Abstract
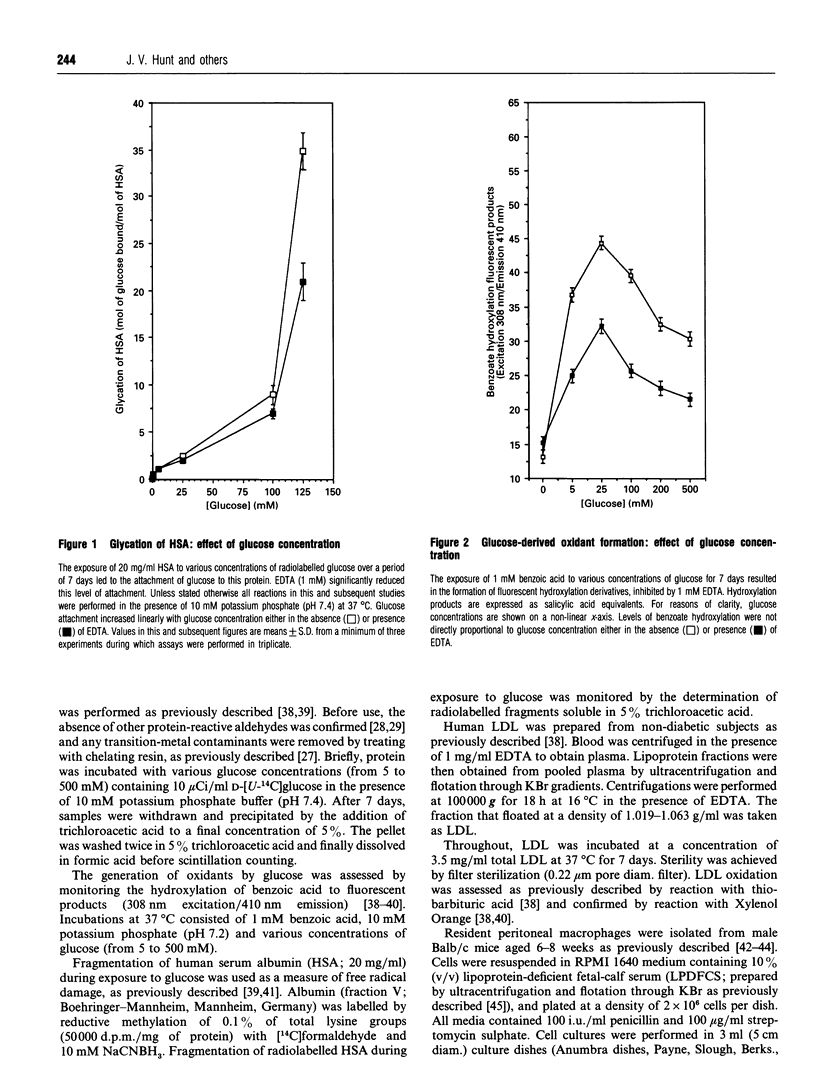
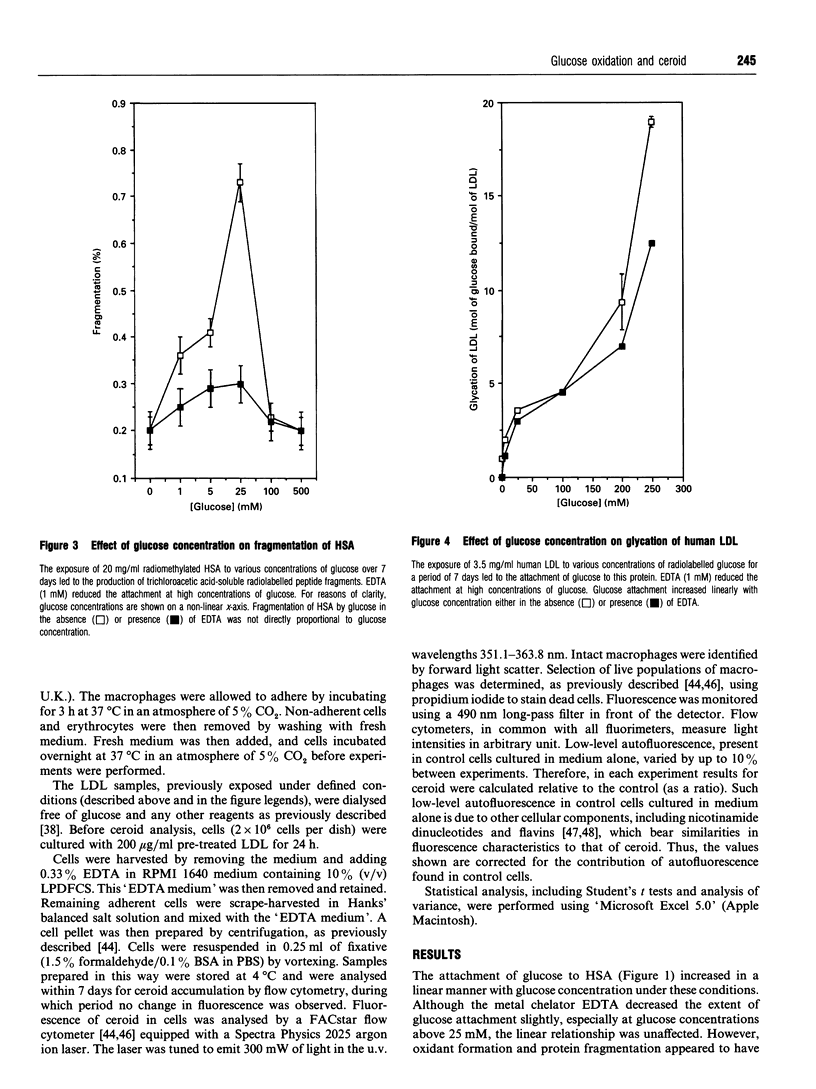
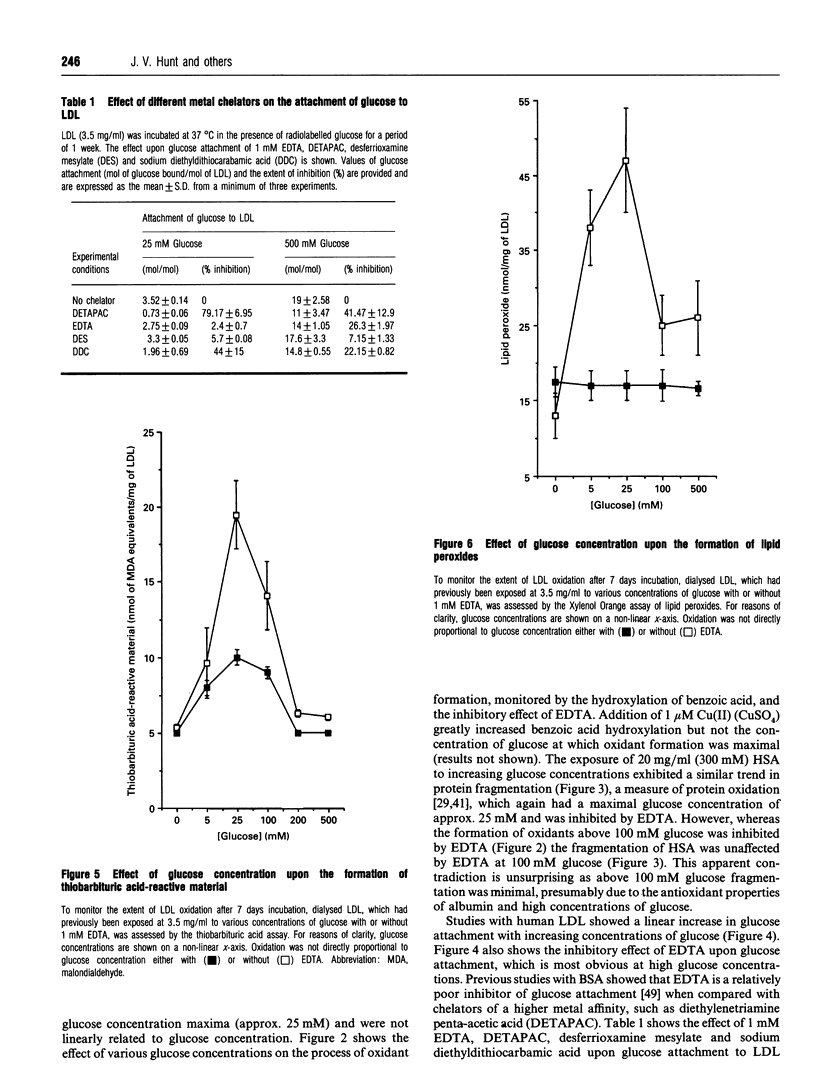
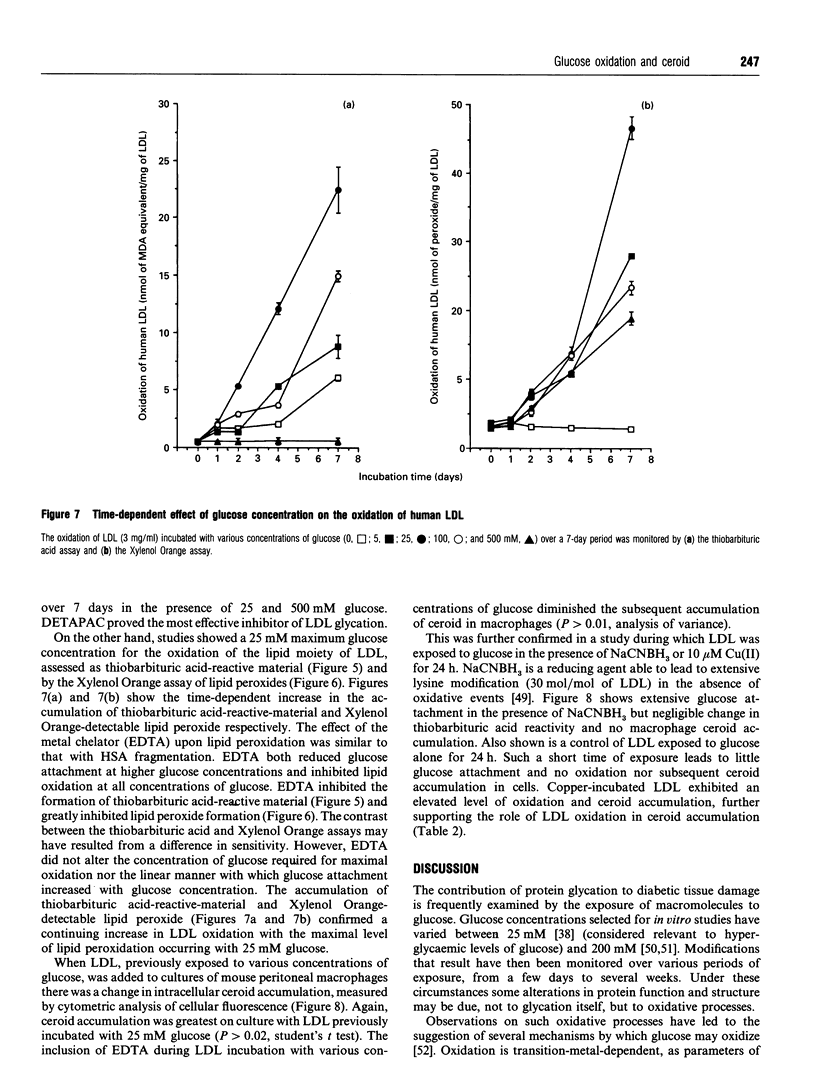
The exposure of proteins to high concentrations of glucose in vitro is widely considered a relevant model of the functional degeneration of tissue occurring in diabetes mellitus. In particular, the enhanced atherosclerosis in diabetes is often discussed in terms of glycation of low-density lipoprotein (LDL), the non-enzymic attachment of glucose to apolipoprotein amino groups. However, glucose can undergo transition-metal-catalysed oxidation under near-physiological conditions in vitro, producing oxidants that possess a reactivity similar to the hydroxyl radical. These oxidants can fragment protein, hydroxylate benzoic acid and induce lipid peroxidation in human LDL. In this study, glycation of LDL in vitro is accompanied by such oxidative processes. However, the oxidation of LDL varies with glucose concentration in a manner which does not parallel changes in protein glycation. Glycation increases in proportion to glucose concentration, whereas in our studies maximal oxidation occurs at a glucose concentration of approx. 25 mM. The modification of LDL resulting from exposure to glucose alters macrophage ceroid accumulation, a process which occurs in the human atherosclerotic plaque. The accumulation of ceroid in macrophages is shown to be related to LDL oxidation rather than LDL glycation, per se, as it too occurs at a maximum of approx. 25 mM. Oxidative sequelae of protein glycation appear to be a major factor in LDL-macrophage interactions, at least with respect to ceroid accumulation. Our observations are discussed in the context of the observed increase in the severity of atherosclerosis in diabetes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Identification of macrophages and smooth muscle cells in human atherosclerosis using monoclonal antibodies. J Pathol. 