Abstract
A central challenge in the quest for precise gene regulation within mammalian cells is the development of regulatory networks that can achieve perfect adaptation—where outputs consistently return to a set baseline post-stimulus. Here, we present such a system that leverages the CRISPR activation (CRISPRa) and anti-CRISPR proteins as two antithetic elements to establish perfect adaptation in mammalian cells and dynamically regulate gene expression. We demonstrate that this system can maintain stable expression levels of target genes in the face of external perturbations, thus providing a robust platform for biological applications. The versatility of our system is further showcased through its integration with endogenous regulatory mechanisms in T cells, such as the NF-κB-mediated immune response, and its ability to program apoptosis responses for precise spatial and temporal control of cellular growth and death. This study not only advances our understanding of gene regulation in mammalian cells but also opens new avenues for therapeutic intervention, particularly in diseases characterized by dysregulated gene expression.
Graphical Abstract
Graphical Abstract.
Introduction
From the feedback loops that govern metabolic pathways to the signal transduction networks that orchestrate cellular responses to external stimuli, nature has evolved a myriad of strategies to ensure stability or homeostasis to counteract fluctuations and perturbations (1–3). This dynamic equilibrium is not only essential for the proper functioning of biological pathways but also an inspiration for engineering novel biological functions (4,5). Engineering such a system will also reciprocally facilitate our understanding of the natural regulatory mechanisms that cells employ to maintain homeostasis (6,7). In addition, adaptive synthetic biological systems hold significant promise for disease therapies, potentially restoring homeostasis when genes are overexpressed or underexpressed abnormally, and also offering new avenues for treating a myriad of diseases, including cancers, inflammations and neurodegenerative disorders (8–12).
By harnessing engineering principles, synthetic biology seeks to create artificial constructs and genetic circuits that can replicate, improve and, ultimately, control biological processes (13–16). The design of synthetic circuits that can achieve dynamic equilibrium or perfect adaptation—a condition where a system’s output returns to a baseline level following a transient response to a stimulus—stands as an important feature in this field. Presently, two adaptive synthetic circuits are widely utilized: incoherent feedforward loops (iFFLs) and negative feedback loops (NFLs). The iFFL comprises three components: part A, which activates in response to an input and subsequently activates downstream parts B and C, with part C inhibiting part B to establish the iFFL (17,18). This loop has been employed in various contexts, such as controlling gene copy number variation (7,19,20) and resource control using CasE (18). However, the iFFL demands precise repression dynamics to achieve robust control (19,20). Furthermore, regulating endogenous genes using iFFL and CasE is challenging due to the need for specific hairpin insertion in the target RNA sequences (18). In contrast, the NFL’s critical feature is its repressive feedback interactions and it has been explored for gene expression control in mammalian cells (21–23). Although simple NFLs are straightforward to implement, they struggle to return to normal expression levels, which is crucial for maintaining homeostasis (16,24).
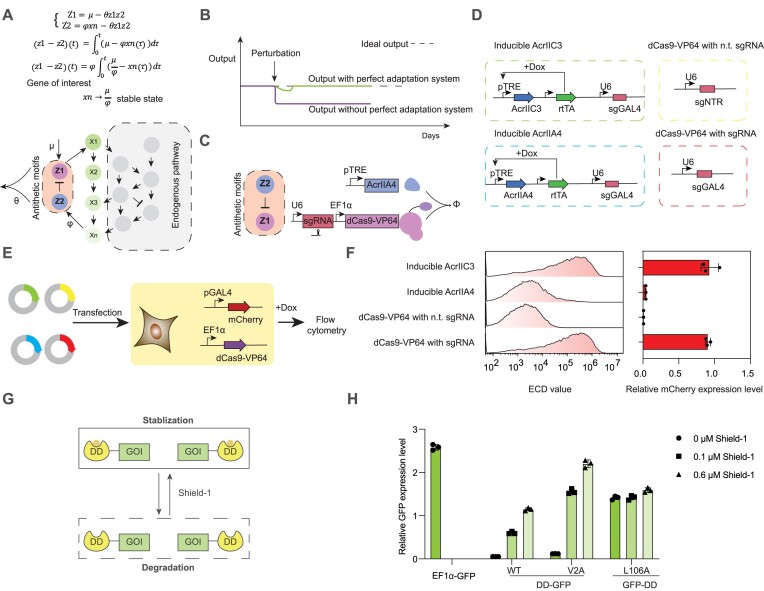
Recently, a more general type of feedback loops, capable of realizing integral feedback and implementing perfect adaptation, was proposed (25). At the core of these systems are two antithetic elements, Z1 and Z2. Z2 is activated by controlled genes, denoted as Xn (rate constant φ), and z2 is its concentration, while Z1, which is constitutively expressed (rate constant μ), and z1 denotes its concentration, activates an intermediary network that ultimately upregulates the controlled genes. By calculating the dynamics of z1 − z2, we can see that as long as the closed-loop circuit is stable, the concentration of the controlled gene of interest Xn will converge to μ/φ, which will be stable and not affected by any perturbations in endogenous pathway or others, and finally realize perfect adaptation (Figure 1A and B). This model of perfect adaptation was first demonstrated in Escherichia coli using the transcriptional regulators sigW and rsiW (26). However, their utility in mammalian cells is constrained. Efforts to attain perfect adaptation in mammalian cells using proteins fused with split inteins encountered obstacles due to the availability of high-performance inteins, potential impacts on protein functionality post-fusion and a lack of programmability (27). An alternative strategy using sense/antisense messenger RNA (mRNA) encountered challenges such as a limited dynamic range, global translational repression and the necessity for stable mRNA (28,29). In addition, this implementation may trigger the stress response in the cell due to the formation of double-stranded RNA (30). In light of these challenges, we propose the CRISPR system as an ideal framework for engineering perfect adaptation mechanisms in mammalian cells. The advent of anti-CRISPRs (Acrs) that selectively inhibit CRISPR proteins, combined with their successful application in mammalian contexts, positions CRISPR and Acrs as the ideal antithetic proteins (31–33). The versatility of single-guide RNA (sgRNA) design further enhances this system, allowing for precise control over endogenous genes and facilitating disease treatment applications.
Figure 1.
Design of antithetic proteins for a perfect adaptation system in mammalian cells. (A) Schematic representation of the perfect adaptation system comprising antithetic controller proteins (Z1, Z2) that regulate the expression level of outputs (Xn) to a desired level (μ/φ) amid uncertainties or perturbations in the endogenous environment as the closed-loop circuit is in steady state. (B) Comparison of output stability in systems without (lower line) and with (upper line) the perfect adaptation system during perturbations. (C) Diagram illustrating CRISPR and anti-CRISPR proteins as the antithetic elements. (D) Validation setup for AcrIIA4 function, detailing plasmid constructs containing sgRNA targeting GAL4 UAS response elements (REs), inducible AcrIIA4 by doxycycline (Dox) and inducible AcrIIC3 by Dox, alongside positive and negative controls [nontargeting (n.t.) sgRNA]. (E) Flow diagram for transient transfection testing AcrIIA4 activity in HEK293T cells containing dCas9-VP64, mCherry under GAL4 UAS promoter as a reporter, using plasmids in panel (D). (F) Flow cytometry measurements of AcrIIA4 and AcrIIC3 activity, normalized with a constitutive promoter (EF1α) expressing GFP. (G) Mechanism of the destabilizing domain (DD) where fusion to a protein leads to its degradation, which can be reversed by the addition of the ligand Shield-1. (H) Analysis of DD domain mutants and terminal fusion effects on protein stability, with fluorescence measurements of DD–GFP and GFP–DD fusions in HEK293T cells treated with varying Shield-1 concentrations, normalized with a constitutive promoter (EF1α) expressing BFP.
