Abstract
In 2010, the United States Human and Health Services (US HHS) and the European Union's (EU) Directorate General for Communications Networks, Content and Technology signed a memorandum of understanding to stimulate cooperation surrounding health-related information communications technology. The key project that emerged from this agreement is the International Patient Summary (IPS), intended to provide succinct clinically relevant patient summaries, which are generalizable and condition-independent, that can be readily used by all clinicians for the care of patients. Although allergies are included in the main information required by the IPS library and framework, it is misrepresented which leads to underdiagnosis or misdiagnosis of patients suffering from allergic and hypersensitivity conditions (A/H). The French and Montpellier World Health Organization (WHO) Collaborating Centres have provided arguments for supporting representation of A/H in the IPS. These are based on the relevance of the new classification of A/H in the WHO International Classification of Diseases 11th version (ICD-11), and the need for alignment of eHealth tools with harmonized health information. We first present the A/H in the IPS initiative with the mission of producing an international information system that can be used globally in electronic health records to standardize clinical diagnoses and facilitate communication between clinicians caring for patients with A/H diseases. It is believed this initiative will provide a strong voice for the allergy community and an effective process for improving the quality of health data that will optimize medical care for our patients worldwide.
Keywords: Allergy, Anaphylaxis, Classification, Coding, Epidemiology, Hypersensitivity, International Patients Summary, International Classification of Diseases, World Health Organization
The International Patients Summary
The International Patient Summary (IPS) perspective
Allergic and hypersensitivity conditions (A/H) are increasing in numbers and complexity worldwide, creating difficult challenges for health care professionals who are initially encountering these patients without a documented medical history. Access to a summary of a patient's clinical data provides health professionals the essential information needed to manage unexpected or unpredicted medical situations, such as anaphylaxis, an acute asthma exacerbation or an acute pneumonia in patients with a beta-lactam allergy. However, this information can also be very useful for providing planned medical care when patients relocate to a different city or country or when they change healthcare payor systems.1,2
With the rapid globalization of healthcare and the need for Digital Health Information System (d-HIS) supported by electronic processes and communications, the United States Human and Health Services (US HHS) and the European Union's (EU) Directorate General for Communications Networks, Content and Technology (DG-CONNECT) signed in 2010 a memorandum of understanding to initiate cooperation for developing health-related information communications technology (ICT). The focus of this transatlantic cooperative agreement was on d-HIS and health ICT.3,4 The key project that emerged from this agreement is the International Patient Summary (IPS), led by the Health Level Seven International (HL7) and the European Committee for Standardization/Comité Européen de Normalisation (CEN) Technical Committee 251 (CEN/TC251) of the European Union.5,6 This ambitious project provides generic solutions to standardize electronic health records globally. CEN/TC 251 is a technical decision-making body working on Health ICT standardization in the European Union7 and HL7 is a not-for-profit organization, providing a set of standards for transferring clinical and administrative data between hospital information systems.8