1985 Jul;146(3):197–204. doi: 10.1002/path.1711460306. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J. E. Autofluorescence of viable cultured mammalian cells. J Histochem Cytochem. 1979 Jan;27(1):36–43. doi: 10.1177/27.1.220325. [DOI] [PubMed] [Google Scholar]
- Ball R. Y., Bindman J. P., Carpenter K. L., Mitchinson M. J. Oxidized low density lipoprotein induces ceroid accumulation by murine peritoneal macrophages in vitro. Atherosclerosis. 1986 May;60(2):173–181. doi: 10.1016/0021-9150(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Ball R. Y., Bindman J. P., Carpenter K. L., Mitchinson M. J. Oxidized low density lipoprotein induces ceroid accumulation by murine peritoneal macrophages in vitro. Atherosclerosis. 1986 May;60(2):173–181. doi: 10.1016/0021-9150(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Ball R. Y., Carpenter K. L., Enright J. H., Hartley S. L., Mitchinson M. J. Ceroid accumulation by murine peritoneal macrophages exposed to artificial lipoproteins. Br J Exp Pathol. 1987 Jun;68(3):427–438. [PMC free article] [PubMed] [Google Scholar]
- Ball R. Y., Carpenter K. L., Enright J. H., Hartley S. L., Mitchinson M. J. Ceroid accumulation by murine peritoneal macrophages exposed to artificial lipoproteins. Br J Exp Pathol. 1987 Jun;68(3):427–438. [PMC free article] [PubMed] [Google Scholar]
- Ball R. Y., Carpenter K. L., Mitchinson M. J. What is the significance of ceroid in human atherosclerosis? Arch Pathol Lab Med. 1987 Dec;111(12):1134–1140. [PubMed] [Google Scholar]
- Baynes J. W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991 Apr;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Beach K. W., Strandness D. E., Jr Arteriosclerosis obliterans and associated risk factors in insulin-dependent and non-insulin-dependent diabetes. Diabetes. 1980 Nov;29(11):882–888. doi: 10.2337/diab.29.11.882. [DOI] [PubMed] [Google Scholar]