Here, we present the design and validation of a synthetic genetic circuit that harnesses the CRISPR activation (CRISPRa) system and anti-CRISPR proteins to establish perfect adaptation in mammalian cells. We have constructed both closed-loop and open-loop configurations to exert control over gene expression. Furthermore, our system can integrate seamlessly with endogenous regulatory mechanisms by designing sgRNAs that target endogenous transcription factors, such as those involved in the NF-κB-mediated immune response in T cells, thus providing intrinsic feedback without external intervention. We have achieved precise and stable modulation of immune cell activity in response to environmental stimuli. Additionally, we programmed apoptosis responses to ensure cellular adaptation, enabling controlled patterns of cell proliferation and death. The capacity of this system to maintain stable gene expression levels amid external disturbances demonstrates the robustness for biological applications, offering a novel strategy for the treatment of diseases characterized by dysregulated gene expression.
Materials and methods
Chemicals and reagents
All the chemicals and reagents used in this study are listed in Supplementary Table S1.
Plasmids and strains
Plasmids were constructed by Gibson assembly using the ClonExpress Ultra One Step Cloning Kit (Vazyme, C115-01). Polymerase chain reactions (PCRs) were performed using 2× Phanta Max Master Mix (Vazyme, P525-01). Endotoxin-free plasmids used for cell experiments were produced by the DH5alpha E. coli strain (AlpalifeBio, KTSM101L) using the HiPure Plasmid Midi Kit (Magen, P1113) according to the manufacturer’s instructions. Key genetic parts used in this study are listed in Supplementary Tables S2–S4. All plasmids used in this study are listed in Supplementary Table S5.
Cell culture
Human embryonic kidney cells (HEK293T) were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, C11995500CP) with 10% fetal bovine serum (FBS; Biological Industries, 04-001-1ACS). Jurkat cells and Daudi cells were cultured in RPMI 1640 medium (Gibco, C11995500CP). Cells were maintained and passaged with standard cell culture techniques and were cultured at 37°C, 5% CO2. Cells used for experiments were passaged before reaching 100% confluence and used within 10 passages. Transient transfections were performed when cell confluence was at 60–70% using Lipo2000 according to the manufacturer’s instructions.
Construction of stable cell lines
HEK293T cells were transfected with PiggyBac constructs in a 24-well plate and then the cells were transferred to a 6-well plate before starting puromycin selection. Puromycin (Solarbio, PB230) was added to a final concentration of 2.5 μg/ml, and the cells were selected for around 1 week. A single cell was then selected by related fluorescence onto a 96-well plate and cultured for 2 weeks for experiments. All single cell lines could be detected by comparison of adding Shield-1 or not, adding Dox or not.
Lentivirus transduction
For the construction of NF-κB RE mCherry reporter Jurkat cells, HEK293FT cells were seeded at 1 × 106 cells per 6-cm dish. A total of 1.875 μg psPAX2 (Addgene, #12260), 0.625 μg pMD2.G (Addgene, #12259) and 2.5 μg transfer plasmid with the desired genes were transfected when the cell confluence reached 60–70%. The medium was changed after 8 h to fresh medium and cultured for 48 h. The supernatant was collected and centrifuged to remove dead cells. For validation of the reporter cells, lentivirus of CAR was transduced in the same manner.
Electroporation of Jurkat cells
1.5 × 106 cells were centrifuged and the supernatant was removed, and then 25 μl of opti-MEM was added together with 3 μg transfer plasmid. The cells were resuspended and added to the electroporation tubes of 20 μl size, and then the voltage was set to be 440 V. After electroporation, cells were added to 1 ml medium in a 24-well plate and cultured for later experiments.
CAR activation of NF-κB RE mCherry reporter Jurkat cells
Two milliliters of supernatant containing CD19-CAR with BFP fluorescence protein was collected as described earlier and added to 1 × 105 NF-κB RE mCherry reporter cells. Twenty-four hours later, the medium was removed and replaced with fresh medium for another 24 h, and then separated into three groups, treated with Daudi cells, naïve Jurkat cells at a 1:1 ratio and a not co-cultured condition as a negative control. Forty-eight hours later, cells were collected and flow cytometry analysis was performed.
Flow cytometry
Cells were washed with phosphate-buffered saline (PBS) and then digested with 0.25% trypsin–EDTA (Gibco, 25200072). Digestion was stopped by adding DMEM with 10% FBS and centrifuged at 500 × g for 5 min. The supernatants were removed and cells were resuspended with 60 μl PBS and analyzed by a Beckman CytoFLEX-S flow cytometer. The flow cytometry data were analyzed by FlowJo. Cells were gated for viability, single cells and positive fluorescence proteins as shown in Supplementary Figure S10.
RNA extraction and RT-qPCR analysis
HEK293T cells were transfected and after 48 h, cells were collected for RNA extraction. Total RNAs were extracted from HEK 293T cells using the HiPure Total RNA Mini Kit (Magen, R4111) according to manufacturer's instructions. The complementary DNAs (cDNAs) were synthesized by reverse-transcription using the Hifair III 1st Strand cDNA Synthesis Kit (gDNA digester plus) (Yeasen, 11139ES60) from 1 μg of RNA and reactions were performed based on the supplier. For RT-qPCR, ChamQ Universal SYBR qPCR Master Mix (Vazyme, Q711) was added to cDNA and primers designed at the gene of interest and GAPDH according to the manufacturer's instructions. GAPDH was used as a control. The primers sequences were as below:
p50-Forward: 5'-TGATTTACTAGCACAAGGAGACAT-3';
p50-Reverse: 5'-TTGCTTCGGTGTAGCCCATT-3';
RELB-Forward: 5'-CATCCTGGACCACTTCCTGCC-3';
RELB-Reverse: 5'-GAACATGTTGCTGCCCACAAG-3';
GAPDH-Forward: 5'-GGAGCGAGATCCCTCCAAAAT-3';
GAPDH-Reverse: 5'-GGCTGTTGTCATACTTCTCATGG-3'.
The conditions of qPCR reactions were as below:
30 s at 95 °C, PCR amplification (10 s at 95 °C, 30 s at 60 °C, 40 total cycles) preceded melt-curve analysis of the product by the CFX Connect Real-Time System (Bio-rad). Ct values were used to calculate changes in expression level relative to GAPDH and control samples using the 2−ΔCt method.
