Abstract
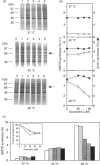
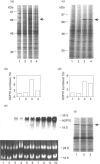
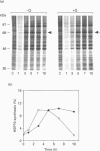
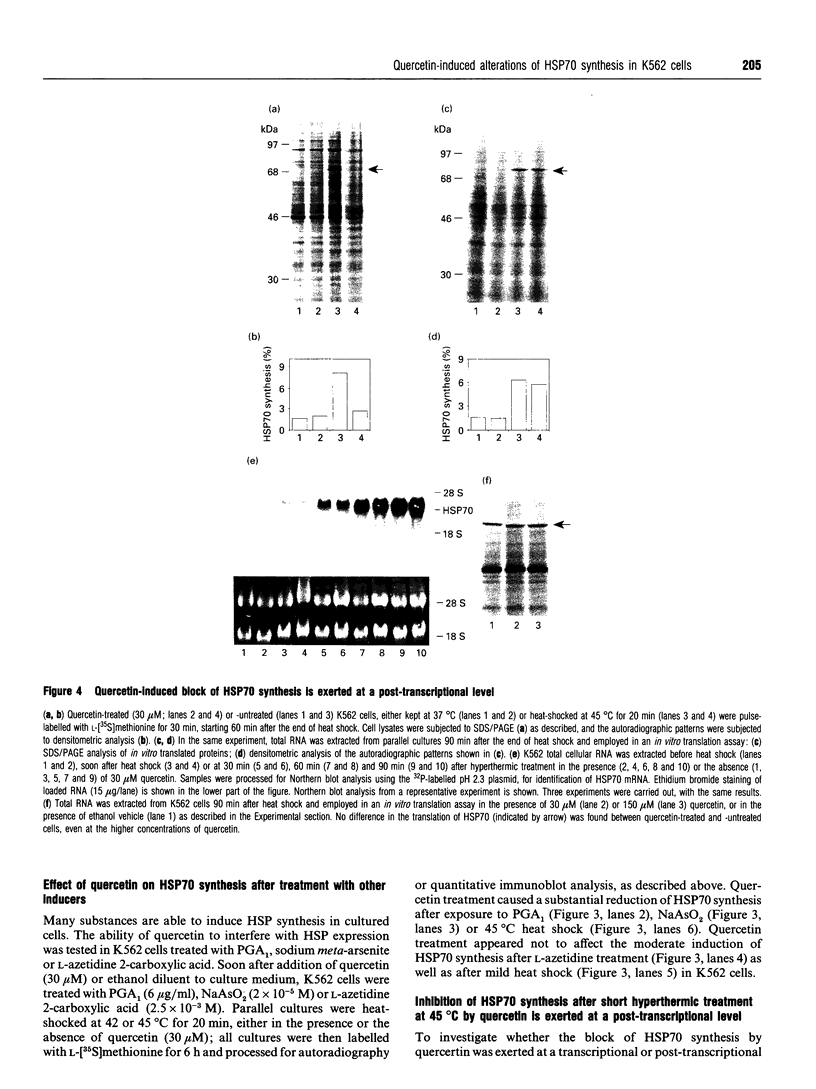
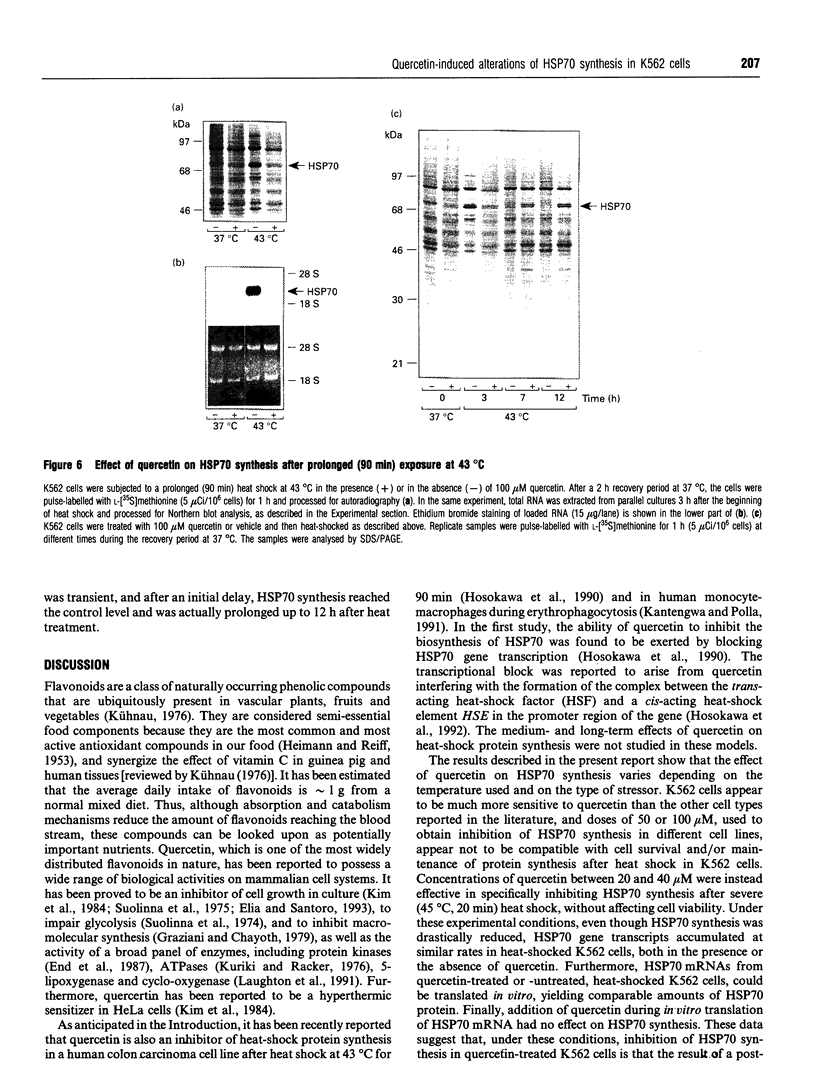
Synthesis of heat-shock proteins (HSPs) is universally induced in eukaryotic and prokaryotic cells by exposure to elevated temperatures or to other types of environmental stress. In mammalian cells, HSPs belonging to the 70 kDa family (HSP70) have a regulatory role in several cellular processes, and have been shown to be involved in the control of cell proliferation and differentiation. Although many types of HSP70 inducers have been identified, only a few compounds, all belonging to the flavonoid group, have been shown to inhibit HSP70 induction. Because inhibitors of HSP70 synthesis could be an important tool with which to study the function of this protein, we have investigated the effect of quercetin, a flavonoid with antiproliferative activity which is widely distributed in nature, on HSP70 synthesis in human K562 erythroleukaemia cells after treatment with severe or mild heat shock and with other inducers. Quercetin was found to affect HSP70 synthesis at more than one level, depending on the conditions used. Indeed, after severe heat shock (45 degrees C for 20 min) treatment with quercetin, at non-toxic concentrations, was found to inhibit HSP70 synthesis for a period of 3-4 h. This block appeared to be exerted at the post-transcriptional level and to be cell-mediated, as the addition of quercetin during translation of HSP70 mRNA in vitro had no effect. After prolonged (90 min) exposure at 43 degrees C, however, quercetin was found to inhibit also HSP70 mRNA transcription. Pretreatment of K562 cells with quercetin had no effect on HSP70 expression, and quercetin needed to be present during induction to be effective. Under all conditions tested, the quercetin-induced block of HSP70 synthesis was found to be transient and, after an initial delay, synthesis of HSP70 reached the control rate and continued at the same level for several hours after the time at which HSP70 synthesis had been turned off in control cells. Finally, inhibition of HSP70 synthesis by quercetin appeared to be dependent on the temperature used and on the type of stressor.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afanas'ev I. B., Dorozhko A. I., Brodskii A. V., Kostyuk V. A., Potapovitch A. I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989 Jun 1;38(11):1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- Amici C., Palamara A. T., Santoro M. G. Induction of thermotolerance by prostaglandin A in human cells. Exp Cell Res. 1993 Aug;207(2):230–234. doi: 10.1006/excr.1993.1188. [DOI] [PubMed] [Google Scholar]
