Abstract
Tartaric acid is one of the characteristic acids in wine, playing a crucial role in wine characteristics. However, superabundant tartaric acid will form insoluble salts and precipitate in the form of crystals, affecting consumers' purchasing appetite. Therefore, tartaric stability is also one of the important indices for controlling the wine quality. At present, the main processing methods for tartaric stability include cold stabilization, ion exchange treatment, electrodialysis and the addition of exogenous components (gum arabic, metatartaric acid, carboxymethyl cellulose, mannoprotein and potassium polyaspartate). This review summarizes and analyzes the origin of tartaric acid in wine, factors influencing the tartaric stability, detection methods, treatments for tartaric stabilization, and the effects of these methods on the sensory quality of wine. Comparing the effects of these methods on wine quality can provide a basis for the further study of tartaric stabilization methods in order to select an appropriate tartaric stabilization method.
Keywords: Wine, Tartaric acid, Tartar, Stabilization, Sensory quality
Highlights
-
•
The sources, processes and influencing factors of tartaric acid in wine are summarized.
-
•
The detection method and stabilization process of tartaric acid in wine are introduced.
-
•
The impact of tartaric acid stabilization on the sensory quality of wine is discussed.
-
•
The effect of tartaric acid stabilization on the aroma and taste of wine needs to be further studied
1. Introduction
Wine is an alcoholic beverage derived from grapes, characterized by a specific alcohol content achieved through the fermentation process in which yeast consumes the sugars present in the grapes, converting them into ethanol. In addition to its appealing flavor and aroma, wine is rich in health-promoting components such as organic acids, phenols, and aromatic compounds, which have been shown to possess antioxidant properties, regulate lipid and lipoprotein metabolism, and lower blood sugar levels (Ivanova-Petropulos et al., 2018; Kang et al., 2023). Consequently, wine has garnered widespread popularity among consumers.
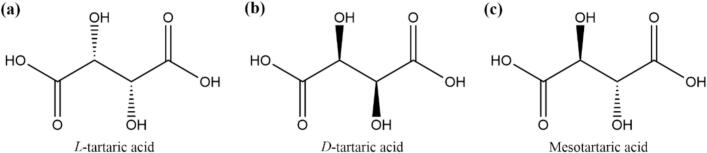
Organic acids represent an essential class of compounds in wine. They enhance the aroma and flavor by softening the taste, reducing astringency, and contributing acidity, while also stabilizing the phenolic substances within the wine, thus serving protective roles in terms of antioxidant activity and color preservation (Volschenk et al., 2006). The primary organic acids found in wine include tartaric acid, citric acid, malic acid, succinic acid, lactic acid, and acetic acid, with tartaric acid being the most abundant. Tartaric acid, also known as 2,3-dihydroxysuccinic acid with the molecular formula C4H6O6, contains two chiral carbon atoms, resulting in three optical isomers: levorotatory, dextrorotatory, and mesoform, which are illustrated in Fig. 1 (Pei et al., 2021). Among grapes, bananas, hawthorns, tamarinds, and various fruits, L-tartaric acid is the sole form present, with grapes containing the highest concentration. In the absence of exogenous additives, the tartaric acid in wine originates entirely from grapes, implying a direct correlation between the tartaric acid content in grapes and that in wine. The concentration of tartaric acid varies significantly among different grape varieties, with wine grapes exhibiting higher levels than both table grapes and those used for juice production (Niu et al., 2022). In addition, tartaric acid remains stable during wine fermentation as it resists metabolism, degradation, or transformation. It is not effectively metabolized by microorganisms unless fermentation temperatures exceed 35 °C (Li et al., 2007; Melino et al., 2009). Consequently, tartaric acid integrates readily into wine, assisting in maintaining acidity, lowering pH, inhibiting bacterial growth, and preserving long-term freshness. Additionally, tartaric acid is vital for strengthening the structure of wine, enhancing its flavor profile, and protecting its color integrity (Yang, 2021).
Fig. 1.
Three optical isomers of tartaric acid.
However, tartaric acid readily combines with potassium (K+) or calcium (Ca2+) in wine to create tartrates. Under wine conditions, tartar tends to precipitate readily and form crystals that attach to the bottom and sides of bottles, or the corks (Tan et al., 2016).
While tartar crystals pose no harm to consumers, they impact the visual appeal of wine, potentially affecting consumers' purchase decisions. Therefore, to prevent tartrate precipitation and inhibit tartrate crystal formation in wine, methods such as cold stabilization, ion exchange treatment, electrodialysis, and the addition of stabilizers are employed prior to bottling (Waterhouse et al., 2016). However, tartaric stabilization treatments inevitably impact the aroma, taste, or color of wine. Hence, striking a balance between the level of tartaric stabilization and its effects on the sensory characteristics of wine is of utmost importance. However, there are currently few studies and reviews on the effects of wine tartar stabilization treatment and wine sensory quality.
This review summarized the origin of tartaric acid in wine, factors influencing the tartaric stability, detection methods for tartaric acid, and treatments for tartaric stabilization. Additionally, the effects of different stabilization methods on the sensory quality of wine were also discussed. The objective was to establish a theoretical basis for choosing a wine tartaric acid stabilization treatment plan and advancing new stabilization technologies.
2. Tartaric acid in grapes and wine
2.1. Tartaric acid in grapes
Tartaric acid is one of the three primary organic acids naturally present in grapes. Initial research indicated that tartaric acid originated in grape leaves, and was later transported and stored in grape berries. However, subsequent studies demonstrated that grape berries also serve as the primary locations for tartaric acid synthesis, notably in the grape pulp. Throughout the development period of grape berries, tartaric acid mainly generates and accumulates in young berries. After veraison, as the berries enlarge and water content rises, the concentration of tartaric acid decreases to 2–6 g/L in grapes (Cao et al., 2021; Li et al., 2007; Waterhouse et al., 2016).
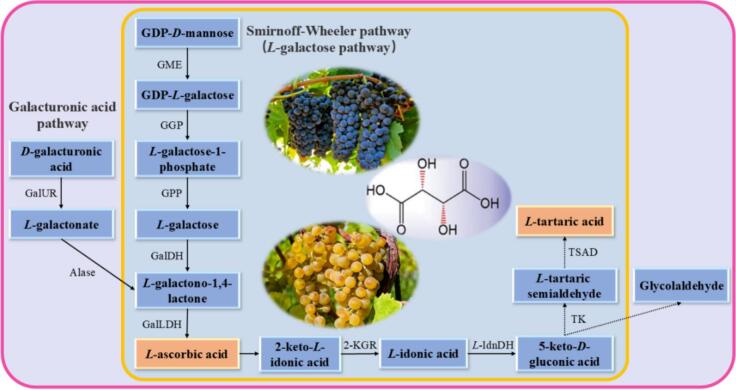
Tartaric acid in grapes is directly converted from the C1-C4 in L-ascorbic acid (Saito & Kasai, 1969). Therefore, the synthesis of tartaric acid involves two main steps: the synthesis of L-ascorbic acid (step 1) and the synthesis of tartaric acid (step 2). Fig. 2 summarizes the proven biosynthetic route of L-tartaric acid during grape growth.
Fig. 2.
Biosynthesis pathways of tartaric acid in grapes. GME, GDP-D-mannose-3′, 5′-epimerase; GGP, GDP-L-galactose phosphorylase; GPP, L-galactose-1-phosphate phosphatase; GalDH, L-galactose dehydrogenase; GalLDH, L-galactono-1,4-lactone dehydrogenase; 2-KGR, 2-keto-L-gulonic acid reductase; L-IdnDH, L-idonate dehydrogenase; TK, Transketolase; TSAD, Tartaric semialdehyde dehydrogenase; GalUR, D-galacturonate reductase; Alase, Aldolactonase. The dotted line indicates the unconfirmed route.
Step 1, the biosynthesis of L-ascorbic acid: There are four known biosynthetic pathways for L-ascorbic acid in plants, the Smirnoff-Wheeler pathway (also known as the L-galactose pathway) (Wheeler et al., 1998), the galacturonic acid pathway (Agius et al., 2003), the gulose pathway (Wolucka & Van Montagu, 2003), and the inositol pathway (Lorence et al., 2004). The confirmed synthesis pathways for L-ascorbic acid in grapes include the Smirnoff-Wheeler pathway and the galacturonic acid pathway, while the existence of the other two pathways in grape berries requires further exploration. In the Smirnoff-Wheeler pathway, GDP-D-mannose acts as the initial metabolite, undergoing isomerization and phosphorylation to form L-galactose, followed by a two-step redox process leading to the production of L-ascorbic acid (Wheeler et al., 1998). The galacturonic acid pathway was first discovered in strawberries (Agius et al., 2003). Cruz-Rus et al. (2010) found D-galacturonate reductase gene (VvGalUR) in Palomino and confirmed a positive correlation between the expression of VvGalUR and the content of ascorbic acid. In the galacturonic acid pathway, L-ascorbic acid can be synthesized from D-galacturonic acid. The primary intermediates are L-galacturonic acid and L-galacturonic acid-1,4-lactone (Agius et al., 2003).
Ascorbic acid biosynthesis in grapes involves multiple pathways throughout fruit development. The Smirnoff-Wheeler pathway serves as the primary route for ascorbic acid production before grape berry ripening, with its significance diminishing as the grapes mature (Li et al., 2023). In contrast, the expression levels of crucial genes in the galacturonic acid pathway, including D-galacturonic acid reductase gene (VvGalUR) and L-galactono-1,4-lactone dehydrogenase gene (VvGalLDH), rise as grapes develop, indicating that this pathway predominantly synthesizes ascorbic acid during ripening (Cruz-Rus et al., 2010). Tartaric acid accumulates primarily at the green stage in grape berries, suggesting that the synthesis of ascorbic acid to produce tartaric acid relies mainly on the Smirnoff-Wheeler pathway.
Step 2, tartaric acid synthesis: The production of tartaric acid commences with the conversion of ascorbic acid into 2-keto-L-gulonic acid through hydrolysis and oxidation. This compound is then catalyzed sequentially by 2-keto-L-gulonic acid reductase, L-iduronic acid dehydrogenase, transketolase, and tartaric semialdehyde dehydrogenase to yield tartaric acid (Cao et al., 2021; Jia et al., 2019).
2.2. Tartaric acid in wine
Tartaric acid is present in three forms in wine: free tartaric acid (H2T), hydrogen tartrate (HT−) and tartrate (T2−). The proportion of H2T, HT− and T2− correlates with the pH level. In the pH range of wine, HT− is the predominant form, making up 50%–70% of the total tartaric acid. HT− and T2− can react with K+ and Ca2+ to form precipitates as potassium hydrogen tartrate (KHT) and calcium tartrate (CaT). Since the content of K+ in wine is 10 times that of Ca2+, the main tartar crystal is KHT (Gonçalves et al., 2003).
The solubility of KHT in wine is influenced by temperature, alcohol content and pH level. Higher alcohol content, lower temperature and higher pH level result in decreased solubility of KHT. During fermentation, as the alcohol content in wine rises, tartrate gradually precipitates, leading to the crystallization of KHT on the inner walls of the fermentation tank. In wine, crystalline KHT appears as right-angled prisms or diamonds (Coulter et al., 2015; Waterhouse et al., 2016).
