Abstract
To isolate melanopsin contributions to retinal sensitivity measured by the post-illumination pupil response (PIPR), controlling for individual differences in non-melanopsin contributions including retinal irradiance is required. When methodologies to negate such differences present barriers, statistical controls have included age, baseline diameter, iris pigmentation, and circadian time of testing. Alternatively, the pupil light reflex (PLR) and calculations estimating retinal irradiance both reflect retinal irradiance, while the PLR also reflects downstream pathways. We reanalyzed data from an observational, correlational study comparing the PIPR across seasons in seasonal affective disorder (SAD) and controls. The PIPR was measured in 47 adults in Pittsburgh, Pennsylvania (25 SAD) over 50 seconds after 1 second of red and blue stimuli of 15.3 log photons/cm2/s. The PLR was within 1 second while PIPR was averaged over 10–40 seconds post-stimulus. Two raters ranked iris pigmentation using a published scale. We evaluated model fit using Akaike’s Information Criterion (AIC) across different covariate sets. The best-fitting models included either estimated retinal irradiance or PLR, and circadian time of testing. The PLR is collected contemporaneously in PIPR studies and is an individually specific measure of nonspecific effects, while being minimally burdensome. This work extends the prior publication by introducing theoretically grounded covariates that improved analytic model fits based on AIC specific to the present methods and sample. Such quantitative methods could be helpful in studies which must balance participant and researcher burden against tighter methodological controls of individual differences in retinal irradiance.
Keywords: melanopsin, pupillometry, sleep, circadian, age, lifespan
Graphical Abstract
Graphical Abstract.
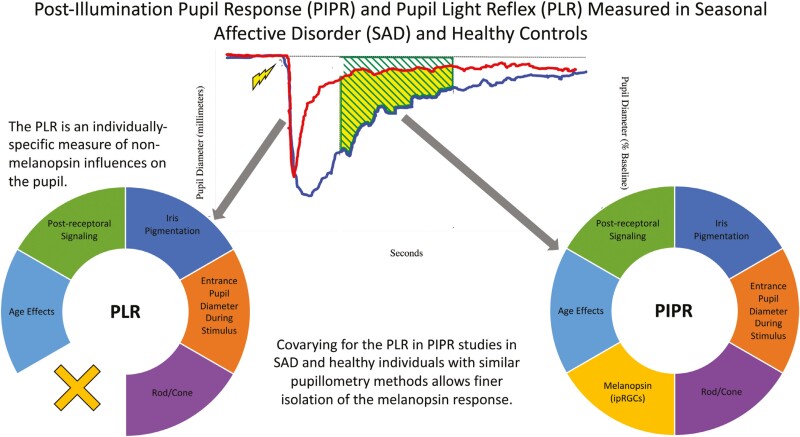
Statement of Significance.
The post-illumination pupil response is poised to become an indispensable method in psychopathology, sleep, and circadian science as it noninvasively measures individual differences in photosensitivity for circadian photoentrainment and direct effects of light on mood and behavior. Seasonal affective disorder (SAD) is an ideal model disorder for studying depression as it relates to sleep and circadian rhythms given it is light-related etiology and seasonal recurrence. This work extends Roecklein et al. (2021) by introducing theoretically grounded covariates that improved Akaike Information Criteria (AIC) values of model fit. Such quantitative methods could be useful in PIPR studies needing to consider the tradeoff of participant and researcher burden against tighter methodological control of individual differences in retinal irradiance.
Individual differences in melanopsin-driven retinal responsivity are hypothesized to influence non-image-forming responses to light, with downstream effects on circadian alignment, sleep, and psychopathology [1]. In order to study melanopsin-driven responses, the post-illumination pupil response (PIPR) [2] should best isolate effects on pupil diameter specific to melanopsin, as opposed to effects that are not primarily driven by, or specific to, melanopsin (i.e. nonspecific). Nonspecific effects include but are not limited to autonomic influences on the pupil, fatigue, attenuation of the stimulus by the lens and pupil diameter, overall rod/cone-driven sensitivity, and other differences in neural processing downstream of the retina [3]. Most PIPR studies have attempted to control or model and statistically adjust for nonspecific effects on pupil diameter.
We tested whether including the pupil light reflex (PLR) as an individual, the built-in measure of retinal irradiance would represent an improvement in quantitative methods for analyzing the PIPR while minimizing participant and experimenter burden. Such improvements in quantitative methods and study design could foster the PIPR’s emergence as an indispensable method in SAD and perhaps other psychiatric populations.
