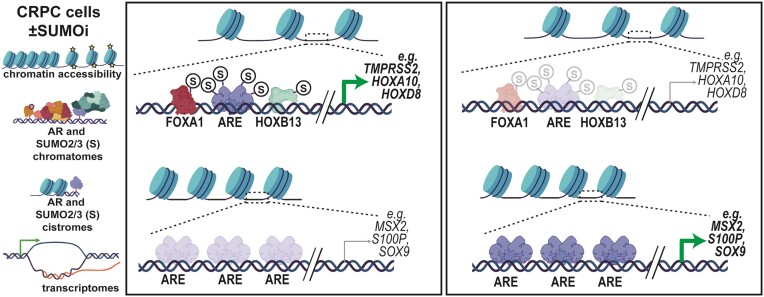
Abstract
The androgen receptor (AR) is pivotal in prostate cancer (PCa) progression and represents a critical therapeutic target. AR-mediated gene regulation involves intricate interactions with nuclear proteins, with many mediating and undergoing post-translational modifications that present alternative therapeutic avenues. Through chromatin proteomics in PCa cells, we identified SUMO ligases together with nuclear receptor coregulators and pioneer transcription factors within the AR’s protein network. Intriguingly, this network displayed a significant association with SUMO2/3. To elucidate the influence of SUMOylation on AR chromatin interactions and subsequent gene regulation, we inhibited SUMOylation using ML-792 (SUMOi). While androgens generally facilitated the co-occupancy of SUMO2/3 and AR on chromatin, SUMOi induced divergent effects dependent on the type of AR-binding site (ARB). SUMOi augmented AR’s pioneer-like binding on inaccessible chromatin regions abundant in androgen response elements (AREs) and diminished its interaction with accessible chromatin regions sparse in AREs yet rich in pioneer transcription factor motifs. The SUMOi-impacted ARBs divergently influenced AR-regulated genes; those associated with AR-mediated activation played roles in negative regulation of cell proliferation, while those with AR-mediated repression were involved in pattern formation. In conclusion, our findings underscore the pervasive influence of SUMOylation in shaping AR’s role in PCa cells, potentially unveiling new therapeutic strategies.
Graphical Abstract
Graphical Abstract.
Introduction
The androgen receptor (AR) is the pivotal transcription factor (TF) in prostate cancer development, progression, and therapeutic approach. While therapies that target androgen signaling demonstrate initial efficacy, resistance emerges, leading to lethal castration-resistant prostate cancer (PCa) (CRPC). The progression to CRPC has been linked to multiple AR mechanisms, including mutants and splice variants, aberrant expression of AR or its coregulator proteins, and gene fusions leading to anomalous androgen regulation of oncogenic TFs (1). Despite the perceived androgen independence of CRPC, the AR remains instrumental for the majority of CRPC tumors' growth and survival. Consequently, second-generation antiandrogens have emerged as primary treatments (2).
Beyond AR, its cognate hormone, and its DNA-binding sites (androgen response elements, AREs), AR’s transcriptional activity relies on a constellation of pioneer TFs and a plethora of coregulators (1). These coregulators function in tandem with AR to amplify or dampen its transcriptional output, contingent on the gene context and specific coregulator involvement. This modulation orchestrates androgen signaling across varying physiological contexts. Thus, targeting unique coregulators or their interactions with AR offers a promising avenue for new and improved PCa therapeutics. The activity and interactions of AR and many of its coregulators are regulated by post-translational modifications (PTMs), such as acetylation, phosphorylation, methylation, ubiquitination and SUMOylation (3). Notably, coregulators also participate in mediating these PTMs (4,5).
SUMOylation has emerged as a widespread and important regulatory mechanism of nuclear proteins, including important TFs, such as the AR (6,7). In SUMOylation, small ubiquitin-like modifier proteins (SUMO) 1, 2 or 3, composed of approximately 100 amino acids, bind covalently to lysine residues within target proteins. This conjugation furnishes target proteins with specific docking sites or novel molecular interfaces for allied proteins, modulating target activity through protein-protein interplay. SUMO2 and SUMO3 are nearly identical, cannot be distinguished by antibodies, and therefore often referred to as SUMO2/3. In contrast, SUMO1 shares a mere ∼50% sequence similarity with SUMO2/3 (8). Notably, cancer cells generally exhibit elevated SUMO2/3 over SUMO1 expression levels (9). SUMOs activated by SAE1/2 heterodimer (E1) are conjugated to target lysines by UBC9 (E2), with SUMO ligases (E3), such as PIAS1, PIAS2 and PIAS3, assisting in the bond formation. SUMOylations are highly dynamic: the bonds are generally rapidly cleaved by a family of SUMO-specific proteases, SENPs (8,10). Interestingly, a recent deep learning model for stratifying PCa patients by their treatment resistance state and evaluating the molecular drivers of treatment resistance emphasized the importance of SUMOylation and SUMO ligases for predicting PCa state (11).
In this study, we employed chromatin-centric proteomics in VCaP cells, representative of an amphicrine CRPC cell phenotype (12). This unveiled the association of PIAS1, -2 and -3 with coregulators in the 5α-dihydrotestosterone (DHT)-induced AR chromatin protein network (chromatome). Intriguingly, our analyses also revealed the AR chromatome's significant entwinement with SUMO2/3. We subsequently evaluated the ramifications of inhibiting SUMOylation using ML-792, a potent SUMO E1 inhibitor (13), on AR chromatin interactions and DHT-mediated gene expression. Our results fortify the notion of SUMOylation as a ‘local SUMO spray’ (14) amplifying interactions within AR’s transcriptional complexes, with profound implications for AR’s chromatin occupancy and transcriptional directives in VCaP cells.
Materials and methods
Reagents and biological resources
Details of used materials are summarized in Supplementary Table S1.
Cell culture and treatments
VCaP cells derived from a vertebral bone metastasis of a patient with CRPC and LNCaP cells derived from a lymph node metastasis were obtained from ATCC, tested negative for mycoplasma, and verified by Institute for Molecular Medicine Finland. VCaP cells were maintained in DMEM supplemented with 10% FBS, 1 U/μl penicillin and 1 μg/ml streptomycin. For downstream experiments, the media was changed to assay medium (DMEM, 5% charcoal-stripped serum) 48 h after seeding cells to appropriate cell culture format, incubated at least 24 h before treatments and treated with vehicle control (CTRL, DMSO), or 1 μM SUMOylation inhibitor, ML-792 (SUMOi) for 24 h. For the last experiment-specific hours cells were exposed to 100 nM 5α-dihydrotestosterone (DHT) or its vehicle control (veh, ethanol) before sample collection. LNCaP cells were maintained in RPMI-1640 supplemented with 10% FBS, 1 U/μl penicillin and 1 μg/ml streptomycin and 2 mM glutamine and for downstream experiments, the media was changed to assay medium containing 5% charcoal-stripped serum. In cell proliferation experiments, also another SUMOi, TAK-981 was used at final concentration of 1 μM.
Immunoblotting
Cell monolayers from SUMOi treated and hormone exposed VCaP cells were washed with ice-cold PBS and collected in PBS complemented with protease inhibitor cocktail (PIC) and N-ethylmaleimide (NEM). Cell pellets were suspended in SDS-PAGE sample buffer complemented with PIC and NEM and heated at 95°C for 5 min before sonicating 2 × 10 s and addition of β-mercaptoethanol (5% final conc.). Before separated on 7.5% SDS-PAGE gels, samples were re-heated for 5 min at 95°C. Proteins were transferred onto nitrocellulose membranes and visualized by antibodies against AR, GAPDH or SUMO2/3. Appropriate horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence detection reagents were used according to the manufacturer's instructions. See the details in the Supplementary Table S1.
Rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) and proteomics data analysis
VCaP cells were divided into 10-cm culture dishes at 5 × 106 cells per dish in maintenance medium as three biological replicates, medium was changed and cells were treated with DMSO or ML-792 as described above and cells exposed to DHT or vehicle for the last 2 h. Chromatin immunoprecipitation (ChIP) was done as described in (15), except that sheared chromatin was first immunoprecipitated overnight by specific antibody for AR, or SUMO2/3 or their controls at 4°C and then captured to magnetic Protein G beads for 24 h at 4°C. Immunoprecipitated chromatin was then washed with RIPA wash buffer and ammonium hydrogen carbonate as described in (16) (see details in Supplementary Table S1).
Beads were then flash frozen and peptide masses were analyzed with high resolution mass spectrometry following the original RIME protocol (16). Briefly, beads were washed with ammonium bicarbonate and sequencing grade modified trypsin was added on to the beads for 15 min with frequent vortexing and left for digestion for overnight in 37°C without further agitation followed by added trypsin for 4 h. Peptide digests in supernatant were quenched with trifluoroacetic acid and finally, desalted with C18 MicroSpin columns as described in (17). The dried peptides were reconstituted in Buffer A, diluted 1:20 HPLC water containing formic acid and loaded onto Evotips (Evosec) by manufacturer's instructions. LC-MS analysis was performed by using the Evosep One liquid chromatography system coupled to a hybrid trapped ion mobility quadrupole TOF mass spectrometer (Bruker timsTOF Pro) via a CaptiveSpray nano-electrospray ion source. Separation was done in 8 cm × 150 μm column with 1.5 μm C18 beads with 60 samples per day methods (21 min gradient time). Mobile phases A and B were 0.1% formic acid in water and 0.1% formic acid in acetonitrile, respectively. The MS analysis was performed in the positive-ion mode using data-dependent acquisition (DDA) in PASEF (18) mode with DDA-PASEF-short_gradient_0.5s-cycletime -method (see also details in Supplementary Table S1).
Raw data were processed with FragPipe v17.1 utilizing MSFragger (19) against reviewed human entries of the UniProtKB database (downloaded 8.3.2022). N-terminal acetylation and oxidation of methionine were used as dynamic modifications. Trypsin was selected as an enzyme, and a maximum of two missed cleavages were allowed. Both instrument and label-free quantification parameters were left to default settings. Results from these steps are Spectral Counts (SPC) values from peptides with FDR < 0.01 from Philosopher (20).
Protein matched data were then analyzed with SAINT (21) using normal rabbit serum (NRS) or IgG as background controls. Proteins having the FDR < 0.01 in any treatment were considered as high confidence members in AR or SUMO2/3 chromatomes within treatment. To compare different treatments, SPC of chromatome members were normalized by bait SPC. Chromatomes were then further categorized into hormone-induced (log2[DHT/veh] > 0.5), and uninduced (log2[DHT/veh] ≤ 0.5) fractions and subdivided into functional groups by GO annotation utilizing UniProt database (22). SUMOi effect on chromatome was analyzed by comparing bait-normalized SPC values and alterations in chromatomes were defined into three association categories by log2(SUMOi + DHT/CTRL + DHT) changes: SUMOi_increased (log2[FC] > 0.5), SUMOi_insensitive (−0.5 ≤ log2[FC] ≤ 0.5) and SUMOi_decreased (log2[FC] < -0.5). Illustrations were prepared with R-studio version 1.2.5033 and R v3.6.0 with ggplot, ImageJ, Cytoscape (v. 3.9.1) and Adobe Illustrator 2022 (v 26.3.1.)