Compared to K+, the quantity of Ca2+ in wine is relatively small (Ough et al., 1982). And the amount of T2− is lower than that of HT− at wine pH. Although T2− can combine with Ca2+ to form soluble CaT (Mckinnon et al., 1994). The content of CaT crystalline is quite low. However, during the long-term storage of wine, some of the dextro CaT (with a solubility of 230 mg/L) is gradually transformed into racemic CaT (with a solubility of 30 mg/L) (Zhao & Wang, 2001). This transformation greatly reduces the solubility of CaT after prolonged storage of wine. Therefore, while avoiding the formation of KHT crystals, the appearance of CaT crystals should also be prevented.
3. Factors affecting the stability of tartaric acid
The process of tartar crystal formation involves crystal nucleation and growth. There are two types of crystal nuclei: homogeneous nucleation, where crystal-forming ions associate in a supersaturated solution through electrostatic interactions, spontaneously creating crystal nuclei; and heterogeneous nucleation, where solid particles mixed in the solution act as crystal nuclei (Barlow & Gregus, 2019). When the crystal nuclei grow, ions diffuse to their surface and crystallize, resulting in the formation of tartaric acid crystals. And several factors can influence tartar formation in wine.
3.1. Wine compositions
3.1.1. The concentrations of K+, Ca2+ and tartaric acid conjugated base in wine
The concentrations of K+, Ca2+ and tartaric acid conjugated base in wine influence the equilibrium for tartrate formation. They are described that K+ and Ca2+ with tartrate according to the equations:
K+ + HT− ⇌ KHT (1).
Ca2+ + T2− ⇌ CaT (2).
Upon reaching a supersaturated state, excess tartrate will precipitate and crystallize. When the concentration of any of these ions decreases, the chemical equilibrium will shift in the opposite direction, leading to the dissolution of tartrate into an ionic form that is less prone to precipitation (Wang, 2004). Therefore, controlling the concentration levels of K+, Ca2+ and tartaric acid conjugated base can effectively prevent the formation of tartar.
3.1.2. Colloidal substances in wine
Wine is a solution that contains various colloidal components, including tannins, proteins, and saccharides, which also affect the precipitation of tartrate (Waterhouse et al., 2016).
(1) Protein and tannin.
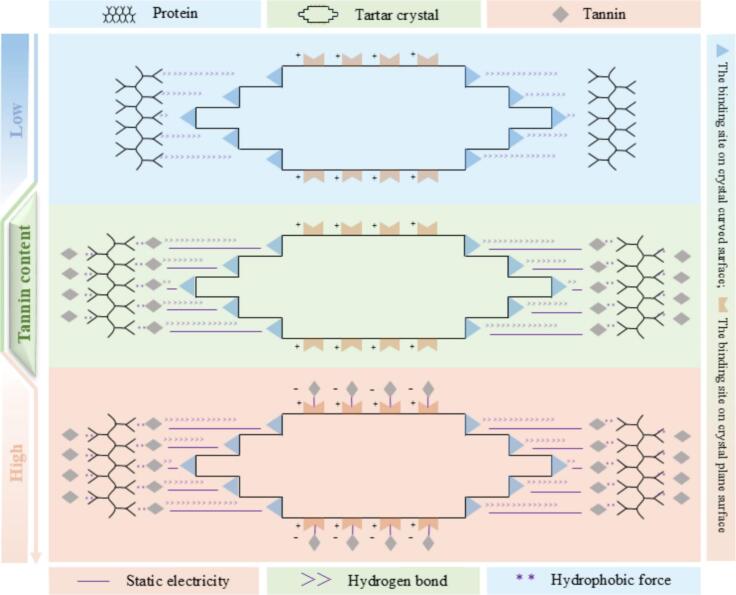
Proteins act as a ‘coating’ to inhibit further growth of tartrate crystal. At a pH of 3.3, proteins partially unfold, exposing more amino acids and increasing the number of binding sites binding to the surface of tartrate crystals. When tannin and protein coexist, they form aggregates centered on protein and covered by tannin, exerting complex effects on tartrate crystallization. In a 1:10 ratio of protein to tannin, the aggregates easily precipitate, diminishing the protective effect of protein on tartrate crystals. As the ratio reaches 1:30, protein is covered by more tannin molecules, forming small and easily soluble aggregates. Additionally, some hydroxyl groups on the tannin molecules not bound to proteins attach to the curved surfaces of the tartrate crystal by hydrogen bonds, providing protection to the crystal. However, tannin, being a weak acid at low pH, carries the same charge as the exposed HT− on the crystal surface, which weakens the hydrogen bond between tannin and the crystal surface due to electrostatic repulsion, leading to poor protection by the protein-tannin polymer. In solutions with high tannin content (protein: tannin = 1:50 or 1:80), tannin may combine with the tartar crystal plane without affecting crystal growth as the crystal plane remains inactive in the growth process. Consequently, the tartrate retention capacity decreases as the protein-to-tannin ratio decreases (Lambri et al., 2014). Fig. 3 demonstrates the binding of protein, tannin and tartar crystal as the tannin content changes. Concerning CaT, proteins and tannins can delay crystal nuclei formation by complexing with Ca2+, yet they do not influence crystal growth (Postel et al., 1984).
Fig. 3.
Schematic diagram of the combination of protein, tannin and tartar crystal (Lambri et al., 2014).
(2) Saccharides.
Saccharide is also colloidal substance found in wine. The two main groups that affect the tartrate stabilization of wine are glycoproteins and uronic acids, such as galacturonic acid, glucuronic acid, polygalacturonic acid, rhamnogalacturonan-I (RG-I), rhamnogalacturonan-II (RG-II), arabinogalactan protein (AGP) and mannoprotein. When 0.5 g/L galacturonic acid, glucuronic acid, and polygalacturonic acid were added to three simulated wine solutions at pH 3.5. Polygalacturonic acid was able to combine with 23% Ca2+ in the solution to form an ‘egg box’ model. This extension increased the CaT crystallization time from 7 to 510 min, inhibiting CaT precipitation. The inhibitory effect of polygalacturonic acid on CaT crystallization decreased as its concentration decreased. Even at a concentration as low as 62.5 mg/L, the inhibitory effect remained noticeable. Galacturonic acid had no significant effect on the induction time of tartrate crystallization but decreased the crystallization rate, while glucuronic acid had no effect on the induction time or rate of tartrate crystallization (McKinnon et al., 1996; Pellerin et al., 2013).
The results of the evaluation of AGP, RG-I, RG-II, and mannoprotein for tartaric acid stabilization showed that RG-I was the most potent inhibitor of CaT precipitation. Consisting of two parallel polymer chains, RG-I formed a cavity capable of sequestering Ca2+. Its carboxyl binding site chelated with Ca2+ to retain it, thereby prolonging the time before CaT precipitation. RG-II also prolonged the induction time of CaT precipitation, although less effectively than RG-I. Although RG-II has the same structural features as RG-I, including two parallel chains and carboxyl binding sites, its larger cavity prevented Ca2+ encapsulation, leading to no isolating effect on Ca2+. Thus, the impact of RG-II on the induction time of CaT precipitation is speculated to be primarily due to its impact on crystal growth via uronic acid. Mannoprotein minimally affected the induction time of CaT precipitation. Typically, the combination of Ca2+ and phosphate occurs at higher pH levels. But at wine pH, this combination is less likely, resulting in mannoprotein having no chelation effect on Ca2+. The ability of AGP to bind Ca2+ was related to the carboxyl group of glucuronic acid in AGP, but an excess of H+ at low pH led to the dissociation of AGP's Ca2+ binding (Gerbaud et al., 1996; Lamport & Varnai, 2013; Leszczuk et al., 2020; Pellerin et al., 2013). In addition, white wine is more prone to tartrate precipitation than red wine due to the lower content of RG-I and RG-II in white wine.
3.1.3. Organic acids in wine
Organic acids in wine can bind with K+ and Ca2+ to compete with tartaric acid, thereby inhibiting the formation of crucial crystalline nuclei. The inhibition sequence of CaT precipitation by organic acids was found to be citric acid > malic acid > lactic acid > succinic acid when various organic acids were added at 2 g/L to wine. Malic acid prolonged the induction time of CaT precipitation by 16 times, whereas lactic acid only did so by 4 times (McKinnon et al., 1995). Furthermore, the solubility of KHT decreases with the reduction of malic acid content in wine (Zhu, 1995). During fermentation, microorganisms metabolize citric acid and malic acid to generate lactic acid and succinic acid. As a result, the inhibitory effect of organic acids on CaT crystal formation diminishes, increasing the likelihood of tartaric precipitation in wine post-fermentation.
3.2. pH
The pH level influences the dissociation state of tartrate. In dry red wine, tartrate exists primarily in molecular form when the pH is below 2.75. As the pH rises, the concentration of HT− also increases. At a pH of 3.6, the proportion of tartrate in ionic form is the highest, and the concentration of tartrate molecules is insufficient to form tartrate crystal nuclei, resulting in stable tartrate in the wine. As the pH rises above 3.6, the concentration of HT− declines continuously, resulting in the formation of T2− (Yu et al., 1999). Consequently, a higher pH value will result in an increased concentration of tartrate in the wine, thereby increasing the risk of tartar formation.
3.3. Temperature
As the primary tartrates in wine, the solubilities of KHT and CaT are influenced by temperature. The formation of crystal nuclei is a reversible process (Gao et al., 1999; Zhu, 1995). As the temperature increases, the solubilities of KHT and CaT also increase, and the crystal nuclei formed in the solution are re-dissolved. Conversely, lower temperature can reduce the solubility of tartrate in wine, promoting its crystallization. Therefore, temperature changes can alter the saturation state of tartrate in wine.
3.4. Alcohol content
The solubility of tartrate is inversely proportional to the alcohol content of the solution. The higher the alcohol content, the lower the solubility of tartrate in the wine, thereby rendering the tartrate more unstable (Wang, 2004). An increase in the alcohol content of the simulated liquor from 10% to 12% or 14% was accompanied by a significant increase in the conductivity drop value and saturation temperature of the simulated liquor (Lambri et al., 2014). This indicates that the stability of tartaric acid in wine is negatively impacted by an increase in alcohol content.
3.5. Oxygen content in wine
Wine typically contains a variety of phenols, which can be divided into two categories based on their structures: flavonoid phenols and non-flavonoid phenols. Flavonoid phenols include flavonoids, tannins and anthocyanidins, while non-flavonoid phenols mainly consist of phenolic acids (Ma et al., 2023). When exposed to aerobic conditions, phenols undergo an oxidation reaction, which ultimately leads to the formation of polymer molecules though polymerization. When the degree of polymerization of the product is high enough, it can precipitate or co-precipitate with protein, which can easily induce the tartrate nucleation in wine, leading to tartaric acid instability (Guo, 2007; Wang, 2004).
4. The detection methods of tartaric acid in wine
4.1. High-performance liquid chromatography (HPLC)
The high-performance liquid chromatography (HPLC) detection method is a widely utilized approach for the identification and quantification of organic acids. Among the most common methods employed in this context are ion chromatography (IC) and reversed-phase high-performance liquid chromatography (RP-HPLC).
4.1.1. IC
IC is a chromatographic technique that utilizes ion exchange resin with low exchange capacity as the stationary phase to separate ionic substances (Hungerford et al., 2023).
The determination of tartaric acid in wine usually uses solid-phase extraction or direct dilution method for pre-processing, combined with an anion analytical column, conductivity detectors and anion suppressors to identify the conductivity signal of the ions (Mu et al., 2022; Song et al., 2018). Song et al. (2018) developed a conductivity suppression-gradient elution ion chromatography method for the determination of 26 organic acids and anions in wine. This method enables the quantification of tartaric acid, succinic acid, maleic acid and other organic acids in wine.