Recent methodological advances may improve our understanding of the relationship between individual differences in sensitivity to light and circadian photoentrainment. Photoresponsivity has been measured since [4] observed light suppression of melatonin in rats, and recent human data demonstrate significant individual differences in suppression of nocturnal melatonin release by light [5]. Convenient but rigorous daytime measures of photoresponsivity are needed in sleep and circadian research. The PIPR largely measures the responsivity of retinal ganglion cells integral to non-visual responses to light. Data suggest these melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs) continuously detect overall environmental light levels for functions such as circadian photoentrainment in rodents and humans [6–12], non-circadian, so-called direct effects of light on mood and alertness in rodents [13], and pupil diameter in humans and non-human primates [2, 14]. These ipRGCs project through the retinohypothalamic tract to brain areas involved in circadian photoentrainment (i.e. suprachiasmatic nucleus, intergeniculate leaflet), the pupil (i.e. olivary pretectal nucleus), mood (parahabenula), sleep (ventrolateral pre optic area and lateral hypothalamus), wakefulness (subparaventricular zone), among other areas involved in learning, body temperature regulation, light aversion (e.g. photophobia), and visual perception and coordination in mammals [15–18].
The PIPR has significant advantages over other measures of photic sensitivity such as melatonin suppression. However, all measures of sensitivity to light can be confounded when nonspecific effects of light differ between individuals. In an important instance, light incident on the retina, or retinal irradiance, is lower in some individuals despite equal light levels at the cornea. First, lens opacity increases with age and preferentially filters blue light [19–21]. Second, the entrance pupil diameter through which a stimulus passes becomes smaller with age throughout adulthood [22, 23], geometrically constraining light entering the eye. Third, light can pass directly through the iris, but more light is absorbed by the melanin in highly pigmented brown irises than sparsely pigmented blue irises [24, 25] Existing PIPR studies vary in approaches to control individual differences in retinal irradiance, complicating interpretation and comparisons between studies. Although multiple variables aim to estimate retinal irradiance (i.e. age, entrance pupil diameter, iris pigmentation, and estimated retinal irradiance), the PLR directly measures retinal irradiance in humans as a “built-in light meter” [24] as well as measuring other nonspecific effects. We used a combination of theoretical rationale and empirical tests of model fit to identify which measures of nonspecific effects should inform the design of experiments measuring the PIPR in SAD and non-depressed individuals.
The post-illumination pupil response
The PIPR has been measured most often in humans with retinal ganglion cell disorders including anterior ischemic optic neuropathy, glaucoma, diabetic retinopathy, and retinitis pigmentosa [26–30]. The PIPR is an ideal measure of retinal responsivity to light in sleep and circadian science due to a few advantages over existing methods: the PIPR can be performed during the day when light is typically experienced, in under 30 minutes; is noninvasive; and does not require expensive melatonin assays. These advantages facilitate its use in research and increase the potential for clinical translation.
Individual differences in the PIPR
Individual differences in circadian timing can arise from the length of the intrinsic period of the central circadian clock, but a second origin is also important—individual differences in photosensitivity for a range of non-visual effects of light. In our previous paper [31], we reported that the PIPR was diminished in individuals with seasonal affective disorder (SAD) during winter compared to summer, and when compared to healthy controls in either season. Specifically, we observed an effect of group (SAD vs. control), season (summer vs. winter), and a group*season interaction. Apart from being evaluated in SAD, the PIPR also has been used in humans to measure retinal sensitivity to light in disorders related to sleep and circadian rhythms [32, 33], substance use [34], nonseasonal mood disorders [28, 35–37], and migraines due to photophobia [19]. While the results of these studies have been largely consistent, and highlight the potential for the PIPR as a transdiagnostic mechanism, analyses varied widely with regard to covariates chosen to model nonspecific effects, and are therefore difficult to compare in magnitude. As the PIPR is increasingly adopted, rigorous, and standardized PIPR methods must be balanced with participant burden.
Nonspecific influences on pupil responses
To compare individuals on melanopsin-driven photosensitivity, studies aim to standardize—or at least estimate and model—retinal irradiance as well as other nonspecific effects across participants. The disadvantage of covarying for nonspecific influences on pupil responses is that this variance may be associated with the PIPR itself. Therefore, the type of future research study should consider the most appropriate covariates to include. For example, if determining an individual’s overall photic sensitivity is the goal, fewer covariates may be ideal. However, another line of investigation aims to understand the unique contribution of melanopsin in mechanistic studies of photic sensitivity. In those cases, covarying for effects like iris pigmentation that are not specific to melanopsin will be more informative.