- Benson R. C., Meyer R. A., Zaruba M. E., McKhann G. M. Cellular autofluorescence--is it due to flavins? J Histochem Cytochem. 1979 Jan;27(1):44–48. doi: 10.1177/27.1.438504. [DOI] [PubMed] [Google Scholar]
- Carpenter K. L., Ballantine J. A., Fussell B., Enright J. H., Mitchinson M. J. Oxidation of cholesteryl linoleate by human monocyte-macrophages in vitro. Atherosclerosis. 1990 Aug;83(2-3):217–229. doi: 10.1016/0021-9150(90)90167-h. [DOI] [PubMed] [Google Scholar]
- Cutler P. Deferoxamine therapy in high-ferritin diabetes. Diabetes. 1989 Oct;38(10):1207–1210. doi: 10.2337/diab.38.10.1207. [DOI] [PubMed] [Google Scholar]
- Deckert T., Poulsen J. E., Larsen M. Prognosis of diabetics with diabetes onset before the age of thirty-one. I. Survival, causes of death, and complications. Diabetologia. 1978 Jun;14(6):363–370. doi: 10.1007/BF01228130. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Gebicki J., Puhl H., Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992 Oct;13(4):341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- Gey K. F., Puska P., Jordan P., Moser U. K. Inverse correlation between plasma vitamin E and mortality from ischemic heart disease in cross-cultural epidemiology. Am J Clin Nutr. 1991 Jan;53(1 Suppl):326S–334S. doi: 10.1093/ajcn/53.1.326S. [DOI] [PubMed] [Google Scholar]
- Herman J. B., Medalie J. H., Goldbourt U. Diabetes, prediabetes and uricaemia. Diabetologia. 1976 Mar;12(1):47–52. doi: 10.1007/BF01221964. [DOI] [PubMed] [Google Scholar]
- Hunt J. V., Bottoms M. A., Mitchinson M. J. Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production. Biochem J. 1993 Apr 15;291(Pt 2):529–535. doi: 10.1042/bj2910529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. V., Carpenter K. L., Bottoms M. A., Carter N. P., Marchant C. E., Mitchinson M. J. Flow cytometric measurement of ceroid accumulation in macrophages. Atherosclerosis. 1993 Jan 25;98(2):229–239. doi: 10.1016/0021-9150(93)90132-e. [DOI] [PubMed] [Google Scholar]