This method is capable of simultaneously detecting multiple organic acids in a sample with high accuracy, sensitivity, and reproducibility. This method is suitable for the analysis of low-content and low-molecular-weight organic acids present in wine. However, due to its lengthy analysis time, it is not optimal for rapid sample detection (Pang et al., 2019).
4.1.2. RP-HPLC
RP-HPLC is employed to separate nonpolar, polar, or ionic compounds depending on the intermolecular bond strengths with the stationary phases (Nair et al., 2022). In the case of wine analysis, employing C18 as the stationary phase, with a mobile phase comprising methanol and a 0.1% phosphoric acid solution, facilitates either gradient or isocratic elution processes. Subsequently, the analysis of tartaric acid content within the sample is viable.
RP-HPLC has diverse applications, including the quantification of tartaric acid in a variety of wines (such as red, white, rose, and ice wines) and fruit wines across different climatic conditions (warm and cold climates) within the concentration range of 0.0313–1.89 g/L (Chahine & Tong, 2019). Besides exhibiting high column efficiency, reproducibility, and selectivity, RP-HPLC is adaptable to most detectors and can simultaneously analyze multiple organic acids in samples. Despite these benefits, the limits of this method include low sensitivity and potential environmental pollution due to the extensive use of mobile phases. To determine small molecular organic acids effectively, it is recommended to utilize eluents with high polarity and low pH value while avoiding continuous analysis of large sample quantities (Pang et al., 2019).
4.2. Capillary electrophoresis (CE)
CE is a liquid-phase separation method that employs a capillary tube as the separation channel and a high-voltage direct current field as the driving force (Pitkanen & Siren, 2022). CE is commonly coupled with either an ultraviolet (UV) detector or a mass spectrometry (MS) detector for the quantification of tartaric acid in wine, which are displayed in Table 1.
Table 1.
The determination of tartaric acid in wine by capillary electrophoresis.
| Detectors | LOD (mg/L) |
LOQ (mg/L) |
Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| UV | 0.38 | 1.31 | Short analysis time | Low sensitivity and poor qualitative ability | (Mato et al., 2007) |
| MS | 1.0 | – | High sensitivity and specificity | Sheath fluid has certain influence on the test results | (Li et al., 2013) |
LOD, the limit of detection; LOQ, the limit of quantification.
CE is a suitable method for the rapid analysis of organic acids owing to its high determination speed, excellent resolution, minimal chemical consumption, and low sample requirement. However, its drawbacks include poor reproducibility and inaccurate quantitative analysis results attributed to the variability in the absolute migration time of solutes (Mato et al., 2007; Pang et al., 2019).
4.3. Gas chromatography (GC)
GC is another commonly utilized method for organic acid detection, with tartaric acid detectable through derivatization in conjunction with GC. Silylation and esterification are the primary derivatization techniques employed for tartaric acid analysis by GC. Analyzing wine using N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) for silylation derivatization combined with GC–MS enables the simultaneous identification of 2 monosaccharides, 8 organic acids, and 14 amino acids. The linear range spanned 109–7000 mg/L, with a LOD of 1.52 μg/L and a LOQ of 4.51 μg/L for tartaric acid (Zhang et al., 2018). Esterification of tartaric acid through iodine lactonization using tetramethylammonium hydroxide, N, N-dimethyl formamide, and iodoethane ensures that the analysis of tartaric acid in homemade and branded wines remains unaffected by sugars, water, pigments, or other wine sample constituents (Du et al., 2007).
GC employs an environmentally friendly gaseous mobile phase for tartaric acid detection. When paired with a highly sensitive selective detector, GC provides the benefit of heightened sensitivity. However, since tartaric acid is a non-volatile acid, pretreatment of the sample is necessary via derivatization, leading to heightened complexity and duration in sample processing (Du et al., 2007; Pang et al., 2019).
5. The treatment method for tartaric acid stability
Tartaric acid stability is defined as its capacity to prevent tartrate precipitation. While tartar itself is harmless to human health and has no effect on the flavor of wine, its appearance resembling shattered glass could be unappealing to consumers. Therefore, winemakers usually adopt one or more methods to stabilize the tartaric acid in wine before it is bottled and sold. The process of stabilizing tartaric acid in wine is commonly categorized into two approaches. The first method entails diminishing the tartrate concentration in wine by removing K+, Ca2+, and the tartaric acid conjugate base via technological procedures, referred to as the ‘subtraction’ tactic. The second method involves introducing external stabilizing agents into the wine to enhance its capability to preserve tartrate and avert tartrate precipitation, identified as the ‘addition’ approach (Li & Xia, 2020).
5.1. The ‘subtraction’ strategy
The ‘subtraction’ method of stabilizing tartaric acid involves diminishing the solubility of tartrate in wine to induce crystallization, followed by filtration to eliminate the precipitated tartrate. This approach decreases the tartrate concentration in wine, ensuring its stability throughout storage and distribution. Primarily, it comprises cold stabilization, ion exchange procedures, and electrodialysis.
5.1.1. Cold stabilization method
The solubility of KHT in wine increases with increasing temperature. When the temperature increases from −4°C to 20°C, the solubility of KHT increases by three times. Following this principle, the wine can be cooled to 0.5°C above the freezing temperature of wine, and stirred continuously for one week to several weeks to make the tartrate supersaturate and precipitate. Then, the wine is filtered at a low temperature to remove the KHT crystals. Subsequently, the wine is gradually returned to storage temperature to achieve an unsaturated state, thereby ensuring the tartaric acid stability in the wine (Li & Xia, 2020; Wei et al., 2019). This method primarily employs three cold treatment methods: long-term treatment, contact stability, and continuous stability. The first method abstains from adding tartaric crystals and relies on extended cold treatment for tartaric acid stabilization. Conversely, the latter two methods expedite cold stabilization by introducing tartaric crystals during the process (Li et al., 2007). The process of cold treatment for wine tartaric acid stabilization is shown in Fig. S1.
The efficacy of cold treatment in stabilizing tartaric acid is contingent upon the specific conditions of the treatment and the characteristics of the wine being treated. Lower temperature, faster cooling rate and longer treatment time facilitate the precipitation of tartrate and enhance the stability of tartaric acid. Additionally, wines with higher clarity and shorter aging time demonstrate a more pronounced stabilizing effect of cold treatment. Furthermore, stirring can facilitate the combination of tartrate and tartar crystal nuclei and accelerate tartar precipitation (Li et al., 2007).
Cold stabilization is the primary, efficient, and commonly employed technique for improving tartaric acid stability in wines. Nonetheless, this method exclusively targets KHT removal without affecting CaT due to minimal changes in CaT solubility, and potentially leads to new wine stability issues. For example, the co-precipitation phenomenon associated with cold stabilization significantly influences the levels of anthocyanins and tannins in wine. The presence of tartrate crystals in cold-stabilized Carignan wines has been demonstrated to contain 0.2%–0.3% anthocyanins and 1.9%–2.5% tannins (Vernhet et al., 1999). Moreover, the cooling and maintenance of low temperature necessitate a significant expenditure of energy. To enhance energy efficiency, cooled wine after filtration can undergo exchange with the wine for cooling through a cold-heat exchange method to optimize energy usage and minimize consumption. Alternatively, the addition of seed crystals can initiate tartrate nuclei formation in the wine, reducing cold stabilization duration (Wei et al., 2019). Furthermore, the crystals generated during the process tend to adhere to the inner walls of tanks, complicating equipment cleaning procedures.
5.1.2. Ion exchange treatment
Ion exchange treatment is the process of removing K+ and Ca2+ from wine by exchanging cations within resin with the corresponding ions in the wine. This technique diminishes the likelihood of tartar precipitation by reducing the levels of K+ and Ca2+ in the wine (Wei et al., 2019). In Fig. S2, the principle and procedure of ion exchange treatment are demonstrated.
The effectiveness of ion exchange treatment in stabilizing tartaric acid is influenced by the resin types and their activation procedures. Ion exchange resins are categorized into anion exchange resins and cation exchange resins. Studies demonstrate that decreasing the levels of K+ and Ca2+ using cation exchange resins yields superior tartaric acid stabilization compared to reducing HT− and T2− contents with anion exchange resins, with fewer adverse effects on wines (Mira et al., 2006). As a result, cation exchange resins are frequently employed for tartaric acid stabilization in wine. Typically, ion exchange resins are activated using an acidic solution, such as sulfuric or hydrochloric acid. A greater volume and concentration of acid applied lead to enhanced tartaric stability in the wine. This is because the H+ attached to the resin can be exchanged with more metal ions in the wine, resulting in a lower K+ and Ca2+ levels and a higher TA content in the wine (Ponce et al., 2018). Nevertheless, the OIV mandates that the pH of wines undergoing cation exchange resin treatment should not decrease by more than 0.3 and must remain above 3.0 (OIV, 2022a). Consequently, when employing cation exchange resins for stabilizing wine tartaric acid, it is crucial to select the correct volume and concentration of the acid utilized. Additionally, the tartaric stability of original wine and the percentage of wine subjected to treatment impact the effectiveness of the process (Mislata et al., 2021). Certain studies suggest that a sherry wine treatment ratio of 10%–15% proves to be adequate (Benítez et al., 2002; Ponce et al., 2018).
Cation exchange resins are employed to improve tartaric stability in wine, leading to a decrease in the concentration of metal ions like Fe3+ and Cu2+, thus effectively averting iron and copper rupture diseases. Additionally, this method boasts low energy consumption. Nevertheless, it diminishes wine anthocyanin content, requires substantial initial equipment investment, and exhibits low efficacy, making it exclusively viable for large wineries (Wei et al., 2019).
5.1.3. Electrodialysis method
Electrodialysis is a process whereby ion exchange membranes exhibit selective permeability to ions in solution under the influence of an external direct current. This enables anions and cations in solution to move in opposite directions and pass through the anion exchange membrane and cation exchange membrane, respectively, with the objective of achieving desalination or concentration (Gu et al., 2022). When wine is subjected to electrodialysis treatment, HT−, T2− and K+, as well as Ca2+, move to opposite electrode direction under the potential difference and are discharged through the selective permeability membrane into polar water chambers. This results in the removal of the effective components that form tartrate (Zhang, 2007). Fig. S3 illustrates the schematic diagram of the electrodialysis method.
The stabilization of tartaric acid through the electrodialysis method is contingent upon fluctuations in both ion concentration and voltage. These factors serve as the driving force behind the removal of ions. The greater the initial concentration of ions that contribute to tartrate formation in wine, the higher the voltage, resulting in more effective tartrate removal (Nuri et al., 2019).
Electrodialysis has been demonstrated to be an effective method for improving the tartaric stability of wine, with minimal impact on the wine body and low energy consumption. Nevertheless, this method exhibits a low treatment efficiency and necessitates a substantial initial investment during the initial stage (Wei et al., 2019). Consequently, electrodialysis is more appropriate for large wineries.
5.2. The ‘addition’ strategy
The ‘addition’ strategy of tartaric stability in wine refers to the addition of stabilizers to the wine in order to inhibit the precipitation of tartrate. The most commonly used stabilizers are gum arabic, metatartaric acid, carboxymethyl cellulose, mannoprotein and potassium polyaspartate. The limits of additives are illustrated in Table 2 and Fig. S4 shows the operation principle of these stabilizers.
Table 2.