To identify the ideal covariates for PIPR studies, dual approaches can be balanced. First, we identified theoretically chosen variables based on their mechanistic relationship with retinal irradiance and non-melanopsin/nonspecific effects on pupil diameter. These include factors affecting retinal irradiance, as well as factors affecting the pupil response to stimuli such as neural circuitry downstream of the ipRGCs to areas controlling pupil diameter (i.e. olivary pretectal nucleus (OPN), Edinger-Westphal nucleus (EW), the cervical ganglia (CG), and innervation of the iris muscles). Other nonspecific factors include prereceptoral light filtering, entrance pupil diameter, and iris pigmentation. Individuals receiving the same light at the cornea may nevertheless receive different levels of light at the retina due to filtering by the lens (i.e. aging effects), the amount of melanin in the iris, and the size of the pupil during stimuli presentation (i.e. entrance pupil diameter).
Age
Retinal irradiance decreases with age in humans due to decreased pupil diameter through which light can pass [38–41] and alterations in the lens proteins which thickens, yellows, and densifies [20, 21]. Lens yellowing preferentially decreases transmission of shorter wavelengths of light (i.e. blue light, 480 nm) [20, 22, 42], from 82% transmission at age 10% to only 23% transmission by age 80 [21, 43] Some empirical studies report a relationship between the PIPR and age in humans [1, 41, 44, 45], possibly due to loss of melanopsin-containing ganglion cells [46], while those not reporting age effects controlled for variables associated with age (i.e. entrance pupil diameter) [47], excluded participants with higher lens opacity [48], or restricted participants to under age 40 [34]. While Kankipati et al. (2010) found that the plateau in PIPR amplitude was independent of age, Herbst et al. (2012) showed that greater PIPR late-AUC from blue light was associated with older age, independent of pupil size and lens transmission. There may be other age-related factors that contribute to the PIPR such as neural circuits downstream of the retina and age-related changes in autonomic regulation. Furthermore, early age-related macular degeneration has been shown to be associated with a less sustained blue PIPR and lower amplitude initial constriction [49]. Therefore, using the PLR instead of a set of covariates (i.e. age, pupil size, and lens transmission) would theoretically account for the aggregate of the above effects, and possibly others. Because light scatter could increase with age, and increase retinal irradiance, while age simultaneously decreases retinal irradiance, individual aggregate measures would be more accurate [50].
Iris pigmentation
Iris pigmentation in humans varies as a function of the type of melanin and the number, size, and shape of melanin-containing cells [51]. Brown irises contain the highest melanin concentration, and blue irises contain the lowest concentration [52]. Previous research has found a lower incidence of retinal diseases, like age-related macular degeneration and uveal melanoma, associated with darker pigmented irises in human participants, likely because melanin attenuates light reaching the retina [53–55]. Higher iris pigmentation interacts with smaller entrance pupil sizes to reduce the amount of light incident on the retina [25, 56]. Data from human participants suggests that light can pass directly through the iris in those with low iris pigmentation, but if iris pigmentation is high enough to block light through the iris, then light penetration is confined to the pupil [25]. We previously reported that iris pigmentation in this sample was associated with the PIPR, but only in older individuals [57].
The pupil light reflex
In contrast to the PIPR which captures the sustained constriction of the pupil 10 to 30–40 seconds post-illumination (“4” & “5” in Figure 1) [58], the PLR in humans is a transient constriction occurring just ~180 milliseconds after stimulus onset and is a function of primarily rod and cone photoreceptors [4]. The PLR reflects total retinal illumination as well as other effects on the pupil that are not specific to melanopsin. The human PLR objectively quantifies individual retinal illumination as a “built-in objective light meter of the eye” [25, 59–61]. Kardon et al. (2013) found that entrance pupil size was associated with retinal illumination in individuals with higher iris pigmentation. Most studies adjust for age and baseline pupil diameter; however, adjusting for PLR (i.e. maximum constriction as a function of baseline pupil diameter) allows for statistical control of the aggregate of nonspecific effects on pupil responses beyond those modeled with age and baseline diameter.
Figure 1.
The post-illumination pupil response and the pupil light reflex. Legend: Pupil light reflex (PLR) in response to 639 nm (red) and 463 nm (blue) narrow bandwidth stimuli of 200 millimeters duration. The most commonly reported PIPR measure (4; AUC late; Adhikari et al., 2015) is indicated in solid shading, along with other important PIPR metrics. Data reflected a single response to red and blue stimuli from a healthy control participant, courtesy of Kathryn Roecklein, Paul Gamlin, Peter Franzen, and Greg Siegle.