ChIP-sequencing, ATAC-sequencing and data analysis
For ChIP-seq VCaP cells were divided into 10-cm culture dishes at 5 × 106 cells per dish as two biological replicates and treated with ML-792 or DMSO as above and DHT or vehicle for last 1 h in prior to chromatin immunoprecipitation, which was prepared as described in (15) with antibodies for AR, SUMO2/3, FOXA1, HOXB13 and H3K27ac and IgG for background. For ATAC-seq VCaP cells were divided and treated as above in prior to nuclear extraction and DNA tagmentation as described in (23,24). The transposed DNA was amplified as described in (25). DNA libraries were prepared (see also details in Supplementary Table S1), and pooled libraries were sequenced with Illumina NextSeq 500 (75SE) and Illumina NextSeq 500 (75PE). ChIP-seq and ATAC-seq data analysis was performed as previously described (15,23–26) with cut offs log2(SUMOi/CTRL) > 1.6 and log2(SUMOi/CTRL) < -1.6 for markedly SUMOi-increased and decreased chromatin sites, respectively. Similarly for the DHT-increased binding, log2(DHT/veh)>1.6 was used as a cut off and for DHT-decreased binding log2(DHT/veh) < -1.6. Changes in binding were considered as moderate, when −1.6 ≤ log2(FC) ≤ 1.6. Typical and super-enhancers were searched with findPeaks command in HOMER (27) using style super.
RNA-seq and data analysis
For RNA-sequencing, VCaP cells (700 000 cells/well on 6-well plates), were treated with ML-792 or DMSO as above and 6 h with DHT or vehicle prior to mRNA isolation according to Monarch and NEB recommendations (see also details in Supplementary Table S1) in three biological replicates. RNA-seq libraries were prepared and pooled libraries were sequenced with Illumina NextSeq 500 (75SE).
In house R pipeline was used to process the sequencing data and raw read data was processed as described in (25) and quality controlled with FastQC and MultiQC. Total count per gene was calculated using TPM normalization. Differentially expressed genes were analyzed with DESeq2 at HOMER v4.11 (27) for all comparisons. Genes with TPM larger than 1.8 (the median from all transcripts across treatments) in at least one sample in any treatment were considered as expressed. Other than protein coding genes were filtered out. Expressed protein-coding genes were considered as differentially SUMOi-downregulated (adj. P-value < 0.01 and log2[SUMOi/CTRL] < -0.5) and upregulated (adj. P-value < 0.01 and log2[SUMOi/CTRL] > 0.5) separately in vehicle treated samples and DHT treated samples. Furthermore, genes were categorized by their DHT-regulation into differentially DHT-down-regulated (adj. P-value < 0.01 and log2[DHT/veh] < −0.5) and DHT-up-regulated (adj. P-value < 0.01 and log2[DHT/veh] > 0.5) within each DHT to vehicle comparison i.e. CTRL + DHT compared to CTRL + veh and SUMOi + DHT compared to SUMOi + veh. For visualization purposes data was first organized ascending by log2(FC[DHT/veh]) in control and secondly by log2(FC [SUMOi/CTRL]) in DHT. Differentially expressed gene sets were subjected to pathway analysis in Metascape (28) with default settings. Data was visualized with R-studio version 1.2.5033 and R v3.6.0, and Adobe Illustrator 2022 (v 26.3.1).
Live-cell imaging
VCaP and LNCaP cells were divided into 96-wells at 30 000 cells/well and 10 000 cells/well, respectively, as five biological replicates in maintenance medium before treatments. After 48 h, the media was changed to assay medium and cells exposed to 100 nM DHT (10 nM for LNCaP cells), 1 μM ML-792, 1 μM TAK-981 or combination treatments (ML-792 + DHT or TAK-891 + DHT). Cells treated with DMSO and ethanol were used as treatment controls. Cells were imaged in IncuCyte S3 Live-Cell Imaging System (Essen BioSciences) for one week with 12 h (VCaP cells) or 8 h (LNCaP cells) intervals. IncuCyte S3 2021C software (Essen BioSciences) was used to count the cell confluence alterations as relative changes to starting point. Significant differences were calculated in GraphPad Prism 5 with Two-way ANOVA and Tukey's multiple comparison posttests comparing all columns. Data were visualized in R-studio version 2023.12.1 and R v4.2.2, utilizing ggplot package with geom_smooth and Adobe Illustrator 2020 version 24.1.1.
Data integration
Sequencing data were integrated with HOMER v4.11 (27) commands mergePeaks, and annotatePeaks.pl. ARBs were associated with SUMOi- and DHT-altered transcriptome and for each cluster the fraction of genes with at least one ARB within 100 kb from TSS was calculated. This fraction was then compared to the control fraction, i.e. the fraction of DHT-regulated genes with no change after SUMOi.
Further integrative analysis of SUMOi altered AR binding sites (ARBs) and transcriptome (SUMOi_DEGs and DHT_DEGs) was executed with BETAplus software package (29) that integrates ChIP-seq data of transcription factors with differential gene expression data to infer direct target genes. Default settings but genome set as hg38 and differential expressed gene FDR Threshold as 0.01 were used. BETAplus is based on a peak-to-gene approach that predicts the function of a DNA-bound factor to the transcriptome. The regulatory potential of AR on different SUMOi-altered ARBs was associated with gene expression. The regulatory potential represents gene's likelihood of being regulated by AR, and it was estimated for each gene. The cumulative distribution function was generated and the groups of up-regulated and down-regulated genes were compared to non-altered genes with one-tailed Kolmogorov–Smirnov test that determines whether these groups are significantly differently regulated by AR relative to the non-altered genes (29). The P-value for regulatory potential was then compared between ARB clusters. Additionally, genes associated with SUMOi-altered ARBs were applied into Metascape (28) for pathway analysis.
Results
The protein network of chromatin-bound AR is intertwined with SUMO2/3 in prostate cancer cells
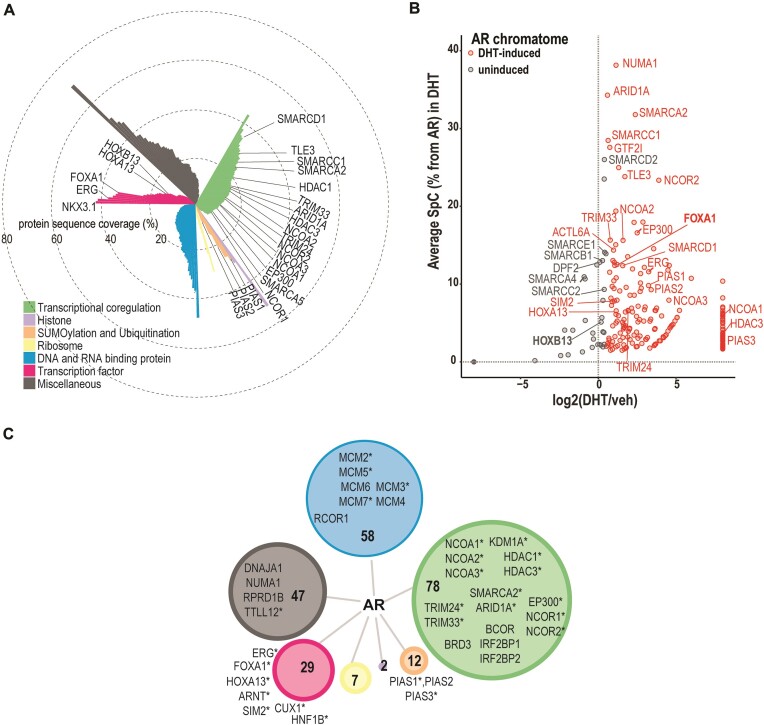
We used chromatin-directed proteomics (RIME, see Materials and methods (16)) to identify AR’s neighboring proteins (chromatome) within approximately 200 bp of AR-binding site. Association of the AR with most (∼81%) of the 234 chromatome proteins was induced by DHT (Figure 1A, B, Supplementary Table S2). As shown in Figure 1C, subdivision of the AR chromatome into GO annotation groups revealed large numbers of TFs, such as ERG, FOXA1, HOXA13, HOXB13 and NKX3.1, transcriptional coregulators, such as nuclear receptor coactivators EP300, NCOA1, NCOA2 and NCOA3, corepressors NCOR1 and NCOR2, BAF complex components ACTL6A, ARID1A, ARID1B, PBRM1, SMARCA2, SMARCC1 and SMARCD1, and histone modifiers, such as HDAC1, HDAC3, KDM1A. Interestingly, nearly half (∼46%) of the AR chromatome proteins have been associated with prostatic diseases in DisGeNET Database 7.0 (30) (examples marked with asterisks in Figure 1c, Supplementary Table S3) and several of them have been shown to be differentially expressed between primary prostate tumor and normal tissue (TCGA data browsed in Xena platform (31), see details in Supplementary Table S2), implying the disease relevance of this resource for AR biology.
Figure 1.
Androgen-dependent protein network of chromatin-bound AR in VCaP cells. (A) Overview of AR chromatome identified with RIME and subdivided into functional groups by GO annotation. Protein sequence coverage percentages are shown as bars in polar coordinated plot and coloring by GO annotation. (B) Chromatome is categorized into androgen-induced (log2[DHT/veh] > 0.5), and uninduced (log2[DHT/veh] ≤ 0.5) fractions. DHT-dependency of AR chromatome in scatterplot with spectral counts normalized by AR as y axis. log2[DHT/veh] = –8 and 8 are inputed values for chromatome members that are identified with vehicle only or with DHT only, respectively. DHT-induced members additionally highlighted red color. (C) AR DHT-dependent chromatome members subdivided into functional groups by their GO annotation. The size of the node represents the numbers of members in the group and coloring by annotation similarly to a. Examples of proteins shown for groups larger than 10 proteins, asterisks mark proteins that have association to prostatic disease in DisGeNet.
