Highlights
-
•
Near-infrared (NIR) angiography to assess intraoperative bowel perfusion can identify anastomoses at risk for AL.
-
•
The use of NIR angiography may reduce AL rates, the need for intestinal diversion and other complications.
-
•
NIR angiography for bowel resection is feasible to implement in gynecologic oncology surgery.
Keywords: Bowel anastomosis, Indocyanine green, Near infrared angiography, Gynecologic surgery
Abstract
Reducing anastomotic leak rates after bowel resection is a priority among patients undergoing gynecologic oncology surgery. While near-infrared (NIR) angiography has been investigated in the colorectal literature, more recent work has demonstrated promising results when used in gynecologic cancer surgery. It has been repeatedly shown to be a safe intervention that can offer real time assessment of bowel perfusion, offering the surgeon the opportunity to act on the results in the hopes of decreasing the risk of complications.
1. Background
Surgical resection has been repeatedly shown to play an integral role in the treatment of advanced gynecologic malignancy. The success of surgical resection in the treatment of advanced uterine or ovarian malignancy has been demonstrated to depend on maximizing the amount of tumor resected with the best survival outcomes being linked to achieving no gross residual (Bristow et al., 2023, Rajkumar et al., 2019). In order to achieve such goals, some form of bowel resection is frequently required. In addition, we often find ourselves performing bowel resections in the setting of bowel obstruction as well as other disease- or treatment- related complications. Given the integral role that bowel resections have on patients in an already fragile state, it is of the utmost importance that we work to minimize their potential for complications. Among these detrimental complications are anastomotic leaks. Rates in the gynecologic literature vary, ranging up to 7 % in patients undergoing debulking surgery for ovarian cancer (Grimm et al., 2017). The mortality rate associated with an anastomotic leak ranges from 3 % up to 21 %, most often secondary to sepsis (Blumetti et al., 2014). In addition anastomotic leaks are associated with increased hospital cost, length of hospital stay, readmission, reoperations, and a longer time to start of adjuvant chemotherapy (Grimm et al., 2017). Given these detrimental impacts, identifying possible interventions to help intra or peri-operatively diminish the risk of an anastomotic leak have undergone investigation.
Among these is the use of near-infrared (NIR) angiography in evaluating bowel anastomosis during a gynecologic oncology surgery. Poor oxygenation due to diminished blood supply is believed to play a role in the risk of an anastomotic leak (Kingham and Pachter, 2009, Vignali et al., 2000). The use of NIR offers the surgeon a method by which to assess anastomotic perfusion intraoperatively. This provides an opportunity to act on abnormal findings intraoperatively, such as the implementation of a diverting ileostomy or revision of anastomosis in the case of abnormal perfusion findings.
2. Technology
Indocyanine green (ICG) has been used in medicine since the late 50 s for a multitude of procedures from measuring cardiac output to studying the anatomy of the retinal vessels. ICG absorbs light in the NIR spectrum between 790 and 805 nm and re‐emits electromagnetic energy at 835 nm, which can be visualized by its fluorescence in the vasculature using NIR irradiation (Alander et al., 2012). Its half‐life in humans is 3–5 min, and the rate of allergic reaction is approximately 1 in 333,000 (Keller et al., 2017, Yamaguchi et al., 2021).
An endoscopic fluorescence imaging system is then used to visualize ICG perfusion. The system provides high‐definition white-light and NIR-light rapid image sequencing, creating a superimposition of NIR-on-white‐light views using false-green signal coloring to allow an enhanced intraoperative assessment of perfusion. ICG perfusion with NIR assessment is an available option whether an open or minimally invasive surgical technique (Keller et al., 2017, Boni et al., 2016). This review will focus on the use of ICG with NIR angiography for low anterior resections, however this technique has additionally been demonstrated in other bowel surgeries such as small bowel anastomosis, sleeve gastrectomy and esophagectomy (Aleassa and El-Hayek, 2020).
3. Technique
This intervention is used to assess perfusion of colonic tissue at two critical steps: the first is to assess the proximal and distal stumps after transection, and the second is for low anterior resections, after completion of the anastomosis to assess the integrity of the mucosal aspect via proctoscopy. Both the NIR angiographic examination of the bowel serosa in the first step, and NIR endoscopic examination of the anastomosed bowel mucosa in the second step promote a comprehensive evaluation of bowel perfusion. This two-step technique was used in the retrospective study by Moukarzel et al. (2020).