- Amici C., Santoro M. G. Suppression of virus replication by prostaglandin A is associated with heat shock protein synthesis. J Gen Virol. 1991 Aug;72(Pt 8):1877–1885. doi: 10.1099/0022-1317-72-8-1877. [DOI] [PubMed] [Google Scholar]
- Amici C., Sistonen L., Santoro M. G., Morimoto R. I. Antiproliferative prostaglandins activate heat shock transcription factor. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6227–6231. doi: 10.1073/pnas.89.14.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler R., Welch W. J., Voellmy R. Heat shock gene regulation by nascent polypeptides and denatured proteins: hsp70 as a potential autoregulatory factor. J Cell Biol. 1992 Jun;117(6):1151–1159. doi: 10.1083/jcb.117.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clerget M., Polla B. S. Erythrophagocytosis induces heat shock protein synthesis by human monocytes-macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1081–1085. doi: 10.1073/pnas.87.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco A., Santoro M. G. Antiviral effect of short hyperthermic treatment at specific stages of vesicular stomatitis virus replication cycle. J Gen Virol. 1993 Aug;74(Pt 8):1685–1690. doi: 10.1099/0022-1317-74-8-1685. [DOI] [PubMed] [Google Scholar]
- End D. W., Look R. A., Shaffer N. L., Balles E. A., Persico F. J. Non-selective inhibition of mammalian protein kinases by flavinoids in vitro. Res Commun Chem Pathol Pharmacol. 1987 Apr;56(1):75–86. [PubMed] [Google Scholar]
- Graziani Y., Chayoth R. Regulation of cyclic AMP level and synthesis of DNA, RNA and protein by quercetin in Ehrlich ascites tumor cells. Biochem Pharmacol. 1979;28(3):397–403. doi: 10.1016/0006-2952(79)90105-9. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Hosokawa N., Hirayoshi K., Kudo H., Takechi H., Aoike A., Kawai K., Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992 Aug;12(8):3490–3498. doi: 10.1128/mcb.12.8.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N., Hirayoshi K., Nakai A., Hosokawa Y., Marui N., Yoshida M., Sakai T., Nishino H., Aoike A., Kawai K. Flavonoids inhibit the expression of heat shock proteins. Cell Struct Funct. 1990 Dec;15(6):393–401. doi: 10.1247/csf.15.393. [DOI] [PubMed] [Google Scholar]
- Kantengwa S., Polla B. S. Flavonoids, but not protein kinase C inhibitors, prevent stress protein synthesis during erythrophagocytosis. Biochem Biophys Res Commun. 1991 Oct 15;180(1):308–314. doi: 10.1016/s0006-291x(05)81293-8. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Kim S. H., Alfieri A. A., Young C. W. Quercetin, an inhibitor of lactate transport and a hyperthermic sensitizer of HeLa cells. Cancer Res. 1984 Jan;44(1):102–106. [PubMed] [Google Scholar]