- Hunt J. V., Dean R. T., Wolff S. P. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988 Nov 15;256(1):205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. V., Simpson J. A., Dean R. T. Hydroperoxide-mediated fragmentation of proteins. Biochem J. 1988 Feb 15;250(1):87–93. doi: 10.1042/bj2500087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. V., Smith C. C., Wolff S. P. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990 Nov;39(11):1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- ILLING E. K. B., GRAY C. H., LAWRENCE R. D. Blood glutathione and non-glucose reducing substances in diabetes. Biochem J. 1951 May;48(5):637–640. doi: 10.1042/bj0480637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings P. E., Chirico S., Jones A. F., Lunec J., Barnett A. H. Vitamin C metabolites and microangiopathy in diabetes mellitus. Diabetes Res. 1987 Nov;6(3):151–154. [PubMed] [Google Scholar]
- Jiang Z. Y., Hunt J. V., Wolff S. P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992 May 1;202(2):384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Karpen C. W., Cataland S., O'Dorisio T. M., Panganamala R. V. Interrelation of platelet vitamin E and thromboxane synthesis in type I diabetes mellitus. Diabetes. 1984 Mar;33(3):239–243. doi: 10.2337/diab.33.3.239. [DOI] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M., Pyörälä K. Lipid and lipoprotein abnormalities in diabetic patients with peripheral vascular disease. Atherosclerosis. 1988 Nov;74(1-2):55–63. doi: 10.1016/0021-9150(88)90191-8. [DOI] [PubMed] [Google Scholar]
- Mateo M. C., Bustamante J. B., Cantalapiedra M. A. Serum, zinc, copper and insulin in diabetes mellitus. Biomedicine. 1978 Apr;29(2):56–58. [PubMed] [Google Scholar]