In the first step, following the transection and immediately prior to planning anastomoses, the surgeon performs the NIR angiography and assesses both the proximal and distal stumps for perfusion. This intervention can be used to assess perfusion of any segment of bowel prior to anastomosis. To assess the perfusion, a bolus of 3–7 ml at a concentration of 2.5 mg/ml of ICG followed by a 10 ml normal saline flush is administered intravenously by the anesthesiologist; recommended maximum dosing is <2 mg/kg (FDA, 2020). Using the endoscopic fluorescence imaging system, the time to ICG visualization should be noted with a goal of less than 60 s to complete perfusion. If perfusion is deemed satisfactory, then surgeon proceeds to perform the anastomoses in accordance with the surgeon's usual techniques (Fig. 1). If perfusion is deemed unsatisfactory, then the surgeon may decide to trim the distal portion of the stump (Fig. 2).
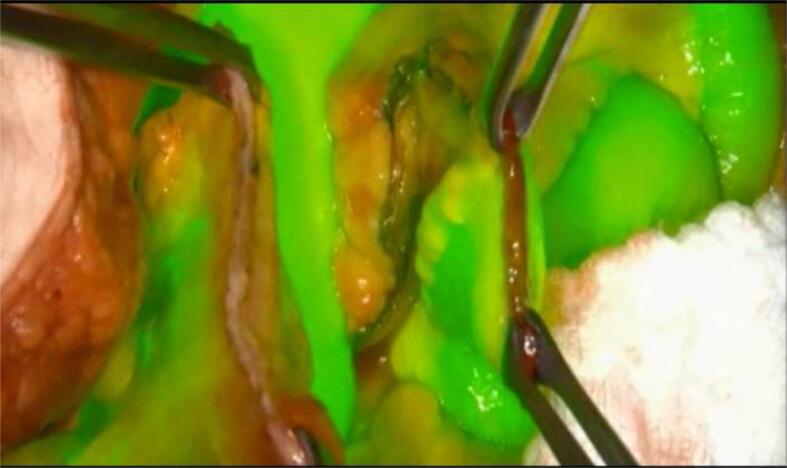
Fig. 1.
ICG should perfuse normal tissue within 60seconds of injection and should be cleared within 10 minutes. Image demonstrates circumferential perfusion throughout colonic stump.
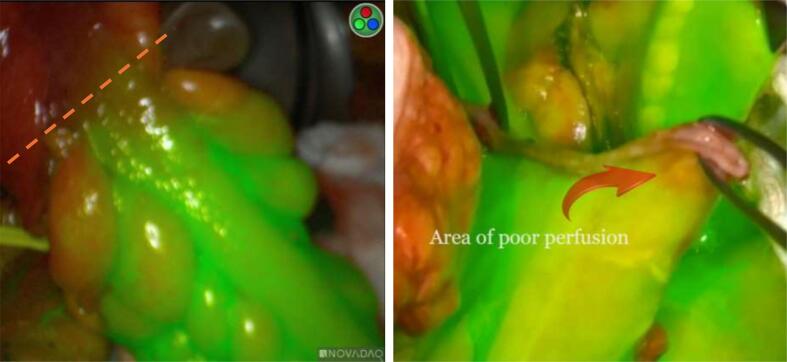
Fig. 2.
Lack of satisfactory perfusion. A) Clear line of demarcation marked by dotted red line. B) Localized to corner of colonic stump.
After completion of the anastomosis, anastomotic assessment is conducted in accordance with the surgeon's preference. For rectosigmoid resections the standard assessment includes visual inspection of tension at the anastomosis, an air bubble test, and, in some cases, visual assessment with rigid proctoscopy. After all anastomoses and standard assessment is complete, then the surgeon can choose to perform an additional assessment using NIR angiography, in step two.
For a rectosigmoid resection, this subsequent assessment is performed via proctoscopy. The endoscopic fluorescence imaging system is inserted into the anus using a disposable endoscope and advanced to the staple line of the anastomosis. The surgeon obtains visualization of the proximal and distal ends of the rectosigmoid anastomosis with visualization of the entire anastomosis. A second bolus of ICG is then re-administered intravenously by the anesthesiologist. The surgeon then assesses in real time the perfusion of the anastomosis. (Video 1,2). The surgeon would then decide on the surgical plan, such as to perform a revision of the anastomosis or perform a diverting ostomy.