Study purpose
The main goal is to identify covariates to statistically control individual differences in nonspecific contributions to the PIPR that minimize experimenter and participant impact while optimizing statistical models when methods to negate such differences are prohibitive. Different combinations of the following covariates estimating nonspecific effects were evaluated. Iris pigmentation was rated using published photographic sets. Pupil diameter during baseline, PLR, and PIPR were measured using infrared pupillometry. We compared statistical models with different covariate sets on Akaike’s information criterion (AIC) reflecting predictive error to determine which models are comparatively better fits. The effects of group and season address the primary research questions of our umbrella SAD research program and have been previously published [1, 46]. In contrast, the primary extension of this work is to test whether using the PLR or estimated retinal irradiance instead of a group of other covariates might (1) be theoretically grounded, and (2) improve analytic models according to AIC criteria for best fit. This could be helpful in similar studies which do not control for all the sources of individual differences in retinal irradiance and studies aiming to isolate the contribution of melanopsin from non-melanopsin influences on pupil diameter. We aim to identify the best covariates from among age, PLR, iris pigmentation, and entrance pupil diameter while minimizing information loss and preserving the explanatory power of the main group and season predictors.
Hypotheses
First, we hypothesized that both iris pigmentation ratings and entrance pupil diameter would predict the PIPR individually and in interaction as first reported by Kardon et al. (2013). Second, we hypothesized that AIC model fit would indicate that the PLR alone explains more variance in the PIPR than the set of indirect measures of nonspecific effects including calculations of estimated retinal irradiance, iris pigmentation, entrance pupil diameter, and age. All analyses included the time of PIPR testing relative to self-reported midsleep as a proxy for individual circadian variation in the PIPR [62, 63].
Methods
Participants
Participants were recruited from the greater Pittsburgh Metropolitan Area through the University of Pittsburgh Pitt + Me research registry. Participants provided informed consent and all study procedures were approved by the University of Pittsburgh Institutional Review Board. Exclusion criteria included sleep–wake disorders (i.e. insomnia disorder that occurs separately from depression), Bipolar Disorder, Psychosis, Post-Traumatic Stress Disorder, Somatoform and Somatic Symptom Disorders, and shift work. We did not exclude individuals with anxiety disorders or ADHD. Individuals were screened for color vision using the Ishihara Test for Color Deficiency (24 plates edition) [64]. Individuals in the control group had no history of mood disorders, while the SAD group met the criteria for unipolar major depressive disorder, with the seasonal pattern on the structured clinical interview for DSM-IV Axis I disorders (SCID-I) [65]. We assessed participants for current medication use at the pupillometry visit. Participants were tested in winter (December 21st–March 21st) and summer (June 21st–September 21st). All participants reported no known eye health problems, and 60% of the sample volunteered for a confirmatory eye exam performed by the University of Pittsburgh Medical Center Eye and Ear Institute.
Calculated retinal irradiance
As reported in the first publication of these data (Roecklein et al., 2021), we measured corneal irradiance using a 50-micron aperture spectrophotometer (USB4000 Fiber Optic Spectrometer; Ocean Optics, Dunedin, FL). We selected red (633 nm; 15.78 nm full-width half-maximum; FWHM; the span in nanometers at half maximum) and blue (468 nm; 22.68 FWHM) stimuli that resulted in calculated retinal irradiance (Mblue = 15.332 log photons/cm2/s, SDblue = 0.101 log photons/cm2/s; Mred = 15.304 log photons/cm2/s, SDred = 0.074 log photons/cm2/s) that was as close as possible. Individual estimates of retinal irradiance for each participant were calculated using corneal irradiance of the stimuli, diameter of the pupil at baseline, and participant age [66], to account for age-related decreases in blue light transmission from the cornea to the retina. Retinal irradiance (Er) was based on corneal irradiance (Ec), the lens transmittance for either red or blue wavelength (T(λ, age)) = 10 − D; where D(λ, age) is the optical media density, the solid angular size of the light screen (Ωscreen = 0.378 sr; screen diameter, d = 16″; screen distance ds = 22″), and the solid angular size of the image on the retina (Ωeye, based on pupil aperture diameter a = 3–5mm, and focal length of the human eye, f = 17.0 mm) [47].
Importantly, the data from Pokorny et al. (1987) reporting age-related corneal transmission indicate that individual deviations from the predicted values are greater than the variation predicted by a linear or polynomial function. For example, the between-participant variation in van Norren & Voss (1974) varied up to 25% from the mean. This is a potential advantage of the PLR as a reflection of retinal irradiance because the PLR is measured before each PIPR and may reflect individual differences in the rate of aging of the cornea [67–69].