It has been agreed that for this project to be successful, a single, common IPS approach, that is readily accessible by clinicians throughout the world, should be implemented so patients who present for healthcare emergently or unexpectedly can be managed more effectively. The IPS specification system emphasizes the importance of generating succinct but generalizable patient summaries, that are clinically relevant to all healthcare providers globally. Thus, the IPS is intended to represent a standardized electronic health record that extracts all essential healthcare information about a patient to ensure safe and secure healthcare wherever they are seen clinically in the world.5,10 The principles of IPS must be: (i) easily implemented, (ii) globally applicable, (iii) widely accessible, and (iv) sustainable.7,10
The International Patient Summary framework
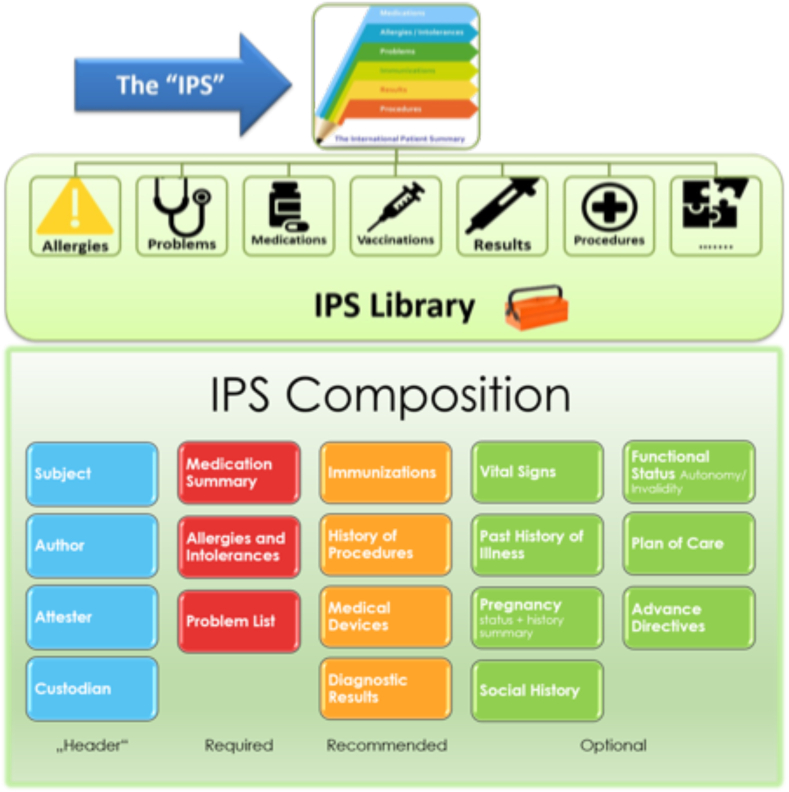
The IPS library structure is composed of a set of robust, well-defined, and potentially reusable core data items (Fig. 1). All inserted data must be agreed upon by the patient and health professional. There are 4 sections that must be completed: 1) header, 2) required data, 3) recommended data, and 4) optional information (Fig. 1). Allergies are included among the primary information required in the IPS library framework.8 However, they are not properly documented, which generates challenges for health care professionals who are firstly encountering A/H patients without a documented medical history.
Fig. 1.
International Patient Summary (IPS) framework
Allergy and hypersensitivity conditions in the International Patient Summary
Scope of the problem
Allergy and hypersensitivity constitute one of the fastest growing non-communicable group of conditions worldwide. According to the World Health Organization (WHO), 50% of the European population may develop an allergy in the next 10 years.9 Since 2012, the Montpellier WHO Collaborating Centre (WHO CC) has led an international scientific and academic initiative to develop a better representation of these conditions in the International Classification of Diseases (ICD)-11.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 This work has been acknowledged internationally by the 6 major international and regional allergy organizations: the American Academy of Allergy Asthma and Immunology (AAAAI), the European Academy of Allergy and Clinical Immunology (EAACI), the World Allergy Organization (WAO), the American College of Allergy Asthma and Immunology (ACAAI), the Asia Pacific Association of Allergy, Asthma and Clinical Immunology (APAAACI), and the Latin American Society of Allergy, Asthma and Immunology (SLAAI).35
The primary outcome of this collaboration was the creation of an “Allergic and Hypersensitivity (A/H) conditions” section for the ICD-11 with changes in the WHO ICD mortality coding rules, that now allow them to be used as underlying causes of death in death certificates.14,36 As a result of this work, in 2018 WHO designated the Montpellier WHO CC to oversee the validation, implementation, and surveillance of this new A/H coding section.
A major limitation was that it did not include health professional communities as stakeholders during the initial conception of these codes. As a result, allergic conditions are still met with significant confusion leading to misdocumentation and miscoding of A/H conditions.8,9
Therefore, the IPS intends to adopt worldwide recognized coding tools to harmonize data and information. It is recommended that the ICD-11 “Allergic and Hypersensitivity conditions” section be used in this process, requiring that the terminology and concepts of the ongoing IPS framework be updated. Since the Montpellier WHO CC is representing the Allergy/Immunology specialty in this initiative, this technical work will be coordinated with the French WHO CC to ensure optimal representation of A/H in the IPS.
The current main key issues that must be addressed are: (I) involvement of health professionals as stakeholders in the IPS development process, as this will likely impact technical views of the IPS data inserted, (II) description of A/H as supplementary data into 3 sections of the IPS (general clinical data, medications, clinical description), (III) update A/H diagnoses that would prevent patients from being underrepresented or not represented, and (IV) update current terminology, to coincide with the evolving knowledge in A/H that will avoid inaccurate or ambiguous diagnoses.