4. Review of the literature & discussion
The use of this technique was originally developed in the colorectal literature where it was demonstrated to be safe with promising results in identifying anastomoses at risk, decreasing leak rates, and improving outcomes (Ris et al., 2018, Jafari et al., 2013, Jafari et al., 2015, Kudszus et al., 2010). Recent studies have described a significant decrease in leak rates with the use of ICG imaging to assess anastomotic perfusion in rectal cancer surgery. The FLAG trial, a randomized control trial (RCT) in Russia investigating the use of NIR angiography for sigmoid or rectal neoplasms, found a decrease in AL rate from 16.3 % to 9.1 % in patients undergoing LAR (Alekseev et al., 2020). A meta-analysis reported a significant decrease in leak rates from 6.1 % to 1.1 % when ICG was used to evaluate anastomotic perfusion (Blanco-Colino and Espin-Basany, 2018). Results from the European IntAct RCT investigating the use of NIR angiography in laparoscopic colorectal cancer surgery are pending (Armstrong et al., 2018). While PILLAR III, a U.S. multicenter trial investigating the use of NIR angiography in LAR for colorectal malignancies, closed early due to slow accrual, the ICG-COLORAL study investigating the use of NIR angiography in benign and malignant colorectal surgery, is ongoing in Finland (Jafari et al., 2021, Kossi, 2018).
More recently the use of this technology has also been investigated during surgical debulking cases for ovarian and uterine carcinoma. In a retrospective review of 133 patients, Moukarzel et al., demonstrated the safety and feasibility of using NIR angiography as an adjunct to standard assessment of primary rectosigmoid anastomosis at the time of surgery for gynecologic malignancy (Moukarzel, 2020). In this study, NIR angiography use was associated with extremely low rates of anastomotic leak: 1.5 % in the NIR group and 4.7 % in the non-NIR cohort. This was accompanied by a lower total rate of intestinal diversion (6.8 % vs 19.9 %), fewer post-operative abscesses (6.0 % vs 15.9 %) and fewer post-operative interventional procedures (9.0 % vs 19.9 %) in the NIR group vs the non-NIR group. Additionally, there were no allergic reactions to ICG or intraoperative complications associated with the use of NIR angiography, further demonstrating the safety of NIR angiography. These findings suggest that the use of NIR angiography reduces complications while resulting in fewer diverting ileostomies and the morbidity associated with them. Currently in the US, there is an ongoing multi-institutional randomized clinical trial investigating the use of this technology in gynecologic cancer surgery (NCT04878094) (Leitao et al., 2024).
5. Barriers to implementation
When considering barriers to implementation, the NIR angiography system and training to safely use this technology is critical. While start-up cost of the NIR angiography system is high, the cost of ICG per patient is minimal (Blanco-Colino and Espin-Basany, 2018). Additionally, the NIR angiography system is used in the gynecologic oncology field for sentinel lymph node mapping, and its use can be modified for the evaluation of bowel anastomosis. A recent cost analysis in colorectal surgery showed a per-case saving of $192 with NIR angiography implementation (Liu et al., 2022). When the costs of NIR angiography are considered against the costs of anastomotic leak including prolonged antibiotic treatment and hospitalization, re-operation and other interventional procedures, NIR angiography implementation can result in significant benefit to the patient and healthcare system.
CRediT authorship contribution statement
Lea A. Moukarzel: Writing – review & editing, Writing – original draft, Conceptualization. Sarah Andres: Writing – review & editing. Oliver Zivanovic: Validation, Supervision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2024.101474.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alander J.T., et al. A review of indocyanine green fluorescent imaging in surgery. International Journal of Biomedical Imaging. 2012;2012:940585-26. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleassa, E.M., El-Hayek, K.M., 2020. Video Atlas of Intraoperative Applications of Near Infrared Fluorescence Imaging.
