Abstract
A fundamental challenge for cystic fibrosis (CF) gene therapy is ensuring sufficient transduction of airway epithelia to achieve therapeutic correction. Hypertonic saline (HTS) is frequently administered to people with CF to enhance mucus clearance. HTS transiently disrupts epithelial cell tight junctions, but its ability to improve gene transfer has not been investigated. Here, we asked if increasing the concentration of NaCl enhances the transduction efficiency of three gene therapy vectors: adenovirus, AAV, and lentiviral vectors. Vectors formulated with 3–7% NaCl exhibited markedly increased transduction for all three platforms, leading to anion channel correction in primary cultures of human CF epithelial cells and enhanced gene transfer in mouse and pig airways in vivo. The mechanism of transduction enhancement involved tonicity but not osmolarity or pH. Formulating vectors with a high ionic strength solution is a simple strategy to greatly enhance efficacy and immediately improve preclinical or clinical applications.
Graphical Abstract
Graphical Abstract.
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease caused by pathogenic variants of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CFTR protein conducts Cl− and HCO3− anions at the apical surface of epithelial cells. Decreased activity or loss of CFTR in the airways leads to a series of complications including bacterial colonization, chronic inflammation, and mucus accumulation. Numerous studies have established that CFTR complementation restores anion transport both in vitro and in vivo (1–3). The advent of the small molecule therapy of elexacaftor, tezacaftor and ivacaftor (ETI), trade name Trikafta, demonstrated that restoring CFTR improves lung function and quality of life in people with CF. Although ETI treatment benefits the majority of people with CF, there remains a pressing need to develop treatments for all people with CF. Gene therapy by CFTR complementation can restore anion channel activity regardless of the mutation. Despite numerous clinical trials (reviewed in (4)), no CF gene therapies have advanced past Phase II trials due, in part, to low efficacy of CFTR gene transfer.
The conducting airways are challenging to transduce using topically applied gene therapy reagents due to an enormous surface area and multiple host defense mechanisms to resist viral vector uptake (4). Vehicle formulations such as lysophosphatidylcholine (LPC) (2,5), EGTA (6,7), methylcellulose (8) and perflurocarbon (9) improve viral vector transduction and subsequent transgene expression in cultured cells and animal models; however, none of these materials are currently FDA approved for pulmonary administration. An ideal vector formulation that enhances gene transfer would be FDA approved and cost effective. Hypertonic saline (HTS) is FDA approved, Generally Recognized As Safe (GRAS), and offers a translational advantage over other vector formulations not currently approved. HTS is commonly aerosolized at 3–7% as a topical lung treatment for people with CF to improve mucociliary transport. HTS transiently disrupts tight junctions (10) and exhibits multiple actions including: a mucolytic agent (disrupting mucus gel), an expectorant (hydrolyzing airway surface liquid), a mucokinetic agent (promoting cough-mediated clearance), and increases ion flux of two thiols (glutathione and thiocyanate) that protect against oxidative injury (reviewed in (11)). Thus, HTS hydrates surface epithelia, breaks ionic bonds, dislodges mucus, and enhances mucociliary clearance (12). Clinical benefits and safety track records of this treatment are well established for people with CF.
Here we screened reagents approved for topical airway delivery. We were interested in reagents which decreased transepithelial resistance to increase access to basolateral receptors and enhance gene transfer. We formulated adenoviral (Ad) vectors, adeno-associated viral (AAV) vectors, or the lentiviral vector human immunodeficiency virus (HIV) with 1–7% NaCl and applied the mixtures to the apical surface of cultured primary human airway epithelial cells (HAE). We found that increasing the NaCl tonicity of the vector formulation correlated with enhanced gene transfer in a dose dependent fashion and functionally restored CFTR anion transport in vitro. Focused entry studies with Ad suggest that vector transduction improves with increasing ionic strength, remains receptor-mediated, and requires a low pH endosomal step. Finally, we asked if vectors delivered in hypertonic saline enhanced gene transfer ex vivo and in vivo. Ad and AAV transduction was significantly increased in pig explants ex vivo. When we delivered Ad with NaCl to both mouse (7% NaCl) and pig airways (5% NaCl), we observed significantly enhanced gene transfer in vivo. Additionally, we aerosolized GP64 pseudotyped HIV lentivirus formulated in 5% NaCl to pig airways and observed widespread reporter gene expression greater than the isotonic saline control. Together, these findings indicate that formulating viral vectors with hypertonic saline for gene addition or gene editing approaches may be a simple means to improve transduction.
Materials and methods
Ethics statement
Primary airway epithelia from human CF and non-CF donors were isolated from discarded tissue, autopsy, or surgical specimens. Cells were provided by The University of Iowa In Vitro Models and Cell Culture Core Repository. Information that could be used to identify a subject was not provided. All studies involving human subjects received University of Iowa Institutional Review Board approval (Protocol #230 167). Mice and pig experimental protocols were reviewed and approved by the University of Iowa Institutional Animal Care and Use Committee (IACUC), in accordance with the United States Department of Agriculture and National Institutes of Health guidelines.
Human airway epithelial cells
The University of Iowa In Vitro Models and Cell Culture Core cultured and maintained human airway epithelial cells (HAE) as previously described (13). Briefly, following enzymatic disassociation of trachea and bronchus epithelia, the cells were seeded onto collagen-coated, polycarbonate Transwell inserts (0.4 μm pore size; surface area = 0.33 cm2; Corning Costar, Cambridge, MA). HAE were submerged in Ultroser G (USG) medium for 24 h (37°C and 5% CO2) at which point the apical media is removed to encourage polarization and differentiation at an air-liquid interface. Transepithelial electrical resistance was measured using an Ohmmeter (Ω·μm2).
Viral vector production and formulation
Vectors were produced by the University of Iowa Viral Vector Core (https://medicine.uiowa.edu/vectorcore). Ad5 and Ad21 CMV-eGFP CMV-mCherry, or F5Tg83-CFTR was produced, purified, and titered as previously described (14). AAV2/2.5T, AAV2/H22 CMV-eGFP or F5Tg83-CFTRΔR was produced by triple transfection. Lentiviral HIV-CMV-eGFP or PGK-CFTR vectors pseudotyped with GP64 were produced by a four-plasmid transfection method as previously described (15,16) and titered using droplet digital PCR (17) and/or by flow cytometry. Similarly, VSVG, JSRV and BaEV pseudotyped lentiviral vectors were made by four-plasmid transfection and titered by flow cytometry. Viral vectors were formulated with indicated NaCl concentrations (presented as final concentrations). The following MOIs were used for each vector: Ad = 250, AAV = 100 000, and HIV = 50. Ad studies were performed by mixing pharmaceutical grade 7.2% NaCl with Ad-GFP (3.6% final). Additional concentrations were made from a 10% NaCl solution of Sodium Chloride (Research Products International, Mount Prospect, IL) in UltraPure Distilled Water (Invitrogen, Waltham, MA). Vectors were formulated with NaCl in a final volume of 50 μl and applied to the apical surface of HAE for 2 h. AAV transduced cultures were pre-treated with indicated concentrations of doxorubicin [1 μM] for 24 h prior to 2 h vector application. Other formulations tested include lysophosphatidylcholine (LPC) (9008–30-4, Millipore Sigma, St. Louis, MO), Ethylene glycol-bis-2-aminoethylether)-N,N,N’N’-tetraacetic acid (EGTA) (Research Products International, Mount Prospect, IL), and surfactant (Infasurf, pharmaceutical grade).
Fluorescence microscopy and flow cytometry
GFP images were acquired using a Keyence All-in-one Fluorescence Microscope BZ-X series (Osaka, Japan). 0.33 cm2 transwells were imaged at 2X magnification. GFP expression was quantified by flow cytometry as previously reported (16,18). Briefly, cells were stained with a fixable LIVE/DEAD stain (Thermo Fisher Scientific, Waltham, MA), lifted in Accutase at 37°C for 30 min, and run through an Attune NxT Flow Cytometer (Thermo Fisher Scientific, Waltham, MA). Cells were treated with the Foxp3/Transcription Factor Staining Buffer Set (Thermo Fisher Scientific, Waltham, MA) according to manufacturer's recommendations and stained for 1 h at 37°C with the following antibodies: NGFR (345 110; 1:600, BioLegend, San Diego, CA), α-tubulin (NB100-69AF405, 1:300, Novus, Centennial, CO) and CD66c (12-0667-42, 1:600, Invitrogen, Waltham, MA). Expression was gated on live cells. Immunofluorescence staining of pig tissue sections was performed using acetylated α-tubulin (D20G3, 1:200, Cell Signaling Technology, Danvers, MA) or cytokeratin 5 (CK5) (Poly19055, 1:500, San Diego, CA). Primary antibodies were detected using a goat anti-rabbit Alexa 546 secondary antibody (A-11035, 1:600, Thermo Fisher Scientific, Waltham, MA).
LDH release assay
LDH release was quantified according to the manufacturer's recommendations (LDH-Glo Cytotoxicity Assay, Promega, Madison, WI). Briefly, basolateral media from each condition was collected in a 96-well plate. The LDH detection reagents were mixed in a 1:1 ratio and applied to each well and incubated for 30 min. Luminescence was recorded using a SpectraMax i3x plate reader.