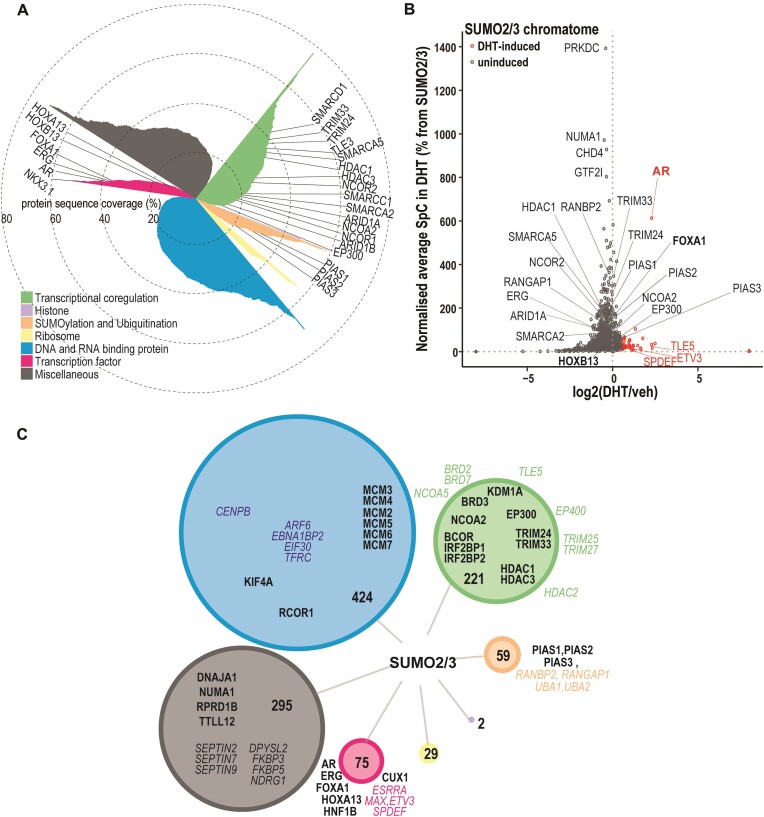
The AR chromatome also included SUMO2, PIAS1, PIAS2 and PIAS3 (Figure 1, Supplementary Table S2), indicating the simultaneous and close presence of SUMOylation machinery components and AR on chromatin. Due to this finding, a positive correlation between AR and SUMO expression in TCGA-PRAD data and association of high expression of SUMOs with a shorter disease-free survival time in PCa (Supplementary Figure S1), we performed complementary RIME to identify SUMO2/3-linked proteins in VCaP cells (Figure 2A, B, Supplementary Table S4). SUMO2/3 RIME was done in the absence and presence of DHT to evaluate effects of AR activation on chromatin SUMOylation in prostate cancer cells. We identified 1105 SUMO2/3-associated proteins which included nearly three times more TFs and transcriptional coregulators than in the AR chromatome (Figure 2C, Supplementary Figure S2a, Supplementary Table S4). Association of only 4% of the SUMO2/3 chromatome proteins was induced by DHT, which is in stark contrast with that of the AR chromatome (Figure 2B, Supplementary Table S4). AR was among these SUMO2/3-associated proteins, likely reflecting the androgen-induced SUMOylation of the receptor and its residence as SUMOylated protein on the chromatin (32). In addition, TFs ETV3 and SPDEF and transcriptional corepressor TLE5 that have been associated with prostatic diseases in DisGeNET Database 7.0 (30) (Supplementary Table S5) are examples of proteins whose association with SUMO2/3 on chromatin was enhanced by DHT. Conversely, DHT notably reduced the presence of almost half (46%) of the proteins in the SUMO2/3 chromatome, as assessed by their reduced spectral counts (SPC) in the presence of DHT. For instance, BAF complex member SMARCC2, histone acetyltransferase EP400 (TIP60) as well as RNA polymerase II subunits RPB1 (POLR2A) and RPB2 (POLR2B) belong to this protein group. Since PIAS1 and PIAS3 are responsible for AR SUMOylation (5,33), the latter effect of DHT on SUMO2/3 chromatome may reflect targeting or recruitment of SUMO2/3 by abundant AR and PIAS proteins, limiting the availability of SUMO2/3 for other proteins.
Figure 2.
Association of SUMO2/3 with chromatin-linked proteins in VCaP cells. (A) Overview of SUMO2/3 chromatome identified with RIME and subdivided into functional groups by GO annotation. Protein sequence coverage percentages are shown as bars in polar coordinated plot and coloring by GO annotation. (B) Chromatome is categorized into androgen-induced (log2[DHT/veh] > 0.5), and uninduced (log2[DHT/veh] ≤ 0.5) fractions and DHT-dependency plotted on scatter plot with SPCs normalized to SUMO2/3. DHT-induced members additionally highlighted red color. (C) SUMO2/3 chromatome members subdivided into functional groups by their GO annotation. Size of the node represents the numbers of members in the group and coloring by annotation similarly to (A). Examples of proteins are shown for groups larger than 30 proteins, SUMO2/3 chromatome members that are found also in the AR chromatome are in bold, others in italics with color matching to the node.
Comparison of the DHT-induced AR chromatome with the SUMO2/3 chromatome revealed that ∼90% of AR chromatome members also associate with SUMO2/3 (Supplementary Figure S2b, Supplementary Table S4), strongly suggesting that most of the AR’s neighboring proteins are SUMOylated. For example, TFs FOXA1, ERG, NKX3.1 and HOXB13, transcriptional coregulators EP300 and NCOA2, BAF complex members SMARCA2, SMARCD1 and SMARCC1, and corepressors NCOR1 and NCOR2 were found in both chromatomes (Supplementary Table S4). This large overlap between the two chromatomes was more than anticipated, although almost one third of AR chromatome members have been implicated as SUMOylation targets or to interact with SUMO2/3 ((34–41), Supplementary Table S2). Taken together, these results underscore the intertwined relationship between AR and the SUMOylation.
Effect of SUMOi on the AR chromatome
The above results let us hypothesize that SUMOylation of AR and/or its chromatome members might modulate the interactions in AR’s chromatin network. To assess the general impact of SUMOylation on AR chromatome in VCaP cells, we exposed them to a small-molecule SUMOylation inhibitor ML-792 (SUMOi, see Materials and methods for details) that selectively blocks SUMO E1 activity. SUMOi led to a general inhibition of SUMOylation with increased levels of unconjugated, free SUMO2/3, as judged by immunoblotting of whole cell extracts with SUMO2/3 antibody. Moreover, SUMOi abolished the slower migrating anti-AR antibody immunoreactive bands indicative of DHT-induced AR SUMOylation. (Supplementary Figure S3a). Also, in accordance with AR as a SUMO2/3 target, SUMOi decreased the association of SUMO2/3 with the AR on chromatin (Supplementary Figure S3b). As expected, SUMOi also reduced SPCs of the majority of SUMO2/3 chromatome components (Supplementary Figure S3c, Supplementary Table S4).
More interestingly, SUMOi also attenuated the association of ∼84% of AR chromatome members with the receptor, as assessed by SPCs (Supplementary Figure S3c and Supplementary Figure S4). However, 30 members of AR chromatome, including TFs FOXA1, HOXA13 and SIM2, and transcriptional coregulators TRIM33 and HDAC1, remained unaffected, and TRIM24 showed even increased association with AR in response to SUMOi. Taken together, these results suggest that SUMOylation acts as a local ‘SUMO spray’ (14) on nearby proteins, which by and large potentiates their associations in transcription complexes with the AR.
Androgen reshapes the SUMO2/3 landscape on chromatin
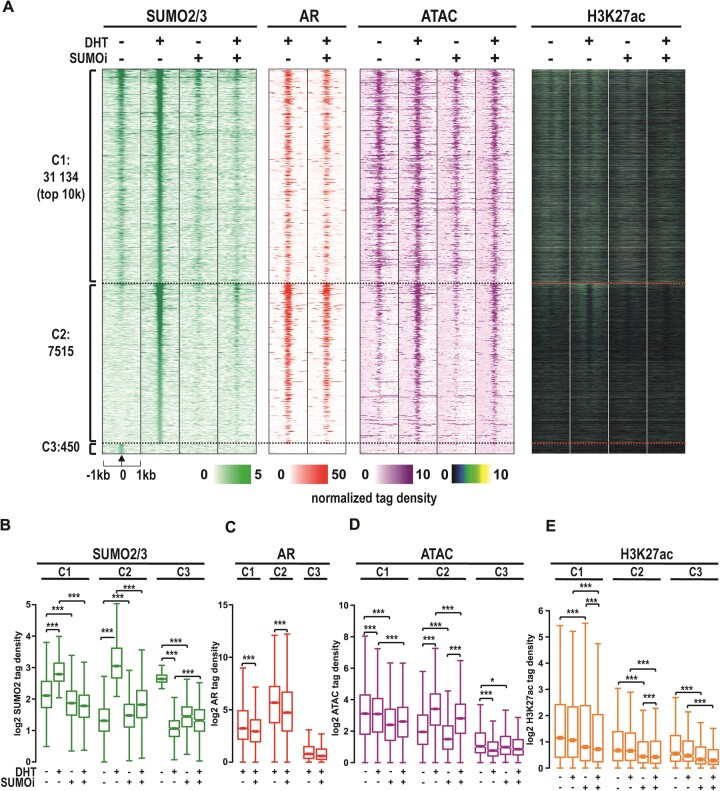
Since the AR chromatome was practically embedded in the SUMO2/3 chromatome and SUMOi had an interesting effect on the AR chromatome, we next assessed the chromatin distribution of SUMO2/3 by ChIP-seq under the same conditions as in the chromatin-directed proteomic assays. In parallel experiments, we analyzed chromatin accessibility (openness) by ATAC-seq and localization of active chromatin histone mark H3K27ac, FOXA1 and HOXB13 by ChIP-seq and compared these data with AR chromatin occupancy (cistrome) data. In the absence of DHT, ChIP-seq yielded 16 627 SUMO2/3 peaks which DHT exposure more than doubled to 38 649 peaks (Supplementary Figure S5a), indicating a remarkable impact of androgen on SUMO2/3 chromatin occupancy in VCaP cells. More detailed clustering analysis of SUMO2/3-binding sites showed that DHT exposure enhanced SUMO2/3 signal moderately (–1.6 < log2FC < 1.6) at 31 134 sites (cluster 1, C1, Figure 3A, B). Instead, a substantial enhancement (log2FC > 1.6) of the signal by DHT exposure was seen at 7515 SUMO2/3-binding sites (C2 in Figure 3A, B), whereas only 450 sites showed decreased signal (log2FC < -1.6) in response to DHT (C3, Figure 3A, B). The inducing impact of DHT on SUMO2/3 signal reflected the AR chromatin occupancy in C1 and C2 (Figure 3C). SUMO ligase PIAS1 ChIP-seq data from the same cells (26) revealed that the PIAS1 chromatin occupancy parallels with the impact of DHT on SUMO2/3 in these clusters (Supplementary Figure S6). Similarly, FOXA1 and HOXB13 chromatin occupancy was higher in the C2 cluster than the C1 (Supplementary Figure S7). However, chromatin openness and the amount of H3K27ac signal was higher in the C1 than the C2 (or the C3) with DHT increasing the chromatin openness clearly only in C2 (Figure 3D, E).
Figure 3.
Effect of SUMOi and androgen on SUMO2/3 landscape on the chromatin. (A) Heatmaps of SUMO2/3 and AR occupancy on chromatin, chromatin accessibility, and H3K27ac marks as normalized tag densities, divided to subcategories by androgen effect in SUMO2/3 signal (C1 = moderately changed – 3 < log2(DHT/veh) < 3, C2 = markedly increased, log2(DHT/veh) > 3 and C3 = markedly decreased, log2(DHT/veh) < -3). For C1 sites only top 10k showed. (B–E) Box blots of tag densities in SUMO2/3, AR, ATAC and H3K27ac in clusters C1–3. Statistical significance for tag density boxplots calculated with One-way ANOVA with Bonferroni post hoc test and shown as asterisks with *P< 0.05, **P< 0.01 and ***P< 0.001.