Cell apoptosis assessment
Morphological changes such as cell shrinkage and rounding can be detected by a microscope (Nikon ECLIPSE Ts2R). Nuclear changes, such as DEVD-like caspase activity, were analyzed by staining with propidium iodide (PI). It is a fluorescent intercalating agent that is used to stain cells and nucleic acids and can be used to differentiate apoptotic and healthy cells. Cells were seeded at around 7 × 104 cells in one well of a 48-well plate 24 h before transient transfection. Cells were transfected with 0.3 μg of constitutive BAX (Bcl-2-associated X protein), DD-BAX, BAX(1–173) and DD-BAX(1–173) and gated with BFP fluorescence protein. Cells were analyzed 48 h post-transfection. Also, stable cells were seeded at around 7 × 104 cells in each well in a 48-well plate and treated with a different amount of Shield-1; after 48 h, cells were then stained with PI for 0.5 h in the dark at 37°C. After 0.5 h, the cells could be imaged with a fluorescence microscope. The other steps are the same as described earlier.
Spatial co-culture
The closed-loop cells and the open-loop cells with BAX were designed as shown in the construction of stable cell lines. A plastic cylinder with an inner diameter of around 7.5 mm was dipped with mineral oil (Beyotime, ST1524) for sealing and placed into the wells of a 12-well plate. 5 × 104 open-loop cells marked with BFP fluorescence protein were seeded inside the cylinder along with around 100 μl medium. 3 × 105 closed-loop cells marked with GFP fluorescence protein were seeded outside the cylinder with 800 μl medium. After being cultured for ∼24 h, the cells adhered to the bottom, and the cylinder was removed from the plate lightly, and Dox was added to the medium with a final concentration at 0.1 μM. The plate was shaken carefully to mix the Dox and avoid resuspending the adhered cells at the same time. After 24 h of culturing, the cells were imaged by a Zeiss LSM980 confocal microscope. The field of view contained 4 × 4 tiles joined by the Zeiss ZEN 3.3 (blue edition) software. After imaging the cells, they were treated with 0.6 μM Shield-1 and 0.1 μM Dox, and cultured for another 24 h. The medium was changed carefully and imaged with a Zeiss LSM980 confocal microscope as described earlier. The plastic cylinder was made with the top of a 1-ml pipette tip (QSP, 112NXLG-Q) and sterilized by autoclave; the mineral oil was sterilized by filtering with a 0.22-μm Nylon filter.
Microscopy data and analysis
Cells were treated as described earlier and imaged by the Zeiss LSM980 confocal microscope and a Nikon microscope (ECLIPSE Ts2R). All fluorescence images were taken directly from living cells without antibody staining. Selected fields of view were imaged for bright-field, PI-positive, GFP and BFP fluorescence, and merged with ImageJ.
Results
Implementation of antithetic elements and perturbations in mammalian cells
To engineer antithetic regulatory elements within mammalian cells, we selected dCas9-VP64 due to its well-characterized regulatory capabilities and selected AcrIIA4 as the anti-CRISPR protein, given its strong inhibitory performance (33) (Figure 1C). We first validated the regulatory functions of AcrIIA4 and dCas9-VP64. Our design included four plasmids: Dox-inducible AcrIIA4, Dox-inducible AcrIIC3 (a nonfunctional Acr variant), a single sgRNA targeting GAL4 REs for positive control and an sgRNA targeting a nontargeted region for negative control (Figure 1D). These plasmids were co-transfected with vectors encoding dCas9-VP64 and mCherry, the latter being a reporter gene under the control of the GAL4 UAS promoter. The transfected HEK293T cells were then treated with Dox to modulate Acr expression, and the reporter gene’s activity was assessed via flow cytometry (Figure 1E). The results demonstrated that AcrIIA4 robustly inhibited the upregulation of gene expression mediated by dCas9-VP64, resulting in an ∼23-fold decrease (Figure 1F). Notably, even in the absence of Dox-induced activation, the basal expression from the pTRE promoter was sufficient to impede dCas9-VP64 activity (Supplementary Figure S1A and B). Further experiments assessing different promoter strengths, including pTRE-GAPDH and pTRE-miniCMV, and varying titers of AcrIIA4 plasmids revealed that minimal quantities of AcrIIA4 could significantly attenuate the upregulation of gene expression by CRISPRa (Supplementary Figure S1C and D). These results demonstrated that AcrIIA4 can serve as a potent and adjustable antithetic element for CRISPRa.
To evaluate the dynamic response of our controlled genes to external stimuli, we engineered a DD fusion strategy to introduce perturbations in our system. The DD—a small protein domain—targets the fused protein for degradation upon expression in mammalian cells (Figure 1G). The presence of a high-affinity ligand, Shield-1, counteracts this effect, stabilizing the protein and rescuing it from degradation (Figure 1G) (34,35). By modulating Shield-1 concentrations, we were able to introduce controlled perturbations to the expression levels of our target genes. Our investigations into the optimal fusion terminal for the DD domain to GFP revealed that its fusion to the N-terminal of the target protein facilitated degradation and subsequent stabilization in proportion to Shield-1 levels (Figure 1H). We also evaluated DD mutants, V2A and L106A, among which the wild-type (WT) DD exhibited superior performance, demonstrating low basal activation and a relatively high activation expression level (Figure 1H). Consequently, we opted to fuse the DD domain to the N-terminal of GFP and selected the WT DD for subsequent experiments (36).
Validation of each component of the perfect adaptation system in stable cell lines
Upon confirming the functionality of the antithetic proteins within our perfect adaptation system and establishing the induction mechanism for perturbations, we proceeded to design the complete genetic circuit. In our designed system, the GAL4-UAS-responsive promoter pGAL4 governs the expression of target genes, including the fluorescent protein GFP and the transcriptional activator rtTA. The sgRNAs were designed to target the pGAL4 promoter, enabling CRISPRa-mediated activation of the downstream genes. The rtTA, in turn, modulates the pTRE promoter, which can be precisely adjusted using the small molecule Dox to modulate the feedback strength and activity range, thereby inducing the function of AcrIIA4. Consequently, when the expression levels of the target genes rise, rtTA amplifies the production of AcrIIA4, which in turn inhibits the function of dCas9-VP64, thereby restoring the expression levels of the target genes to their baseline state (Figure 2A). Additionally, we incorporated an open-loop control system lacking the pTRE-AcrIIA4 element to disrupt the feedback loop, serving as a negative control (Figure 2B).
Figure 2.