Dextran permeability assay
Tight junction permeability was assessed by passage of 3000–5000 average molecular weight Fluorescein isothiocyanate-dextran (FD4, Sigma-Aldrich, St. Louis, MO) from the apical to basolateral side of well-differentiated HAE. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (Sigma-Aldrich, St. Louis, MO) serves as a positive control for membrane permeabilization and was pre-treated (50 μM) overnight prior to addition of dextran. 250 mM EGTA pre-treatment for 2 h was used for a positive experimental tight junction disruption control. Cells were left untreated or pre-treated with NaCl ranging from 1–7% for 2 h. At the time of the assay, pre-treatments were removed and fresh basolateral media was added. 100 μl of 1 mg/ml dextran was applied apically for 30 min at 37°C. Basolateral media was assessed for fluorescent units using a SpectraMax i3x plate reader.
SEM
Scanning electron microscopy (SEM) samples were processed by the Johns Hopkins Institute for Biomedical Sciences Microscope Facility as a fee for service (https://microscopy.jhmi.edu/Services/EMCostStruct.html).
Electrophysiology
Short circuit current (Isc) and conductance (Gsc) was measured as previously described (16). Briefly, cells were pre-stimulated with forskolin and IBMX (F&I) overnight and their bioelectric properties were quantified by Ussing chamber analysis. Assay protocol is as follows: amiloride, 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS), F&I, and GlyH-101 (GlyH). Results are reported as change in short circuit current (ΔIsc) and conductance (ΔGsc) in response to F&I or GlyH.
Western blot
Protein from HAE transduced with Ad-CFTR alone or Ad-CFTR + NaCl (3.6%) was harvested using RIPA buffer. 20 μg of each sample was loaded on a criterion Tris-glycine 4–20% gel and run at 110 V for 1 h and transferred overnight onto a PVDF membrane at 110 μA at 4°C. After membrane blocking, each membrane was probed for using the following primary antibodies: mouse anti-CFTR 596 (1:1000) (UNC) or CAR anti-rabbit (CXADR) (1:100) (PA5-31175, Invitrogen, Waltham, MA) was applied for 2 h. Following TBST washes, the LiCor secondary antibody was used at 1:10 000 for 30 min. The membrane was imaged using an Odyssey imager.
Sugar tonicity and ionic versus non-ionic osmolytes
KCl, mannitol and N-methyl-d-glucamine (NMDG) gluconate were formulated 1–7% and co-delivered with Ad-GFP (MOI = 250) for 2 h apically. Molarities and osmolarities are presented in Table 1. Sodium chloride (NaCl), mannitol, sodium gluconate, and potassium chloride (KCl) were made with equal percentages (3.6%) or equal molarity (1.23 mOsmol/l) to NaCl as shown in Table 2. Each solution was mixed with Ad-GFP (MOI = 250) and applied apically to HAE for 2 h. GFP expression was quantified by flow cytometry 5 days post-transduction.
Table 1.
Molarity and osmolarity of NaCl, KCl, mannitol and NMDG gluconate up to 7%
| Tonicity | Isotonic | Hypertonic | ||||||
|---|---|---|---|---|---|---|---|---|
| DMEM | ||||||||
| % NaCl | 0.64 % | 1% | 2% | 3% | 4% | 5% | 6% | 7% |
| Molarity | 110 mM | 170 mM | 340 mM | 510 mM | 680 mM | 860 mM | 1.03 mM | 1.2 M |
| Osmolarity | 219 mOsmol/l | 342 mOsmol/l | 684 mOsmol/l | 1 027 mOsmol/l | 1 369 mOsmol/l | 1 711 mOsmol/l | 2 053 mOsmol/l | 2 693 mOsmol/l |
| % KCl | 0.4% | 1% | 2% | 3% | 4% | 5% | 6% | 7% |
| Molarity | 50 mM | 130 mM | 270 mM | 400 mM | 540 mM | 670 mM | 800 mM | 940 mM |
| Osmolarity | 107 mOsmol/l | 268 mOsmol/l | 537 mOsmol/l | 805 mOsmol/l | 1 073 mOsmol/l | 1 341 mOsmol/l | 1 610 mOsmol/l | 1 878 mOsmol/l |
| % mannitol | 1% | 2% | 3% | 4% | 5% | 6% | 7% | |
| Molarity | 55 mM | 110 mM | 170 mM | 220 mM | 270 mM | 330 mM | 380 mM | |
| Osmolarity | 55 mOsmol/l | 110 mOsmol/l | 170 mOsmol/l | 220 mOsmol/l | 270 mOsmol/l | 330 mOsmol/l | 380 mOsmol/l | |
| % NMDG gluconate | 1% | 2% | 3% | 4% | 5% | 6% | 7% | |
| Molarity | 50 mM | 100 mM | 150 mM | 200 mM | 260 mM | 310 mM | 360 mM | |
| Osmolarity | 342 mOsmol/l | 684 mOsmol/l | 1 027 mOsmol/l | 1 369 mOsmol/l | 1 711 mOsmol/l | 2 053 mOsmol/l | 3 693 mOsmol/l | |
Tonicity, molarity, and osmolarity of DMEM and 1–7% NaCl, KCl, mannitol and N-methyl-d-glucamine (NMDG) gluconate. Hypertonic indicated a NaCl solution >0.9%. DMEM has additional inorganic salts (i.e. CaCl2, MgSO4, KCl and NaHCO3) and is considered isotonic.
Table 2.
Molarity and osmolarity of ionic and non-ionic osmolytes
| 3.6% NaCl | 0.9 % NaCl | 0.9% NaCl + mannitol | 3.6% NaGluconate | 3.6% KCl | |
|---|---|---|---|---|---|
| Molecular weight | 58.44 g/mol | 58.44 g/mol | Mannitol: 182.172 g/mol | 590 g/mol | 74.55 g/mol |
| Tonicity (%) | 3.6% | 0.9% | 16% mannitol | 3.6% | 3.6% |
| Molarity | 620 mM | 150 mM | 620 mM | 61 mM | 480 mM |
| Osmolarity | 1 230 mOsmol/l | 310 mOsmol/l | 1 230 mOsmol/l | 120 mOsmol/l | 970 mOsmol/l |
Molecular weight, tonicity, molarity and osmolarity for NaCl (0.9% and 3.6%), mannitol, NaGluconate and KCl are presented.
CXADR knockout (KO)
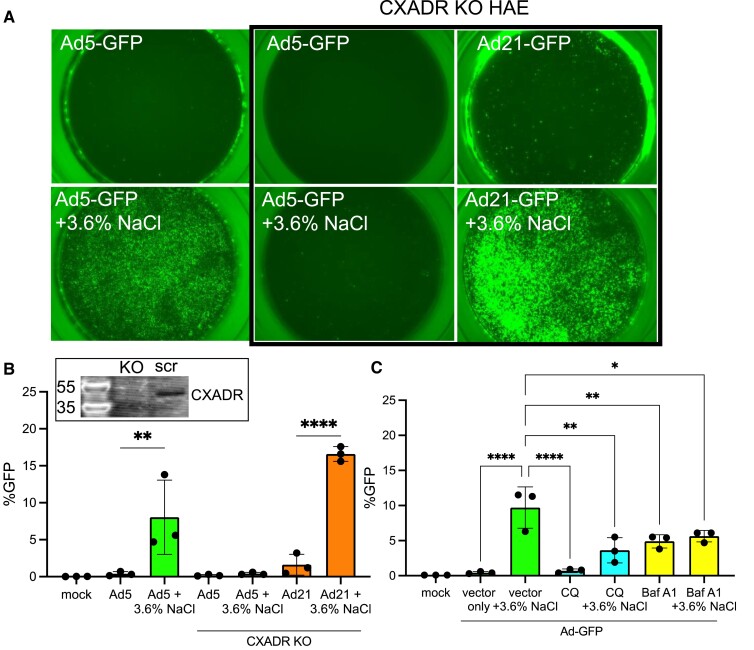
CRISPR/Cas9 was used to knockout the coxsackie and adenovirus receptor (CXADR) by nucleofection (Lonza, Basel Switzerland). Well-differentiated HAE were lifted in TrypLE and electroporated with ribonucleoproteins (RNP). CXADR Exon 4 was targeted with the following guide RNAs: gRNA 1: ACGTAACATCTCGCACCTGAAGG and gRNA 2: AGTACCTGCTAACCATGAAGTGG (19). Alt-R S.p. HiFi Cas9 nuclease (1 081 058, IDT, Coralville, IA) was mixed with crRNA and trcrRNA duplex to form the RNP. RNP and cells were mixed with the nucleofector solution and electroporated using program U-024 and seeded in a 6 well plate in Pneumacult ExPlus media. 2 days later, cells were lifted and reseeded on a Corning 3413 collagen coated membranes. Cells were maintained in Pneumacult ALI-M media for 3 weeks to differentiate. Ad5-GFP (CXADR-dependent) and Ad21-GFP (CXADR-independent) were formulated with DMEM or NaCl (3.6%) and applied to the apical surface of HAE for 2 h. GFP expression was quantified 5 days post-transduction by flow cytometry.
Inhibitors of endosomal acidification
Endosomal acidification inhibitors chloroquine (CQ) (200 μM) (C6628, Millipore Sigma, St. Louis, MO) and bafilomycin A1 (Baf A1) (1 μM) (19 148, Millipore Sigma, St. Louis, MO) were used to pre-treat HAE for 2 h prior to Ad-GFP transduction in the presence or absence of NaCl (3.6%). GFP was quantified by flow cytometry 5 days post-transduction.
Pig explants transduction
0.25 cm2 tracheal explants from newborn pigs were cultured on Surgifoam (1972, Ethicon, Raritan, NJ) for 3–5 days prior to transduction. Ad5-mcherry (1 × 109 TU), AAVH22-GFP (2 × 1010 vg) were applied with or without NaCl (5%) to the apical surface of the trachea by inverting the trachea onto a tissue culture dish containing the viral solution for 2 h then returned to an upright position on Surgifoam. 5 days post-transduction, tracheal explants were mounted on a microscope slide and imaged for fluorescent expression using confocal microscopy. Expression was quantified using Image J (FIJI) by measuring fluorescence intensity per high power field.
