem J. 1992 Jul 1;285(Pt 1):173–180. doi: 10.1042/bj2850173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., Neal G. E. Resistance to aflatoxin B1 is associated with the expression of a novel aldo-keto reductase which has catalytic activity towards a cytotoxic aldehyde-containing metabolite of the toxin. Cancer Res. 1993 Sep 1;53(17):3887–3894. [PubMed] [Google Scholar]
- Hayes J. D., Kerr L. A., Peacock S. D., Cronshaw A. D., McLellan L. I. Hepatic glutathione S-transferases in mice fed on a diet containing the anticarcinogenic antioxidant butylated hydroxyanisole. Isolation of mouse glutathione S-transferase heterodimers by gradient elution of the glutathione-Sepharose affinity matrix. Biochem J. 1991 Jul 15;277(Pt 2):501–512. doi: 10.1042/bj2770501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Anomalous electrophoretic behaviour of the glutathione S-transferase Ya and Yk subunits isolated from man and rodents. A potential pitfall for nomenclature. Biochem J. 1986 Aug 1;237(3):731–740. doi: 10.1042/bj2370731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Use of immuno-blot techniques to discriminate between the glutathione S-transferase Yf, Yk, Ya, Yn/Yb and Yc subunits and to study their distribution in extrahepatic tissues. Evidence for three immunochemically distinct groups of transferase in the rat. Biochem J. 1986 Feb 1;233(3):779–788. doi: 10.1042/bj2330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Purification and characterization of glutathione S-transferases P, S and N. Isolation from rat liver of Yb1 Yn protein, the existence of which was predicted by subunit hybridization in vitro. Biochem J. 1984 Dec 15;224(3):839–852. doi: 10.1042/bj2240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T., Irokawa N., Ono S., Ohmori S., Ueno K., Kitagawa H., Satoh T. Difference in the effects of phenobarbital and 3-methylcholanthrene treatment on subunit composition of hepatic glutathione S-transferase in male and female rats. Xenobiotica. 1987 Feb;17(2):127–137. doi: 10.3109/00498258709043923. [DOI] [PubMed] [Google Scholar]
- Ito N., Fukushima S., Tsuda H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit Rev Toxicol. 1985;15(2):109–150. doi: 10.3109/10408448509029322. [DOI] [PubMed] [Google Scholar]
- Iwaki M., Kitagawa T., Akamatsu Y., Aibara K. Cytotoxic effects of aflatoxin B1 and its association with cellular components in chicken embryo primary cultured cells. Biochim Biophys Acta. 1990 Aug 17;1035(2):146–153. doi: 10.1016/0304-4165(90)90109-a. [DOI] [PubMed] [Google Scholar]
- Judah D. J., Davies R., Hayes J. D., McLellan L. I., Neal G. E. The primary and secondary metabolism of aflatoxin B1 by rat hepatocytes cultured on matrigel. Toxicol Appl Pharmacol. 1994 Mar;125(1):27–33. doi: 10.1006/taap.1994.1045. [DOI] [PubMed] [Google Scholar]
- Judah D. J., Hayes J. D., Yang J. C., Lian L. Y., Roberts G. C., Farmer P. B., Lamb J. H., Neal G. E. A novel aldehyde reductase with activity towards a metabolite of aflatoxin B1 is expressed in rat liver during carcinogenesis and following the administration of an anti-oxidant. Biochem J. 1993 May 15;292(Pt 1):13–18. doi: 10.1042/bj2920013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotlikar P. D., Raj H. G., Bohm L. S., Ho L. L., Jhee E. C., Tsuji K., Gopalan P. A mechanism of inhibition of aflatoxin B1-DNA binding in the liver by phenobarbital pretreatment of rats. Cancer Res. 1989 Feb 15;49(4):951–957. [PubMed] [Google Scholar]
- MILLER E. C., MILLER J. A., BROWN R. R., MACDONALD J. C. On the protective action of certain polycyclic aromatic hydrocarbons against carcinogenesis by aminoazo dyes and 2-acetylaminofluorene. Cancer Res. 1958 May;18(4):469–477. [PubMed] [Google Scholar]
- Mandel H. G., Manson M. M., Judah D. J., Simpson J. L., Green J. A., Forrester L. M., Wolf C. R., Neal G. E. Metabolic basis for the protective effect of the antioxidant ethoxyquin on aflatoxin B1 hepatocarcinogenesis in the rat. Cancer Res. 1987 Oct 1;47(19):5218–5223. [PubMed] [Google Scholar]
- McCusker F. M., Boyce S. J., Mantle T. J. The development of glutathione S-transferase subunits in rat liver. Sensitive detection of the major subunit forms of rat glutathione S-transferase by using an e.l.i.s.a. method. Biochem J. 1989 Sep 1;262(2):463–467. doi: 10.1042/bj2620463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan L. I., Harrison D. J., Hayes J. D. Modulation of glutathione S-transferases and glutathione peroxidase by the anticarcinogen butylated hydroxyanisole in murine extrahepatic organs. Carcinogenesis. 1992 Dec;13(12):2255–2261. doi: 10.1093/carcin/13.12.2255. [DOI] [PubMed] [Google Scholar]