Dissecting the perfect adaptation system components in stable cell lines. (A) Closed-loop circuit: This system comprises two antithetic proteins induced by Dox and a controlled gene of interest, which includes rtTA and GFP with an N-terminal DD domain. Perturbations are introduced by reducing Shield-1, leading to the degradation of rtTA and GFP. (B) Open-loop circuit: Serving as a negative control, this system lacks the antithetic protein AcrIIA4, rendering it incapable of maintaining stability after Shield-1 reduction. (C) Flow diagram detailing the construction of stable cell lines for the experiment. (D) GFP expression changes in a stable cell line when inducible AcrIIA4 inhibits dCas9-VP64, normalized with a constitutive promoter (EF1α) expressing mCherry, across increasing Dox concentrations for three experimental replicates. (E) GFP expression shifts upon fusing the DD domain to both rtTA and GFP proteins, normalized with a constitutive promoter (EF1α) expressing mCherry, with cells treated with or without Shield-1 and increasing Dox amounts for three experimental replicates. (P-values were calculated using unpaired t-test with Welch’s correction, ****P< 0.0001.)
Prior to system integration, we verified the functionality of each component in stable cell lines. The system elements were introduced into HEK293T cells using PiggyBac transposition (Figure 2C). Initially, we assessed whether rtTA could regulate AcrIIA4 protein expression in a Dox-dependent manner. We observed that constitutive rtTA expression could be modulated by Dox, with 0.1 μM resulting in an ∼7-fold decrease and 0.3 μM yielding an 8-fold decrease in GFP expression (Figure 2D). Additionally, we tested different Dox concentrations using mCherry as the reporter and determined that 0.1 and 0.3 μM were sufficient for our experiments (Supplementary Figure S2A). Furthermore, the DD domain was fused to either the N or C terminal of the rtTA protein. We found that only the N-terminal fusion was responsive to Shield-1 induction (Supplementary Figure S2B). We next evaluated the impact of the DD domain on rtTA and GFP expression levels within the system. Fusing the DD domain solely to rtTA resulted in the absence of rtTA expression without Shield-1, thereby preventing AcrIIA4 activation and leading to elevated GFP expression levels. Conversely, the introduction of Shield-1 restored rtTA functionality, which in turn regulated AcrIIA4 and reduced GFP expression with increased Dox concentrations (Supplementary Figure S3A). When the DD domain was fused exclusively to GFP, the absence of Shield-1 led to minimal GFP expression, which increased and became Dox-responsive upon Shield-1 addition (Supplementary Figure S3B). Finally, fusing the DD domain to both rtTA and GFP proteins demonstrated that the DD domain did not adversely affect their expression, and the system was responsive to both Shield-1 and Dox (Figure 2E). These results collectively affirm that all components of the perfect adaptation system are functional in the stable cell lines.
Evaluating the stability of the perfect adaptation system under perturbations
We next proceeded to assess the robustness of the entire perfect adaptation system in the face of perturbations. We engineered both closed-loop and open-loop PiggyBac constructs for integration into HEK293T cell lines, resulting in two distinct cell populations (Figure 3A). Analysis of the closed-loop circuit revealed that the expression level of GFP could be finely tuned by varying the concentrations of Dox and Shield-1 (Figure 3B). Dox modulated the expression of AcrIIA4, while Shield-1 interacted with the DD fused to the N-terminal of rtTA and GFP, preventing protein degradation. The closed-loop cells also exhibited stable GFP expression levels over extended periods, when subjected to different Dox concentrations (Figure 3C). We quantified the GFP expression in both closed-loop and open-loop configurations under varying degrees of perturbation by altering Shield-1 concentrations from 0.6 to 0.1 μM, or to 0.05 μM. In multiple Dox scenarios, the controlled gene within the closed loop maintained near-constant expression levels post-perturbation, in contrast to the open loop, which exhibited a significant decrease in fluorescence, approaching an 80% reduction (Figure 3D).
Figure 3.
Implementation of the synthetic and hybrid perfect adaptation systems. (A) Diagrams of closed-loop and open-loop circuit constructs, with the closed loop containing antithetic proteins dCas9 and AcrIIA4 (Dox-tunable), and controlled genes GFP and rtTA fused with the DD domain. The open loop lacks the AcrIIA4 protein. (B) Heatmap showing GFP fluorescence response in the closed-loop circuit to different Shield-1 and Dox concentrations across multiple replicates. (C) Dynamic GFP expression response in the closed-loop circuit to varying Dox concentrations, measured by flow cytometry, normalized with a constitutive promoter (EF1α) expressing mCherry. (D) Output states of closed- and open-loop circuits 24 h post-perturbation, normalized to the pre-perturbation level for each set of induction condition (The “No perturbation” condition indicates that the cells were pre-treated with 0.6 μM Shield-1 and either 0.1 μM Dox or 0.3 μM Dox prior to applying the perturbations. This means that the “No perturbation” condition was identical for both perturbation scenarios, using the same plate of cells.). (E) Flow diagram for dynamic perturbation and response detection in both circuit types. (F) Dynamic GFP expression response in closed- and open-loop circuits treated with Dox and subjected to decreasing Shield-1 concentrations, normalized to the pre-perturbation level. Representative images and GFP fluorescence distributions of closed-loop (exposure time 100 ms) (G) and open-loop (exposure time 500 ms) (H) HEK293T cells on day 6.
To delineate the dynamic response curve, we cultured the two stable cell lines from day 0, introducing 0.6 μM Shield-1 and 0.1 μM Dox concurrently. GFP fluorescence was monitored via flow cytometry at 24-h intervals. On day 3, we induced a perturbation by reducing the Shield-1 concentration to 0.1 μM and continued monitoring (Figure 3E). Our findings indicated that the expression levels of the controlled gene (GFP) rapidly reverted to baseline in the closed loop and remained stable over prolonged durations, in stark contrast to the significant decline observed in the open loop (Figure 3F). Direct microscopic observation of GFP intensity corroborated these results, and the closed loop sustained consistent GFP intensity post-perturbation, whereas the open loop exhibited a marked decrease. In addition, the fluorescence distributions revealed a significant shift in the open-loop circuit after perturbation compared to the closed one (Figure 3G and H). These results underscore the closed loop’s capacity to compensate for rtTA loss and maintain system equilibrium despite ongoing perturbations.
In addition, for the expression of target genes, we explored two different configurations: one involving the fusion of GFP to rtTA via a self-cleaving 2A peptide (Supplementary Figure S4A) and another employing separate promoters for GFP and rtTA expression (Figure 2A and B). Both implementations showed perfect adaptation and maintained gene expression stability under perturbation (Supplementary Figure S4B–D). The former design showed lower expression levels of the target gene due to the usage of 2A peptide (Supplementary Figure S5). These varied constructs again demonstrated the robustness of our system and provided alternative methods for regulating gene expression levels for diverse targets.