Adenoviral transduction of mouse airways
6–8 week old Balb/c mice were sedated with ketamine/xylazine (87.5 + 2.5 mg/kg). Ad-CMV-firefly luciferase was delivered intratracheally with 1 × 109 TU formulated with NaCl (0.9% or 7% final) in a 50 μl volume. 5 days later mice were given intraperitoneal d-luciferin (50 227, Millipore Sigma, St. Louis, MO) imaged for luminescence expression using the Xenogen IVIS-200.
Viral gene transfer in pig airways
Newborn pigs were sedated using isoflurane and viral vector formulated with NaCl (0.9% or 5% final) and aerosolized 2 ml intratracheally using a MADgic Laryngo-Tracheal Mucosal Atomization Device (Teleflex, Morrisville, NC). Doses for each vector for each pig were as follows: 2.8 × 1010 infectious genomic units (IGU) of helper-dependent Ad-CMV-GFP and 7 × 108 TU of GP64 HIV-CMV-GFP. Doses were prepared by mixing a 1.8% NaCl or 10% NaCl stock 1:1 with 1 ml of vector. 1 week later, pigs were humanely euthanized and lungs were analyzed for GFP expression. Lungs were systematically divided and fixed in 4% paraformaldehyde, subjected to a sucrose gradient, and embedded in OCT for cryosectioning. Sectioned lung slides were mounted using DAPI and imaged using a Keyence All-in-one Fluorescence Microscope BZ-X series (Osaka, Japan).
Statistics
Student's two-tailed t-test, one-way analysis of variance (ANOVA) with Tukey's multiple comparison test, or two-way ANOVA with Dunnett's multiple comparison test were used to analyze differences in mean values between groups. Results are expressed as mean ± SEM. P values ≤0.05 were considered significant. R2 values in Figure 1E represent exponential growth of nonlinear regression.
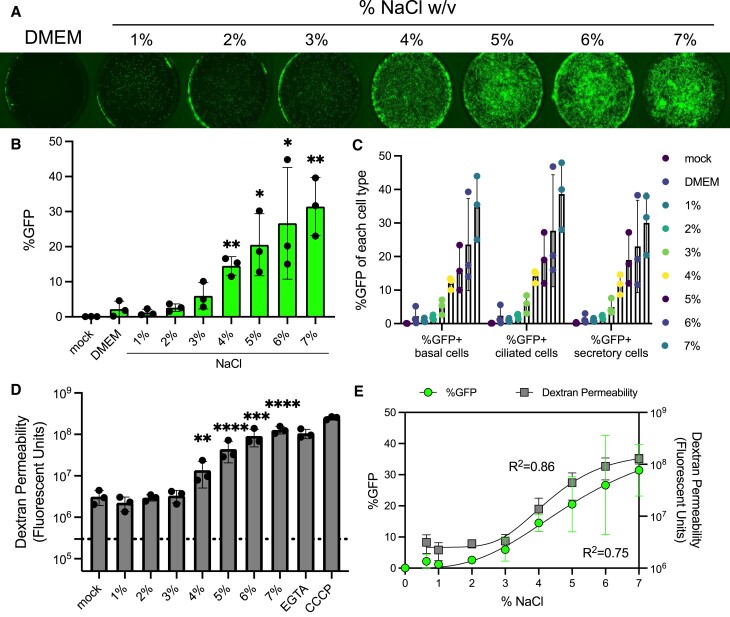
Figure 1.
Increasing saline tonicity enhances apical Ad gene transfer in airway epithelial cells. (A) Ad-CMV-eGFP (MOI = 250) was co-delivered to HAE with DMEM or NaCl ranging from 1–7% (final concentration) and imaged 5 days post-transduction. 0.33 cm2 transwells were imaged at 2×. (B) Percentage of GFP+ cells in each condition quantified by flow cytometry. (C) Percentages of GFP+ cells by cell type from 1–7% NaCl. (D) Tight junction permeability was measured by the passage of dextran from the apical to basolateral media. HAE were left untreated or treated with NaCl (1–7%) for 2 h. Following the treatment, apical dextran was applied and basolateral media was collected 30 min later and fluorescent units were measured using a plate reader. (E) Correlative %GFP (R2= 0.75) from Figure 1B and Dextran Permeability (R2= 0.86) from Figure 1D were plotted together. N = 3, *P< 0.05, **P< 0.005, ***P< 0.0005, ****P< 0.00005. Statistical differences were determined by one-way ANOVA.
Results
Not all vehicles that decrease transepithelial resistance enhance gene transfer
Disrupting the epithelial cell tight junctions allows Ad5-based viral vectors access to their basolaterally localized coxsackie and adenovirus receptor (CXADR) (20). Unless otherwise specified, these studies use the Ad5 serotype. We screened several compounds for their ability to reduce the transepithelial electrical resistance (TEER) of HAE and improve transduction of an Ad vector. First, reagents were applied to the apical surface of HAE for 5 min and then TEER was measured using an ohmmeter. Consistent with previous reports, lysophosphatidylcholine (LPC) (0.1%) and EGTA (250 mM) reduced TEER and increased gene transfer (2,6). The clinically approved reagents doxorubicin (5 μM), NaCl (3.6%), and surfactant (Infasurf, 35 mg/ml) all decreased TEER (Supplementary Figure S1A). In separate cultures, Ad-GFP (MOI = 250) in 50 μl of DMEM combined 1:1 with the test reagents was applied to the apical surface for 2 h. 5 days post-treatment, cells were imaged by fluorescence microscopy. Doxorubicin, surfactant, and 0.9% NaCl (PBS) did not enhance Ad gene transfer. Of note, the 3.6% NaCl formulation (mixed 1:1 from 7.2%) conferred remarkable transduction (Supplementary Figure S1B). Based on this initial observation, this concentration of NaCl was selected for the many of the subsequently described Ad-based studies.
Increasing saline tonicity enhances gene transfer in a dose and time dependent manner
To determine if increasing saline tonicity further improves Ad transduction in HAE, we performed a dose-response of 1–7% NaCl formulation (final concentration, Table 1) with Ad-GFP (MOI = 250). We observed a dose-dependent increase in transduction efficiency with increasing NaCl tonicity (Figure 1A, B). Basal, secretory, and ciliated cells were all transduced in a dose-dependent manner (Figure 1C, Supplementary Figure S2A). HAE permeability to dextran also increased in the presence of 4–7% NaCl (Figure 1D), which correlated with increasing GFP expression (Figure 1E). The highest concentrations of NaCl (5–7%) reduced cell viability as determined by flow cytometry (Supplementary Figure S2B) and increased LDH release (Supplementary Figure S2C). To visualize that cell surface integrity and ciliation remained intact, we performed scanning electron microscopy (SEM) on HAE that were untreated or treated with NaCl (3.6%) for 2 h and observed no overt physical differences compared to the no NaCl control (Supplementary Figure S2D). The therapeutic range of NaCl delivered to patients by aerosolization is up to 7% (21); however, based on the cell viabilty results, we restricted the delivered concentration of NaCl to 3.5–4.5% for subsequent in vitro studies.
We next asked how the length of exposure to NaCl or Ad impacted transduction in cultured HAE. As depicted schematically (Supplementary Figure S3A), HAE were incubated with constant Ad-GFP for 120 min and varied times for NaCl (3.6%) at 5, 15, 30, 60 or 120 min. In parallel, HAE were incubated with constant NaCl (3.6%) and varied Ad-GFP incubation times. GFP expression was visualized 5 days post-transduction and quantified by flow cytometry (Supplementary Figure S3B). The %GFP + cells increased incrementally with increasing temporal exposure to either Ad-GFP or NaCl, with significant increase at 1 h Ad-GFP with constant NaCl (3.6%) exposure and maximal expression after a 2 h co-treatment. Given these time-dependent results, we established a standard 2 h co-delivery protocol.