As expected, SUMOi clearly decreased the number of SUMO2/3 peaks (Supplementary Figure S5b, c), abolishing also all or most of the enhancing effect of DHT on the SUMO2/3 signal at C1 and C2, respectively (Figure 3B). SUMOi thus resulted in severe hypoSUMOylation on the chromatin, but it did not totally erase the SUMO2/3 from the chromatin. Additionally, SUMOi diminished the AR and FOXA1 chromatin occupancy, chromatin accessibility and H3K27ac marks in the C1 and the C2 (Figure 3C–E, Supplementary Figure S7). In sum, these results indicate that the SUMO2/3 landscape on chromatin in VCaP cells responds to extracellular signals, as DHT exposure of the cells markedly reshapes the landscape. Since androgen had only a minor effect on the association of coregulators or TFs with SUMO2/3 in chromatin proteomics assays, the DHT-reshaped SUMO2/3 chromatin landscape is likely to reflect the AR-dependent recruitment of SUMOylated coregulators and TFs onto the chromatin as well as the chromatin occupancy of SUMOylated AR.
SUMOylation modifies the chromatin interactions of AR in a binding site-selective fashion
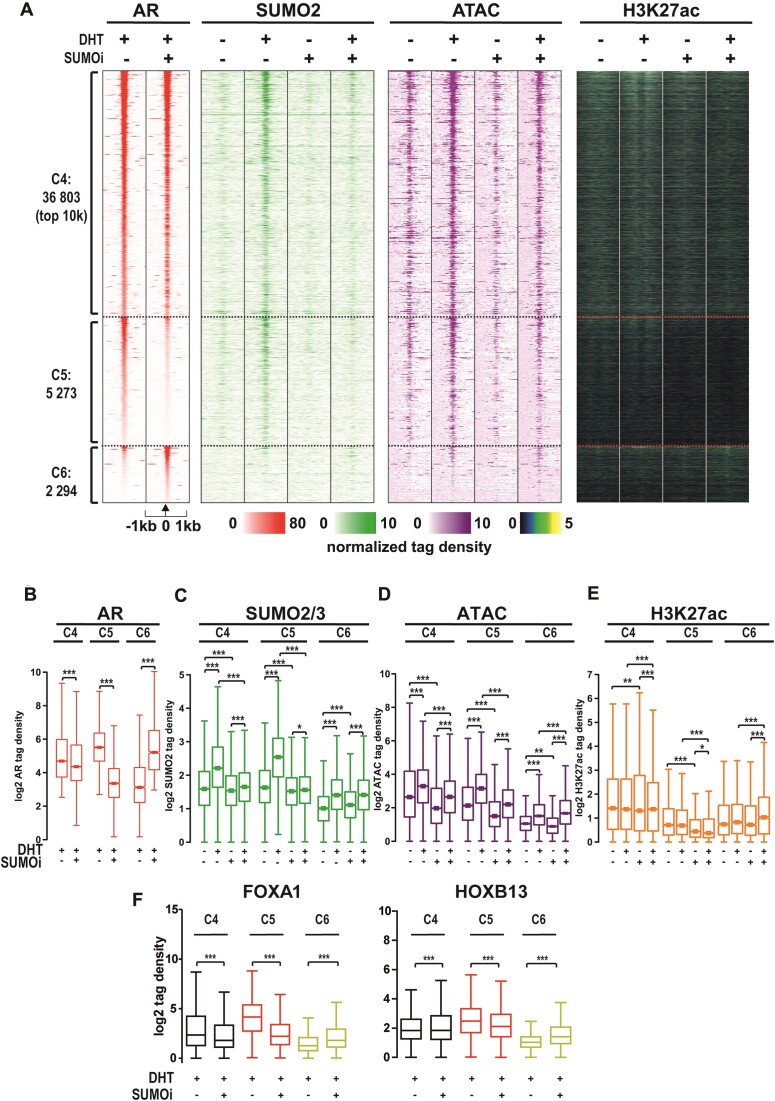
We next analyzed the effect of SUMOi on the AR chromatin occupancy. Clustering of AR-binding chromatin sites (ARBs) revealed that SUMOi had only moderate attenuating effect on the AR chromatin occupancy on the majority (∼83%) of ARBs (C4, Figure 4A, B), whereas the occupancy was substantially decreased on 5273 sites (C5, Figure 4a-b), and contrastingly increased on 2294 ARBs (C6, Figure 4A, B, example IGV tracks of TMPRSS2 and KLK locus in Supplementary Figure S8). SUMOi abolished the enhancing effect of DHT on SUMO2/3 chromatin occupancy in C4 and C5, but not in C6 (Figure 4C), which is in line with the overlap of C4 and C5 ARBs with SUMO2/3-binding site clusters in Figure 3, particularly with C2, and the lack of overlap of C6 ARBs with the SUMO2/3-binding clusters (Supplementary Figure S9). SUMOi also reduced chromatin openness in the presence of DHT in C4 and C5, but not in C6 (Figure 4D), whereas its effects on H3K27ac signal were less clear (Figure 4E). These results indicate that the ability of SUMOylation to promote AR chromatin occupancy is paralleled with its effect on chromatin openness. At chromatin sites where the modification inhibits the AR chromatin occupancy, this parallel is not evident.
Figure 4.
SUMOylation inhibition modifies AR occupancy on chromatin. (A) Heatmap of AR’s normalized tag densities on moderately SUMOi-affected ARBs (C4, – 3 < log2[SUMOi/CTRL] < 3), clearly SUMOi-attenuated ARBs (C5, log2[SUMOi/CTRL] < −3) and SUMOi-enhanced ARBs (C6, 3 < log2[SUMOi/CTRL]) together with SUMO2/3 occupancy, chromatin accessibility and H3K27ac data. (B–E) Box plots of AR, SUMO2/3, ATAC and H3K27ac tag densities in clusters C4–C6. (F) Chromatin occupancy of FOXA1 and HOXB13 in the presence and absence of DHT and SUMOi in clusters C4–C6 as box plots of tag densities. Statistical significance for tag density box plots calculated with one-way ANOVA with Bonferroni post hoc test and shown as asterisks with *P< 0.05, **P< 0.01 and ***P< 0.001.
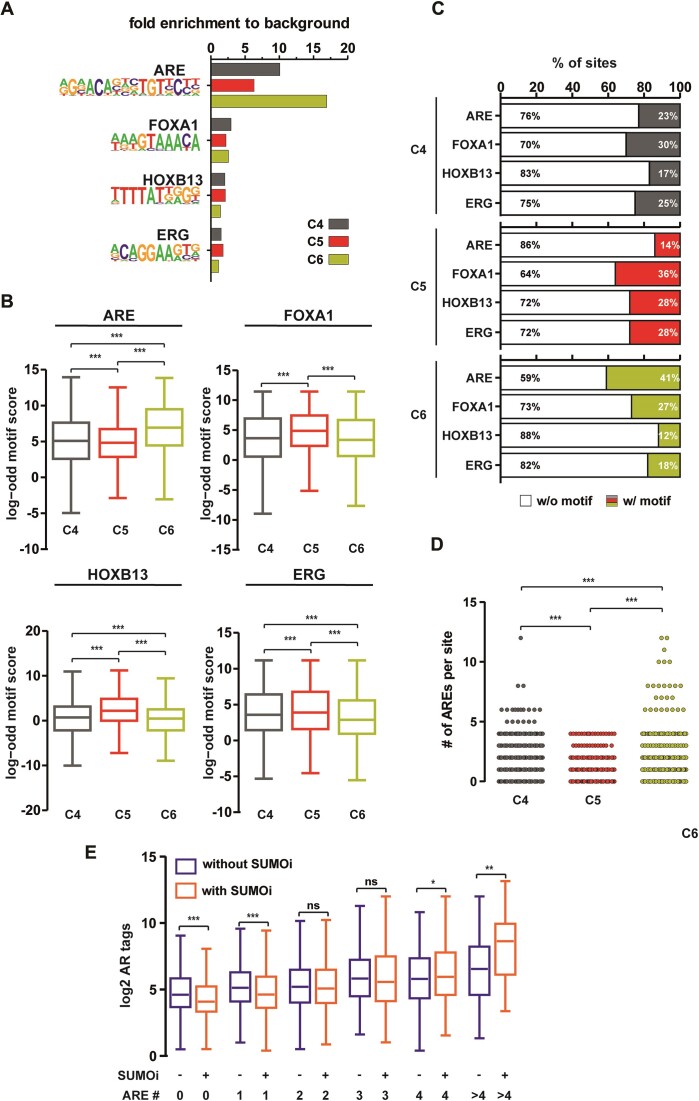
We were next interested in whether differential pioneer TF occupancy could be associated with the different SUMOi-affected ARB clusters. ChIP-seq of FOXA1 and HOXB13 showed that the chromatin occupancy of these TFs is higher in C5 than in C4, being lowest in C6 (Figure 4f, Supplementary Table S6). In line with the effect of SUMOi on the AR in these clusters, SUMOi attenuated the chromatin occupancy of FOXA1 and HOXB13 in C4 and C5, but it enhanced the occupancy in C6. Detailed analysis of the occurrence of TF binding motifs in these clusters revealed differences in the enrichment of AR (ARE), FOXA1, HOXB13 and ERG binding motifs (Figure 5A–C). Analysis of their fold enrichment over background, motif scores and relative portions within the ARBs, all supported the highest occurrence of AREs in C6 and its lowest occurrence in C5. In contrast, the occurrence of FOXA1, HOXB13 and ERG motifs increased in the order of C6 > C4 > C5 (Figure 5C). Moreover, counts of the actual numbers of AREs within each ARB revealed a clear difference between the C5 and the C6, as no ARE clusters harboring more 5 AREs were visible in C5 in contrast to C6 ARBs (Figure 5D). Interestingly, SUMOi decreased AR occupancy on sites harboring less than two AREs, whereas it increased the occupancy on sites with four or more AREs (Figure 5E, an example of 12 ARE-harboring ARB site in Supplementary Figure S10). Analysis of the genomic distribution of ARBs reproduced a preference towards intronic and intergenic regions (42), suggesting that SUMOi by and large affects the occupancy of AR at enhancers (Supplementary Figure S11a). Similarly, FOXA1 and HOXB13 were enriched in intergenic and intronic regions (Supplementary Figure S11b, c). When calling larger regions (12.5 kb) with ‘stitched’ AR peaks as defined in (43), interestingly, a larger amount of super-enhancer regions was detected in C6 than in C4 or C5 (Supplementary Figure S12). Collectively, these results imply that SUMOylation inhibits the receptor binding onto ARBs with multiple ‘perfect’ AREs, fitting with the pivotal role of SUMOylation in the regulation of synergy control motifs in the AR (3,44). Otherwise, SUMOylation enhances AR binding to ARBs associated with AR’s pioneer TFs.