The hybrid perfect adaptation system integrating endogenous immune regulatory elements in T cells to keep NF-κB stable in T-cell stimulation
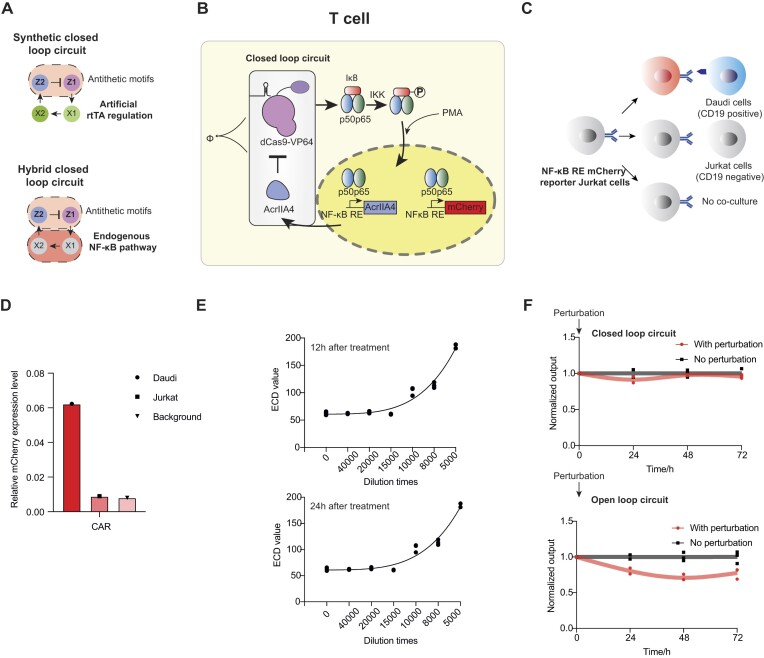
Utilizing dCas9-VP64 within our perfect adaptation system offers the distinct advantage of regulating a variety of endogenous genes through simple sgRNA modification. In addition, we can replace the rtTA feedback branch with an endogenous transcription regulator, thereby crafting a hybrid perfect adaptation system (Figure 4A). Here, we explored the potential of integration of this system with the endogenous NF-κB pathway in T cells, aiming to establish a hybrid system perfectly adapted for immune regulation. In the canonical NF-κB pathway, activation is triggered by the degradation of IκB following stimulation, such as exposure to NF-κB activators like PMA/ionomycin (37). This degradation process is mediated by IκB kinase, which phosphorylates IκB, leading to its ubiquitination and subsequent degradation (38). The liberated NF-κB then migrates to the nucleus, binds to the NF-κB RE and activates downstream genes (Figure 4B). In T-cell stimulation, keeping NF-κB expression level stable is significant to avoid T-cell exhaustion and avoid cytokine storm.
Figure 4.
Hybrid perfect adaptation system in T cells. (A) The diagram compares the hybrid perfect adaption system and synthetic closed-loop circuit. (B) Integration of the endogenous NF-κB canonical pathway with the perfect adaptation system. (C) Co-culture methods in CAR activation validation. (D) Validation of the reporter cell line functionality with CAR activation, normalized with a constitutive promoter (EF1α) expressing BFP. (E) Validation of the reporter cell line functionality with variable PMA/ionomycin concentrations over different time points. (F) Dynamic response of the hybrid closed- and open-loop circuits post-electroporation and induction with diluted PMA/ionomycin, followed by perturbation and subsequent expression level monitoring at set intervals, normalized to the pre-perturbation level.
To achieve stable NF-κB expression and perfect adaptation, we tailored our system with sgRNAs targeting a subunit of NF-κB and re-engineered the promoter of AcrIIA4 to an NF-κB RE. Upon PMA/ionomycin addition, NF-κB binds to the NF-κB RE, triggering AcrIIA4 activation and establishing the perfect adaptation system. To monitor NF-κB expression levels, we introduced an NF-κB RE mCherry reporter into the Jurkat T cells (Figure 4B). Initial transient transfection of the reporter gene yielded suboptimal results (Supplementary Figure S6A), prompting us to develop a stable Jurkat T-cell line harboring the reporter gene (Supplementary Figure S6B). Validation of this reporter cell line through CAR activation assays in co-culture experiments or PMA/ionomycin stimulation at various concentrations and time points confirmed its effectiveness in reflecting NF-κB expression levels (Figure 4C–E).
Subsequently, we designed sgRNAs targeting different NF-κB subunits (39) (Supplementary Figure S7A and B), assessed their activity and selected the most effective sgRNA targeting the p50 subunit (Supplementary Figure S7C). Electroporation of closed-loop, open-loop and negative control plasmids into the reporter Jurkat T-cell line revealed that open-loop cells exhibited higher mCherry expression levels compared to negative controls, indicating enhanced NF-κB activation via CRISPRa. Conversely, closed-loop cells displayed lower expression levels than open-loop cells, signifying the functional role of AcrIIA4 in the system (Supplementary Figure S7D). To test the system’s resilience to perturbations, we cultured cells with closed and open loops in a 10 000-fold dilution of PMA/ionomycin for 12 h (time zero), followed by a 20 000-fold dilution to decrease input signals and introduce perturbation, and monitored expression levels at 24-h intervals. The cells harboring the closed loop demonstrated more stable expression levels compared to those with the open loop (Figure 4F), validating the applicability of our hybrid perfect adaptation system for the regulation of endogenous immune regulation pathways in T cells.
Chemical regulation of apoptosis via Shield-1
We next extended our ability to control more intricate cellular processes such as apoptosis. BAX, a member of the BCL2 protein family, plays a pivotal role in the apoptosis pathway. BAX facilitates mitochondrial membrane permeabilization by interacting with BH3-only proteins, leading to the release of apoptogenic molecules such as cytochrome c. This cascade activates caspase-3, culminating in cell apoptosis (Figure 5A). Abnormal overexpression of BAX in normal cells will lead to cell apoptosis (40–42) and results in many diseases such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease (11). Given that aberrant overexpression of BAX can trigger apoptosis and is implicated in various neurodegenerative diseases, maintaining its cellular levels is of paramount importance.
Figure 5.
Shield-1 modulation of BAX expression and cell apoptosis. (A) The mechanism by which BAX induces apoptosis in mammalian cells. (B) Schematic of Shield-1-induced DD-BAX and chemical-controlled cell death and survival. (C) Constructs used to assay the BAX and DD-BAX effects on apoptosis in response to Shield-1. (D) Flow diagram for the BAX-related apoptosis experiment in response to Shield-1. (E) Comparison of BAX- and DD-BAX-induced cell death, with or without Shield-1, alongside a backbone plasmid serving as a negative control. (F) Representative images depicting BAX-related apoptosis. Representative dead cells are shown with black arrows. The scale bar is 100 μm. (P-values were calculated using unpaired t-test with Welch’s correction, *P< 0.05.)