- McLellan L. I., Hayes J. D. Differential induction of class alpha glutathione S-transferases in mouse liver by the anticarcinogenic antioxidant butylated hydroxyanisole. Purification and characterization of glutathione S-transferase Ya1Ya1. Biochem J. 1989 Oct 15;263(2):393–402. doi: 10.1042/bj2630393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan L. I., Kerr L. A., Cronshaw A. D., Hayes J. D. Regulation of mouse glutathione S-transferases by chemoprotectors. Molecular evidence for the existence of three distinct alpha-class glutathione S-transferase subunits, Ya1, Ya2, and Ya3, in mouse liver. Biochem J. 1991 Jun 1;276(Pt 2):461–469. doi: 10.1042/bj2760461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Harris J. M., Gilmore K. S., Coles B., Kensler T. W., Ketterer B. Quantitation of tissue- and sex-specific induction of rat GSH transferase subunits by dietary 1,2-dithiole-3-thiones. Carcinogenesis. 1993 Apr;14(4):567–572. doi: 10.1093/carcin/14.4.567. [DOI] [PubMed] [Google Scholar]
- Neal G. E., Metcalfe S. A., Legg R. F., Judah D. H., Green J. A. Mechanism of the resistance to cytotoxicity which precedes aflatoxin B1 hepatocarcinogenesis. Carcinogenesis. 1981;2(5):457–461. doi: 10.1093/carcin/2.5.457. [DOI] [PubMed] [Google Scholar]
- O'Brien K., Moss E., Judah D., Neal G. Metabolic basis of the species difference to aflatoxin B1 induced hepatotoxicity. Biochem Biophys Res Commun. 1983 Jul 29;114(2):813–821. doi: 10.1016/0006-291x(83)90854-9. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Windle J. J., Morrow J. F., Benson A. M., Talalay P. Increased synthesis of glutathione S-transferases in response to anticarcinogenic antioxidants. Cloning and measurement of messenger RNA. J Biol Chem. 1983 Feb 10;258(3):2052–2062. [PubMed] [Google Scholar]
- Perry A. C., Jones R., Niang L. S., Jackson R. M., Hall L. Genetic evidence for an androgen-regulated epididymal secretory glutathione peroxidase whose transcript does not contain a selenocysteine codon. Biochem J. 1992 Aug 1;285(Pt 3):863–870. doi: 10.1042/bj2850863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988 Sep 1;48(17):4776–4782. [PubMed] [Google Scholar]
- Ramsdell H. S., Eaton D. L. Mouse liver glutathione S-transferase isoenzyme activity toward aflatoxin B1-8,9-epoxide and benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide. Toxicol Appl Pharmacol. 1990 Sep 1;105(2):216–225. doi: 10.1016/0041-008x(90)90183-u. [DOI] [PubMed] [Google Scholar]
- Ramsdell H. S., Eaton D. L. Mouse liver glutathione S-transferase isoenzyme activity toward aflatoxin B1-8,9-epoxide and benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide. Toxicol Appl Pharmacol. 1990 Sep 1;105(2):216–225. doi: 10.1016/0041-008x(90)90183-u. [DOI] [PubMed] [Google Scholar]
- Raney K. D., Meyer D. J., Ketterer B., Harris T. M., Guengerich F. P. Glutathione conjugation of aflatoxin B1 exo- and endo-epoxides by rat and human glutathione S-transferases. Chem Res Toxicol. 1992 Jul-Aug;5(4):470–478. doi: 10.1021/tx00028a004. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Reddy J. K. Peroxisome proliferation and hepatocarcinogenesis. Carcinogenesis. 1987 May;8(5):631–636. doi: 10.1093/carcin/8.5.631. [DOI] [PubMed] [Google Scholar]
- Reinhart J., Pearson W. R. The structure of two murine class-mu glutathione transferase genes coordinately induced by butylated hydroxyanisole. Arch Biochem Biophys. 1993 Jun;303(2):383–393. doi: 10.1006/abbi.1993.1299. [DOI] [PubMed] [Google Scholar]
- Robaire B., Hales B. F. Regulation of epididymal glutathione S-transferases: effects of orchidectomy and androgen replacement. Biol Reprod. 1982 May;26(4):559–565. doi: 10.1095/biolreprod26.4.559. [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., King R. G., Paulson K. E., Pickett C. B. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990 May;87(10):3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore T. H., Morton M. R., Pickett C. B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991 Jun 25;266(18):11632–11639. [PubMed] [Google Scholar]
- Sato K., Kitahara A., Yin Z., Waragai F., Nishimura K., Hatayama I., Ebina T., Yamazaki T., Tsuda H., Ito N. Induction by butylated hydroxyanisole of specific molecular forms of glutathione S-transferase and UDP-glucuronyltransferase and inhibition of development of gamma-glutamyl transpeptidase-positive foci in rat liver. Carcinogenesis. 1984 Apr;5(4):473–477. doi: 10.1093/carcin/5.4.473. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. Chemoprevention of cancer. Cancer Res. 1985 Jan;45(1):1–8. [PubMed] [Google Scholar]