- Mitchinson M. J., Ball R. Y., Carpenter K. H., Enright J. H., Brabbs C. E. Ceroid, macrophages and atherosclerosis. Biochem Soc Trans. 1990 Dec;18(6):1066–1069. doi: 10.1042/bst0181066. [DOI] [PubMed] [Google Scholar]
- Mitchinson M. J., Hothersall D. C., Brooks P. N., De Burbure C. Y. The distribution of ceroid in human atherosclerosis. J Pathol. 1985 Feb;145(2):177–183. doi: 10.1002/path.1711450205. [DOI] [PubMed] [Google Scholar]
- Mitchinson M. J. Insoluble lipids in human atherosclerotic plaques. Atherosclerosis. 1982 Oct;45(1):11–15. doi: 10.1016/0021-9150(82)90167-8. [DOI] [PubMed] [Google Scholar]
- Mykkänen L., Laakso M., Penttilä I., Pyörälä K. Asymptomatic hyperglycemia and cardiovascular risk factors in the elderly. Atherosclerosis. 1991 Jun;88(2-3):153–161. doi: 10.1016/0021-9150(91)90077-g. [DOI] [PubMed] [Google Scholar]
- Noto R., Alicata R., Sfogliano L., Neri S., Bifarella M. A study of cupremia in a group of elderly diabetics. Acta Diabetol Lat. 1983 Jan-Mar;20(1):81–85. doi: 10.1007/BF02629133. [DOI] [PubMed] [Google Scholar]
- Parums D. V., Brown D. L., Mitchinson M. J. Serum antibodies to oxidized low-density lipoprotein and ceroid in chronic periaortitis. Arch Pathol Lab Med. 1990 Apr;114(4):383–387. [PubMed] [Google Scholar]
- Phelps G., Chapman I., Hall P., Braund W., Mackinnon M. Prevalence of genetic haemochromatosis among diabetic patients. Lancet. 1989 Jul 29;2(8657):233–234. doi: 10.1016/s0140-6736(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Rimm E. B., Stampfer M. J., Ascherio A., Giovannucci E., Colditz G. A., Willett W. C. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993 May 20;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Cottam G. L. Glycosylation of LDL decreases its ability to interact with high-affinity receptors of human fibroblasts in vitro and decreases its clearance from rabbit plasma in vivo. Biochim Biophys Acta. 1982 Nov 12;713(2):199–207. doi: 10.1016/0005-2760(82)90237-5. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Okamura T., Cottam G. L. Measurement of receptor-independent metabolism of low-density lipoprotein. An application of glycosylated low-density lipoprotein. Eur J Biochem. 1983 Apr 5;131(3):535–538. doi: 10.1111/j.1432-1033.1983.tb07294.x. [DOI] [PubMed] [Google Scholar]