NaCl-mediated Ad transduction restores anion transport
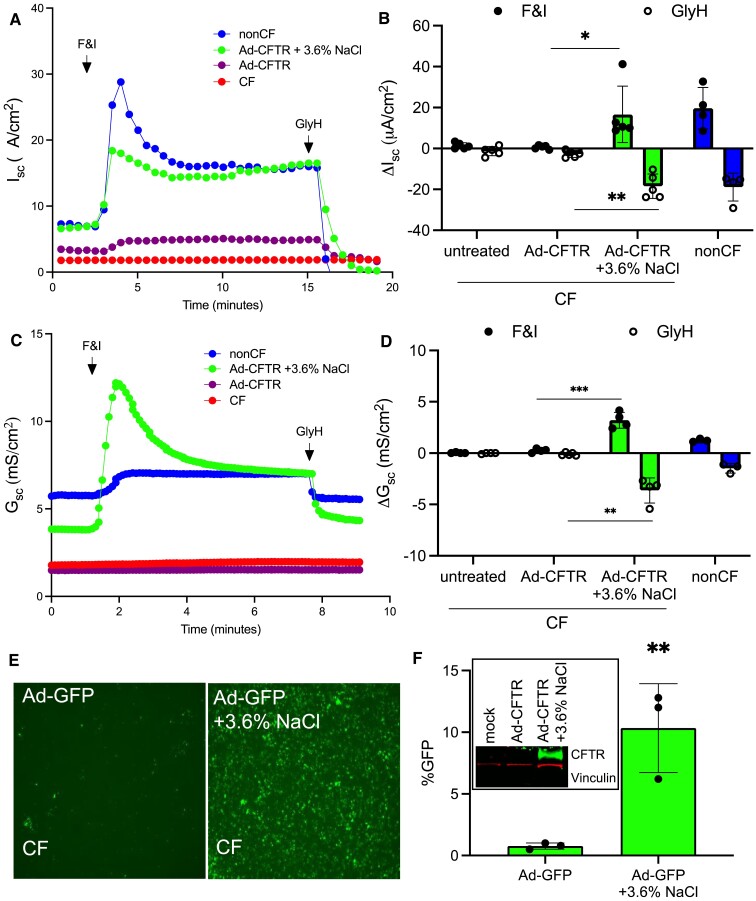
We hypothesized that the NaCl conferred increase in transduction efficiency would enhance Ad-CFTR complementation of anion transport in CF HAE. We next co-delivered Ad-CFTR formulated with NaCl (3.6%) to the apical surface of primary CF HAE for 2 h. Five days post-delivery, bioelectric properties were measured in Ussing chambers as previously reported (22). Without NaCl (3.6%) formulation, no change in Cl− conductance was observed in response to the CFTR agonists forskolin and IBMX (F&I) or CFTR inhibitor GlyH in cultured primary CF epithelia treated with Ad-CFTR. In contrast, cells that received Ad-CFTR and NaCl (3.6%) showed wild-type levels of Cl− secretion as measured by short circuit current (Figure 2A,B) and conductance (Figure 2C, D). Parallel CF airway cultures were transduced as indicated to determine transgene expression by microscopy (Figure 2E) and measured by flow cytometry (Figure 2F) or western blot (Figure 2F, inset). These results suggest that NaCl formulation of Ad-CFTR improved gene transfer and conferred wild-type levels of CFTR mediated anion transport in CF HAE.
Figure 2.
Apically applied Ad-CFTR + 3.6% NaCl restores anion transport defect in CF airway epithelial cells. (A, B) Short circuit current measurements of CF HAE alone or treated with Ad-CFTR or Ad-CFTR + 3.6% NaCl (MOI = 250) compared to non-CF. Responses to Forskolin and IBMX (F&I) and GlyH-101 (GlyH) are reported. (C, D) Short circuit conductance in response to F&I and GlyH (N = 5). (E) Representative images of parallel CF cultures transduced with Ad-GFP (MOI = 250) and (F) quantified by flow cytometry (N = 3). Inset: representative western blot of CF HAE untreated or transduced with Ad-CFTR or Ad-CFTR + 3.6% NaCl. Western blot was probed for CFTR (green) and Vinculin (red) (loading control). *P< 0.05, **P< 0.005, ***P< 0.0005. Statistical differences were determined by Student's t-test or one-way ANOVA.
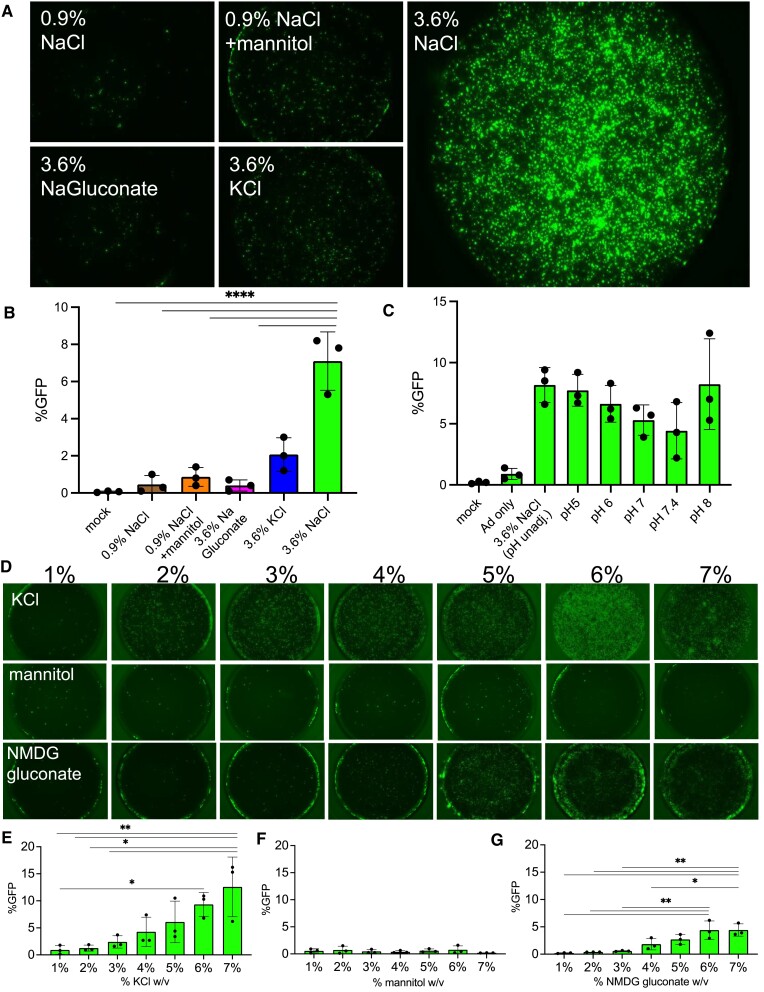
Impact of osmolarity, pH, and ionic strength on NaCl enhanced gene transfer
To investigate possible mechanisms for the enhanced transduction by NaCl formulations, we compared 3.6% NaCl to the ionic osmolytes 3.6% NaC6H11O7 (NaGluconate), 3.6% KCl, 0.9% NaCl and 0.9% NaCl with the non-ionic osmolyte mannitol of equivalent osmolarity to the NaCl (3.6%) formulation (Table 2). The indicated regents were co-delivered with Ad-GFP apically to HAE for 2 h. Microscopy and flow cytometry quantification was performed five days later. NaCl (3.6%) conferred the greatest increase in transduction efficiency (Figure 3A,B), whereas equivalent tonicity (3.6%) or osmolarity (620 mM, 1 230 mOsmo/l) with ionic or non-ionic osmolytes did not significantly enhance gene transfer. We next determined if the pH of NaCl formulation impacts transduction. Given that the unadjusted pH of 3.6% NaCl in deionized water is ∼6, we adjusted the pH of the NaCl (3.6%) solution to 5, 6, 7, 7.4 and 8. Following Ad-GFP delivery, and flow cytometry quantification protocols in HAE, no significant differences in %GFP + cells were observed across this pH range (Figure 3C). To investigate another potential mechanism, we asked if ionic strength was responsible for enhanced vector transduction. We compared KCl (lower ionic strength than NaCl for a given percent solution), mannitol (uncharged sugar) and N-methyl-d-glucamine gluconate (charged sugar) at 1–7% tonicities (Table 1 and Figure 3D). We observed dose-dependent increases with KCl and N-methyl-d-glucamine gluconate whereas mannitol did not enhance gene transfer (Figure 3E–G). The formulation was not dependent on osmolarity or pH, but ionic strength increased the Ad transduction efficiency.
Figure 3.
Entry is dependent on ionic strength but not osmolarity, osmolytes, or pH. (A) Isotonic NaCl (0.9%), Isotonic NaCl + the non-ionic osmolyte mannitol with equivalent osmolarity (620 mM, 1 230 mOsmol/l) to 3.6% NaCl, NaGluconate (3.6%), and KCl (3.6%) were co-delivered apically to HAE with Ad-GFP (MOI = 250) for 2 h. 5 days post-transduction, cells were imaged and (B) GFP expression was quantified by flow cytometry. (C) Ad-GFP was co-delivered with NaCl (3.6%) at pH 5, 6, 7, 7.4 and 8 for 2 h. 5 day post-transduction GFP was quantified by flow cytometry. (D) KCl, mannitol, or N-methyl-D-glucamine (NMDG) gluconate 1–7% was formulated with Ad-GFP (MOI = 250) and applied to the apical surface of HAE for 2 h. Cultures were imaged 5 days post-transduction and GFP expression was quantified by flow cytometry for (E) KCl, (F) mannitol, or (G) NMDG gluconate. N = 3, *P< 0.05, **P< 0.005, ****P< 0.00005. Statistical differences were determined by one-way ANOVA.
NaCl-mediated gene transfer remains receptor dependent and requires a low pH endosome
Next, we investigated whether NaCl enhanced Ad5 transduction is receptor dependent, using CRISPR/Cas9 RNPs to knockout (KO) the Ad5 receptor CXADR in primary human airway basal cells (19,23,24). The electroporated primary cells were seeded on transwell membranes and cultured at an air-liquid interface until well-differentiated (∼21 days). CXADRKO cells and donor-matched HAE were transduced with Ad5-GFP and Ad21-GFP in the presence or absence of NaCl (3.6%). Ad21 enters airway cells through a CXADR-independent mechanism using CD46 as a cellular receptor (25). Ad5 + NaCl did not transduce CXADRKO epithelia, whereas Ad21 + NaCl readily transduced CXADRKO cells (Figure 4A, B). Following receptor engagement and internalization, Ad5 transduction requires escape from low pH endosomes. Inhibiting endosomal acidification with chloroquine (CQ) or bafilomycin A1 (Baf A1) significantly decreased the NaCl enhanced transduction (Figure 4C). These results indicate NaCl enhanced transduction requires receptor engagement and low pH endosomal release.
Figure 4.