We first fused DD to the N-terminal of BAX, enabling us to modulate BAX levels using Shield-1. This approach simulates the effects of various stressors on apoptosis through chemical regulation (Figure 5B). We evaluated three plasmid constructs: constitutive BAX expression, DD-BAX with and without Shield-1 induction, and a negative control backbone (Figure 5C). Post-transfection, cells were stained with PI to assess apoptosis (Figure 5D). Our results showed that N-terminal fusion of DD to BAX, upon Shield-1 induction, led to cell death comparable to that of constitutively expressed BAX (Figure 5E and Supplementary Figure S8A). Microscopic examination post-transfection revealed pronounced cell rounding, a hallmark of cell death, in both constitutively expressed BAX cells and inducible BAX cells with Shield-1. A construct containing constitutive BFP fluorescence protein was co-transfected to cells to indicate transfection efficiency (Figure 5F). Additionally, we explored an alternative BAX construct with amino acid deletion at positions 174–192, referred to as BAX(1–173), that was reported to induce apoptosis as well (41). However, the apoptotic effect of the deletion BAX was markedly reduced, with almost no apoptotic cells observed in DD-deletion BAX even with Shield-1 present (Supplementary Figure S8B and C). Consequently, we proceeded with the full-length BAX in further experiments. These results collectively suggest that Shield-1 can function as an artificial regulator for the apoptosis-related factor BAX, offering a novel approach to control its expression and cellular apoptosis in response to environmental stimuli.
Implementation of the perfect adaptation system for spatial and temporal control of apoptosis
We next adapted the closed-loop and open-loop circuits to modulate the apoptosis-related pathway, substituting GFP with the pro-apoptotic protein BAX (Figure 6A and B). We established stable cell lines featuring both loops, distinguishing the closed loop with GFP fluorescence and the open loop with BFP fluorescence (Figure 6C). These cell lines were subjected to treatments with varying concentrations of Shield-1 (0, 0.1 and 0.6 μM) and monitored for apoptosis using PI staining at 10 μM (Figure 6D and E). In the absence of Dox to initiate the antithetic components, apoptosis was observed in both loops upon treatment with 0.6 μM Shield-1, confirming the functional expression of BAX. A dose-dependent increase in apoptotic cells was noted with escalating Shield-1 levels, indicating that Shield-1 can modulate apoptosis intensity within both loops, serving as an effective perturbation agent (Figure 6D and E).
Figure 6.
Regulation of spatial and temporal cell apoptosis by the perfect adaptation system. (A) Diagram of the closed-loop feedback control system for cell apoptosis, with antithetic proteins regulating the apoptosis-related gene BAX. (B) Diagram of the open-loop circuit disabled by the deletion of the AcrIIA4 protein. (C) Construction of stable cell lines for both circuits, each marked with different fluorescent proteins. (D) Representative images of open-loop HEK293T cells treated with varying Shield-1 concentrations and stained with PI at 10 μM. The scale bar is 100 μm. (E) Representative images of closed-loop HEK293T cells treated with different Shield-1 levels and stained with PI at 10 μM. The scale bar is 100 μm. (F) Schematic and images of spatially cultured closed-loop and open-loop cells. The scale bar is 2000 μm. (G) Representative images of spatially cultured closed-loop and open-loop cells with different amounts of Shield-1. The scale bar is 2000 μm.
Considering the significant role of apoptosis in the development of patterns in nature, we sought to create distinct apoptotic patterns by co-culturing both cell lines in a single well, positioning the open-loop cells in a central circle surrounded by the closed-loop cells (Figure 6F). Initial treatment with 0 μM Shield-1 and 0.1 μM Dox for 24 h resulted in no apoptosis. Subsequent exposure to 0.6 μM Shield-1 and 0.1 μM Dox for an additional 24 h induced apoptosis predominantly in the open-loop cells, while the closed-loop cells remained unaffected (Figure 6F). This differential response was visually confirmed by the presence of GFP fluorescence in the closed loop and the absence of BFP fluorescence in the open loop post-treatment, suggesting that the perfect adaptation system can effectively normalize BAX overexpression in healthy cells without impacting neighboring cells. Moreover, by fine-tuning Shield-1 levels, we could precisely control apoptosis in the open-loop cells to achieve various degrees of pattern formation (Figure 6G). Additionally, we monitored apoptosis levels before and after perturbations with varying Dox concentrations. Remarkably, even at 0.05 μM Dox, the closed-loop cell line remained viable (Supplementary Figure S9), showcasing the system’s extensive regulatory capability for patterned apoptosis. These results collectively demonstrate our ability to achieve spatial and temporal control over cell growth and death by integrating perfect adaptation with apoptosis mechanisms.
Discussion
In this study, we have engineered a robust perfect adaptation system within mammalian cells, showcasing its versatility across various applications. The system’s efficiency is attributed to the synergistic application of CRISPRa and anti-CRISPR proteins, which together form a robust antithetic regulatory mechanism. Beyond the modulation of individual genes, we also demonstrated that our system can adeptly integrate with and govern the endogenous network, proving valuable for homeostatic maintenance in both immune and apoptotic responses.
Excessive apoptosis is often indicative of pathological cellular processes. In scenarios such as ischemic stroke or organ transplantation, the inhibition of the apoptotic pathway is crucial for the survival of healthy cells. Various animal models have demonstrated that mitigating Bcl-2-associated apoptosis can effectively address these challenges (12). For instance, the overexpression of Bcl-XL, a Bcl-2 family protein, during hematopoietic stem cell transplantation has been shown to preserve a significant number of donor cells (43). Similarly, the deletion of BAX and BAK proteins has yielded promising results in preventing motor neuron degeneration, offering potential therapeutic avenues for amyotrophic lateral sclerosis and related motor neuron diseases (44). The capability of our system to normalize and stabilize BAX expression highlights its potential applications in preventing aberrant apoptosis linked to various diseases.
The prevalence of transcriptional factor dysregulation in disease pathogenesis underscores the importance of our system. Approximately 19% of the estimated 1600 transcriptional factors encoded in the human genome are implicated in disease phenotypes (45), from cancer to metabolic and cardiovascular disorders. The dysregulation of these factors, such as MYC, MYB, p53 and FOXO1, drives tumorigenesis and is associated with aberrant cellular proliferation (46–51). Similarly, the STAT family’s overexpression is linked to inflammatory and autoimmune conditions (52–54), while alterations in HNF1α, HNF4α or NEUROD1 are related to diabetes (55), and GATA4 or Nkx2–5 mutations are connected to cardiovascular diseases (56). The challenge in targeting transcriptional factors for drug development lies in their ‘undruggable’ nature due to intrinsic disorder and the absence of defined binding pockets (57). The use of dCas9-VP64 expands the targeting capability to different genomic regions, enabling the regulation of endogenous genes and transcriptional factors through custom sgRNA designs. While the current system features constitutive expression of dCas9-VP64, future iterations could incorporate inducible promoters to enhance adjustability, providing a broader range of control over gene expression levels for tailored applications. Also, the large size of delivery presents difficulties for in vivo applications, and addressing this issue remains a research frontier.
With the rapid advancement of CRISPR and anti-CRISPR tools, we anticipate that these technologies will significantly enhance the robustness and applicability of our system. In addition, our perfect adaptation system offers a versatile platform for the substitution of rtTA with other transcriptional factors to suit various biological contexts. By integrating an endogenous transcriptional factor of interest, such as demonstrated in our study with NF-κB, and incorporating DNA binding sequences at the promoter of AcrIIA4, our system can counteract abnormal gene expression. This approach holds the promise of maintaining transcriptional factor stability and preventing the onset of associated diseases, marking a significant advance in the field of gene regulation therapy.