Allowable amounts of stabilizers in wine (g/L).
| Additive Institutions |
GA | MAT | CMC | MP | KPA | References |
|---|---|---|---|---|---|---|
| USA | 1.9 | Nad | Ad | 0.4 | Nad | (United States, Code of Federal Regulations, 2015) |
| Australia | Ad | Ad | Ad | 0.4 | 0.1 | (Australia Government, 2021) |
| OIV | 0.3 | 0.1 | 0.2 | Ad | 0.1 | (OIV, 2022b, OIV, 2022c, OIV, 2022d, OIV, 2022e, OIV, 2022f) |
| China | Nad | Nad | Nad | Nad | 0.3 | (GB 2760–2014, 2014; National Health Commission of the People's Republic of China, 2023) |
GA, gum arabic; MAT, metatartaric acid, CMC, carboxymethyl cellulose; MP, mannoprotein; KPA, potassium polyaspartate; Ad, allowed but no clear limits; Nad, not allowed to use.
5.2.1. Gum arabic stabilizer
Gum arabic, the secretion product of Acacia senegal (Linn.) Willd, is composed of hydrophobic proteins and macromolecular polysaccharides and their calcium, magnesium and potassium salts (Prasad et al., 2022). Gum arabic can combine with tartrate to form a polymerand, which is a hydrophilic layer on the surface of the polymer. This increases the solubility of tartrate in wine, inhibits the growth of crystal nuclei, and maintains the metastable state of wine (Wei et al., 2019). This stabilizer is typically employed prior to bottling and filtration. However, it is inadvisable to employ gum arabic in wines that require aging or long-term storage, as it impedes the formation of natural precipitates in long-term storage wines, resulting in their emulsification (Li et al., 2007).
5.2.2. Metatartaric acid stabilizer
Metatartaric acid, a polyester resulting from the esterification of tartaric acid molecules, forms soluble complexes with K+ and Ca2+ of tartrate in wine, enhancing tartrate solubility. It envelops minute tartrate crystals in wine, impeding their growth and ensuring stability and non-precipitation (Huang, 1997). Metatartaric acid is usually employed after the final filtration and prior to bottling of wines (OIV, 2022e). Metatartaric acid acts as a potent inhibitor of tartrate crystal nucleation with minimal cost and impact on wine characteristics (Wei et al., 2019). However, prolonged storage or high temperature can trigger hydrolysis of metatartaric acid, converting it to tartaric acid, compromising tartaric stability (Huang, 1997). The stabilizing effect of metatartaric acid is also wine-dependent. Red wine stored at 20°C for 5 months with 100 mg/L metatartaric acid showed instability due to hydrolysis, while white wine remained stable for up to 12 months under the same conditions (Bosso et al., 2015). Consequently, metatartaric acid is better suited for high-demand wines.
5.2.3. Carboxymethyl cellulose stabilizer
Carboxymethyl cellulose, a harmless polysaccharide substance resistant to heat and acid, carries a negative charge in wine (Tang & Lu, 2020). It competes with HT− to bind with K+, lowering the concentration of KHT in the wine and maintaining the tartrate in an unsaturated state. Moreover, carboxymethyl cellulose interacts with the positive charges on KHT crystal surfaces, decreasing the free ions essential for crystal growth, thereby delaying crystal growth (Gerbaud et al., 2010).
A higher concentration of carboxymethyl cellulose in wine leads to improved inhibition of tartar crystallization. Additionally, its effectiveness in inhibiting tartaric crystallization correlates with its degree of polymerization (DP) and substitution degree (SD). The DP serves as a crucial metric for assessing polymer molecular size. The higher the molecular weight of carboxymethyl cellulose, the greater the DP and viscosity, with polymerization and folding of molecular chains reducing its solubility in wine and weakening its stabilizing effect on tartrate. In Cabernet Sauvignon red wine with an 80 mg/L addition of carboxymethyl cellulose, a lower DP enhances the tartrate stabilizing effect. The SD value indicates the quantity of carboxylic acid groups (-COOH) in carboxymethyl cellulose, with higher SD value providing more cation-binding sites, enhancing its competitive interaction with HT− and improving the tartaric stability effect in wine. Carboxymethyl cellulose with an SD value between 0.60 and 0.95 is suitable for use in wine applications (Bosso et al., 2010; Filipe-Ribeiro et al., 2021; Guise et al., 2014; Hou, 2019; Wei et al., 2019).
The carboxymethyl cellulose additive offers a cost-effective solution and long-lasting effectiveness. White wine with 40 mg/L carboxymethyl cellulose maintained tartaric stability for at least 1 year (Bosso et al., 2015). Currently, carboxymethyl cellulose is widely employed as a tartaric stabilizing agent in the production of high-quality and premium wines. But the addition of carboxymethyl cellulose affects the color and turbidity of red wines. As a result, the OIV restricts the usage of carboxymethyl cellulose to white wines and sparkling wines exclusively (OIV, 2022c). Furthermore, carboxymethyl cellulose has the potential to interact with proteins in wine, leading to haziness. Hence, wines intended for carboxymethyl cellulose treatment need to be protein-stabilized (Dabare et al., 2023).
5.2.4. Mannoprotein stabilizer
Mannoprotein, a soluble glycoprotein extracted from the yeast cell wall, has the ability to adsorb outside the crystal nuclei, preventing further crystal growth, thus contributing to stabilizing tartrate in wine (Wei et al., 2019).
The stabilizing efficacy of mannoprotein stabilizer varies based on the dosage and the wine type under treatment. Comparing the tartaric stability outcomes of 150–350 mg/L mannoprotein, white wine treated with 250 mg/L mannoprotein exhibited minimal K+ loss and the highest tartaric stability (Zhou & Zhou, 2011). Conversely, due to the abundant presence of protective colloids in red wines, the addition of mannoprotein does not notably impact tartaric stability (Pascotto et al., 2021).
Mannoprotein usage in white wines not only effectively enhances stability of tartaric acid, but also improves protein stability and boosts the organoleptic quality of the wine (Juniora et al., 2020). However, employing mannoprotein for tartaric stability in red wines has constraints. The use of saponite, gelatin and bentonite in the finning process may reduce mannoprotein level in the wine. Therefore, mannoprotein is typically applied for enhancing tartaric stability in white or rose wines (OIV, 2022b).
5.2.5. Potassium polyaspartate stabilizer
Potassium polyaspartate is a macromolecular amino acid polymer composed of L-aspartic acid units, featuring active groups like peptide bonds and carboxyl groups (Galbusera et al., 2017). Known for its chelating, dispersing, and adsorptive capabilities, potassium polyaspartate finds utility as a scale and corrosion inhibitor, fertilizer synergist, and enhancer in industries like papermaking, printing, dyeing, and laundry (Li et al., 2019). In winemaking, potassium polyaspartate is crucial in preventing the formation of tartar crystals by modifying the surface properties of tartrate crystals (Wei et al., 2019). To address high colloidal instability in red wines, it is recommended to perform pretreatment with bentonite as the final step prior to bottling (OIV, 2022f).
Research indicates that potassium polyaspartate effectively inhibits the formation of tartrate crystals. The stabilizing effect on tartaric acid increases proportionally with the addition of potassium polyaspartate. However, even at the lower dose of 100 mg/L, tartaric stability is achieved in both red and white wines (Bosso et al., 2015; Bosso, Motta, Panero, Lucini, & Guaita, 2020; Bosso, Motta, Panero, Petrozziello, et al., 2020). Storage temperature is the most important factor influencing the efficacy of potassium polyaspartate. However, even at 40°C, the inhibition of tartar crystallization by potassium polyaspartate is still maintained for at least 45 days (Canuti et al., 2019).
5.3. The testing method of tartaric stability
After the treatment for tartaric stabilization, an evaluation needs to be conducted to assess the stability of tartaric acid. This evaluation is typically performed using cold stability testing, conductivity micro-contact method, saturation temperature method, or ion concentration product method. The comparison of advantages and disadvantages of these methods are shown in Table 3 (Li & Xia, 2020; Liu, Lu, et al., 2008).
Table 3.
Advantages and disadvantages of tartaric acid stability testing methods.
| Method | Advantages | Disadvantages | |
|---|---|---|---|
| Cold treatment | Freezing method |
|
|
| Refrigeration method |
|
|
|
| Conductivity-micro-contact |
|
|
|
| Saturation temperature |
|
|
|
| Ion concentration product |
|
|
|
5.3.1. Cold stability testing method
The cold stability testing method to assess the stability of tartaric acid in wine is based on the fact that the solubility of KHT decreases in wine as the temperature decreases (Li & Xia, 2020). The wine for testing is cooled and kept at a low temperature, and then observed for precipitation with the naked eye to ascertain if the wine is in a stable tartaric acid state.
The cold stability testing methods include freezing and refrigerating. The former involves freezing the wine below its freezing point for 4 to 16 h, while the latter involves refrigerating the wine at 1°C above its freezing point for several days to weeks (Zoecklein et al., 1990). The specific duration of low-temperature treatment varies depending on the alcohol content of the wine, the experience of winemakers, and the desired cold stability level of the production (Chen & Pan, 2009; Li & Xia, 2020). If there is no crystallization after cold treatment, it indicates that the tartaric acid in the wine tested is stable.
5.3.2. Conductivity micro-contact method
Conductivity is a measure of the ability of a substance to conduct electricity, indicating how easily electrical charge moves through a material (Zhang et al., 2020). The change in conductivity before and after the tartaric acid stabilization treatment signals the changes in the number of ions in the wine. Conductivity tests are conducted on the wine before and after stabilization treatment, and significant differences imply former instability of tartaric acid in the wine. Conversely, if the change in conductivity is less than 100 μS/cm for red wine and 150 μS/cm for white wine, the state of tartaric acid in wine is considered stable (Bosso, Motta, Panero, Petrozziello, et al., 2020).
When the tartar stability of wine is determined by the conductivity-micro-contact method, first, tartar seeds (the addition amount is usually 4 g/L and the crystal size is in the range of 50–100 μm) need to be added to the wine at −4-0°C to induce KHT crystallization, thus reducing the number of ions and the conductivity (Lasanta & Gomez, 2012; Li & Xia, 2020). If the tartaric stability is good, KHT does not easily reach the supersaturated state and the conductivity changes little after liquor treatment. On the other hand, if the tartaric stability is not good, KHT will easily reach supersaturated state, the concentration of ions in the liquor will obviously decrease, and the conductivity will change greatly after treatment. Therefore, the tartaric stability of wine should be tested by measuring the conductivity change of wine before and after treatment with a conductivity meter (Bosso et al., 2016).
It should be noted that the solubility of tartrate in wine is also affected by temperature, so the conductivity of wine should be measured at the lowest possible temperature. If the test temperature is higher than the storage temperature of the wines tested, it may still be unstable (Li & Xia, 2020). Generally, the determination temperature of white wine is 0°C and that of red wine is 4–5°C (Xue, 2002). However, it has been found that the conductivity-micro-contact method is suitable only for the determination of tartaric stability at the initial stage of tartaric stabilization treatment of wine, and is not suitable for the detection of tartaric stability during aging, for reasons that have not yet been explored (Bosso, Motta, Panero, Petrozziello, et al., 2020; Mislata et al., 2021).
5.3.3. Saturation temperature method
The saturation temperature (TS) of wine is defined the critical temperature at which tartrate reaches saturation (Benítez et al., 2003). For a given wine, the higher the tartrate content, the higher the critical TS. It is commonly accepted that tartaric acid is stable when the TS of white wine is below 12.5°C, and that of red wine is below 22°C (dos Santos et al., 2002).