NaCl-mediated transduction requires receptor and low pH entry step. Ad5 utilizes CXADR for entry and Ad21 utilizes CD46. (A) CRISPR/Cas9 with gRNA targeted to CXADR was electroporated into HAE and allowed to re-differentiate at air liquid interface for 3 weeks. In parallel with non-electroporated cells from the same donor, Ad5-GFP and Ad21-GFP were applied apically to HAE with or without NaCl for 2 h. Cultures were imaged 5 days post-transduction and representative images are shown. (B) GFP was quantified by flow cytometry. Inset: Western blot for CXADR in parallel on CXADR KO or scrambled airway epithelial cultures. (C) HAE were pre-treated with endosomal acidification inhibitors chloroquine (CQ) (200 μM) or bafilomycin A1 (1 μM) for 2 h. Next, Ad5-GFP (MOI = 250) was delivered with or without NaCl (3.6%) for 2 h. GFP expression was quantified by flow 5 days post-transduction. N = 3, *P< 0.05, **P< 0.005, ****P< 0.00005. Statistical differences were determined by one-way ANOVA.
NaCl formulations enhance lentiviral and AAV gene transfer
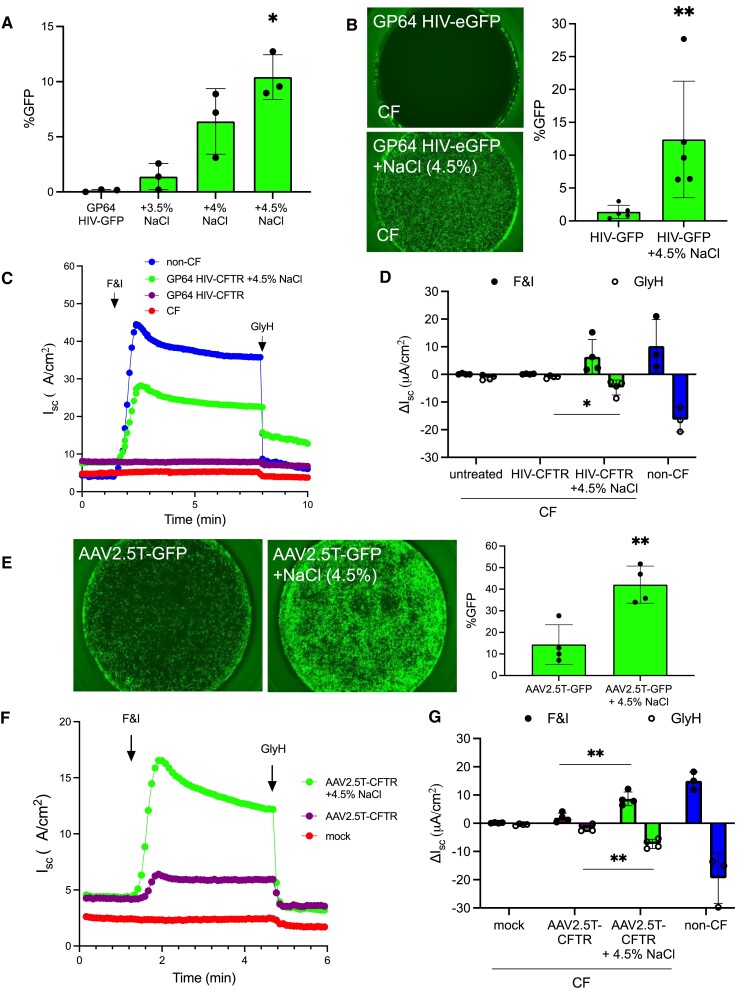
We next asked if formulating lentiviral or adeno-associated viral (AAV) vectors with increasing concentrations of NaCl enhanced transduction. From the Ad-based studies, we learned that ≤3% NaCl had little benefit on transduction while 5% NaCl formulation was noted to have negative effects on HAE integrity in vitro. Therefore, we focused our experimental design on a range of 3.5–4.5% NaCl. An HIV-based viral vector pseudotyped with the baculoviral GP64 envelope glycoprotein (26) was applied to the apical surface of HAE with 3.5, 4 or 4.5% NaCl. A dose-dependent increase in GFP expression was observed in non-CF HAE (Figure 5A) and we therefore selected 4.5% NaCl for lentiviral and AAV studies. Primary human CF cultures that received GP64 HIV-GFP (MOI = 50) also showed remarkable levels of transduction (Figure 5B). Notably, in the presence of NaCl (4.5%), GP64 HIV-CFTR restored the CFTR-dependent anion transport in primary CF HAE. Without NaCl formulation, improvements in CFTR-dependent currents were not observed (Figure 5C, D). Interestingly, NaCl-mediated entry did not enhance transduction of all pseudotyped lentiviral vectors. A screen of additional envelopes from Vesicular Stomatitis Virus (VSVG) (27), Jaagsiekte Sheep Retrovirus (JSRV) (28), and Baboon Endogenous Virus (BaEV) (29) in the presence or absence of NaCl (4.5%) revealed that only GP64 and VSVG showed enhanced transduction (Supplementary Figure S4).
Figure 5.
Increasing saline tonicity enhances GP64 pseudotyped lentiviral and AAV transduction. (A) GP64 pseudotyped HIV-CMV-eGFP (MOI = 50) was co-delivered to HAE with DMEM, 3.5%, 4%, or 4.5% NaCl for 2 h and then removed. 1 week post-transduction GFP expression was quantified by flow cytometry. (B) Parallel CF cultures were transduced with GP64 HIV-CMV-eGFP. GFP expression was visualized by fluorescence microscopy and quantified by flow cytometry 5 days later. (C, D) As described in Figure 2, CF HAE were transduced with GP64-HIV-CFTR (MOI = 50) formulated with DMEM or 4.5% NaCl for 2 h and incubated for 1 week. Short circuit current of F&I and GlyH in untreated or treated CF cells compared to non-CF. (E) AAV with the 2.5T capsid expressing CMV-eGFP was co-delivered (MOI = 100 000) with DMEM or 4.5% NaCl for 2 h. HAE were pre-treated with doxorubicin (1 μM) for 24 h prior to transduction. 2 weeks post-transduction, airway cultures were imaged and GFP was quantified by flow cytometry. (F, G) CF airway cultures were treated overnight with doxorubicin (1 μM) and the next day cells were treated with AAV2.5T-CFTR alone or with 4.5% NaCl for 2 h. Two weeks post-transduction, electrical properties were measured by Ussing chambers as described in (C, D). N = 3, *P< 0.05, **P< 0.005. Statistical differences were determined by Student's t-test or one-way ANOVA.
To test the effect of NaCl formulation on AAV-based vectors, we selected the AAV2.5T capsid based on previously established airway tropism (30). Similar to Ad and lentiviral studies, we applied AAV-GFP (MOI = 100 000 vg/cell) to the apical surface of HAE for 2 h with or without NaCl (4.5%). Doxorubicin (1 μM) was added to the basolateral media 24 h prior to the 2 h AAV application. A 2 h application was chosen to be consistent with the Ad and HIV protocols, however we and others have shown that overnight applications of AAV are effective in the absence of NaCl (31). Microscopy, flow cytometry quantification, and Ussing chamber assays were performed 2 weeks post-transduction. The epithelia treated with NaCl (4.5%) formulated AAV-GFP achieved significantly higher GFP expression (Figure 5E) and increased anion channel activity in responses to F&I and GlyH (Figure 5F, G). These results confirm that transduction with NaCl formulation is enhanced across all three viral vector systems.
Increased saline tonicity enhances gene transfer ex vivo and in vivo
We next asked if formulating vectors in NaCl (5%) would enhance gene transfer in an ex vivo model. Using freshly excised newborn pig tracheal explants maintained on Surgifoam (32), we applied Ad-GFP or AAVH22-GFP (a pig-tropic AAV capsid) (33) ± NaCl (5%) to the apical surface for 2 h. One week later, tissues were fixed and imaged by confocal microscopy. For both Ad and AAV, NaCl (5%) formulation resulted in improved gene transfer efficacy that was particularly striking using low power microscopy (Supplementary Figure S5A, right panels). In the vector only condition, no appreciable expression was observed at low power, however expression was seen at higher magnifications (Supplementary Figure S5A, left panels). Fluorescence was quantified by transduction area per high power field. Transduction with both Ad and AAVH22 was markedly enhanced in ex vivo tracheal explants when formulated with NaCl (5%) (Supplementary Figure S5B).