During the course of this study, a similar system was also constructed by another group and reported online while our own study and corresponding manuscript preparation was in its final stages (58).
Supplementary Material
Acknowledgements
We thank Xuebin Liao for kind gift of the Jurkat cell line. We apologize to authors whose work cannot be cited owing to referencing restrictions.
Author contributions: S.Z. conceptualized and supervised the project. S.Z. and Y.Z. designed the experiments and analyzed the data. Y.Z. performed the experiments. Y.Z. wrote the first draft. S.Z. wrote the final manuscript.
Contributor Information
Yichi Zhang, School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China.
Shuyi Zhang, School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China; State Key Laboratory of Molecular Oncology, School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China; Center for Synthetic and Systems Biology, Tsinghua University, Beijing 100084, China; Beijing Frontier Research Center for Biological Structure, Tsinghua University, Beijing 100084, China.
Data availability
Data and materials used in this study are available from the corresponding author upon reasonable request. All plasmid sequences are provided in supplementary materials and uploaded to GenBank. Accession numbers are provided in Supplementary Table S5.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
National Natural Science Foundation of China [32171416 and U22A20552 to S.Z.]; Ministry of Science and Technology of the People’s Republic of China [2023YFA0915601 and 2021YFA0911000 to S.Z.]; Tsinghua University Dushi Plan Foundation (to S.Z.); Beijing Frontier Research Center for Biological Structure (to S.Z.). Funding for open access charge: National Natural Science Foundation of China [32171416].
Conflict of interest statement. None declared.
References
- 1. Nielsen A.A., Segall-Shapiro T.H., Voigt C.A. Advances in genetic circuit design: novel biochemistries, deep part mining, and precision gene expression. Curr. Opin. Chem. Biol. 2013; 17:878–892. [DOI] [PubMed] [Google Scholar]
- 2. Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986; 322:697–701. [DOI] [PubMed] [Google Scholar]
- 3. Shine M., Gordon J., Schärfen L., Zigackova D., Herzel L., Neugebauer K.M. Co-transcriptional gene regulation in eukaryotes and prokaryotes. Nat. Rev. Mol. Cell Biol. 2024; 25:534–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marchisio M.A., Stelling J. Automatic design of digital synthetic gene circuits. PLoS Comput. Biol. 2011; 7:e1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Purcell O., Lu T.K. Synthetic analog and digital circuits for cellular computation and memory. Curr. Opin. Biotechnol. 2014; 29:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller M., Hafner M., Sontag E., Davidsohn N., Subramanian S., Purnick P.E., Lauffenburger D., Weiss R. Modular design of artificial tissue homeostasis: robust control through synthetic cellular heterogeneity. PLoS Comput. Biol. 2012; 8:e1002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frei T., Khammash M. Adaptive circuits in synthetic biology. Curr. Opin. Syst. Biol. 2021; 28:100399. [Google Scholar]
- 8. Kemmer C., Gitzinger M., Daoud-El Baba M., Djonov V., Stelling J., Fussenegger M Self-sufficient control of urate homeostasis in mice by a synthetic circuit. Nat. Biotechnol. 2010; 28:355–360. [DOI] [PubMed] [Google Scholar]
- 9. Smole A., Lainšček D., Bezeljak U., Horvat S., Jerala R. A synthetic mammalian therapeutic gene circuit for sensing and suppressing inflammation. Mol. Ther. 2017; 25:102–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mattson M.P. Pathways towards and away from Alzheimer’s disease. Nature. 2004; 430:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mattson M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000; 1:120–130. [DOI] [PubMed] [Google Scholar]
- 12. van Delft M.F., Chappaz S., Khakham Y., Bui C.T., Debrincat M.A., Lowes K.N., Brouwer J.M., Grohmann C., Sharp P.P., Dagley L.F. et al. A small molecule interacts with VDAC2 to block mouse BAK-driven apoptosis. Nat. Chem. Biol. 2019; 15:1057–1066. [DOI] [PubMed] [Google Scholar]
- 13. Gardner T.S., Cantor C.R., Collins J.J. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000; 403:339–342. [DOI] [PubMed] [Google Scholar]
- 14. Elowitz M.B., Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000; 403:335–338. [DOI] [PubMed] [Google Scholar]
- 15. Nielsen A.A.K., Der B.S., Shin J., Vaidyanathan P., Paralanov V., Strychalski E.A., Ross D., Densmore D., Voigt C.A. Genetic circuit design automation. Science. 2016; 352:aac7341. [DOI] [PubMed] [Google Scholar]
- 16. Ma W., Trusina A., El-Samad H., Lim W.A., Tang C. Defining network topologies that can achieve biochemical adaptation. Cell. 2009; 138:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mangan S., Alon U. Structure and function of the feed-forward loop network motif. Proc. Natl Acad. Sci. U.S.A. 2003; 100:11980–11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones R.D., Qian Y., Siciliano V., DiAndreth B., Huh J., Weiss R., Del Vecchio D. An endoribonuclease-based feedforward controller for decoupling resource-limited genetic modules in mammalian cells. Nat. Commun. 2020; 11:5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bleris L., Xie Z., Glass D., Adadey A., Sontag E., Benenson Y. Synthetic incoherent feedforward circuits show adaptation to the amount of their genetic template. Mol. Syst. Biol. 2011; 7:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Segall-Shapiro T.H., Sontag E.D., Voigt C.A. Engineered promoters enable constant gene expression at any copy number in bacteria. Nat. Biotechnol. 2018; 36:352–358. [DOI] [PubMed] [Google Scholar]
- 21. Bloom R.J., Winkler S.M., Smolke C.D. Synthetic feedback control using an RNAi-based gene-regulatory device. J. Biol. Eng. 2015; 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guinn M.T., Balázsi G. Noise-reducing optogenetic negative-feedback gene circuits in human cells. Nucleic Acids Res. 2019; 47:7703–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang J., Lee J., Land M.A., Lai S., Igoshin O.A., St-Pierre F. A synthetic circuit for buffering gene dosage variation between individual mammalian cells. Nat. Commun. 2021; 12:4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenfeld N., Elowitz M.B., Alon U. Negative autoregulation speeds the response times of transcription networks. J. Mol. Biol. 2002; 323:785–793. [DOI] [PubMed] [Google Scholar]
- 25. Briat C., Gupta A., Khammash M. Antithetic integral feedback ensures robust perfect adaptation in noisy biomolecular networks. Cell Syst. 2016; 2:15–26. [DOI] [PubMed] [Google Scholar]
- 26. Aoki S.K., Lillacci G., Gupta A., Baumschlager A., Schweingruber D., Khammash M. A universal biomolecular integral feedback controller for robust perfect adaptation. Nature. 2019; 570:533–537. [DOI] [PubMed] [Google Scholar]