The principle allows for the use of the conductivity-saturation temperature method to assess the stability of tartaric acid in wine. The wine undergoes division into two portions: one containing 4 g/L tartrate seeds and the other without seeds. A conductivity meter is employed to track the changes in conductivity of both samples as the temperature varies from 5°C to 25°C. Subsequently, temperature and conductivity change curves are plotted, and the TS of the sample is identified at the point of clear divergence between the two curves (Li et al., 2020). Ultimately, the stability of tartaric acid in wine is determined by the TS value of the sample.
5.3.4. Ion concentration product method
For insoluble salts, the ion concentration product (CP) is the product of the actual concentrations of the constituent ions, while the solubility product (Ksp) is the product of the concentrations of the constituent ions when the insoluble salt reaches equilibrium for precipitation and dissolution in solution (Yang et al., 2024). And the CPs of KHT and CaT are expressed as the two formulas respectively:
| (3) |
| (4) |
where ‘[]’ represents the actual concentration of ions in g/L.
For a given solution matrix and temperature, the Ksp is a constant. The CP can be compared with the Ksp to predict the direction of reactions, thus evaluating the difficulty of wine to produce tartar precipitation (Chattaraj et al., 2021).
In order to test the tartaric stability, it is necessary to determine the concentrations of K+, Ca2+, HT− and T2− in the wine and calculate the CP(KHT) and CP(CaT), respectively. Furthermore, the Ksp of two tartrates can be determined by consulting the table according to the temperature of the wine. If the CP is greater than the Ksp, precipitation will occur. Conversely, if the CP is less than the Ksp, precipitation will not occur.
6. Effect of tartaric stabilization on the wine sensory
6.1. The turbidity of wine
Turbidity is a crucial indicator of the value of wine as a commodity. As long as the quality of the wine remains uncompromised, a lower turbidity value is indicative of superior quality. The efficacy of different tartaric stability treatments on wine turbidity varies.
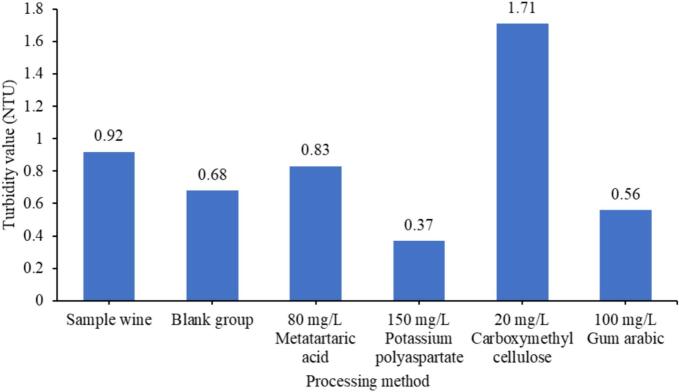
To evaluate the effect of each tartar stabilization treatment on the wine turbidity, two control groups were established using Cabernet Sauvignon dry red wine without a stabilizer and with 80 mg/L metatartaric acid added, and the experimental groups were comprised of wines with 50–200 mg/L gum arabic, 20–80 mg/L carboxymethyl cellulose, and 50–200 mg/L potassium polyaspartate added. The control and experimental groups samples were subjected to a 7-day treatment at a temperature of −4°C, after which the turbidity of each sample was analyzed (Fig. 4). The results demonstrated that, in comparison to the two control groups, potassium polyaspartate exhibited a notable reduction in wine turbidity, with a corresponding decrease in turbidity observed with increasing potassium polyaspartate addition. Conversely, the addition of carboxymethyl cellulose was found to elevate wine turbidity, accompanied by an increase in turbidity with rising carboxymethyl cellulose concentration. The addition of 50–150 mg/L gum arabic was found to reduce the turbidity of wine, with the lowest turbidity observed at 100 mg/L. However, an increase in gum arabic concentration to 200 mg/L resulted in a significant increase in turbidity, reaching 1.24 NTU, which was found to be significantly higher than the two control groups (Zhang et al., 2019). Consequently, when employing gum arabic as a stabilizer for tartar, it is imperative to consider the impact of the addition amount on the turbidity of the wine.
Fig. 4.
Effects of different treatment methods on wine turbidity. The concentration of each additive was the additive concentration that can reduce the turbidity of wine to the minimum (Zhang et al., 2019).
The wine treated by electrodialysis will exhibit varying degrees of turbidity and flocculent precipitation following a one-day period of storage at −4°C. However, there is no evidence of crystal precipitation, which may be caused by cold unstable pigmented substances. As the storage period is extended, the turbidity of the wine will increase (Yan et al., 2007). For wines with high tartrate saturation, it is necessary to add a high concentration of mannoprotein to inhibit the formation of tartrate crystals. However, the high concentration of mannoprotein is prone to flocculation in the wine, which can result in cloudiness, particularly when the addition amount of mannoprotein exceeds 200 mg/L (Gerbaud et al., 2010; Li et al., 2003). In addition to tartaric acid salts, colloidal compounds such as proteins and pigments also cause turbidity in wine. Consequently, when implementing a tartaric acid stabilization treatment, it is essential to consider whether this method will introduce additional turbidity substances and whether there are other substances that will cause turbidity in the wine. In such instances, it is necessary to perform the corresponding treatments.
6.2. The color of wine
Color is the most intuitive sensory characteristic and quality index of wine, which directly affects acceptance of consumers, and is a preliminary evaluation indicator of wine quality. The color of wine is typically quantified by measuring its chromaticity.
The application of electrodialysis and gum arabic stabilizer can enhance the color of wine. During electrodialysis, some of the sulfite ions in the wine that are bound to the pigments are removed by the electric field force, thus releasing the pigments and improving the color of the wine (Yan et al., 2007). The chromaticity value of the wine increases with the concentration of gum arabic added, although high concentrations of gum arabic (200 mg/L) can result in increased turbidity of red wines, which can have a detrimental effect on the wine (Claus et al., 2014; Zhang et al., 2019).
The ion exchange resin method and the treatment of wines with potassium polyaspartate stabilizer have no significant effect on the color of the wines. Although ion exchange resins also adsorb anthocyanins, which results in a reduction of anthocyanins in wine, the use of cation exchange resins with H+ can lower the pH of the treated wines, thereby increasing the chromaticity of anthocyanins and counteracting the effect of anthocyanin loss on the sensory color of wines (Ibeas et al., 2015). The addition of potassium polyaspartate stabilizer (50–200 mg/L), in conjunction with cold stabilization treatment, ensures the stability of tartaric acid in wine, while having no significant effect on the chromaticity values of the wine. It can be observed that the potassium polyaspartate stabilizer exerts a protective effect on the color of red wine (Zhang et al., 2019).
Both cold stabilization and carboxymethyl cellulose stabilizer treatment result in a reduction of the chromaticity value of red wine. During the cooling process of red wine subjected to cold stabilization, certain color-forming substances precipitates. As the low-temperature treatment time increases, the chromaticity value continues to decrease (Yan et al., 2007; Zhang et al., 2007). As for the addition of carboxymethyl cellulose, it may neutralize positively charged pigment substances, resulting in the formation of larger colloidal particles that are aggregated. As the concentration of carboxymethyl cellulose increases, the chromaticity value decreases (Sommer et al., 2016; Zhang et al., 2019).
6.3. The aroma of wine
The wine aroma is a crucial factor in the evaluation of wine quality. The alcohol content, pH value, temperature, and wine matrix influence the volatilization of aroma compounds and the perception of aroma substances (Villamor & Ross, 2013). It is also the case that tartar stabilization can affect the aroma intensity and aroma characteristics of wine. However, related research is very limited, with only studies on the effects of adding mannoprotein, electrodialysis and cold stabilization on wine aroma.
The addition of mannoprotein alters the volatility of aroma compounds, thereby enhancing the perceived fullness and aroma intensity of wine (Li, 2000). Adding 0.25 g/L and 0.30 g/L mannoprotein to Cabernet Sauvignon wine has been demonstrated to significantly enhance the fruity aroma of the wine, with notable increases in the concentrations of ethyl propionate, ethyl butyrate, diethyl succinate and ethyl caprylate. Furthermore, the utilization of mannoprotein can also elevate the concentration of phenethyl alcohol and hexanoic acid in wine, and enhance the rose and cheese aromas of wine (Men, 2015).
The wines treated with cation exchange resins exhibited a lower pH. A reduction in pH can facilitate acid-catalyzed esterification or hydrolysis reactions (Makhotkina et al., 2012; Makhotkina & Kilmartin, 2012; Ramey & Ough, 1980). Therefore, the cation-exchanged Tempranillo wines exhibited elevated concentrations of fragrant compounds following six months of aging, when compared to the control group. These compounds included hexyl acetate, isobutanol, 2-phenylethanol, ethyl isovalerate, and diethyl succinate. Moreover, the concentrations of ethyl isovalerate and diethyl succinate exhibited a gradual increase with the enhancement of the penetration rate of the cation exchange resin treatment. These indicate that the cation exchange resin method is effective in increasing the content of aroma substances in red wines during the aging process (Mislata et al., 2021). The wines that underwent cold treatment exhibited analogous changes in their aroma compounds, in a manner analogous to those observed in the wines treated with cation exchange resin. The aroma compounds of Riesling wines exhibited minimal changes following the conclusion of the cold treatment. However, after 12 months of aging, the cold-treated group exhibited a lower pH than the control group. Furthermore, the main components, including esters, hypromellose, terpenes, furfural, and others, exhibited a significant increase in the cold-treated wines, particularly the characteristic aroma substance of Riesling wines, 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN). The content of TDN in the wines exhibited a gradual increase with increasing cold treatment time. It can be postulated that the lower pH contributed to the conversion of some aroma precursors into volatiles in the wines (Xia et al., 2022).
6.4. The taste of wine
Tartaric acid, being the predominant organic acid in wine, significantly impacts its tartness and acidity. If the content and composition of other organic acids remain constant, a higher concentration of tartaric acid leads to increased sourness in the wine. Regardless of the pH level, elevating the tartaric acid concentration will elevate the perceived acidity of the wine (Sowalsky & Noble, 1998). Subsequently, employing cold treatment, electrodialysis, and ion exchange resin processes will decrease the tartaric acid content in wine, leading to a simultaneous reduction in sourness.
Simultaneously, variations in tartaric acid levels and resulting pH changes can impact the astringency of wine. The astringency in wine primarily results from the interaction between tannins and salivary proteins, leading to sediment formation. Below 3.0 g/L tartaric acid, increased tartaric acid concentrations elevate tannin-salivary protein complexes, encouraging salivary protein sedimentation, leading to enhanced granularity and oral friction, and increasing astringency. Within the 3.0–7.0 g/L range of tartaric acid content, wine astringency diminishes progressively, while heightened acidity masks astringency perception in the mouth. pH primarily influences astringency by regulating salivary layer removal. Reduced pH levels enhance the removal process of salivary layer. Astringency intensifies with decreasing pH levels. Therefore, the dynamics of tartaric acid concentration visibly influence both acidity and astringency in wine (Zhao et al., 2023). A notable pH reduction in cation exchange resin treatment may elevate wine astringency. Furthermore, cold treatment can elevate the dissolved oxygen content of wine, facilitating the interaction of oxygen with tannins and tyrosine, which in turn reduces the perception of rawness, greenness, and bitterness (Liu, Zhang, et al., 2008).