Current status and perspectives
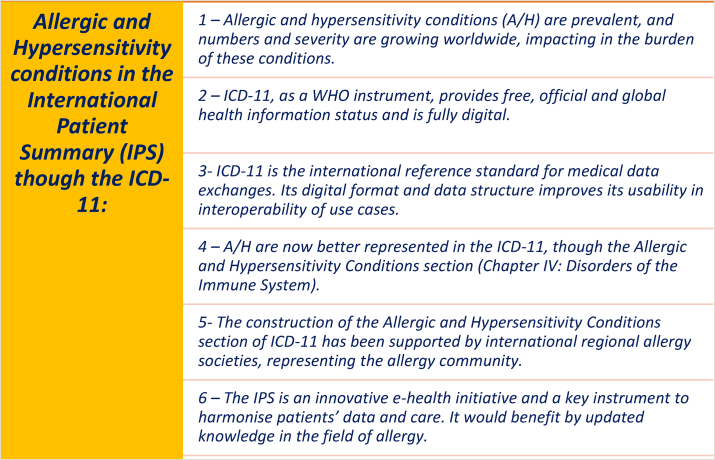
Reasons supporting the inclusion of A/H as IPS though ICD-11 have been submitted, which include their relevance to the new WHO ICD-11 A/H classification system and the need to align to d-HIS tools so health information can be harmonized (Table 1).
Table 1.
Key arguments for a better representation of allergic and hypersensitivity conditions (A/H) in the International Patient Summary (IPS) through the ICD-11
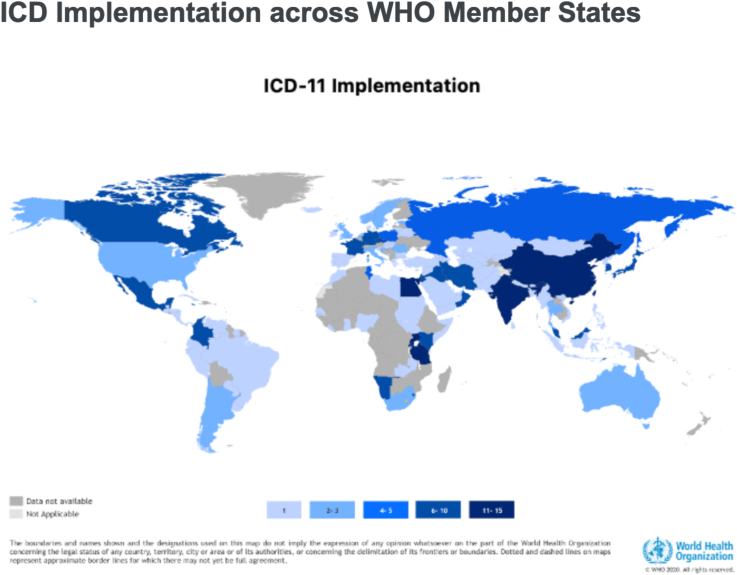
The need for recording diseases and mortality data, understanding the evolution of clinical disorders, and tracking areas of interest/investments in the health system, were key factors that originally drew attention for the need to develop a consensus generalizable classification and coding system allowing for worldwide alignment. Many different classifications and terminologies systems including ICD have previously emerged. The ICD is the global health information standard to capture morbidity and mortality statistics (MMS) that is maintained and periodically revised by WHO. The ICD is used as a public health tool to aggregate epidemiological statistics to substantiate decision-making and allocation of resources for both management and research by more than 75% of countries. The ICD-11 has been proposed and fully endorsed during the last World Health Assembly (May 2019) and its implementation is ongoing worldwide (Fig. 2).36 Like ICD-10, ICD-11 is a semantic standard for medical data exchange. The new fully digitalized version is designed to improve ICD usability through compatible electronic interfaces.
Fig. 2.
Current status of the global implementation of the ICD-11 according to the World Health Organization, 2023 (43)
The consolidated new A/H section in the IPS through the ICD-11 will now allow A/H be recognized as the clinical conditions requiring specific documentation and management. By allowing inclusion of all the relevant diagnostic A/H terms into the ICD-11, WHO has recognized their importance to clinicians, epidemiologists, researchers, statisticians, health care planners, and other stakeholders.28 Work is also ongoing to include allergen codes into the ICD-11 to ensure accurate classification of the aetiology/triggers for A/H conditions.