- Sato Y., Hotta N., Sakamoto N., Matsuoka S., Ohishi N., Yagi K. Lipid peroxide level in plasma of diabetic patients. Biochem Med. 1979 Feb;21(1):104–107. doi: 10.1016/0006-2944(79)90061-9. [DOI] [PubMed] [Google Scholar]
- Som S., Basu S., Mukherjee D., Deb S., Choudhury P. R., Mukherjee S., Chatterjee S. N., Chatterjee I. B. Ascorbic acid metabolism in diabetes mellitus. Metabolism. 1981 Jun;30(6):572–577. doi: 10.1016/0026-0495(81)90133-5. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Hennekens C. H., Manson J. E., Colditz G. A., Rosner B., Willett W. C. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993 May 20;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Witztum J. L. Glucosylation of low-density lipoproteins to an extent comparable to that seen in diabetes slows their catabolism. Diabetes. 1984 Feb;33(2):130–134. doi: 10.2337/diab.33.2.130. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Specific macrophage receptor activity for advanced glycosylation end products inversely correlates with insulin levels in vivo. Diabetes. 1988 Apr;37(4):456–461. doi: 10.2337/diab.37.4.456. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Mahoney E. M., Branks M. J., Fisher M., Elam R., Steinberg D. Nonenzymatic glucosylation of low-density lipoprotein alters its biologic activity. Diabetes. 1982 Apr;31(4 Pt 1):283–291. doi: 10.2337/diab.31.4.283. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem J. 1987 Jul 1;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. P., Jiang Z. Y., Hunt J. V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10(5):339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]