7. The development of new technologies for tartaric acid stabilization
7.1. The combination of cold treatment and additive stabilizers
While stabilizers effectively stabilize tartaric acid, they are ineffective against cold-labile substances in wine. Hence, combining cold treatment with adding stabilizers can compensate for the limitations of the ‘addition’ strategy. Furthermore, the sequence of adding stabilizers and conducting cold treatment impacts the efficacy of wine tartar stabilization. Adding 200 mg/L mannoprotein to Chardonnay wine and freezing it at −4°C for 7 days yielded a similar tartaric acid stabilization effect as adding 40 mg/L of mannoprotein after freezing it at −4°C for 4 days. Similarly, introducing 150 mg/L of mannoprotein to Riesling wine and freezing it at −4°C for 7 days resulted in a tartaric acid stabilization effect equivalent to adding 30 mg/L of mannoprotein following freezing at −4°C for 4 days (He et al., 2016). Evidently, incorporating mannoprotein stabilizer post cold treatment in white wine efficiently reduces cold stabilization duration and minimizes the required stabilizer quantity, consequently reducing overall costs and stabilization time.
7.2. Plasma surface modification technology
Plasma surface modification technology involves applying nanoscale coatings with chemically reactive functional groups to inert substrates. This process enables these surfaces with specific chemical functionalities to extract unwanted substances from wine (Vandenabeele & Lucas, 2020). Plasma surface modification technology is currently extensively utilized across various industries like aerospace, semiconductors, printing, packaging, and textiles (Zhang et al., 2023). Recently, this technology has found application in eliminating protein haze in white wines as well (Mierczynska-Vasilev et al., 2020).
Dabare et al. (2023) chose allylamine, acrylic acid, and 2-methyl-2-oxazoline as the precursors for plasma polymerization to be applied to the substrates of silicon wafers and stainless steel sheets. All three plasma polymers demonstrated efficacy in eliminating KHT crystals from white wines at a temperature of 15°C, leading to an average reduction of 22% in K+ concentration and 23% in tartaric acid concentration. The affinity of these plasma coatings for KHT was ranked as allylamine >2-methyl-2-oxazoline > acrylic acid in cold and heat-unstable white wines. Besides the composition of the plasma polymers, the efficiency of this method in KHT crystal removal from wines was associated with the duration of contact between the plasma polymers and the wine. It was observed that only 8%–43% of surface crystals were covered by the three plasma polymers after a week of contact; however, the coverage could reach 60%–90% after a month, indicating greater effectiveness in KHT crystal removal.
In comparison to conventional cold treatment, this technique effectively eliminates protein haze from the wine without altering the wine's phenolic content, in addition to stabilizing tartaric acid. It offers the benefit of energy savings and cost reduction. Furthermore, functionalized surfaces fall under the category of processing aids, thus potentially being exempt from ingredient labeling requirements that apply to approved additives, indicating promising growth opportunities.
8. Conclusions
Tartaric acid stabilization treatment and testing are essential process operations conducted prior to wine bottling to prevent the formation of precipitates caused by tartar. This is crucial to maintain the quality of the wine during storage and ensure its market value. This study provides a comprehensive overview of research advancements concerning the properties of wine tartaric acid, methods for stabilization, techniques for stability assessment, and explores the impacts of various stabilization methods on wine characteristics such as turbidity, color, aroma, and taste. Despite this progress, there is a notable lack of research focusing on how different stabilization methods influence wine quality attributes, especially regarding aroma and taste profiles. Consequently, it is recommended that the development of tartaric stabilization processes should aim to integrate diverse methods and explore innovative technologies to offer more choices in the winemaking process.
Funding Information
National Natural Science Foundation of China, Grant/Award Numbers: 32102314; 2024 Beijing University of Agriculture Open Project, Grant/Award Numbers: KFKT-2024025.
CRediT authorship contribution statement
Wenwen Cui: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Xiaoqin Wang: Resources, Methodology, Formal analysis. Shuang Han: Methodology, Investigation. Wentao Guo: Methodology, Investigation. Nan Meng: Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization. Jinchen Li: Supervision, Funding acquisition. Baoguo Sun: Supervision, Funding acquisition. Xinke Zhang: Supervision, Funding acquisition.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101728.
Appendix A. Supplementary data
Supplementary material
Data availability
The data that has been used is confidential.
References
- Agius F., González-Lamothe R., Caballero J.L., Muñoz-Blanco J., Botella M.A., Valpuesta V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nature Biotechnology. 2003;21(2):177–181. doi: 10.1038/nbt777. [DOI] [PubMed] [Google Scholar]
- Australia Government, Federal Register of Legislation . Australia New Zealand Food Standards Code-Schedule 15-Substances that may be used as food additives. 2021. [Google Scholar]
- Barlow D.A., Gregus J. The kinetics of homogeneous and two-step nucleation during protein crystal growth from solution. International Journal of Chemical Kinetics. 2019;51(11):840–847. doi: 10.1002/kin.21313. [DOI] [Google Scholar]
- Benítez J.G., Macías V.M.P., Gorostiaga P.S., López R.V., Rodríguez L.N. Comparison of electrodialysis and cold treatment on an industrial scale for tartrate stabilization of sherry wines. Journal of Food Engineering. 2003;58(4):373–378. doi: 10.1016/s0260-8774(02)00421-1. [DOI] [Google Scholar]
- Benítez J.G., Macías V.M.P., Pazo J.A.S., Rodríguez L.P. Industrial development of proton exchange for tartrate stabilization of sherry wines. European Food Research and Technology. 2002;214(5):418–422. doi: 10.1007/s00217-002-0504-3. [DOI] [Google Scholar]
- Bosso A., Motta S., Panero L., Lucini S., Guaita M. Use of potassium polyaspartate for stabilization of potassium bitartrate in wines: Influence on colloidal stability and interactions with other additives and enological practices. Journal of Food Science. 2020;85(8):2406–2415. doi: 10.1111/1750-3841.15342. [DOI] [PubMed] [Google Scholar]
- Bosso A., Motta S., Panero L., Petrozziello M., Asproudi A., Lopez R., Guaita M. Use of polyaspartates for the tartaric stabilisation of white and red wines and side effects on wine characteristics. OENO ONE. 2020;54(1):15–26. doi: 10.20870/oeno-one.2020.54.1.2527. [DOI] [Google Scholar]
- Bosso A., Motta S., Petrozziello M., Guaita M., Asproudi A., Panero L. Validation of a rapid conductimetric test for the measurement of wine tartaric stability. Food Chemistry. 2016;212:821–827. doi: 10.1016/j.foodchem.2016.06.044. [DOI] [PubMed] [Google Scholar]
- Bosso A., Panero L., Petrozziello M., Sollazzo M., Asproudi A., Motta S., Guaita M. Use of polyaspartate as inhibitor of tartaric precipitations in wines. Food Chemistry. 2015;185:1–6. doi: 10.1016/j.foodchem.2015.03.099. [DOI] [PubMed] [Google Scholar]
- Bosso A., Salmaso D., De Faveri E., Guaita M., Franceschi D. The use of carboxymethylcellulose for the tartaric stabilization of white wines, in comparison with other oenological additives. Vitis. 2010;49(2):95–99. [Google Scholar]
- Canuti V., Cappelli S., Picchi M., Zanoni B., Domizio P. Effects of high temperatures on the efficacy of potassium polyaspartate for tartaric stabilization in wines. American Journal of Enology and Viticulture. 2019;70(3):332–337. doi: 10.5344/ajev.2019.18077. [DOI] [Google Scholar]
- Cao H., Shu H., Shao J., Zhang H., Ma C. Research progress on biosynthesis of tartaric acid in grape berries. China Fruits. 2021;04:8–13. doi: 10.16626/j.cnki.issn1000-8047.2021.04.003. [DOI] [Google Scholar]
- Chahine S., Tong A.Z. Effect of climatic conditions on organic acid composition of some wines obtained from different sources. AIMS Agriculture and Food. 2019;4(1):27–40. doi: 10.3934/agrfood.2019.1.27. [DOI] [Google Scholar]
- Chattaraj A., Blinov M.L., Loew L.M. The solubility product extends the buffering concept to heterotypic biomolecular condensates. Elife. 2021;10 doi: 10.7554/eLife.67176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Pan L. A preliminary study on the stability of tartaric acid salts in wines from Hexi corridor region. Sino-overseas Grapevine & Wine. 2009;11:58–60. [Google Scholar]
- Claus H., Tenzer S., Sobe M., Schlander M., Koenig H., Froehlich J. Effect of carboxymethyl cellulose on tartrate salt, protein and colour stability of red wine. Australian Journal of Grape and Wine Research. 2014;20(2):186–193. doi: 10.1111/ajgw.12070. [DOI] [Google Scholar]
- Coulter A.D., Holdstock M.G., Cowey G.D., Simos C.A., Smith P.A., Wilkes E.N. Potassium bitartrate crystallisation in wine and its inhibition. Australian Journal of Grape and Wine Research. 2015;21:627–641. doi: 10.1111/ajgw.12194. [DOI] [Google Scholar]
- Cruz-Rus E., Botella M.A., Valpuesta V., Carmen Gomez-Jimenez M. Analysis of genes involved in L-ascorbic acid biosynthesis during growth and ripening of grape berries. Journal of Plant Physiology. 2010;167(9):739–748. doi: 10.1016/j.jplph.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Dabare P.R., Reilly T., Mierczynski P., Bindon K., Vasilev K., Mierczynska-Vasilev A. A novel solution to tartrate instability in white wines. Food Chemistry. 2023;422 doi: 10.1016/j.foodchem.2023.136159. [DOI] [PubMed] [Google Scholar]
- Du X., Zhou X., Yu L., Tang B., Liu K. Study on the determination of polybasic organic acids in wine by derivatization gas chromatographic method. Journal of southwest Minzu university(natural science edition) 2007;06:1365–1368. [Google Scholar]
- Filipe-Ribeiro L., Milheiro J., Guise R., Vilamarim R., Fraga J.B., Martins-Gomes C.…Cosme F. Efficiency of carboxymethylcellulose in red wine tartaric stability: Effect on wine phenolic composition, chromatic characteristics and colouring matter stability. Food chemistry. 2021:360. doi: 10.1016/j.foodchem.2021.129996. [DOI] [PubMed] [Google Scholar]
- Galbusera C., Casalegno C., Marroncelli S., Triulzi G., Santos J., Corsini E., Restani P. Toxicologic evaluation of potassium polyaspartate (A-5D K/SD): Genotoxicity and subchronic toxicity. Food and Chemical Toxicology. 2017;109:452–464. doi: 10.1016/j.fct.2017.09.036. [DOI] [PubMed] [Google Scholar]