The ICD-11 has a multi-hierarchical online framework, is multilingual, and allows delivering more details to the diseases. It enables diagnoses to be linked to a range of parameters by the addition of one or more “extensions”, classified under the “Extension codes” X chapter in a process termed post-coordination. WHO has been promoting this classification strategy in which a stem entity (eg, anaphylaxis) can be more fully defined by linking it to a range of different value sets including severity, anatomical location, and causal agent.36
The representation of A/H in the WHO ICD-11 coding system is a key health, political, and economic advancement that advocates for the best management and prevention of A/H across different countries. It is believed that A/H inclusion in the ICD-11 coding system will result in greater recognition of these conditions that will generate novel perspectives in the upcoming years. Furthermore, this initiative will ensure that health professionals have easy access to standardized patient electronic health records anywhere in the world.34
Regular technical work has begun in France, and changes have been proposed at the most recent IPS EU business meeting. The next steps of this initiative are to establish bilateral discussions with the EU and US IPS, request modification of current international guidelines for the electronic exchange of health data,4 implement updates of the ICD-11 A/H diagnoses in the IPS, and initiate the validation process by the allergy community.
Conclusion
Development of the A/H IPS, which is an international standardized information system for use in electronic health records, that facilitates communication among all physicians and other healthcare providers interested in allergy, has been initiated. Although in the early stages of execution, it is strongly believed that this initiative will provide a strong voice to the global allergy community and that the outcomes of this process will improve heath data and optimize care for patients worldwide. In addition, implementation of ICD-11 in the IPS will also contribute on the A/H documentation.
Abbreviations
AAAAI: American Academy of Allergy Asthma and Immunology; ACAAI: American College of Allergy Asthma and Immunology; APAAACI: Asia Pacific Association of Allergy, Asthma and Clinical Immunology; CEN: European Committee for Standardization; CEN/TC251: European Committee for Standardization Technical Committee 251; DG-CONNECT: Directorate General for Communications Networks, Content and Technology; EAACI: European Academy of Allergy and Clinical Immunology; EU: European Union; HL7: Health Level Seven International-7; ICD: International Classification of Diseases; ICT: Health Information and Communications Technology; IPS: International Patient Summary; SLAAI: Latin American Society of Allergy, Asthma and Immunology; US/HHS: United States Human and Health Services; WAO: World Allergy Organization; WHA: World Heal Assembly; WHO: World Health Organization; WHO CC: World Health Organization Collaborating Centre.
Funding
See acknowledgments section for individual author funding.
Availability of data and materials
Not applicable.
Authors’ contributions
The first and last authors contributed to the construction of the document. All the authors critically revised and approved the final version of the manuscript and agree to be accountable for all the aspects of the work.
Ethics approval
Not applicable.
Authors’ consent for publication
All the authors consent for publication in WAO Journal.
Declaration of competing interest
The authors declare that they do not have any conflict of interests related to the contents of this article.
PI and consultant for: Novartis, Genentech, Sanofi Regeneron, Astra Zeneca/Amgen, GSK, Allakos, Escient/Incyte, Celldex, Areteia, Takeda/Shire, CSL Behring, Biocryst, Pharming, Kalvista, Ionis, Intellia, Biomarin, Astria, Jaspar.
Acknowledgments
We thank very much the World Health Organization International Classification of Diseases Team for their support, namely: Robert Jakob, Nenad Friedrich Ivan Kostanjsek, Eva Krpelanova and Carine Alsokhn. Luciana Kase Tanno received an unrestricted ANS grant through CHRUM administration and a research AllerGOS grant.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Johansson S.G., Bieber T., Dahl R., et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the world allergy organization, october 2003. J Allergy Clin Imunnol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos N.G., Agache I., Bavbek S., et al. Research needs in allergy: an EAACI position paper, in collaboration with EFA. Clin Transl Allergy. 2012;2:21. doi: 10.1186/2045-7022-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Patient Summary webpage (available: https://international-patient-summary.net/, accessed July 2023).
- 4.European commission, digital strategy webpage (available: https://digital-strategy.ec.europa.eu/en/news/european-standard-digital-patient-summary-has-been-approved, accessed July 2023).
- 5.HealthIT webpage (available: https://www.healthit.gov/topic/global-digital-health-partnership/international-patient-summary, accessed July 2023).