Figure 5.
Motif analysis of AR chromatin-binding sites differentially affected by SUMOi. (A) Fold enrichment bar plot for ARE, FOXA1, HOXB13 and ERG motifs for ARB clusters in Figure 4. (B) Comparison of log odd motif scores between ARB clusters of motifs in (C). Motif coverage in percentages from all ARBs within each cluster. (D) Number of AREs in each site within clusters. (E) Box blots of AR chromatin occupancy with and without SUMOi, depending on the number of AREs per site. Statistical significance for boxplots calculated with One-way ANOVA with Bonferroni post hoc test and shown as asterisks with *P< 0.05, **P< 0.01 and ***P< 0.001.
SUMOylation modulates the transcriptional output of prostate cancer cells
To complement the above genomic data, we analyzed the effect of SUMOi on the transcriptome of VCaP cells by RNA-seq. Principal component analysis showed that the four exposures (vehicle control, DHT, SUMOi and SUMOi + DHT) all form clearly separate groups (Supplementary Figure S13a), demonstrating that SUMOi influences both androgen-dependent and -independent transcription programs. Overall, SUMOi significantly altered the expression of 3945 genes of which ∼57% were downregulated and ∼43% were upregulated by SUMOi (SUMOi_dnDEGs and SUMOi_upDEGs, respectively, Supplementary Figure S13b, c). This indicates that SUMOylation is not merely a repressing PTM in VCaP cells. Pathway analysis in Metascape (28) revealed that in the absence of DHT, SUMOi-affected genes are involved in e.g. ribosome biogenesis and regulation of cytoskeleton organization, whereas in the presence of DHT, genes involved in mitotic prometaphase and cell population proliferation are affected (Supplementary Figure S13d, Supplementary Table S7). SUMOylation therefore impacts important cellular pathways in a pathway selective fashion.
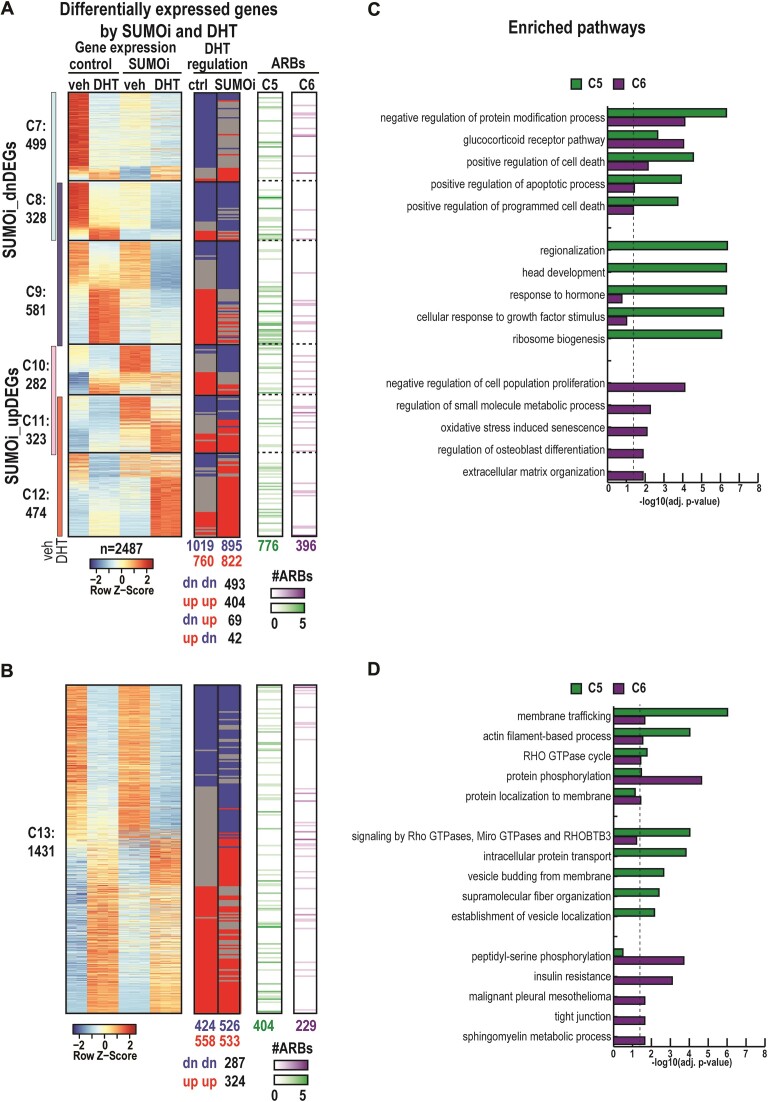
We subsequently focused on DHT-dependent gene regulation. A remarkable portion (∼63%) of SUMOi_DEGs were also differentially DHT-regulated either in control conditions (CTRL + DHT versus CTRL + veh) or became DHT-regulated after SUMOi (SUMOi + DHT vs. SUMOi + veh). SUMOi decreased the expression of 1408 DHT-regulated genes and increased that of 1079 genes (C7–C9 and C10–C12, respectively, Figure 6a). A group of 1431 DHT-regulated genes was not affected by SUMOi (C13, Figure 6b). SUMOi did not change the direction of DHT regulation, i.e. induction or repression of most genes. However, in clusters C7–C12, SUMOi changed the direction of DHT-regulation of 111 genes, blunted the DHT regulation of 771 genes and 709 genes became DHT-regulated (shared genes shown at the bottom of the heatmaps in Figure 6A, B). Metascape pathway analysis of DHT-regulated SUMOi-DEGs revealed that SUMOylation for example influences expression of genes involved in pathways of cancer, tube morphogenesis, protein processing in endoplasmic reticulum, and response to hormone (Supplementary Table S7).
Figure 6.
Effect of SUMOi on AR-regulated gene expression and its association with SUMOi-affected AR chromatin-binding sites in VCaP cells. (A) The leftmost heatmap represents z-scores of SUMOi- and androgen-regulated DEGs in SUMOi dn-regulated clusters (C7–C9) and SUMOi up-regulated clusters (C10–C12). In the middle, there is information of whether each gene is DHT-regulated in control and/or after SUMOi. DHT-up-regulation is shown in red and DHT-dn-regulation in blue. On the right, gene-associated ARBs from SUMOi-altered clusters C5 and C6 are shown in green and purple, respectively. The color intensity indicates the number of ARBs within ±100 kb from TSS of each gene (B). Heatmaps and associations as in a but for SUMOi-unaffected DHT-regulated genes (C13). (C, D) Metascape pathway analysis for genes associating with SUMOi-attenuated ARBs (C5, in green) and SUMOi-enhanced ARBs (C6, in purple). Top5 enriched pathways shown as bar graph of adj. P-values for C5 and C6-shared pathways (topmost part of the bar plot), for pathways enriching significantly only with genes associating with C5 (middle part), for pathways enriching significantly only with genes associating with C6 (bottom part) in clusters C7–C12 (C) and C13 (D). Dashed line indicates significance threshold (adj. P-value) <0.05 after Benjamini–Hochberg multiple correction in-built in Metascape (28).
As many of the SUMOylation-affected pathways were involved in cell proliferation regulation, we next studied whether the effects of SUMOi on gene expression are reflected in altered growth of PCa cells. To that end, we used automated live-cell imaging, measuring cell confluency as a proxy for cell growth. In these experiments, we used also TAK-981, a pharmacologically improved SUMOi currently in clinical trials (45), in the presence and absence of DHT. As shown in Supplementary Figure S14a, both ML-792 and TAK-981 similarly and totally abolished the stimulatory effect of DHT on VCaP cell growth, with both compounds being capable of attenuating the cell growth also in the absence of androgen. Similarly, the growth of another AR positive cell line, LNCaP cells was sensitive to TAK-981 and ML-792, albeit their response to SUMOi in the presence of DHT was delayed in comparison to that of VCaP cells (Supplementary Figure S14b). Transcriptome analysis showed that SUMOi (ML-792) affected a much smaller group of genes in LNCaP than VCaP cells (Supplementary Figure S15), but cell growth regulation-linked pathways showed enrichment among genes affected by SUMOi also in LNCaP cells. On the other hand, there were clear differences in the enrichment of SUMOi-affected pathways between the cell lines, and e.g. two cell cycle- and senescence-associated transcripts, CDKN1A and CDKN2A (46), were regulated by androgen and SUMOi in VCaP cells but not in LNCaP cells (Supplementary Figure S15e). These results indicate a wide, but target gene- and context-dependent role of SUMOylation in the regulation gene expression and the growth of PCa cells.
Association of SUMOi-affected gene expression with AR chromatin binding
Next, we predicted how differently SUMOi-responsive ARBs (C4–C6) regulate the gene expression. We first associated the ARBs with the transcriptome and observed that SUMOi-attenuated ARBs (C5 in Figure 4) were often associated with SUMOi-repressed genes, whereas SUMOi-induced ARBs (C6 in Figure 4) associated with SUMOi-activated ones (Supplementary Figure S16). We continued the integrative analysis by utilizing the Binding and Expression Target Analysis (BETAplus) tool (29) that associated ARBs within ±100 kb from TSS of DEGs to further predict their regulatory function. This integration of our AR ChIP-seq data with up- and downregulated genes in different treatments (DHT and SUMOi) revealed that most genes in C7–C13 have at least one ARB associated within 100 kb from TSS (Figure 6A, B, Supplementary Figure S17a). The predicted regulation function of AR on ARBs, which can be either gene activation (upregulation) or repression (downregulation), decreased from C4 ARBs via C5 ARBs to C6 ARBs (Supplementary Figure S17B–D). C4 ARBs were similarly associated with repression and activation functions in all DEG categories, and SUMOi did not clearly influence these functions. ARBs in C5 were more strongly associated with activation than repression, and the situation was reversed in SUMOi-treated cells (compare the graphs between DHT_DEGs in CTRL and DHT_DEGs in SUMOi, Supplementary Figure S17b). In line with Supplementary Figure S16 analysis, the C5 ARBs were strongly associated with genes that SUMOi represses in DHT-treated cells, whereas the C6 ARBs associated merely with the activation function (SUMOi_DEGs in DHT in Supplementary Figure S17b–d). These integrative data indicate that the effects of SUMOi on AR chromatin occupancy are reflected in the gene regulatory function of AR.
To gain more biological insight into the above integrated data, we exploited pathway analysis of DEGs that associated with C5 or C6 ARBs. The analysis revealed interesting differences between genes associated with SUMOi-repressed ARBs (C5) and SUMOi-induced ARBs (C6) (Figure 6C, D). For example, the top pathway among the C5 ARB-associated genes was regionalization (i.e. the pattern formation), whereas that among the C6 ARB-associated genes was negative regulation of cell population proliferation (Figure 6C, Supplementary Table S7. Moreover, the C5 and C6 ARBs that associate with SUMOi-affected genes (C7–C12) enriched in different pathways than those that associate with the genes unaffected by SUMOi (C13) (Figure 6D). Based on TCGA-PRAD data, both regionalization and negative regulation of cell population proliferation pathways include several genes that are differentially expressed between normal prostates and primary prostate tumors (Supplementary Figure S18, Supplementary Table S7), suggesting that SUMOylation can contribute to de-regulated gene expression linked to PCa.