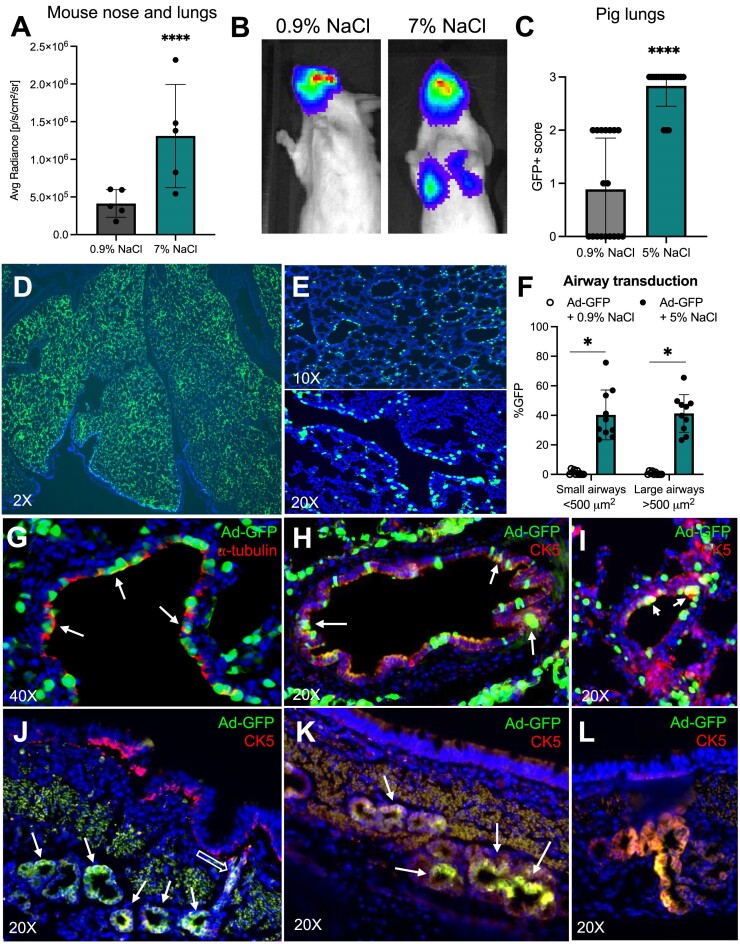
Improving viral vector transduction in vivo has broad implications for advancing gene therapy for lung diseases. We asked if vectors formulated with NaCl (7%) improved gene transfer to mouse lungs. Ad-luciferase was formulated with NaCl (7%) or NaCl (0.9%) and delivered intratracheally. 5 days post-delivery, animals treated with vectors formulated in 7% NaCl demonstrated significantly increased luciferase expression in the nose and lung relative to saline control mice (Figure 6A, B). Nasal expression that exceeds lung expression following vector delivery via tracheal intubation is a reproducible phenomenon in mice (34). The observed improved lung expression with NaCl (7%) could have implications for studies that rely on mouse lung delivery.
Figure 6.
Increased saline tonicity enhances transduction of mouse and pig airways. (A) 6–8 week old Balb/c mice received intratracheal Ad-luciferase formulated with NaCl (0.9% or 7% final concentration). Mice were imaged by IVIS 5 days after delivery (N = 5). (B) Representative images of luciferase expression in mouse nose and lungs quantified in (A). (C–L) Ad-GFP was formulated 1:1 with 1.8% (0.9% final concentration) or 10% NaCl (5% final concentration) and aerosolized intratracheally to newborn pigs. 5 days after delivery, lungs were divided into 20 regions as previously reported2. (C) Combined GFP + scores (0 = no expression, 1 = low expression, 2 = moderate expression, and 3 = high expression). (D, E) Lungs were fixed, processed, sectioned, and counterstained with DAPI. Representative 10X and 20X images of small and large airways from 5% NaCl condition shown. (F) GFP + cells quantified in small (<500 μm2) and large (>500 μm2) in pigs that received Ad-GFP formulated with 0.9% or 5% NaCl. (G–L) Representative images of cell types transduced in 5% NaCl condition. (G) Transduced ciliated cells detected by immunofluorescence using acetylated ⍺-tubulin. (H–L) Cytokeratin 5 (CK5) staining representing basal cells transduced in large airways (H) and small airways (I). (J–L) Representative images of submucosal gland transduction; empty arrow pointing to transduced ductal cells. Arrows indicate Ad-GFP expression in counterstained ⍺-tubulin or CK5 cells. Pig experiment: n = 1 animal per condition. 10 slides per lung were evaluated. *P< 0.05. ****P< 0.00005. Statistical differences were determined by Student's t-test.
To determine cell types transduced in vivo, we focused on a pig model that more closely recapitulates the anatomy and cell biology of human airways. Newborn pigs received Ad-GFP via intratracheal delivery with 0.9% NaCl or 5% NaCl. Five days after delivery, lungs were collected and GFP expression was visualized under a fluorescence dissecting microscope (Supplementary Figure S6A). Lungs were divided into 20 regions for systematic quantification as previously described(2) (Supplementary Figure S6B) and scored based on level of expression (0 = no expression, 1 = low expression, 2 = moderate expression, and 3 = high expression) (Supplementary Figure S6C). Formulation of Ad-GFP with NaCl (5%) resulted in a significantly increased the GFP + score compared to 0.9% NaCl (Figure 6C, Supplementary Figure S6C). The same assay was used to demonstrate that GP64 HIV-GFP formulated with NaCl (5%) significantly enhanced transduction over 0.9% NaCl (Supplementary Figure S6E-G).
Supplementary Figure S6D shows representative images of NaCl (5%) treated lung sections at 2× and 10×, respectively. GFP expression was widespread among airways of all sizes (Figure 6D,E). Both large (>500 μm2) and small (<500 μm2) airways demonstrated significantly more airway transduction than Ad-GFP (0.9% NaCl) (Figure 6F). Indeed, we observed transduction of ciliated cells (Figure 6G) as well as cytokeratin 5 (CK5)+ basal cells of the large (Figure 6H) and small (Figure 6I) airways. Additionally, we observed high levels of transduction in submucosal glands, including transduction of submucosal ductal cells (Figure 6J–L). In summary, formulating either Ad or lentiviral vectors in 5–7% NaCl enhanced airway and alveolar gene transfer in mice and pigs.
Discussion
Here, we show that NaCl (3.6–7%) enhanced the transduction efficiency of Ad, AAV and lentiviral vectors. This improved transduction restored functional CFTR anion transport in CF HAE across all three vector platforms. For adenovirus vectors, the NaCl formulation enhanced transduction remained receptor dependent, requiring a low pH endosomal escape. These in vitro findings of enhanced transduction also translated to two animal models where formulating Ad or lentiviral vectors with 5% or 7% NaCl increased transgene expression. Given that 3–7% NaCl is routinely aerosolized in people with CF (35), these findings have important implications for improving viral vector transduction for CF and other lung diseases. Formulating viral vectors with 3.6–7% NaCl could boost therapeutic efficacy, lower the therapeutic index, and overcome previous expression threshold limitations for pulmonary gene therapy.
Formulations including LPC (5), EGTA (6,7), perfluorocarbon (9) and methylcellulose (8) have been instrumental in in vitro and pre-clinical in vivo studies to improve gene transfer efficiency. Interestingly, the discovery that Ad-based viral vectors use a basolateral receptor and gene transfer is boosted by tight junction disruption came to light after the first CF gene therapy clinical trials (4). Since none of the above mentioned formulations are currently FDA approved for lung administration, each reagent would require a separate evaluation for co-administration with a gene therapy vector. CF gene therapy clinical trials performed to date include vehicles with a 150 mM NaCl concentration, which is equivalent to 0.9% isotonic NaCl (reviewed in (4)). Viral vectors including AAV and lentiviral platforms are under investigation for CF gene therapy applications. 4D Molecular Therapeutics began a Phase I AAV clinical trial in 2022 (clinicaltrials.gov) and Spirovant and Carbon Biosciences are generating preclinical data to pursue clinical trials with AAV and bocavirus vectors. Historically, achieving sufficient gene transfer for phenotypic correction has been challenging (4). Efforts to overcome these challenges include directed evolution for capsid (30) and envelope design (26), cassette modification including promoter (36), cDNA (37) and polyadenylation signal, codon optimization (27), and immunomodulation (reviewed in (4)). Using a formulation generally recognized as safe for use in people with CF such as NaCl (3–7%) could eliminate a hurdle for approval and advancement of a gene therapy reagent.
Beyond efficient, widespread delivery and cellular transduction, gene therapy for lung disease faces additional challenges. Innate and adaptive immune barriers may limit long-term expression from a viral vector (38,39). Pre-existing neutralizing antibodies or acquired immunity to viral capsids or envelope proteins may prevent re-administration (40). Transiently blocking immune function at the time delivery, capsid evolution, PEGylation, and serotype switching are strategies to head-off these obstacles (41,42). Gradual loss of functional correction as a consequence of turnover of corrected cells is another potential concern. We show here that NaCl formulation enhances access to basal progenitor cells. Correcting sufficient stem cells by gene addition with integrating vectors such as lentivirus, piggyBac/Ad, or piggyBac/AAV (1–3) would be expected to provide life-long correction for the patient. Alternatively, delivery of base editors, prime editors, or prime-editing-assisted site-specific integrase gene editing tools (43) to stem cells using non-integrating vehicles such as Ad, AAV or LNPs would also be expected to provide life-long correction (18,24,44).
The effects of increased saline tonicity formulation on viral vector transduction have not been previously investigated. To our knowledge, only one relevant report suggests that increased saline tonicity does not enhance SARS-CoV-2 viral entry (45). In screening several lentiviral envelope pseudotypes (Supplementary Figure S4) we observed that vectors with the GP64 and VSVG envelopes benefited from NaCl (4.5%) formulation, while the beta-retrovirus envelopes JSRV or BaEV did not. JSRV uses the GPI-linked apical receptor Hyal2 (46) while the BaEV uses SLC1A4 and SLC1A5 as receptors. Although these receptors are present in HAE, transduction was not enhanced with NaCl formulation. These results suggest that entry pathways may influence the effectiveness of NaCl formulations. Indeed, different viruses have differing entry routes, including receptor-mediated endocytosis or fusion at the plasma membrane. Ad, AAV, GP64, and VSVG use receptor mediated endocytosis for entry and were enhanced by NaCl. The BeEV and JSRV confer membrane fusion at the cell surface (47) for lentiviral entry and were not enhanced by NaCl. Based on this correlation, we postulate that NaCl may, in part, enhance transduction in settings where entry involves endosomal related mechanisms. We are actively pursuing this line of research. Further, it may be that some receptors, co-receptors, and other binding or entry factors may be affected by the presence of high NaCl while others are not. We speculate that virus like particles (VLPs) (48) pseudotyped with envelopes such as VSVG or GP64 would also be enhanced when formulated with hypertonic saline.