- Gao N., Li X., Yang F. Studies on the organic acids and reducing acids of grapes and wines. Sino-overseas Grapevine & Wine. 1999;04:6–11. doi: 10.13414/j.cnki.zwpp.1999.04.002. [DOI] [Google Scholar]
- GB 2760–2014 . 2014. Food Safety National Standards-Standards for the use offood additives. [Google Scholar]
- Gerbaud, Gabas N., Laguerie C., Blouin J., Pellerin P. Effect of wine polysaccharides on the nucleation of potassium hydrogen tartrate in model solutions. Chemical Engineering Research and Design. 1996;74(7):782–790. doi: 10.1016/S0923-0467(97)80004-8. [DOI] [Google Scholar]
- Gerbaud V., Gabas N., Blouin J., Crachereau J.C. Study of wine tartaric salt stabilization by addition of carboxymethylcellulose (CMC). Comparison with the « protective colloïds » effect. OENO one. 2010;44(3):231–242. [Google Scholar]
- Gonçalves F., Fernandes C., dos Santos P.C., de Pinho M.N. Wine tartaric stabilization by electrodialysis and its assessment by the saturation temperature. Journal of Food Engineering. 2003;59(2–3):229–235. doi: 10.1016/s0260-8774(02)00462-4. [DOI] [Google Scholar]
- Gu M., Wang Y., Wan D., Shi Y., He Q. Electrodialysis ion-exchange membrane bioreactor (EDIMB) to remove nitrate from water: Optimization of operating conditions and kinetics analysis. Science of the Total Environment. 2022;839 doi: 10.1016/j.scitotenv.2022.156046. [DOI] [PubMed] [Google Scholar]
- Guise R., Filipe-Ribeiro L., Nascimento D., Gessa O., Nunes F.M., Cosme F. Comparison between different types of carboxylmethylcellulose and other oenological additives used for white wine tartaric stabilization. Food Chemistry. 2014;156:250–257. doi: 10.1016/j.foodchem.2014.01.081. [DOI] [PubMed] [Google Scholar]
- Guo A. Unpublished Doctorate; Northwest A&F University: 2007. Mechansisms of oxidative browning of wine and antioxidant alternative to sulfur dioxide in wine. [Google Scholar]
- He Z., Li Z., Zhao Q., Li P., Quan Y. Effects of yeast polysaccharides Mp60 on the quality of wine in process of post treatment. Food Research and Development. 2016;37(08):33–36. [Google Scholar]
- Hou R. Unpublished Master; Yantai University: 2019. Study on the effect of sodium carboxymethyl cellulose (CMC) on the tartrate stabilization of wines. [Google Scholar]
- Huang Y. Use of metatartaric acid to inhibit the formation of tartrate precipitates in bottled wines. Liquor-making Science & Technology. 1997;01:62. doi: 10.13746/j.njkj.1997.01.027. [DOI] [Google Scholar]
- Hungerford N.L., Yates H.S.A., Smith T.J., Fletcher M.T. Organic acid profiles of Australian stingless bee honey samples determined by ion chromatography. Journal of Food Composition and Analysis. 2023:122. doi: 10.1016/j.jfca.2023.105466. [DOI] [Google Scholar]
- Ibeas V., Correia A.C., Jordao A.M. Wine tartrate stabilization by different levels of cation exchange resin treatments: Impact on chemical composition, phenolic profile and organoleptic properties of red wines. Food Research International. 2015;69:364–372. doi: 10.1016/j.foodres.2015.01.003. [DOI] [Google Scholar]
- Ivanova-Petropulos V., Naceva Z., Sandor V., Makszin L., Deutsch-Nagy L., Berkics B., Stafilov T., Kilar F. Fast determination of lactic, succinic, malic, tartaric, shikimic, and citric acids in red Vranec wines by CZE-ESI-QTOF-MS. Electrophoresis. 2018;39(13):1597–1605. doi: 10.1002/elps.201700492. [DOI] [PubMed] [Google Scholar]
- Jia Y., Burbidge C.A., Sweetman C., Schutz E., Soole K., Jenkins C.…Ford C.M. An aldo-keto reductase with 2-keto-l-gulonate reductase activity functions in l-tartaric acid biosynthesis from vitamin C in Vitis vinifera. Journal of Biological Chemistry. 2019;294(44):15932–15946. doi: 10.1074/jbc.RA119.010196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniora W.J.F.L., Nadai C., Rolled L., Gulaoe E.D.S., Leaoe M.H.M.D.R., Giacomini A.…Vincenzi S. Influence of the mannoproteins of different strains of Starmerella bacillaris used in single and sequential fermentations on foamability, tartaric and protein stabilities of wines. OENO ONE. 2020;54(2):231–243. doi: 10.20870/oeno-one.2020.54.2.2948. [DOI] [Google Scholar]
- Kang Q., Sun J., Wang B., Sun B. Wine, beer and Chinese baijiu in relation to cardiovascular health: The impact of moderate drinking. Food Science and Human Wellness. 2023;12(1):1–13. doi: 10.1016/j.fshw.2022.07.013. [DOI] [Google Scholar]
- Lambri M., Colangelo D., Dordoni R., De Faveri D.M. The effects of different protein:Tannin ratios on the tartrate-holding capacity of wine model solutions. Food Research International. 2014;62:441–447. doi: 10.1016/j.foodres.2014.03.044. [DOI] [Google Scholar]
- Lamport D.T.A., Varnai P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytologist. 2013;197(1):58–64. doi: 10.1111/nph.12005. [DOI] [PubMed] [Google Scholar]
- Lasanta C., Gomez J. Tartrate stabilization of wines. Trends in Food Science & Technology. 2012;28(1):52–59. doi: 10.1016/j.tifs.2012.06.005. [DOI] [Google Scholar]
- Leszczuk A., Kalaitzis P., Blazakis K.N., Zdunek A. The role of arabinogalactan proteins (AGPs) in fruit ripening-a review. Horticulture Research. 2020;7(1) doi: 10.1038/s41438-020-00397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Role of polysaccharides on wine aroma and tartaric stabilization. Liquor Making. 2000;05:64–66. [Google Scholar]
- Li H., Wang H., Yuan C., Wang S. Science Press; Beijing: 2007. Wine technology. [Google Scholar]
- Li H., Yang X., Hu B., Chen X., Chen H. Study on effects of mannoprotein added to wine tartar stability. Food Science. 2003;10:104–107. [Google Scholar]
- Li M., Su J., Yang H., Feng L., Wang M., Xu G., Shao J., Ma C. Grape tartaric acid: Chemistry, function, metabolism, and regulation. Horticulturae. 2023;9(11) doi: 10.3390/horticulturae9111173. [DOI] [Google Scholar]
- Li X., Xia G. Review on wine cold stability test methods and research progress. Sino-overseas Grapevine & Wine. 2020;02:58–63. doi: 10.13414/j.cnki.zwpp.2020.02.012. [DOI] [Google Scholar]
- Li Y., Li S., Sun D., Zhu K. Synthesis of potassium polyaspartic acid. Shandong chemical industry. 2019;48(03):1–2. doi: 10.19319/j.cnki.issn.1008-021x.2019.03.002. [DOI] [Google Scholar]
- Li Y., Liu Y., Lu L., Li X., Wang F. Simultaneous determination of eight organic acids in grape wines by capillary electrophoresis-electrospray ionization mass spectrometry. Journal of Chinese Mass Spectrometry Society. 2013;34(05):288–293. [Google Scholar]
- Liu T., Zhang J., Yan J., Xing K. Discussion on new frozen method of red wine. Liquor Making. 2008;05:77–79. [Google Scholar]
- Liu Z., Lu X., Wu J., Huo X., Hou X. Study on the determination methods of tartar stability of wine. Sino-overseas Grapevine & Wine. 2008;04:12–15. [Google Scholar]
- Lorence A., Chevone B.I., Mendes P., Nessler C.L. Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiology. 2004;134(3):1200–1205. doi: 10.1104/pp.103.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Xiong R., Wei X., Wei Z., Yu K., Xie L., Yu X., Huang H. Formation mechanism of phenols during winemaking and the influencing factors: A review. Food Research and Development. 2023;44(13):195–201. [Google Scholar]
- Makhotkina O., Kilmartin P.A. Hydrolysis and formation of volatile esters in New Zealand sauvignon blanc wine. Food Chemistry. 2012;135(2):486–493. doi: 10.1016/j.foodchem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- Makhotkina O., Pineau B., Kilmartin P.A. Effect of storage temperature on the chemical composition and sensory profile of sauvignon blanc wines. Australian Journal of Grape and Wine Research. 2012;18(1):91–99. doi: 10.1111/j.1755-0238.2011.00175.x. [DOI] [Google Scholar]
- Mato I., Suarez-Luque S., Huidobro J.F. Simple determination of main organic acids in grape juice and wine by using capillary zone electrophoresis with direct UV detection. Food Chemistry. 2007;102(1):104–112. doi: 10.1016/j.foodchem.2006.05.002. [DOI] [Google Scholar]
- Mckinnon A.J., Scollary G.R., Solomon D.H., Williams P.J. The mechanism of precipitation of calcium L(+)-tartrate in a model wine solution. Colloids and Surfaces A Physicochemical and Engineering Aspects. 1994;82(3):225–235. doi: 10.1016/0927-7757(93)02636-S. [DOI] [Google Scholar]
- McKinnon A.J., Scollary G.R., Solomon D.H., Williams P.J. The influence of wine components on the spontaneous precipitation of calcium L(+)-tartrate in a model wine solution. American Journal of Enology and Viticulture. 1995;46(4):509–517. [Google Scholar]
- McKinnon A.J., Williams P.J., Scollary G.R. Influence of uronic acids on the spontaneous precipitation of calcium L-(+)-tartrate in a model wine solution. Journal of Agricultural and Food Chemistry. 1996;44(6):1382–1386. doi: 10.1021/jf950111v. [DOI] [Google Scholar]
- Melino V., Soole K., Ford C. Ascorbate metabolism and the developmental demand for tartaric and oxalic acids in ripening grape berries. BMC Plant Biology. 2009;9(1):145. doi: 10.1186/1471-2229-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y. Unpublished Master; Qilu University of Technology: 2015. Study on the effect of yeast mannose glycoprotein on the quality of wine. [Google Scholar]
- Mierczynska-Vasilev A., Qi G., Smith P., Bindon K., Vasilev K. Regeneration of magnetic nanoparticles used in the removal of pathogenesis-related proteins from white wines. Foods. 2020;9(1) doi: 10.3390/foods9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H., Leite P., Ricardo-Da-Silva J.M., Curvelo-Garcia A.S. Use of ion exchange resins for tartrate wine stabilization. Journal International Des Sciences De La Vigne Et Du Vin. 2006;40(4):223–246. [Google Scholar]
- Mislata A.M., Puxeu M., Nart E., de Lamo S., Ferrer-Gallego R. Preliminary study of the effect of cation-exchange resin treatment on the aging of tempranillo red wines. Lwt-Food Science and Technology. 2021:138. doi: 10.1016/j.lwt.2020.110669. [DOI] [Google Scholar]
- Mu Y., Wu Y., Wang X., Hu L., Ke R. Determination of 10 organic acids in alcoholic products by ion chromatography-tandem mass spectrometry. Chinese Journal of Chromatography. 2022;40(12):1128–1135. doi: 10.3724/sp.J.1123.2022.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R.S., Billa N., Morris A. A validated reverse-phase high performance liquid chromatography (RP-HPLC) method for the quantification of gamma- tocotrienol in tocotrienol rich fractions of crude palm oil. Current Nutrition & Food Science. 2022;18(1):75–81. doi: 10.2174/1573401317666210803155717. [DOI] [Google Scholar]
- National Health Commission of the People's Republic of China . 2023. Announcement on 28 kinds of 'three new foods' such as Pseudo-Enteric Membrane Stringy Bacteria. [Google Scholar]