- 6.European commission eHealth standards webpage (available: https://www.ehealth-standards.eu/, accessed July 2023).
- 7.Health Level Seven International webpage (available: https://www.hl7.org/, accessed July 2023).
- 8.Health Informatics, International Patient Summary (available: https://health.ec.europa.eu/system/files/2019-07/ev_20190611_co123_en_0.pdf, accessed July 2023).
- 9.Prevention of Allergy and Asthma (available: https://apps.who.int/iris/bitstream/handle/10665/68361/WHO_NMH_MNC_CRA_03.2.pdf, accessed July 2023).
- 10.Tanno L.K., Ganem F., Demoly P., Toscano C.M., Bierrenbach A.L. Undernotification of anaphylaxis deaths in Brazil due to difficult coding under the ICD-10. Allergy. 2012;67:783–789. doi: 10.1111/j.1398-9995.2012.02829.x. [DOI] [PubMed] [Google Scholar]
- 11.Tanno L.K., Calderon M.A., Goldberg B.J., Akdis C.A., Papadopoulos N.G., Demoly P. Categorization of allergic disorders in the new world health organization international classification of diseases. Clin Transl Allergy. 2014;4:42. doi: 10.1186/2045-7022-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demoly P., Tanno L.K., Akdis C.A., et al. Global classification and coding of hypersensitivity diseases – an EAACI – WAO survey, strategic paper and review. Allergy. 2014;69:559–570. doi: 10.1111/all.12386. [DOI] [PubMed] [Google Scholar]
- 13.Tanno L.K., Calderon M.A., Goldberg B.J., et al. Constructing a classification of hypersensitivity/allergic diseases for ICD-11 by crowdsourcing the allergist community. Allergy. 2015;70:609–615. doi: 10.1111/all.12604. [DOI] [PubMed] [Google Scholar]
- 14.Tanno L.K., Calderon M.A., Demoly P. On behalf the joint allergy academies. New allergic and hypersensitivity conditions section in the international classification of diseases-11. Allergy Asthma Immunol Res. 2016;8:383–388. doi: 10.4168/aair.2016.8.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanno L.K., Calderon M., Papadopoulos N.G., Demoly P. Mapping hypersensitivity/allergic diseases in the International Classification of Diseases (ICD)-11: cross-linking terms and unmet needs. Clin Transl Allergy. 2015;5:20. doi: 10.1186/s13601-015-0063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanno L.K., Calderon M.A., Demoly P. On behalf the joint allergy academies. Making allergic and hypersensitivity conditions visible in the international classification of diseases-11. Asian Pac Allergy. 2015;5:193–196. doi: 10.5415/apallergy.2015.5.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanno L.K., Calderon M.A., Demoly P. On behalf the Joint Allergy Academies. Optimization and simplification of the allergic and hypersensitivity conditions classification for the ICD-11. Allergy. 2016;71:671–676. doi: 10.1111/all.12834. [DOI] [PubMed] [Google Scholar]
- 18.Tanno L.K., Calderon M.A., Papadopoulos N.G., et al. Revisiting desensitization and allergen immunotherapy concepts for the international classification of diseases (ICD)-11. J Allergy Clin Immunol Pract. 2016;4:643–649. doi: 10.1016/j.jaip.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Tanno L.K., Calderon M.A., Li J., Casale T., Demoly P. Updating Allergy/Hypersensitivity diagnostic procedures in the WHO ICD-11 revision. J Allergy Clin Immunol Pract. 2016;4:650–657. doi: 10.1016/j.jaip.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Tanno L.K., Calderon M.A., Papadopoulos N.G., et al. Joint Allergy Academies. Surveying the new allergic and hypersensitivity conditions chapter of the International classification of diseases (ICD)-11. Allergy. 2016;71:1235–1240. doi: 10.1111/all.12945. [DOI] [PubMed] [Google Scholar]
- 21.Tanno L.K., Calderon M., Demoly P., Joint Allergy Academies Supporting the validation of the new allergic and hypersensitivity conditions section of the world health organization international classification of diseases-11. Asia Pac Allergy. 2016;6:149–156. doi: 10.5415/apallergy.2016.6.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanno L.K., Calderon M., Sublett J.L., Casale T., Demoly P., Joint Allergy Academies Smoothing the transition from international classification of diseases, tenth revision, clinical modification to international classification of diseases, eleventh revision. J Allergy Clin Immunol Pract. 2016;4:1265–1267. doi: 10.1016/j.jaip.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Tanno L.K., Bierrenbach A.L., Calderon M.A., et al. Decreasing the undernotification of anaphylaxis deaths in Brazil through the International Classification of Diseases (ICD)-11 revision. Allergy. 2017;72:120–125. doi: 10.1111/all.13006. [DOI] [PubMed] [Google Scholar]