We originally suspected the mechanism for NaCl-mediated enhanced transduction was due to the osmotic strength of the formulation. We know that the airway surface liquid volume is regulated in part by water movement through the cell and that osmotically driven water permeability is regulated by aquaporins to maintain airway epithelial cell homeostasis (49,50). When a hypertonic solution is applied to epithelia, an electrochemical gradient generates a driving force for fluid movement across the cell membrane, causing hypertonic shock, followed by an adaptive regulatory increase in cell volume. The non-ionic osmolyte mannitol did not enhance transduction. Instead, we learned that ionic strength is a key determinant in the mechanism by which NaCl formulation enhanced vector transduction. We speculate that NaCl formulation may enhance transduction, in part, by increasing receptor access by disrupting tight junctions or by enhancing endosomal escape. We observed that NaCl, KCl, and the charged sugar N-methyl-d-glucamine enhanced transduction in a dose-dependent manner, although NaCl had the greatest impact in a given percent solution. These findings suggest that Na+ regulation of the airway extracellular environment has the greatest impact on viral transduction (51).
As mentioned in the Results, a concentration of 3.6% final concentration of NaCl was serendipitously chosen for most of the studies using Ad because it was initially diluted 1:1 from a 7.2% pharmaceutical grade stock solution. For in vitro studies in HAE, our dose response results suggest that 4.5% NaCl may be the best compromise between efficacy and ultimate toxicity at doses of NaCl > 6%. This was true for all vectors tested. Of note, attempts to improve gene transfer of cultured cells on plastic using hypertonic saline resulted in lifting of the cells. While we did not test all concentrations of NaCl in vivo, we speculate that we would see a dose-response. We used the in vitro results and common clinical dosages to choose the concentration of NaCl for our in vivo formulations. Our results demonstrate that 5–7% NaCl formulations had a positive impact on gene transfer to the lungs of mice and pigs. In ongoing and future studies we will further refine the optimal NaCl concentration for delivery to different animal models.
The goal of this study was to investigate whether FDA approved agents could improve lung gene therapy efficacy. Hypertonic saline is a routinely used therapy for people with CF and other lung conditions and could be rapidly adopted in vector formulations. We show evidence that formulating Ad, AAV or lentiviral vectors with NaCl could advance gene therapy development for CF. Importantly, this finding has broad implications for other genetic lung diseases such as alveolar type 2 deficiencies (ABCA3 Deficiency, Surfactant Protein B Deficiency) or Primary Ciliary Dyskinesia.
Supplementary Material
Acknowledgements
We thank Ian Thornell for the insightful conversations which helped us identify ionic strength as the mechanism for NaCl-mediated vector transduction. We thank Amber Vu for her work on lentiviral envelopes and Soumba Traore for guidance on CXADR knockout of HAE. We thank Linda Powers, Brie Hilken, and Nick Gansemer for orchestrating the pig experiments and delivering the viral vector. We thank Ian Thornell, Lynda Ostedgaard, Cami Hippee, Lorellin Durnell and Stephanie Clark for their critical review of this manuscript.
Author contributions: Conceptualization: A.L.C., P.B.M., P.L.S.; Methodology: A.L.C., L.M.L., K.N., C.M.B.; Investigation: A.L.C.; Visualization: A.L.C., P.B.M., P.L.S.; Funding acquisition: P.B.M., P.L.S.; Project administration: A.L.C.; Supervision: P.B.M., P.L.S.; Writing – original draft: A.L.C.; Writing – review & editing: A.L.C., P.B.M., P.L.S.
Contributor Information
Ashley L Cooney, University of Iowa, Stead Family Department of Pediatrics; Iowa City, IA 52242, USA; University of Iowa, Pappajohn Biomedical Institute; Iowa City, IA 52242, USA.
Laura Marquez Loza, University of Iowa, Stead Family Department of Pediatrics; Iowa City, IA 52242, USA; University of Iowa, Pappajohn Biomedical Institute; Iowa City, IA 52242, USA.
Kenan Najdawi, University of Iowa, Stead Family Department of Pediatrics; Iowa City, IA 52242, USA; University of Iowa, Pappajohn Biomedical Institute; Iowa City, IA 52242, USA.
Christian M Brommel, University of Iowa, Stead Family Department of Pediatrics; Iowa City, IA 52242, USA; University of Iowa, Pappajohn Biomedical Institute; Iowa City, IA 52242, USA.
Paul B McCray, Jr., University of Iowa, Stead Family Department of Pediatrics; Iowa City, IA 52242, USA University of Iowa, Pappajohn Biomedical Institute; Iowa City, IA 52242, USA; University of Iowa, Center for Gene Therapy; Iowa City, IA 52242, USA.
Patrick L Sinn, University of Iowa, Stead Family Department of Pediatrics; Iowa City, IA 52242, USA; University of Iowa, Pappajohn Biomedical Institute; Iowa City, IA 52242, USA; University of Iowa, Center for Gene Therapy; Iowa City, IA 52242, USA.
Data availability
There are no new data associated with this article.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
National Institutes of Health [R01HL133089 to P.L.S.]; National Institutes of Health [P01HL152960 to P.B.M.]; National Institutes of Health [R01HL171035 to P.L.S., P.B.M.]; National Institutes of Health [P01HL51670 to P.B.M.]; National Institutes of Health [F31 HL152500 to L.M.L.]; Cystic Fibrosis Foundation [SINN22G0 to P.L.S.]; Cystic Fibrosis Foundation [SINN23G0 to P.L.S.]; Cystic Fibrosis Foundation [STOLTZ19 to P.L.S.]; Emily's Entourage grant (to P.L.S.); University of Iowa Center for Gene Therapy, National Institutes of Health grant [P30DK54759 to P.L.S.]; Roy J. Carver Chair in Pulmonary Research (to P.B.M.); American Society of Gene and Cell Therapy Career Development Award (to A.L.C.); Boomer Esiason Foundation grant (to A.L.C.), Vertex Mentored Research Innovation Award (to A.L.C.), WellsLee Foundation (to P.B.M.). Funding for open access charge: National Institutes of Health [R01HL133089].
Conflict of interest statement. P.B.M. is on the SAB for Spirovant Science, Inc.
References
- 1. Cooney A.L., Abou Alaiwa M.H., Shah V.S., Bouzek D.C., Stroik M.R., Powers L.S., Gansemer N.D., Meyerholz D.K., Welsh M.J., Stoltz D.A.et al.. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight. 2016; 1:e88730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooney A.L., Singh B.K., Loza L.M., Thornell I.M., Hippee C.E., Powers L.S., Ostedgaard L.S., Meyerholz D.K., Wohlford-Lenane C., Stoltz D.A.et al.. Widespread airway distribution and short-term phenotypic correction of cystic fibrosis pigs following aerosol delivery of piggyBac/adenovirus. Nucleic Acids Res. 2018; 46:9591–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooney A.L., Thornell I.M., Singh B.K., Shah V.S., Stoltz D.A., McCray P.B. Jr, Zabner J., Sinn P.L. A novel AAV-mediated gene delivery system corrects CFTR function in pigs. Am. J. Respir. Cell Mol. Biol. 2019; 61:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooney A.L., McCray P.B. Jr, Sinn P.L. Cystic Fibrosis gene therapy: looking back, looking forward. Genes (Basel). 2018; 9:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cmielewski P., Anson D.S., Parsons D.W.. Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length. Respir. Res. 2010; 11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang G., Zabner J., Deering C., Launspach J., Shao J., Bodner M., Jolly D.J., Davidson B.L., McCray P.B. Jr. Increasing epithelial junction permeability enhances gene transfer to airway epithelia In vivo. Am. J. Respir. Cell Mol. Biol. 2000; 22:129–138. [DOI] [PubMed] [Google Scholar]
- 7. Wang G., Davidson B.L., Melchert P., Slepushkin V.A., van Es H.H., Bodner M., Jolly D.J., McCray P.B. Jr. Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J. Virol. 1998; 72:9818–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sinn P.L., Shah A.J., Donovan M.D., McCray P.B. Jr. Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am. J. Respir. Cell Mol. Biol. 2005; 32:404–410. [DOI] [PubMed] [Google Scholar]
- 9. Kazzaz J.A., Strayer M.S., Wu J., Malone D.J., Koo H.C., Shaffer T.H., Davis J.M., Strayer D.S., Wolfson M.R.. Perfluorochemical liquid-adenovirus suspensions enhance gene delivery to the distal lung. Pulm. Med. 2011; 2011:918036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hogman M., Mork A.C., Roomans G.M.. Hypertonic saline increases tight junction permeability in airway epithelium. Eur. Respir. J. 2002; 20:1444–1448. [DOI] [PubMed] [Google Scholar]
- 11. Elkins M.R., Bye P.T.. Mechanisms and applications of hypertonic saline. J. R. Soc. Med. 2011; 104:S2–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tarran R., Donaldson S., Boucher R.C.. Rationale for hypertonic saline therapy for cystic fibrosis lung disease. Semin. Respir. Crit. Care Med. 2007; 28:295–302. [DOI] [PubMed] [Google Scholar]
- 13. Karp P.H., Moninger T.O., Weber S.P., Nesselhauf T.S., Launspach J.L., Zabner J., Welsh M.J.. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol. Biol. 2002; 188:115–137. [DOI] [PubMed] [Google Scholar]
- 14. Anderson R.D., Haskell R.E., Xia H., Roessler B.J., Davidson B.L.. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000; 7:1034–1038. [DOI] [PubMed] [Google Scholar]