Discussion
Development of chromatin-directed proteomics has enabled unbiased identification of TFs’ chromatin protein networks (chromatomes, protein complements in the vicinity of chromatin-bound TFs) (25,47,48). Rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) for analysis of protein complexes method used in this work is based on capture of formaldehyde-crosslinked chromatin (16). Preservation of transient interactions in chromatin protein complexes has been shown to be best achieved by crosslinking complexes in their native state before the affinity capture. Like all antibody- and crosslinking-based proteomics methods, RIME has its limitations, e.g. concerning the specificity of antibody and the modifying effect of cross-linking agents on certain amino acid residues, leading to possible epitope modification-masking and low abundancy and complexity problem in MS spectra (49) (50). The antibodies used in our RIME analyses have been robustly validated and shown to perform excellently with crosslinked chromatin (15,25,26,34,42,51,52). Despite its limitations, RIME captured in VCaP CRPC cells nearly two hundred proteins that associate in an androgen-dependent manner with chromatin-bound AR. These proteins had reasonable spectral counts and protein peptide coverages and passed tight statistical tests (see Materials and methods). These AR-associated proteins play diverse roles ranging from pioneer TFs like FOXA1 and HOXB13 via coregulators like EP300, NCOA1/2/3 and NCOR1/2 and chromatin remodeling BAF complex components to DNA replication licensing factors like MCM2/3/4/5/6/7 and proteins involved in RNA processing and splicing like DDX23/46, PRP4 and STRAP. This offers a panoramic view of the chromatin milieu that AR resides in and underpins the receptor's multifaceted interactions on chromatin, which may offer alternative therapeutic avenues. Interestingly, small-molecule-targeting of the AR chromatome components, EP300 and SMARCA2, has already shown great promise in preclinical models of PCa (53,54). The presence of SUMO2 and PIAS proteins of SUMOylation machinery alongside AR on chromatin lead us to show that over a thousand proteins are linked to SUMO2/3 on chromatin in prostate cancer cells. Importantly, these RIME-identified chromatomes of AR and SUMO2/3 are of considerable relevance to prostate cancer biology and anticipated to serve as crucial foundations for forthcoming research.
The large overlap between androgen-induced AR chromatome and SUMO2/3 chromatome strongly suggests that most AR-associated proteins are SUMOylated. This supports the idea that SUMOylation can have a profound influence on the protein-protein interactions of AR on the chromatin and on the transcriptional output of the prostate cancer cells. In keeping with this notion, exposure of the PCa cells to the SUMOylation inhibitor ML-792 altered the association of AR with most of its chromatome members. This supports the idea of group SUMOylation i.e. ’SUMO spray’ as a determinant of protein complex stability or assembly (14), here, as a determinant of AR transcription complexes. This fits with the substantial remodeling of the SUMO2/3 chromatin landscape upon androgen exposure, which reflects the occupancy of SUMOylated AR on the chromatin and an AR-dependent recruitment of SUMOylated TFs and coregulators onto the chromatin. It is worth noting that only a very restricted number of the chromatome members display increased SUMOylation after AR activation, strongly suggesting that TFs and coregulators are recruited to chromatin in an AR-induced manner, but their SUMO2/3 modification is largely independent of the receptor activation.
Our results also shed light on how SUMOylation modulates AR’s binding landscape on chromatin. Strikingly, the effect of SUMOylation on AR chromatin occupancy was not uniform across ARBs. While AR binding was moderately affected by SUMOi on the majority of the ARBs, a significant proportion exhibited either substantial decreases or increases in receptor occupancy. This selective and context-dependent modulatory effect is reminiscent of previous findings where disruption of TFs’ SUMOylation sites differentially influenced their occupancy at various binding sites (7,15,52). Based on our current data, SUMOylation differently dictates AR’s preference for certain compositions and patterns of its binding motifs. Our unbiased data indicate that SUMOylation inhibits the receptor binding to ARBs with multiple ‘perfect’ AREs, which agrees with the critical role of SUMOylation in regulating synergy control motifs, as initially determined by ectopic reporter gene assays (44). This synergy control may represent a mechanism for restricting the strength of AR-driven transcription. Moreover, the binding and enrichment patterns of AR-, FOXA1- and HOXB13- and ERG-binding motifs across differentially SUMOi-affected ARB clusters, along with the changes in AR occupancy upon SUMOi, strongly suggest a DNA-binding motif- and AR-neighboring TF-dependent mechanism for the SUMOylation-mediated regulation of AR chromatin occupancy. In contrast to the synergy control motif concept of AREs, SUMOylation strongly enhanced AR’s occupancy on ARBs that were relatively scarce in AREs but enriched in pioneer TFs FOXA1 and HOXB13. Prior studies have affirmed FOXA1’s importance in mediating AR’s chromatin interactions and AR-driven transcriptional landscape in PCa (34,55,56). Like AR, FOXA1 has been shown to interact with PIAS1 and be subject to PIAS1-promoted SUMOylation (26,34), which influences PIAS1’s chromatin binding and activity with the AR (34). Furthermore, the FOXA1’s SUMOylation status is relevant to its competence to influence proliferation of PCa cells (34). Moreover, differences in chromatin openness across ARB clusters that were differentially influenced by SUMOylation suggest that changes in the chromatin environment are connected to the impact of SUMOylation on AR interactions.
Integration of our RNA-seq and ChIP-seq data offers important novel insights into the ways SUMOylation modulates the transcriptional output of VCaP cells. The extensive intertwining of AR and SUMOylation is clearly reflected in the transcriptional output of VCaP cells. Our RNA-seq data underscore the diverse impact SUMOylation has on transcriptional processes, both in androgen-dependent and -independent manners. In contrast to initial observations (3,32), SUMOylation does not act as a predominantly repressive PTM; instead, it modulates gene expression in both upregulated and downregulated directions. The pathway analysis further reinforces the concept of SUMOylation's selectivity. By differentially impacting cellular pathways based on the presence or absence of DHT, SUMOylation demonstrates a unique capacity to fine-tune cellular processes. The enrichment of pathways, such as ribosome biogenesis, cytoskeleton organization, and mitotic prometaphase indicates SUMOylation's influence on a wide range of cellular functions. Of particular interest is the revelation that SUMOylation impacts pathways like cellular senescence when associated with DHT-upregulation. Given the links between senescence and tumor suppression (6,57), this finding raises interesting questions about the potential therapeutic applications of modulating SUMOylation in prostate cancer therapy.
Most of the genes that were impacted by DHT and SUMOi, associated with nearby ARB. Integration of AR ChIP-seq data with the RNA-seq data provided more insight into connection of SUMOi-affected ARBs with alterations in the VCaP cell transcriptome. The pathway analysis of genes associated with different ARBs revealed a nuanced picture: while the SUMOylation-enhanced (C5) ARB-associated genes predominantly revolved around pattern formation, the SUMOylation-repressed (C6) ARB-associated genes leaned towards negative regulation of cell proliferation. Importantly, both pathways contained genes that can differentiate normal from tumorous prostate tissue. The pattern formation is regulated by WNT/β-catenin, TGF-β and FGF signaling during embryogenesis and tumorigenesis (58) and includes HOX genes (HOXA10, HOXA11 and HOXD8 in our data) that govern lobe development in prostate (59). Many HOX genes are dysregulated in PCa and due to their pro-proliferation and anti-apoptotic roles, represent potential therapeutic targets (59,60). The negative regulation of cell proliferation pathway includes genes SOX4, SOX9 and MSX2 that are of particular interest for PCa biology. SOX9 is essential for prostate development and its overexpression has been associated with prostate tumorigenesis (61,62). SOX4 is linked to promotion of epithelial-to-mesenchymal transition, a process crucial for cancer metastasis (63,64). SOX4 expression has been correlated with increased cell migration and invasion also in PCa (61,63,64). As a part of bone morphogenetic pathway, MSX2 has been associated especially with the bone metastases of the PCa (65). The potential oncogenic or tumor-suppressive roles of these pathways suggest SUMOylation's regulatory role in prostate cancer progression.
SUMO pathway components are typically upregulated in cancers. This may lead to enhanced SUMOylation, which has been suggested to protect the functionality of gene programs and signaling pathways instrumental for cancer cells to survive and proliferate in adverse conditions (6). Fittingly, SUMOylation inhibitors ML-792 and TAK-981, a SUMOi in clinical trials, efficiently suppressed the growth of model PCa cells. Therefore, SUMOi might be worth testing with second generation antiandrogens to evaluate its potential as a component in combination therapies for PCa. Our genome-wide data hint at a potential symbiotic relationship between AR-driven transcriptional programs and SUMOylation in PCa, which is in line with a recent deep learning modeling highlighting the significance of SUMOylation and SUMO ligases in predicting PCa treatment resistance and patient stratification (11). With the AR pathway playing a crucial role in PCa progression, it is conceivable that enhanced SUMOylation serves to stabilize or bolster this pathway, thereby driving the carcinogenesis process forward. Further supporting this notion is the evidence implicating dysregulation of SUMOylation in prostate carcinogenesis and tumor progression (66,67). High SUMO protein expression is associated with poorer disease-free survival (Supplementary Figure S1). AR-associated SUMO ligase PIAS1 in turn shows an elevated expression in PCa, promotes proliferation and apoptosis (68) and predicts poorer survival for patients (69). Additionally, it is upregulated by androgens in CRPC cells (26). Similar to PIAS1, elevated expression of PIAS3 has been reported in prostate tumors (70) which, like PIAS1, may modulate AR activity (26,71), potentially influencing the course of the disease. Also deregulated expression of SENP1 that is capable of cleaving AR-SUMO conjugates and stimulating AR activity (72) has been linked to PCa progression (73). The dysregulation of the SUMOylation system in cancer might not just be a passive consequence but could actively contribute to the initiation and progression of the disease.
In conclusion, this study brings to light the pivotal role of SUMOylation in shaping the AR-associated chromatin landscape in PCa cells. The extensive overlap and interaction between AR and SUMO2/3 systems suggests that they are tightly intertwined in guiding the transcriptional program of PCa cells. The modulation of AR chromatin interactions and the resultant transcriptional outputs by SUMOylation highlights the complex regulatory mechanisms at play, which can have profound implications in prostate cancer progression and therapy. Future research with a focus on therapeutic interventions targeting this AR-SUMO crosstalk may offer potential novel avenues for prostate cancer treatment.