- Niu S., Liu C., Liu Q., Fan X., Zhang Y., Sun L., Zhang X., Jiang J. Composition and contents of organic acids in different grape germplasms. Food Science. 2022;43(12):228–234. [Google Scholar]
- Nuri A.O., Rajab A.S., Raşit Ö.M. Fractional factorial design application for the determination of affecting parameters on the generation of KOH and HCl from simulated wastewater solution by bipolar membrane electrodialysis abstract. European journal of science and technology. 2019:866–873. doi: 10.31590/EJOSAT.646850. [DOI] [Google Scholar]
- OIV . 2022. International Code of Oenological Practices. Acidification by cation exchanger treatment (sections 3.1.1.5) [Google Scholar]
- OIV . 2022. International Code of Oenological Practices. Treatment of wines with yeast mannoproteins (sections 3.3.13) [Google Scholar]
- OIV . 2022. International Code of Oenological Practices. Treatment with Cellulose gums-Carboxymethylcellulose (sections 3.3.14) [Google Scholar]
- OIV . 2022. International Code of Oenological Practices. Treatment with gum arabic (sections 3.3.6) [Google Scholar]
- OIV . 2022. International Code of Oenological Practices. Treatment with metatartaric acid (sections 3.3.7) [Google Scholar]
- OIV . 2022. International Code of Oenological Practices. Treatment with potassium polyaspartate (sections 3.3.15) [Google Scholar]
- Ough C.S., Cromwell E.A., Benz J. Metal content of California wines. Journal of Food Science. 1982;47(3):825–828. doi: 10.1111/j.1365-2621.1982.tb12724.x. [DOI] [Google Scholar]
- Pang M., Cai S., Liu Q. Research progress on the analysis methods of organic acids in wine. Journal of Food Safety & Quality. 2019;10(06):1588–1593. [Google Scholar]
- Pascotto K., Leriche C., Caille S., Violleau F., Boulet J.-C., Geffroy O., Levasseur-Garcia C., Cheynier V. Study of the relationship between red wine colloidal fraction and astringency by asymmetrical flow field-flow fractionation coupled with multi-detection. Food Chemistry. 2021:361. doi: 10.1016/j.foodchem.2021.130104. [DOI] [PubMed] [Google Scholar]
- Pei H., Chen F., Guo W., Jia Q., Guo R., Liu N., Mo Z. Chiral nitrogen-doped graphene quantum dot electrochemical sensor for recognition of tartaric acid isomers. Journal of the Electrochemical Society. 2021;168(6) doi: 10.1149/1945-7111/ac0942. [DOI] [Google Scholar]
- Pellerin P., Doco T., Scollary G.R. The influence of wine polymers on the spontaneous precipitation of calcium tartrate in a model wine solution. International Journal of Food Science and Technology. 2013;48(12):2676–2682. doi: 10.1111/ijfs.12264. [DOI] [Google Scholar]
- Pitkanen E.M., Siren H.M.M. Capillary zone electrophoresis of lipoarabinomannan by multi-layered concentration. Journal of Separation Science. 2022;45(4):945–959. doi: 10.1002/jssc.202100357. [DOI] [PubMed] [Google Scholar]
- Ponce F., Mirabal-Gallardo Y., Versari A., Felipe Laurie V. The use of cation exchange resins in wines: Effects on pH, tartrate stability, and metal content. Ciencia E Investigacion Agraria. 2018;45(1):82–92. doi: 10.7764/rcia.v45i1.1911. [DOI] [Google Scholar]
- Postel W., Meier B., Graf T. Studies on kinetics of crystallization of calcium tartrate in wine. Mitteilungen Klosterneuburg, Rebe und Wein, Obstbau und Fruechteverwertung. 1984;34(3):102–107. [Google Scholar]
- Prasad N., Thombare N., Sharma S.C., Kumar S. Gum arabic-a versatile natural gum: A review on production, processing, properties and applications. Industrial Crops and Products. 2022;187 doi: 10.1016/j.indcrop.2022.115304. [DOI] [Google Scholar]
- Ramey D.D., Ough C.S. Volatile ester hydrolysis or formation during storage of model solutions and wines. Journal of Agricultural and Food Chemistry. 1980;28(5):928–934. doi: 10.1021/jf60231a021. [DOI] [Google Scholar]
- Saito K., Kasai Z. Tartaric acid synthesis from L-ascorbic acid-1-14C in grape berries. Phytochemistry. 1969;8(11):2177–2182. doi: 10.1016/s0031-9422(00)88177-7. [DOI] [Google Scholar]
- dos Santos P.C., Gonçalves F., De Pinho M.N. Optimisation of the method for determination of the temperature of saturation in wines. Analytica Chimica Acta. 2002;458(1):257–261. doi: 10.1016/s0003-2670(01)01557-4. [DOI] [Google Scholar]
- Sommer S., Dickescheid C., Harbertson J.F., Fischer U., Cohen S.D. Rationale for haze formation after carboxymethyl cellulose (CMC) addition to red wine. Journal of Agricultural and Food Chemistry. 2016;64(36):6879–6887. doi: 10.1021/acs.jafc.6b02479. [DOI] [PubMed] [Google Scholar]
- Song W., Li Z., Liu B., Jing W., Ye J., Li L., Li J. Simultaneous determination of 26 organic acids and anions in grape wine by ion chromatography. Liquor-making Science & Technology. 2018;04:106–111. doi: 10.13746/j.njkj.2017316. [DOI] [Google Scholar]
- Sowalsky R.A., Noble A.C. Comparison of the effects of concentration, pH and anion species on astringency and sourness of organic acids. Chemical Senses. 1998;23(3):343–349. doi: 10.1093/chemse/23.3.343. [DOI] [PubMed] [Google Scholar]
- Tan Q., Leng Y., Wang J., Huang C., Yuan Y. Correlation and prediction of the solubility of the racemic tartaric acid-ethanol-water system with the NRTL model. Journal of Molecular Liquids. 2016;216:476–483. doi: 10.1016/j.molliq.2016.01.080. [DOI] [Google Scholar]
- Tang Y., Lu X. Research progress of application of edible film composite biological preservative in the preservation of fruits and vegetables. Food Research and Development. 2020;41(18):213–218. [Google Scholar]
- United States, Code of Federal Regulations . 2015. Title 27-alcohol, tobacco products and firearms, chapter I, subchapter a, part 24 (Wine) [Google Scholar]
- Vandenabeele C.R., Lucas S. Technological challenges and progress in nanomaterials plasma surface modification - a review. Materials Science & Engineering R-Reports. 2020;139 doi: 10.1016/j.mser.2019.100521. [DOI] [Google Scholar]
- Vernhet A., Dupre K., Boulange-Petermann L., Cheynier V., Pellerin P., Moutounet M. Composition of tartrate precipitates deposited on stainless steel tanks during the cold stabilization of wines. Part I. White wines. American Journal of Enology and Viticulture. 1999;50(4):391–397. [Google Scholar]
- Villamor R.R., Ross C.F. In: Annual review of food science and technology. Doyle M.P., Klaenhammer T.R., editors. Vol. 4. 2013. Wine matrix compounds affect perception of wine aromas; pp. 1–20. [DOI] [PubMed] [Google Scholar]
- Volschenk H., Vuuren H.J.J.V., Viljoen-Bloom M. Malic acid in wine: Origin, function and metabolism during vinification. South African Journal of Enology and Viticulture. 2006;27(2):123–136. [Google Scholar]
- Wang S. Mechanism of tartar formation in wine and methods to tartar stabilization. Liquor Making. 2004;05:60–61. [Google Scholar]
- Waterhouse A.L., Sacks G.L., Jeffery D.W. 2016. Understanding wine chemistry: John Wiley & Sons. [Google Scholar]
- Wei H., Tian D., Zhang Q. Introduction to cold stability in wine production. Liquor-making Science & Technology. 2019;09:78–83. doi: 10.13746/j.njkj.2019006. [DOI] [Google Scholar]
- Wheeler G.L., Jones M.A., Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393(6683):365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Wolucka B.A., Van Montagu M. GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. Journal of Biological Chemistry. 2003;278(48):47483–47490. doi: 10.1074/jbc.M309135200. [DOI] [PubMed] [Google Scholar]
- Xia N., Cheng H., Yao X., Pan Q., Meng N., Yu Q. Effect of cold stabilization duration on organic acids and aroma compounds during Vitis vinifera L. cv. Riesling wine bottle storage. Foods. 2022;11(9) doi: 10.3390/foods11091179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J. Stability of potassium hydrogen tartrate in wine. Sino-overseas Grapevine & Wine. 2002;05:54–55. [Google Scholar]
- Yan B., Chen X., Li W. Application of electrodialysis to improve the cold stability of wine. China Brewing. 2007;03:25–27. [Google Scholar]
- Yang M., Tan L., Batchelor-McAuley C., Compton R.G. The solubility product controls the rate of calcite dissolution in pure water and seawater. Chemical Science. 2024;15(7):2464–2472. doi: 10.1039/d3sc04063a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Discussion on organic acids in wine and their determination. China food safety magazine. 2021;29:187–188. doi: 10.16043/j.cnki.cfs.2021.29.113. [DOI] [Google Scholar]
- Yu J., Lin K., Zhu S. Reexamination of the expression equation for the stability of tartaric acid. Food Science. 1999;09:34–36. [Google Scholar]
- Zhang B. Removal of tartaric acid from wine by electrodialysis. Sino-overseas Grapevine & Wine. 2007;06:53–54. [Google Scholar]
- Zhang J., Zhou S., Wang K., Han W. Influence of stabilization techniques on total phenol and color intensity of red wine. Liquor Making. 2007;03:66–67. [Google Scholar]
- Zhang M., Zhu L., Wang J., Ye N., Dai S., Yu S., Wu Y. Surface modification of biomedical metals by double glow plasma surface alloying technology: A review of recent advances. Journal of Materials Research and Technology-Jmr&T. 2023;24:3423–3452. doi: 10.1016/j.jmrt.2023.04.003. [DOI] [Google Scholar]
- Zhang N., Xia H., Zhang J. Effects of three stabilizers on cold stability of cabernet sauvignon. Food and fermentation industries. 2019;45(17):32–39. doi: 10.13995/j.cnki.11-1802/ts.020872. [DOI] [Google Scholar]
- Zhang W., Chen X., Wang Y., Wu L., Hu Y. Experimental and modeling of conductivity for electrolyte solution systems. ACS Omega. 2020;5(35):22465–22474. doi: 10.1021/acsomega.0c03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Lan Y., Zhu B., Xiang X., Duan C., Shi Y. Changes in monosaccharides, organic acids and amino acids during cabernet sauvignon wine ageing based on a simultaneous analysis using gas chromatography-mass spectrometry. Journal of the Science of Food and Agriculture. 2018;98(1):104–112. doi: 10.1002/jsfa.8444. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Du G., Wang S., Zhao P., Cao X., Cheng C., Liu H., Xue Y., Wang X. Investigating the role of tartaric acid in wine astringency. Food Chemistry. 2023;403 doi: 10.1016/j.foodchem.2022.134385. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang Y. Process investigation on calcium stability in wines. Sino-overseas Grapevine & Wine. 2001;04:47–48. [Google Scholar]
- Zhou P., Zhou Y. Influence of mannoprotein on the stability of wine. Sino-overseas Grapevine & Wine. 2011;07:57–59. doi: 10.13414/j.cnki.zwpp.2011.07.023. [DOI] [Google Scholar]
- Zhu B. China Light Industry Press; Beijing: 1995. Wine industry handbook. [Google Scholar]
- Zoecklein B.W., Fugelsang K.C., Gump B.H., Nury F.S. Van Nostrand Reinhold; New York NY, USA: 1990. Production wine analysis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data that has been used is confidential.