- 15. Sinn P.L., Coffin J.E., Ayithan N., Holt K.H., Maury W.. Lentiviral vectors pseudotyped with filoviral glycoproteins. Methods Mol. Biol. 2017; 1628:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooney A.L., Thurman A.L., McCray P.B., Pezzulo A.A., Sinn P.L. Lentiviral vectors transduce lung stem cells without disrupting plasticity. Mol. Ther. Nucleic Acids. 2021; 25:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y., Bergelson S., Feschenko M.. Determination of lentiviral infectious titer by a novel droplet digital PCR method. Hum. Gene. Ther. Methods. 2018; 29:96–103. [DOI] [PubMed] [Google Scholar]
- 18. Cooney A.L., Brommel C.M., Traore S., Newby G.A., Liu D.R., McCray P.B. Jr, Sinn P.L. Reciprocal mutations of lung-tropic AAV capsids lead to improved transduction properties. Front. Genome Ed. 2023; 5:1271813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ortiz-Zapater E., Bagley D.C., Hernandez V.L., Roberts L.B., Maguire T.J.A., Voss F., Mertins P., Kirchner M., Peset-Martin I., Woszczek G.et al.. Epithelial coxsackievirus adenovirus receptor promotes house dust mite-induced lung inflammation. Nat. Commun. 2022; 13:6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Excoffon K.J., Gansemer N.D., Mobily M.E., Karp P.H., Parekh K.R., Zabner J.. Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PLoS One. 2010; 5:e9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terlizzi V., Masi E., Francalanci M., Taccetti G., Innocenti D.. Hypertonic saline in people with cystic fibrosis: review of comparative studies and clinical practice. Ital. J. Pediatr. 2021; 47:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooney A.L., Singh B.K., Sinn P.L.. Hybrid nonviral/viral vector systems for improved piggyBac DNA transposon in vivo delivery. Mol. Ther. 2015; 23:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lei L., Traore S., Romano Ibarra G.S., Karp P.H., Rehman T., Meyerholz D.K., Zabner J., Stoltz D.A., Sinn P.L., Welsh M.J.et al.. CFTR-rich ionocytes mediate chloride absorption across airway epithelia. J. Clin. Invest. 2023; 133:e171268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krishnamurthy S., Traore S., Cooney A.L., Brommel C.M., Kulhankova K., Sinn P.L., Newby G.A., Liu D.R., McCray P.B.. Functional correction of CFTR mutations in human airway epithelial cells using adenine base editors. Nucleic Acids Res. 2021; 49:10558–10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sirena D., Lilienfeld B., Eisenhut M., Kalin S., Boucke K., Beerli R.R., Vogt L., Ruedl C., Bachmann M.F., Greber U.F.et al.. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 2004; 78:4454–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinn P.L., Hwang B.Y., Li N., Ortiz J.L.S., Shirazi E., Parekh K.R., Cooney A.L., Schaffer D.V., McCray P.B. Jr. Novel GP64 envelope variants for improved delivery to human airway epithelial cells. Gene Ther. 2017; 24:674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marquez Loza L.I., Cooney A.L., Dong Q., Randak C.O., Rivella S., Sinn P.L., McCray P.B. Jr. Increased CFTR expression and function from an optimized lentiviral vector for cystic fibrosis gene therapy. Mol. Ther. Methods Clin. Dev. 2021; 21:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sinn P.L., Penisten A.K., Burnight E.R., Hickey M.A., Williams G., McCoy D.M., Mallampalli R.K., McCray P.B.. Gene transfer to respiratory epithelia with lentivirus pseudotyped with Jaagsiekte sheep retrovirus envelope glycoprotein. Hum. Gene Ther. 2005; 16:479–488. [DOI] [PubMed] [Google Scholar]
- 29. Girard-Gagnepain A., Amirache F., Costa C., Levy C., Frecha C., Fusil F., Negre D., Lavillette D., Cosset F.L., Verhoeyen E.. Baboon envelope pseudotyped LVs outperform VSV-G-LVs for gene transfer into early-cytokine-stimulated and resting HSCs. Blood. 2014; 124:1221–1231. [DOI] [PubMed] [Google Scholar]
- 30. Excoffon K.J., Koerber J.T., Dickey D.D., Murtha M., Keshavjee S., Kaspar B.K., Zabner J., Schaffer D.V.. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl. Acad. Sci. U.S.A. 2009; 106:3865–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L.N., Karp P., Gerard C.J., Pastor E., Laux D., Munson K., Yan Z., Liu X., Godwin S., Thomas C.P.et al.. Dual therapeutic utility of proteasome modulating agents for pharmaco-gene therapy of the cystic fibrosis airway. Mol. Ther. 2004; 10:990–1002. [DOI] [PubMed] [Google Scholar]
- 32. Lee-Ferris R.E., Okuda K., Galiger J.R., Schworer S.A., Rogers T.D., Dang H., Gilmore R., Edwards C., Nakano S., Cawley A.M.et al.. Prolonged airway explant culture enables study of health, disease, and viral pathogenesis. 2024; bioRxiv doi:05 February 2024, preprint: not peer reviewed 10.1101/2024.02.03.578756. [DOI]
- 33. Steines B., Dickey D.D., Bergen J., Excoffon K.J., Weinstein J.R., Li X., Yan Z., Abou Alaiwa M.H., Shah V.S., Bouzek D.C.et al.. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight. 2016; 1:e88728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oakland M., Maury W., McCray P.B. Jr, Sinn P.L. Intrapulmonary versus nasal transduction of murine airways with GP64-pseudotyped viral vectors. Mol. Ther. Nucleic Acids. 2013; 2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elkins M.R., Robinson M., Rose B.R., Harbour C., Moriarty C.P., Marks G.B., Belousova E.G., Xuan W., Bye P.T.National Hypertonic Saline in Cystic Fibrosis Study, G. . A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N. Engl. J. Med. 2006; 354:229–240. [DOI] [PubMed] [Google Scholar]
- 36. Yan Z., Sun X., Feng Z., Li G., Fisher J.T., Stewart Z.A., Engelhardt J.F.. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum. Gene Ther. 2015; 26:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ostedgaard L.S., Meyerholz D.K., Vermeer D.W., Karp P.H., Schneider L., Sigmund C.D., Welsh M.J.. Cystic fibrosis transmembrane conductance regulator with a shortened R domain rescues the intestinal phenotype of CFTR-/- mice. Proc. Natl. Acad. Sci. U.S.A. 2011; 108:2921–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCarron A., Cmielewski P., Drysdale V., Parsons D., Donnelley M.. Effective viral-mediated lung gene therapy: is airway surface preparation necessary?. Gene Ther. 2023; 30:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrari S., Geddes D.M., Alton E.W.. Barriers to and new approaches for gene therapy and gene delivery in cystic fibrosis. Adv. Drug. Deliv. Rev. 2002; 54:1373–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mendell J.R., Connolly A.M., Lehman K.J., Griffin D.A., Khan S.Z., Dharia S.D., Quintana-Gallardo L., Rodino-Klapac L.R.. Testing preexisting antibodies prior to AAV gene transfer therapy: rationale, lessons and future considerations. Mol. Ther. Methods Clin. Dev. 2022; 25:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mingozzi F., High K.A.. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013; 122:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muhuri M., Maeda Y., Ma H., Ram S., Fitzgerald K.A., Tai P.W., Gao G.. Overcoming innate immune barriers that impede AAV gene therapy vectors. J. Clin. Invest. 2021; 131:e143780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pandey S., Gao X.D., Krasnow N.A., McElroy A., Tao Y.A., Duby J.E., Steinbeck B.J., McCreary J., Pierce S.E., Tolar J.et al.. Efficient site-specific integration of large genes in mammalian cells via continuously evolved recombinases and prime editing. Nat. Biomed Eng. 2024; 10.1038/s41551-024-01227-1. [DOI] [PubMed] [Google Scholar]
- 44. Muhuri M., Levy D.I., Schulz M., McCarty D., Gao G.. Durability of transgene expression after rAAV gene therapy. Mol. Ther. 2022; 30:1364–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Machado R.R.G., Glaser T., Araujo D.B., Petiz L.L., Oliveira D.B.L., Durigon G.S., Leal A.L., Pinho J.R.R., Ferreira L.C.S., Ulrich H.et al.. Inhibition of severe acute Respiratory syndrome coronavirus 2 replication by hypertonic saline solution in lung and kidney epithelial cells. ACS Pharmacol. Transl. Sci. 2021; 4:1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rai S.K., DeMartini J.C., Miller A.D.. Retrovirus vectors bearing jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J. Virol. 2000; 74:4698–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Overbaugh J., Miller A.D., Eiden M.V.. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol. Rev. 2001; 65:371–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Banskota S., Raguram A., Suh S., Du S.W., Davis J.R., Choi E.H., Wang X., Nielsen S.C., Newby G.A., Randolph P.B.et al.. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell. 2022; 185:250–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kreda S.M., Gynn M.C., Fenstermacher D.A., Boucher R.C., Gabriel S.E.. Expression and localization of epithelial aquaporins in the adult human lung. Am. J. Respir. Cell Mol. Biol. 2001; 24:224–234. [DOI] [PubMed] [Google Scholar]
- 50. Verkman A.S., Matthay M.A., Song Y.. Aquaporin water channels and lung physiology. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000; 278:L867–L879. [DOI] [PubMed] [Google Scholar]
- 51. Bartoszewski R., Matalon S., Collawn J.F.. Ion channels of the lung and their role in disease pathogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017; 313:L859–L872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no new data associated with this article.