Supplementary Material
Acknowledgements
Eija Korhonen, Merja Räsänen and Saara Pirinen are thanked for their technical expertise and support in cell culture and lab maintenance, and Sini Miettinen, Salla Keskitalo, Antti Tuhkala and Tanja Turunen from the HiLIFE-Proteomics Unit for their expertise in MS runs and peptide analyses of RIME samples. The EMBL GeneCore (Heidelberg, Germany) is greatly acknowledged for deep sequencing, and the UEF Bioinformatics Center (University of Eastern Finland, Finland) for providing computational infrastructure. UEF Cell and Tissue Imaging Unit (University of Eastern Finland, Finland), Biocenter Kuopio and Biocenter Finland are acknowledged for support in live-cell imaging experiments.
Author contributions: K.M.L., V.P. and J.J.P. designed the research. K.M.L., V.V. and N.A. prepared samples and executed experiments. K.M.L. and V.P. prepared sequencing libraries. M.V. lab provided the Mass spectrometry facilities and analyzed raw MS data. K.M.L., V.V. and V.P. analyzed data. K.M.L. and V.P. executed data integration. K.M.L., V.P., E.A.N. and J.J.P. wrote the paper with others’ contribution.
Contributor Information
Kaisa-Mari Launonen, Institute of Biomedicine, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland.
Vera Varis, Institute of Biomedicine, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland.
Niina Aaltonen, Institute of Biomedicine, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland.
Einari A Niskanen, Institute of Biomedicine, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland.
Markku Varjosalo, Institute of Biotechnology, HiLIFE, University of Helsinki, Helsinki, Finland; HiLIFE-Proteomics Unit, University of Helsinki, Helsinki, Finland.
Ville Paakinaho, Institute of Biomedicine, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland.
Jorma J Palvimo, Institute of Biomedicine, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland.
Data availability
This paper utilized existing data to complement observations. The Cancer Genome Atlas Prostate Adenocarcinoma (TCGA-PRAD) data were interpreted and visualized for the angle of SUMOi altered signatures in clinical prostate cancer with Xena Platform (31) and GEPIA (74). The DisGeNET Database 7.0 (30) was utilized to investigate protein association with prostatic diseases. Publicly available ChIP-seq data were obtained from GEO database with accession number GSE56086 (PIAS1).
Newly generated MS data have been deposited to the ProteomeXchange Consortium via the PRIDE (75) partner repository with the dataset identifier PXD039179. ATAC-seq, ChIP-seq and RNA-seq datasets have been submitted to GEO database (76) with accession code GSE211164 and for LNCaP cell RNA-seq data with accession code GSE216549.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
UEF Doctoral Program of Molecular Medicine; Paavo Koistinen foundation [202000004 to K.M.L.]; Finnish Cultural Foundation [00220596 to K.M.L.]; Cancer Foundation Finland [62-5909 to K.M.L.]; Research Council of Finland (333866 to J.J.P.); and Sigrid Jusélius Foundation. Funding for open access charge: the Research Council of Finland.
Conflict of interest statement. None declared.
References
- 1. Burris T.P., de Vera I.M.S., Cote I., Flaveny C.A., Wanninayake U.S., Chatterjee A., Walker J.K., Steinauer N., Zhang J., Coons L.A.et al.. International union of basic and clinical pharmacology. CXIII: nuclear receptor superfamily – update 2023. Pharmacol. Rev. 2023; 75:1233–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shafi A.A., Yen A.E., Weigel N.L.. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol. Ther. 2013; 140:223–238. [DOI] [PubMed] [Google Scholar]
- 3. Poukka H., Karvonen U., Janne O.A., Palvimo J.J.. Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1). Proc. Natl. Acad. Sci. U.S.A. 2000; 97:14145–14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coffey K., Robson C.N.. Regulation of the androgen receptor by post-translational modifications. J. Endocrinol. 2012; 215:221–237. [DOI] [PubMed] [Google Scholar]
- 5. Kotaja N., Karvonen U., Jänne O.A., Palvimo J.J.. PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol. Cell. Biol. 2002; 22:5222–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seeler J.-S., Dejean A.. SUMO and the robustness of cancer. Nat. Rev. Cancer. 2017; 17:184–197. [DOI] [PubMed] [Google Scholar]
- 7. Rosonina E. A conserved role for transcription factor sumoylation in binding-site selection. Curr. Genet. 2019; 65:1307–1312. [DOI] [PubMed] [Google Scholar]
- 8. Pichler A., Fatouros C., Lee H., Eisenhardt N.. SUMO conjugation – a mechanistic view. Biomol. Concepts. 2017; 8:13–36. [DOI] [PubMed] [Google Scholar]
- 9. Bouchard D., Wang W., Yang W.C., He S., Garcia A., Matunis M.J.. SUMO paralogue-specific functions revealed through systematic analysis of human knockout cell lines and gene expression data. Mol. Biol. Cell. 2021; 32:1849–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vertegaal A.C.O. Signalling mechanisms and cellular functions of SUMO. Nat. Rev. Mol. Cell Biol. 2022; 23:715–731. [DOI] [PubMed] [Google Scholar]
- 11. Elmarakeby H.A., Hwang J., Arafeh R., Crowdis J., Gang S., Liu D., AlDubayan S.H., Salari K., Kregel S., Richter C.et al.. Biologically informed deep neural network for prostate cancer discovery. Nat. 2021; 598:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Labrecque M.P., Coleman I.M., Brown L.G., True L.D., Kollath L., Lakely B., Nguyen H.M., Yang Y.C., da Costa R.M.G., Kaipainen A.et al.. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Invest. 2019; 130:4492–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He X., Riceberg J., Soucy T., Koenig E., Minissale J., Gallery M., Bernard H., Yang X., Liao H., Rabino C.et al.. Probing the roles of SUMOylation in cancer cell biology by using a selective SAE inhibitor. Nat. Chem. Biol. 2017; 13:1164–1171. [DOI] [PubMed] [Google Scholar]
- 14. Psakhye I., Jentsch S.. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell. 2012; 151:807–820. [DOI] [PubMed] [Google Scholar]
- 15. Paakinaho V., Kaikkonen S., Makkonen H., Benes V., Palvimo J.J.. SUMOylation regulates the chromatin occupancy and anti-proliferative gene programs of glucocorticoid receptor. Nucleic Acids Res. 2014; 42:1575–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohammed H., Taylor C., Brown G.D., Papachristou E.K., Carroll J.S., D’Santos C.S. Rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) for analysis of chromatin complexes. Nat. Protoc. 2016; 11:316–326. [DOI] [PubMed] [Google Scholar]
- 17. Liu X., Salokas K., Weldatsadik R.G., Gawriyski L., Varjosalo M.. Combined proximity labeling and affinity purification−mass spectrometry workflow for mapping and visualizing protein interaction networks. Nat. Protoc. 2020; 15:3182–3211. [DOI] [PubMed] [Google Scholar]
- 18. Meier F., Brunner A.-D., Koch S., Koch H., Lubeck M., Krause M., Goedecke N., Decker J., Kosinski T., Park M.A.et al.. Online parallel accumulation–Serial fragmentation (PASEF) with a novel trapped ion mobility mass spectrometer*. Mol. Cell. Proteomics. 2018; 17:2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu F., Haynes S.E., Teo G.C., Avtonomov D.M., Polasky D.A., Nesvizhskii A.I.. Fast quantitative analysis of timsTOF PASEF data with MSFragger and IonQuant. Mol. Cell. Proteomics. 2020; 19:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. da Veiga Leprevost F., Haynes S.E., Avtonomov D.M., Chang H.-Y., Shanmugam A.K., Mellacheruvu D., Kong A.T., Nesvizhskii A.I.. Philosopher: a versatile toolkit for shotgun proteomics data analysis. Nat. Methods. 2020; 17:869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi H., Larsen B., Lin Z.Y., Breitkreutz A., Mellacheruvu D., Fermin D., Qin Z.S., Tyers M., Gingras A.C., Nesvizhskii A.I.. SAINT: probabilistic scoring of affinity purification-mass spectrometry data. Nat. Methods. 2011; 8:70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The UniProt Consortium UniProt: the Universal Protein knowledgebase in 2023. Nucleic Acids Res. 2023; 51:D523–D531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buenrostro J.D., Wu B., Chang H.Y., Greenleaf W.J.. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 2015; 109:21.29.1–21.29.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paakinaho V., Johnson T.A., Presman D.M., Hager G.L.. Glucocorticoid receptor quaternary structure drives chromatin occupancy and transcriptional outcome. Genome Res. 2019; 29:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Launonen K.M., Paakinaho V., Sigismondo G., Malinen M., Sironen R., Hartikainen J.M., Laakso H., Visakorpi T., Krijgsveld J., Niskanen E.A.et al.. Chromatin-directed proteomics-identified network of endogenous androgen receptor in prostate cancer cells. Oncogene. 2021; 40:4567–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toropainen S., Malinen M., Kaikkonen S., Rytinki M., Jaaskelainen T., Sahu B., Janne O.A., Palvimo J.J.. SUMO ligase PIAS1 functions as a target gene selective androgen receptor coregulator on prostate cancer cell chromatin. Nucleic Acids Res. 2015; 43:848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heinz S., Benner C., Spann N., Bertolino E., Lin Y.C., Laslo P., Cheng J.X., Murre C., Singh H., Glass C.K.. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010; 38:576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K.. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019; 10:1523–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S., Sun H., Ma J., Zang C., Wang C., Wang J., Tang Q., Meyer C.A., Zhang Y., Liu X.S.. Target analysis by integration of transcriptome and ChIP-seq data with BETA. Nat. Protoc. 2013; 8:2502–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piñero J., Ramírez-Anguita J.M., Saüch-Pitarch J., Ronzano F., Centeno E., Sanz F., Furlong L.I.. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020; 48:D845–D855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldman M.J., Craft B., Hastie M., Repečka K., McDade F., Kamath A., Banerjee A., Luo Y., Rogers D., Brooks A.N.et al.. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020; 38:675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rytinki M., Kaikkonen S., Sutinen P., Paakinaho V., Rahkama V., Palvimo J.J.. Dynamic SUMOylation is linked to the activity cycles of androgen receptor in the cell nucleus. Mol. Cell. Biol. 2012; 32:4195–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishida T., Yasuda H.. PIAS1 and PIASxalpha function as SUMO-E3 ligases toward androgen receptor and repress androgen receptor-dependent transcription. J. Biol. Chem. 2002; 277:41311–41317. [DOI] [PubMed] [Google Scholar]
