Abstract
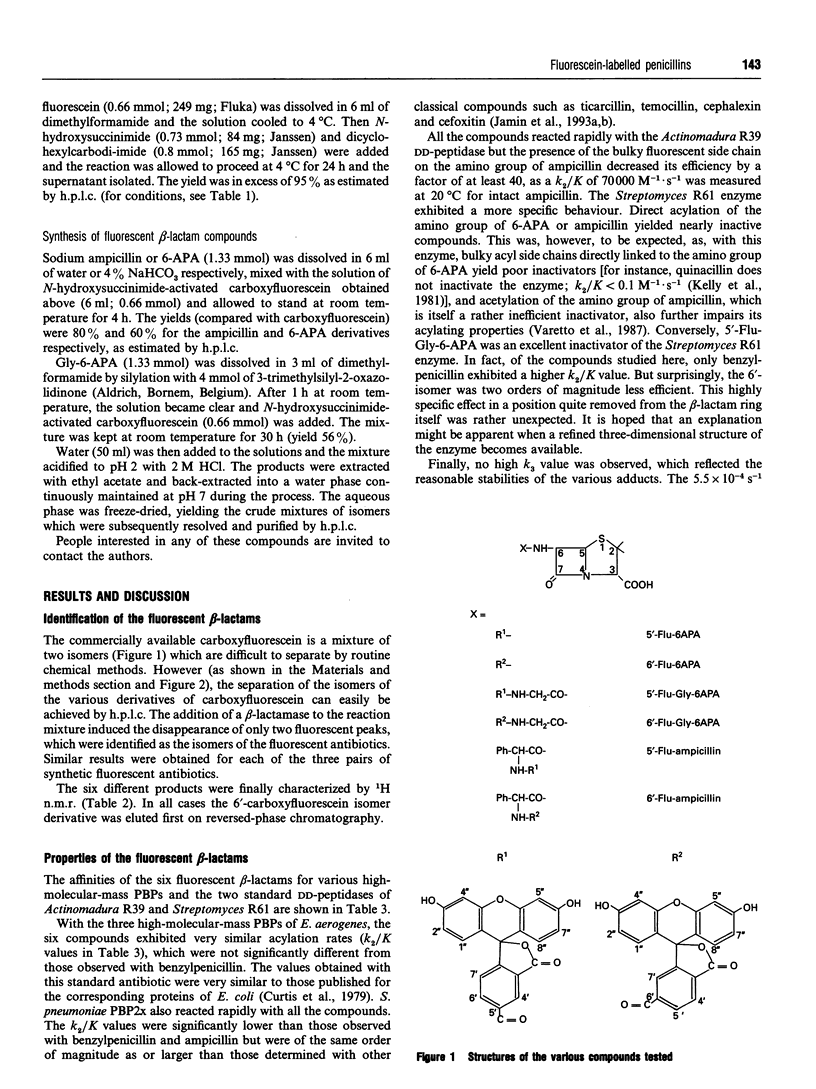
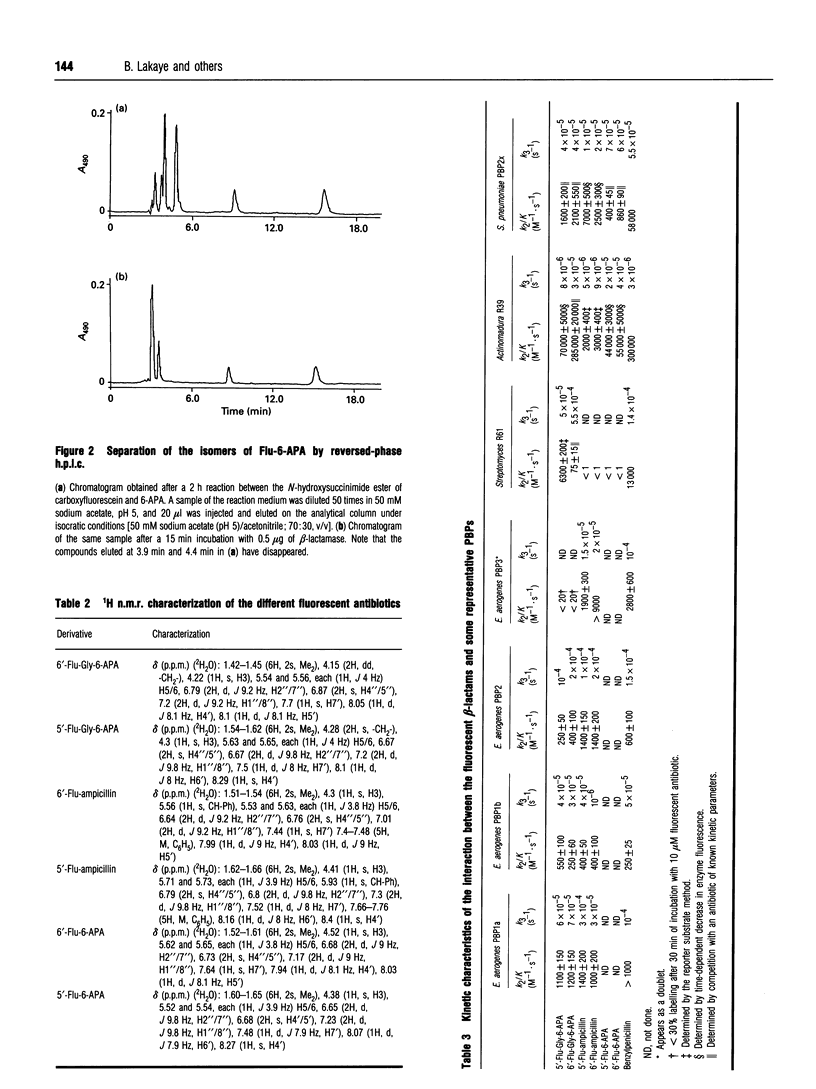
The synthesis and properties of six fluorescein-labelled penicillins are reported. The two isomers of fluoresceyl-glycyl-6-amino-penicillanic acid are probably the best compounds to use for detection of all the penicillin-binding proteins (PBPs) present in a bacterial membrane preparation. However, the derivatives of ampicillin were much more efficient against Enterobacter aerogenes PBP3. The two isomers obtained when a commercial mixture of the two isomers of carboxyfluorescein was used most often exhibited similar properties, but the Streptomyces R61 extracellular DD-peptidase was only efficiently acylated by the 5'-carboxyfluorescein derivative of glycyl-6-aminopenicillanic acid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellido F., Veuthey C., Blaser J., Bauernfeind A., Pechère J. C. Novel resistance to imipenem associated with an altered PBP-4 in a Pseudomonas aeruginosa clinical isolate. J Antimicrob Chemother. 1990 Jan;25(1):57–68. doi: 10.1093/jac/25.1.57. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., Rudd C., White A. J. Inducible type I beta-lactamases of gram-negative bacteria and resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1986 Jan;17(1):51–61. doi: 10.1093/jac/17.1.51. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979 Nov;16(5):533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester F., Joris B., Reckinger G., Bellefroid-Bourguignon C., Frère J. M., Waley S. G. Automated analysis of enzyme inactivation phenomena. Application to beta-lactamases and DD-peptidases. Biochem Pharmacol. 1987 Jul 15;36(14):2393–2403. doi: 10.1016/0006-2952(87)90609-5. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Iwatsubo M. Kinetics of interaction between the exocellular DD-carboxypeptidase-transpeptidase from Streptomyces R61 and beta-lactam antibiotics. A choice of models. Eur J Biochem. 1975 Sep 15;57(2):343–351. doi: 10.1111/j.1432-1033.1975.tb02307.x. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Leyh-Bouille M., Ghuysen J. M., Perkins H. R. Interaction between beta-lactam antibiotics and exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R61. Eur J Biochem. 1974 Dec 16;50(1):203–214. doi: 10.1111/j.1432-1033.1974.tb03889.x. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Moreno R., Ghuysen J. M. Molecular weight, amino acid composition and physicochemical properties of the exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R39. Biochem J. 1974 Oct;143(1):233–240. doi: 10.1042/bj1430233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galleni M., Lakaye B., Lepage S., Jamin M., Thamm I., Joris B., Frère J. M. A new, highly sensitive method for the detection and quantification of penicillin-binding proteins. Biochem J. 1993 Apr 1;291(Pt 1):19–21. doi: 10.1042/bj2910019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadonou A. M., Jamin M., Adam M., Joris B., Dusart J., Ghuysen J. M., Frère J. M. Importance of the His-298 residue in the catalytic mechanism of the Streptomyces R61 extracellular DD-peptidase. Biochem J. 1992 Mar 1;282(Pt 2):495–500. doi: 10.1042/bj2820495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin M., Damblon C., Millier S., Hakenbeck R., Frère J. M. Penicillin-binding protein 2x of Streptococcus pneumoniae: enzymic activities and interactions with beta-lactams. Biochem J. 1993 Jun 15;292(Pt 3):735–741. doi: 10.1042/bj2920735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin M., Hakenbeck R., Frere J. M. Penicillin binding protein 2x as a major contributor to intrinsic beta-lactam resistance of Streptococcus pneumoniae. FEBS Lett. 1993 Sep 27;331(1-2):101–104. doi: 10.1016/0014-5793(93)80305-e. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Frère J. M., Klein D., Ghuysen J. M. Interaction between non-classical beta-lactam compounds and the Zn2+-containing G and serine R61 and R39 D-alanyl-D-alanine peptidases. Biochem J. 1981 Oct 1;199(1):129–136. doi: 10.1042/bj1990129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laible G., Spratt B. G., Hakenbeck R. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1991 Aug;5(8):1993–2002. doi: 10.1111/j.1365-2958.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R., Frère J. M., Ghuysen J. M. Fluorescence and circular dichroism studies on the Streptomyces R61 DD-carboxypeptidase-transpeptidase. Penicillin binding by the enzyme. Biochem J. 1973 Nov;135(3):493–505. doi: 10.1042/bj1350493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras G., el Kharroubi A., van Beeumen J., Coeme E., Coyette J., Ghuysen J. M. Characterization of an Enterococcus hirae penicillin-binding protein 3 with low penicillin affinity. J Bacteriol. 1990 Dec;172(12):6856–6862. doi: 10.1128/jb.172.12.6856-6862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Varetto L., Frère J. M., Nguyen-Distèche M., Ghuysen J. M., Houssier C. The pH dependence of the active-site serine DD-peptidase of Streptomyces R61. Eur J Biochem. 1987 Feb 2;162(3):525–531. doi: 10.1111/j.1432-1033.1987.tb10671.x. [DOI] [PubMed] [Google Scholar]