- Kuriki Y., Racker E. Inhibition of (Na+, K+)adenosine triphosphatase and its partial reactions by quercetin. Biochemistry. 1976 Nov 16;15(23):4951–4956. doi: 10.1021/bi00668a001. [DOI] [PubMed] [Google Scholar]
- Köller M., König W. 12-Hydroxyeicosatetraenoic acid (12-HETE) induces heat shock proteins in human leukocytes. Biochem Biophys Res Commun. 1991 Mar 29;175(3):804–809. doi: 10.1016/0006-291x(91)91636-q. [DOI] [PubMed] [Google Scholar]
- König W., Köller M., Brom J. Priming mechanisms and induction of heat shock proteins in human polymorphonuclear granulocytes induced by eicosanoids and cytokines. Eicosanoids. 1992;5 (Suppl):S39–S41. [PubMed] [Google Scholar]
- Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- Laughton M. J., Evans P. J., Moroney M. A., Hoult J. R., Halliwell B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing ability. Biochem Pharmacol. 1991 Oct 9;42(9):1673–1681. doi: 10.1016/0006-2952(91)90501-u. [DOI] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I., Sarge K. D., Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992 Nov 5;267(31):21987–21990. [PubMed] [Google Scholar]
- Muñoz A., Alonso M. A., Carrasco L. Synthesis of heat-shock proteins in HeLa cells: inhibition by virus infection. Virology. 1984 Aug;137(1):150–159. doi: 10.1016/0042-6822(84)90018-7. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Healy A. M., Wojno W. C., Krane S. M. Hormone 1 alpha,25-dihydroxyvitamin D3 modulates heat shock response in monocytes. Am J Physiol. 1987 Jun;252(6 Pt 1):C640–C649. doi: 10.1152/ajpcell.1987.252.6.C640. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Garaci E., Amici C. Prostaglandins with antiproliferative activity induce the synthesis of a heat shock protein in human cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8407–8411. doi: 10.1073/pnas.86.21.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. K., Yu J. Accumulation of a heat shock-like protein during differentiation of human erythroid cell line K562. Nature. 1984 Jun 14;309(5969):631–633. doi: 10.1038/309631a0. [DOI] [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T. T. Stress protein systems of mammalian cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- Suolinna E. M., Buchsbaum R. N., Racker E. The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res. 1975 Jul;35(7):1865–1872. [PubMed] [Google Scholar]
- Suolinna E. M., Lang D. R., Racker E. Quercetin, an artificial regulator of the high aerobic glycolysis of tumor cells. J Natl Cancer Inst. 1974 Nov;53(5):1515–1519. doi: 10.1093/jnci/53.5.1515. [DOI] [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Benedetti A., Baglioni C. Translational regulation of the synthesis of a major heat shock protein in HeLa cells. J Biol Chem. 1986 Nov 25;261(33):15800–15804. [PubMed] [Google Scholar]