- Alekseev M., et al. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: results of the FLAG randomized trial. Colorectal Disease. 2020;22(9):1147–1153. doi: 10.1111/codi.15037. [DOI] [PubMed] [Google Scholar]
- Armstrong G., et al. IntAct: intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: a randomized controlled trial. Colorectal Disease. 2018;20(8):O226–O234. doi: 10.1111/codi.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Colino R., Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Techniques in Coloproctology. 2018;22(1):15–23. doi: 10.1007/s10151-017-1731-8. [DOI] [PubMed] [Google Scholar]
- Blumetti J., et al. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World Journal of Surgery. 2014;38(4):985–991. doi: 10.1007/s00268-013-2340-y. [DOI] [PubMed] [Google Scholar]
- Boni L., et al. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surgical Endoscopy. 2016;30(7):2736–2742. doi: 10.1007/s00464-015-4540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow R.E., et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. Journal of Clinical Oncology. 2023;41(25):4065–4076. doi: 10.1200/JCO.22.02765. [DOI] [PubMed] [Google Scholar]
- FDA, 2020. Indocyanine green, FDA, Editor.
- Grimm C., et al. The impact of type and number of bowel resections on anastomotic leakage risk in advanced ovarian cancer surgery. Gynecologic Oncology. 2017;146(3):498–503. doi: 10.1016/j.ygyno.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Jafari M.D., et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surgical Endoscopy. 2013;27(8):3003–3008. doi: 10.1007/s00464-013-2832-8. [DOI] [PubMed] [Google Scholar]
- Jafari M.D.M.D., et al. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. Journal of the American College of Surgeons. 2015;220(1):82–92.e1. doi: 10.1016/j.jamcollsurg.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Jafari M.D., et al. Perfusion assessment in left-sided/low anterior resection (PILLAR III): a randomized, controlled, parallel, multicenter study assessing perfusion outcomes with pinpoint near-infrared fluorescence imaging in low anterior resection. Diseases of the Colon and Rectum. 2021;64(8):995–1002. doi: 10.1097/DCR.0000000000002007. [DOI] [PubMed] [Google Scholar]
- Keller D.S., Ishizawa T., Cohen R., Chand M. Indocyanine green fluorescence imaging in colorectal surgery: overview, applications, and future directions. The Lancet. Gastroenterology & Hepatology. 2017;2(10):757–766. doi: 10.1016/S2468-1253(17)30216-9. [DOI] [PubMed] [Google Scholar]
- Kingham T.P.M.D., Pachter H.L.M.D.F. Colonic anastomotic leak: risk factors, diagnosis, and treatment. Journal of the American College of Surgeons. 2009;208(2):269–278. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Kossi, J., 2018. Indocyanine Green Fluorescence Imaging in Prevention of Colorectal Anastomotic Leakage (ICG-COLORAL). 2018 03/27/2023 [cited 2024; Available from: https://clinicaltrials.gov/study/NCT03602677#study-overview].
- Kudszus S., Roesel C., Schachtrupp A., Höer J.J. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck's Archives of Surgery. 2010;395(8):1025–1030. doi: 10.1007/s00423-010-0699-x. [DOI] [PubMed] [Google Scholar]
- Leitao M.M., et al. ARIA II: a randomized controlled trial of near-infrared angiography during RectosIgmoid resection and anastomosis in women with ovarian cancer. International Journal of Gynecological Cancer. 2024;34(7):1098–1101. doi: 10.1136/ijgc-2024-005395. [DOI] [PubMed] [Google Scholar]
- Liu R.Q., et al. Cost analysis of indocyanine green fluorescence angiography for prevention of anastomotic leakage in colorectal surgery. Surgical Endoscopy. 2022;36(12):9281–9287. doi: 10.1007/s00464-022-09166-1. [DOI] [PubMed] [Google Scholar]
- Moukarzel L.A., et al. The impact of near-infrared angiography and proctoscopy after rectosigmoid resection and anastomosis performed during surgeries for gynecologic malignancies. Gynecologic Oncology. 2020;158(2):397–401. doi: 10.1016/j.ygyno.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar S., et al. Advanced stage (IIIC/IV) endometrial cancer: role of cytoreduction and determinants of survival. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2019;234:26–31. doi: 10.1016/j.ejogrb.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Ris F., et al. Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surgical Endoscopy. 2018;28(7):2221–2226. doi: 10.1007/s00464-014-3432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali A., et al. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Diseases of the Colon & Rectum. 2000;43(1):76–82. doi: 10.1007/BF02237248. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., et al. Use of near-infrared imaging using indocyanine green associates with the lower incidence of postoperative complications for intestinal and mesenteric injury. Scientific Reports. 2021;11(1):23880–23887. doi: 10.1038/s41598-021-03361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.