- 34. Sutinen P., Rahkama V., Rytinki M., Palvimo J.J.. Nuclear mobility and activity of FOXA1 with androgen receptor are regulated by SUMOylation. Mol. Endocrinol. Baltim. Md. 2014; 28:1719–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khanna-Gupta A. Sumoylation and the function of CCAAT enhancer binding protein alpha (C/EBPα). Blood Cells. Mol. Dis. 2008; 41:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ji Z., Degerny C., Vintonenko N., Deheuninck J., Foveau B., Leroy C., Coll J., Tulasne D., Baert J.-L., Fafeur V.. Regulation of the Ets-1 transcription factor by sumoylation and ubiquitinylation. Oncogene. 2007; 26:395–406. [DOI] [PubMed] [Google Scholar]
- 37. Fattet L., Ay A.-S., Bonneau B., Jallades L., Mikaelian I., Treilleux I., Gillet G., Hesling C., Rimokh R.. TIF1γ requires sumoylation to exert its repressive activity on tgfβ signaling. J. Cell Sci. 2013; 126:3713–3723. [DOI] [PubMed] [Google Scholar]
- 38. Rossitto M., Déjardin S., Rands C.M., Le Gras S., Migale R., Rafiee M.-R., Neirijnck Y., Pruvost A., Nguyen A.L., Bossis G.et al.. TRIM28-dependent SUMOylation protects the adult ovary from activation of the testicular pathway. Nat. Commun. 2022; 13:4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Appikonda S., Thakkar K.N., Shah P.K., Dent S.Y.R., Andersen J.N., Barton M.C.. Cross-talk between chromatin acetylation and SUMOylation of tripartite motif–containing protein 24 (TRIM24) impacts cell adhesion. J. Biol. Chem. 2018; 293:7476–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen X., Qin Y., Zhang Z., Xing Z., Wang Q., Lu W., Yuan H., Du C., Yang X., Shen Y.et al.. Hyper-SUMOylation of ERG is essential for the progression of acute myeloid leukemia. Front. Mol. Biosci. 2021; 8:652284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oughtred R., Rust J., Chang C., Breitkreutz B.-J., Stark C., Willems A., Boucher L., Leung G., Kolas N., Zhang F.et al.. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. Publ. Protein Soc. 2021; 30:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toropainen S., Niskanen E.A., Malinen M., Sutinen P., Kaikkonen M.U., Palvimo J.J.. Global analysis of transcription in castration-resistant prostate cancer cells uncovers active enhancers and direct androgen receptor targets. Sci. Rep. 2016; 6:33510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whyte W.A., Orlando D.A., Hnisz D., Abraham B.J., Lin C.Y., Kagey M.H., Rahl P.B., Lee T.I., Young R.A.. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013; 153:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iñiguez-Lluhí J.A., Pearce D.. A common motif within the negative regulatory regions of multiple factors inhibits their transcriptional synergy. Mol. Cell. Biol. 2000; 20:6040–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Langston S.P., Grossman S., England D., Afroze R., Bence N., Bowman D., Bump N., Chau R., Chuang B.-C., Claiborne C.et al.. Discovery of TAK-981, a first-in-class inhibitor of SUMO-activating enzyme for the treatment of cancer. J. Med. Chem. 2021; 64:2501–2520. [DOI] [PubMed] [Google Scholar]
- 46. Noren Hooten N., Evans M.K.. Techniques to induce and quantify cellular senescence. J. Vis. Exp. 2017; 7:55522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paltoglou S., Das R., Townley S.L., Hickey T.E., Tarulli G.A., Coutinho I., Fernandes R., Hanson A.R., Denis I., Carroll J.S.et al.. Novel androgen receptor coregulator GRHL2 exerts both oncogenic and antimetastatic functions in prostate cancer. Cancer Res. 2017; 77:3417–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stelloo S., Nevedomskaya E., Kim Y., Hoekman L., Bleijerveld O.B., Mirza T., Wessels L.F.A., van Weerden W.M., Altelaar A.F.M., Bergman A.M.et al.. Endogenous androgen receptor proteomic profiling reveals genomic subcomplex involved in prostate tumorigenesis. Oncogene. 2018; 37:313–322. [DOI] [PubMed] [Google Scholar]
- 49. van Mierlo G., Vermeulen M.. Chromatin proteomics to study epigenetics — Challenges and opportunities. Mol. Cell. Proteomics. 2021; 20:100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klockenbusch C., O’Hara J.E., Kast J.. Advancing formaldehyde cross-linking towards quantitative proteomic applications. Anal. Bioanal. Chem. 2012; 404:1057–1067. [DOI] [PubMed] [Google Scholar]
- 51. Rafiee M.R., Girardot C., Sigismondo G., Krijgsveld J.. Expanding the circuitry of pluripotency by selective isolation of chromatin-associated proteins. Mol. Cell. 2016; 64:624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sutinen P., Malinen M., Heikkinen S., Palvimo J.J.. SUMOylation modulates the transcriptional activity of androgen receptor in a target gene and pathway selective manner. Nucleic Acids Res. 2014; 42:8310–8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xiao L., Parolia A., Qiao Y., Bawa P., Eyunni S., Mannan R., Carson S.E., Chang Y., Wang X., Zhang Y.et al.. Targeting SWI/SNF ATPases in enhancer-addicted prostate cancer. Nature. 2022; 601:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen Z., Wang M., Wu D., Zhao L., Metwally H., Jiang W., Wang Y., Bai L., McEachern D., Luo J.et al.. Discovery of CBPD-409 as a highly potent, selective, and orally efficacious CBP/p300 PROTAC degrader for the treatment of advanced prostate cancer. J. Med. Chem. 2024; 67:5351–5372. [DOI] [PubMed] [Google Scholar]
- 55. Copeland B.T., Du J., Pal S.K., Jones J.O.. Factors that influence the androgen receptor cistrome in benign and malignant prostate cells. Mol. Oncol. 2019; 13:2616–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sahu B., Laakso M., Ovaska K., Mirtti T., Lundin J., Rannikko A., Sankila A., Turunen J.P., Lundin M., Konsti J.et al.. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011; 30:3962–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sheng Z., Zhu J., Deng Y., Gao S., Liang S.. SUMOylation modification-mediated cell death. Open Biol. 11:210050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Copeland J., Wilson K., Simoes-Costa M.. Micro-managing pattern formation: miRNA regulation of signaling systems in vertebrate development. FEBS J. 2022; 289:5166–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Javed S., Langley S.E.M.. Importance of HOX genes in normal prostate gland formation, prostate cancer development and its early detection. BJU Int. 2014; 113:535–540. [DOI] [PubMed] [Google Scholar]
- 60. Morgan R., Boxall A., Harrington K.J., Simpson G.R., Michael A., Pandha H.S.. Targeting HOX transcription factors in prostate cancer. BMC Urol. 2014; 14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grimm D., Bauer J., Wise P., Krüger M., Simonsen U., Wehland M., Infanger M., Corydon T.J.. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2020; 67:122–153. [DOI] [PubMed] [Google Scholar]
- 62. Huang Z., Hurley P.J., Simons B.W., Marchionni L., Berman D.M., Ross A.E., Schaeffer E.M.. Sox9 is required for prostate development and prostate cancer initiation. Oncotarget. 2012; 3:651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang L., Li Y., Yang X., Yuan H., Li X., Qi M., Chang Y.W.Y., Wang C., Fu W., Yang M.et al.. ERG-SOX4 interaction promotes epithelial-mesenchymal transition in prostate cancer cells. Prostate. 2014; 74:647–658. [DOI] [PubMed] [Google Scholar]
- 64. Liu Y., Zeng S., Jiang X., Lai D., Su Z.. SOX4 induces tumor invasion by targeting EMT-related pathway in prostate cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017; 39:1010428317694539. [DOI] [PubMed] [Google Scholar]
- 65. Koeneman K.S., Yeung F., Chung L.W.. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999; 39:246–261. [DOI] [PubMed] [Google Scholar]
- 66. Yang Y., Xia Z., Wang X., Zhao X., Sheng Z., Ye Y., He G., Zhou L., Zhu H., Xu N.et al.. Small-molecule inhibitors targeting protein SUMOylation as novel anticancer compounds. Mol. Pharmacol. 2018; 94:885–894. [DOI] [PubMed] [Google Scholar]
- 67. Wang Y., Yu J.. Dissecting multiple roles of SUMOylation in prostate cancer. Cancer Lett. 2021; 521:88–97. [DOI] [PubMed] [Google Scholar]
- 68. Hoefer J., Schäfer G., Klocker H., Erb H.H.H., Mills I.G., Hengst L., Puhr M., Culig Z.. PIAS1 Is increased in Human prostate cancer and enhances proliferation through inhibition of p21. Am. J. Pathol. 2012; 180:2097–2107. [DOI] [PubMed] [Google Scholar]
- 69. Puhr M., Hoefer J., Eigentler A., Dietrich D., Van Leenders G., Uhl B., Hoogland M., Handle F., Schlick B., Neuwirt H.et al.. PIAS1 is a determinant of poor survival and acts as a positive feedback regulator of AR signaling through enhanced AR stabilization in prostate cancer. Oncogene. 2016; 35:2322–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang L., Banerjee S.. Differential PIAS3 expression in human malignancy. Oncol. Rep. 2004; 11:1319–1324. [PubMed] [Google Scholar]
- 71. Junicho A., Matsuda T., Yamamoto T., Kishi H., Korkmaz K., Saatcioglu F., Fuse H., Muraguchi A.. Protein inhibitor of activated STAT3 regulates androgen receptor signaling in prostate carcinoma cells. Biochem. Biophys. Res. Commun. 2000; 278:9–13. [DOI] [PubMed] [Google Scholar]
- 72. Kaikkonen S., Jääskeläinen T., Karvonen U., Rytinki M.M., Makkonen H., Gioeli D., Paschal B.M., Palvimo J.J.. SUMO-specific protease 1 (SENP1) reverses the hormone-augmented SUMOylation of androgen receptor and modulates gene responses in prostate cancer cells. Mol. Endocrinol. 2009; 23:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang Q., Xia N., Li T., Xu Y., Zou Y., Zuo Y., Fan Q., Bawa-Khalfe T., Yeh E.T.H., Cheng J.. SUMO-specific protease 1 promotes prostate cancer progression and metastasis. Oncogene. 2013; 32:2493–2498. [DOI] [PubMed] [Google Scholar]
- 74. Tang Z., Kang B., Li C., Chen T., Zhang Z.. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic. Acids. Res. 2019; 47:W556–W560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martens L., Hermjakob H., Jones P., Adamsk M., Taylor C., States D., Gevaert K., Vandekerckhove J., Apweiler R.. PRIDE: the proteomics identifications database. Proteomics. 2005; 5:3537–3545. [DOI] [PubMed] [Google Scholar]
- 76. Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M.et al.. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 2013; 41:D991–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This paper utilized existing data to complement observations. The Cancer Genome Atlas Prostate Adenocarcinoma (TCGA-PRAD) data were interpreted and visualized for the angle of SUMOi altered signatures in clinical prostate cancer with Xena Platform (31) and GEPIA (74). The DisGeNET Database 7.0 (30) was utilized to investigate protein association with prostatic diseases. Publicly available ChIP-seq data were obtained from GEO database with accession number GSE56086 (PIAS1).
Newly generated MS data have been deposited to the ProteomeXchange Consortium via the PRIDE (75) partner repository with the dataset identifier PXD039179. ATAC-seq, ChIP-seq and RNA-seq datasets have been submitted to GEO database (76) with accession code GSE211164 and for LNCaP cell RNA-seq data with accession code GSE216549.