- 27. Anastassov S., Filo M., Chang C.-H., Khammash M. A cybergenetic framework for engineering intein-mediated integral feedback control systems. Nat. Commun. 2023; 14:1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frei T., Chang C.-H., Filo M., Arampatzis A., Khammash M. A genetic mammalian proportional–integral feedback control circuit for robust and precise gene regulation. Proc. Natl Acad. Sci. U.S.A. 2022; 119:e2122132119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nejepinska J., Malik R., Wagner S., Svoboda P. Reporters transiently transfected into mammalian cells are highly sensitive to translational repression induced by dsRNA expression. PLoS One. 2014; 9:e87517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paget M., Cadena C., Ahmad S., Wang H.-T., Jordan T.X., Kim E., Koo B., Lyons S.M., Ivanov P., Mu X. et al. Stress granules are shock absorbers that prevent excessive innate immune responses to dsRNA. Mol. Cell. 2023; 83:1180–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pawluk A., Amrani N., Zhang Y., Garcia B., Hidalgo-Reyes Y., Lee J., Edraki A., Shah M., Sontheimer E.J., Maxwell K.L. et al. Naturally occurring off-switches for CRISPR–Cas9. Cell. 2016; 167:1829–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rauch B.J., Silvis M.R., Hultquist J.F., Waters C.S., McGregor M.J., Krogan N.J., Bondy-Denomy J. Inhibition of CRISPR–Cas9 with bacteriophage proteins. Cell. 2017; 168:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura M., Srinivasan P., Chavez M., Carter M.A., Dominguez A.A., La Russa M., Lau M.B., Abbott T.R., Xu X., Zhao D. et al. Anti-CRISPR-mediated control of gene editing and synthetic circuits in eukaryotic cells. Nat. Commun. 2019; 10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonger K.M., Chen L.-c., Liu C.W., Wandless T.J. Small-molecule displacement of a cryptic degron causes conditional protein degradation. Nat. Chem. Biol. 2011; 7:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Egeler E.L., Urner L.M., Rakhit R., Liu C.W., Wandless T.J. Ligand-switchable substrates for a ubiquitin–proteasome system. J. Biol. Chem. 2011; 286:31328–31336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maynard-Smith L.A., Chen L.-c., Banaszynski L.A., Ooi A.L., Wandless T.J. A directed approach for engineering conditional protein stability using biologically silent small molecules. J. Biol. Chem. 2007; 282:24866–24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brignall R., Cauchy P., Bevington S.L., Gorman B., Pisco A.O., Bagnall J., Boddington C., Rowe W., England H., Rich K. et al. Integration of kinase and calcium signaling at the level of chromatin underlies inducible gene activation in T cells. J. Immunol. 2017; 199:2652–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017; 2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perkins N.D. The diverse and complex roles of NF-κB subunits in cancer. Nat. Rev. Cancer. 2012; 12:121–132. [DOI] [PubMed] [Google Scholar]
- 40. Pastorino J.G., Chen S.-T., Tafani M., Snyder J.W., Farber J.L. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J. Biol. Chem. 1998; 273:7770–7775. [DOI] [PubMed] [Google Scholar]
- 41. Finucane D.M., Bossy-Wetzel E., Waterhouse N.J., Cotter T.G., Green D.R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 1999; 274:2225–2233. [DOI] [PubMed] [Google Scholar]
- 42. Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008; 9:47–59. [DOI] [PubMed] [Google Scholar]
- 43. Kollek M., Voigt G., Molnar C., Murad F., Bertele D., Krombholz C.F., Bohler S., Labi V., Schiller S., Kunze M. et al. Transient apoptosis inhibition in donor stem cells improves hematopoietic stem cell transplantation. J. Exp. Med. 2017; 214:2967–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reyes N.A., Fisher J.K., Austgen K., VandenBerg S., Huang E.J., Oakes S.A. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. J. Clin. Invest. 2010; 120:3673–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lambert S.A., Jolma A., Campitelli L.F., Das P.K., Yin Y., Albu M., Chen X., Taipale J., Hughes T.R., Weirauch M.T. The human transcription factors. Cell. 2018; 172:650–665. [DOI] [PubMed] [Google Scholar]
- 46. Flavahan W.A., Gaskell E., Bernstein B.E. Epigenetic plasticity and the hallmarks of cancer. Science. 2017; 357:eaal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramsay R.G., Gonda T.J. MYB function in normal and cancer cells. Nat. Rev. Cancer. 2008; 8:523–534. [DOI] [PubMed] [Google Scholar]
- 48. Lin C.Y., Lovén J., Rahl P.B., Paranal R.M., Burge C.B., Bradner J.E., Lee T.I., Young R.A. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012; 151:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schuijers J., Manteiga J.C., Weintraub A.S., Day D.S., Zamudio A.V., Hnisz D., Lee T.I., Young R.A. Transcriptional dysregulation of MYC reveals common enhancer-docking mechanism. Cell Rep. 2018; 23:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brown C.J., Lain S., Verma C.S., Fersht A.R., Lane D.P. Awakening guardian angels: drugging the p53 pathway. Nat. Rev. Cancer. 2009; 9:862–873. [DOI] [PubMed] [Google Scholar]
- 51. Henley M.J., Koehler A.N. Advances in targeting ‘undruggable’ transcription factors with small molecules. Nat. Rev. Drug Discov. 2021; 20:669–688. [DOI] [PubMed] [Google Scholar]
- 52. Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 2018; 18:309–324. [DOI] [PubMed] [Google Scholar]
- 53. Sikorski K., Czerwoniec A., Bujnicki J.M., Wesoly J., Bluyssen H.A. STAT1 as a novel therapeutical target in pro-atherogenic signal integration of IFNγ, TLR4 and IL-6 in vascular disease. Cytokine Growth Factor Rev. 2011; 22:211–219. [DOI] [PubMed] [Google Scholar]
- 54. Walford H.H., Doherty T.A. STAT6 and lung inflammation. JAKSTAT. 2013; 2:e25301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mitchell S.M., Frayling T.M. The role of transcription factors in maturity-onset diabetes of the young. Mol. Genet. Metab. 2002; 77:35–43. [DOI] [PubMed] [Google Scholar]
- 56. Kohli S., Ahuja S., Rani V. Transcription factors in heart: promising therapeutic targets in cardiac hypertrophy. Curr. Cardiol. Rev. 2011; 7:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu J., Perumal N.B., Oldfield C.J., Su E.W., Uversky V.N., Dunker A.K. Intrinsic disorder in transcription factors. Biochemistry. 2006; 45:6873–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mallozzi A., Fusco V., Ragazzini F., di Bernardo D. The CRISPRaTOR: a biomolecular circuit for Automatic Gene Regulation in Mammalian Cells with CRISPR technology. 2024; bioRxiv doi:30 March 2024, preprint: not peer reviewed 10.1101/2024.03.30.587417. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials used in this study are available from the corresponding author upon reasonable request. All plasmid sequences are provided in supplementary materials and uploaded to GenBank. Accession numbers are provided in Supplementary Table S5.