- 24.Tanno L.K., Ansotegui I., Demoly P. Globalization and anaphylaxis. Curr Opin Allergy Clin Immunol. 2018;18:365–369. doi: 10.1097/ACI.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 25.Tanno L.K., Molinari N., Bruel S., et al. Joint Allergy Academies Field-testing the new anaphylaxis' classification for the WHO international classification of diseases (ICD)-11 revision. Allergy. 2017 May;72(5):820–826. doi: 10.1111/all.13093. [DOI] [PubMed] [Google Scholar]
- 26.Tanno L.K., Sublett J.L., Meadows J.A., et al. Joint Allergy Academies Perspectives of the international classification of diseases (ICD)-11 in allergy clinical practice in the United States of America. Ann Allergy Asthma Immunol. 2017 Feb;118(2):127–132. doi: 10.1016/j.anai.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Tanno L.K., Chalmers R.J., Calderon M.A., Aymé S., Demoly P., on behalf the Joint Allergy Academies Reaching multidisciplinary consensus on classification of anaphylaxis for the eleventh revision of the World Health Organization's (WHO) International Classification of Diseases (ICD-11) Orphanet J Rare Dis. 2017 Mar 16;12(1):53. doi: 10.1186/s13023-017-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanno L.K., Calderon M.A., Linzer JFSr, Chalmers R.J.G., Demoly P., on behalf of the Joint Allergy Academies Collaboration between specialties for respiratory allergies in the international classification of diseases (ICD)-11. Respir Res. 2017, Feb 10;18(1):34. doi: 10.1186/s12931-017-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanno L.K., Haahtela T., Calderon M.A., Cruz A., Demoly P. Joint Allergy Academies. Implementation gaps for asthma prevention and control. Respir Med. 2017 Sep;130:13–19. doi: 10.1016/j.rmed.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Tanno L.K., Torres M.J., Castells M., Demoly P. Joint Allergy Academies. What can we learn in drug allergy management from World Health Organization's International classifications? Allergy. 2018 May;73(5):987–992. doi: 10.1111/all.13335. [DOI] [PubMed] [Google Scholar]
- 31.Tanno L.K., Demoly P. Lessons of drug allergy management through the world health organization's international classification of diseases (ICD)-11. Curr Treatment Opin in Allergy. 2018 March 5;(1):52–59. [Google Scholar]
- 32.Tanno L.K., Demoly P., on behalf of the Joint Allergy Academies How can the world health organization's international classification of diseases (ICD)-11 change the clinical management of anaphylaxis? Expet Rev Clin Immunol. 2018 Sep;11:1–4. doi: 10.1080/1744666X.2018.1520094. [DOI] [PubMed] [Google Scholar]
- 33.Tanno L.K., Demoly P., Joint Allergy Academies Are outcome measures in allergic diseases relevant for the WHO's International Classification of Diseases in allergology? Curr Opin Allergy Clin Immunol. 2019 Jun;19(3):198–203. doi: 10.1097/ACI.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 34.Tanno L.K., Chalmers R., Bierrenbach A.L., et al. on behalf Joint Allergy Academies Changing the history of anaphylaxis mortality statistics througth the world health organization's international classification of diseases (ICD)-11. J Allergy Clin Immunol. 2019 Sep;144(3):627–633. doi: 10.1016/j.jaci.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Tanno L.K., Casale T., Papadopoulos N.G., et al. A call to arms of specialty societies to review the WHO International Classification of Diseases, Eleventh Revision terms appropriate for the diseases they manage: the example of the Joint Allergy Academies. Allergy Asthma Proc. 2017 Jul 1;38(4):54–55. doi: 10.2500/aap.2017.38.4063. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization, ICD-11 Beta Draft website. (cited, available: http://apps.who.int/classifications/icd11/browse/l-m/en January 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.