Pupillometry
Participants were in dim light (<25 lux) for 60 minutes, followed by 11 minutes of dark adaptation, and pupils were freely reactive without pharmacological dilation. The stimuli were presented in the horizontal plane for 1 second, followed by 1.5–3-minute interstimulus intervals. The interstimulus interval is longer after blue due to sustained pupil constriction. Four red and four blue stimuli were alternated, and pupil diameter was recorded at 60 Hz with an EYE-TRAC ® 6000 pupillometer (Applied Science Laboratories Inc., Bedford, MA). The stimulus was projected onto a 16″ diameter Mylar diffuser at 22″ from the chin and forehead rest of the pupillometer, resulting in binocular stimuli centered on approximately 42 degrees of the visual field (Focal length 27 mm, F-number 4.5). We used the ASL reference card for measuring pupil diameter with black dots ranging from 2.0 to 10.0 mm in 0.5 mm increments placed at the position of the eye in the ASL headset to generate a function to convert data from ASL units to mm. In Roecklein et al. (2021), retinal irradiance was calculated based on age and entrance pupil diameter, and individual PIPR results were statistically adjusted by calculated retinal irradiance. This was not done in the present study so the magnitude of the effects on the PIPR due to age, iris pigmentation, and pupil diameter could be estimated. Stimulus characteristics are presented in Table 1. The ENLIGHT checklist is a consensus-based reporting guideline for studies on the impact of light on non-visual physiology [71]. Applicable elements from the ENLIGHT checklist are included in the methods, and the completed checklist is provided in Supplementary Materials. Artifacts including blinks were removed from the data using standard methods (sliding window; MATLAB, MathWorks, Inc., Natick, MA) [72]. Baseline pupil diameter was averaged across the 6.5 seconds prior to the stimulus and is included to model the effect of the diameter of the pupil upon entrance of the stimulus (i.e. “entrance diameter”).
Table 1.
Stimulus Characteristics
| Red | Blue | |
|---|---|---|
| Photon flux | 5.01 × 1013 photons/cm²/s | 5.01 × 1013 photons/cm²/s |
| Log photon flux | 13.7 log10 photons/cm²/s | 13.7 log10 photons/cm²/s |
| Melanopic lux | 0.05 lux | 144.07 lux |
| Irradiance | 15.73 µW/cm² | 161.04 µW/cm² |
| Peak spectral irradiance | 635 nm | 470 nm |
| Narrowband peak | 468 nm | 633 nm |
| Narrowband FWHM | 23 nm | 16 nm |
Values were calculated using the toolbox from Lucas et al. (2014) [70]. FWMH is full width maximum half.
To minimize potential rod and cone contributions captured up to 6 seconds post-illumination [3], the PIPR was calculated as the average diameter (i.e. area under the curve) from 10–40 seconds post-illumination (Late AUC) [73]. We expressed the diameter of red and blue responses in both millimeters and as a percentage of baseline diameter. We then calculated the difference between the red and blue stimuli, termed the Net PIPR.
The initial PLR was calculated both in millimeters and as a percent of baseline (%) for comparison in model fit. The acute minimum constriction of the pupil in the 200 millimeters post-stimulus was averaged across the red (PLRred) separately from the blue (PLRblue) trials.
Testing time
Time of testing relative to midsleep was included as a covariate because the PIPR and pupil diameter vary across the day, rising shortly after the wake and then falling 3–5 hours prior to the onset of melatonin release under dim light (DLMO) [62, 63], which occurs in most individuals at approximately 8–10:00 pm [74]. Due to participant work schedules and time for DLMO assay return, we were not able to standardize testing by circadian time. Testing time (TT) of the PIPR ranged from 10:00 am to 6:48 pm (M = 1:30 pm; SD = 144 minutes) in our sample, with 75% of the sample completing the assessment prior to 2:45 pm. Participants completed the self-report Pittsburgh Sleep Quality Index [75] and reported their typical bedtime and waketime over the past month. We calculated midsleep time to approximate individual variation in circadian time [76, 77], and calculated the minutes between midsleep and PIPR TT.
Iris pigmentation
Digital photos of participants’ irises under consistent indoor fluorescent lighting conditions were classified by two raters using the Franssen et al. (2008) scale, a set of 24 standardized iris photos ranging from least pigmentation (1) to most pigmentation (24) [78]. Both raters (AMK, KAR) scored each participant’s iris photo separately, and consensus was determined for any discrepant ratings and used in analyses. The intraclass correlation coefficient measure of inter-rater reliability was excellent at 0.976, 95% CI: (0.954–0.987), p < .001 [79].
Statistical analysis
We performed multilevel regression to accommodate repeated measures across seasons within participants predicting the PIPR. Because the best model is the one that explains the greatest amount of variation including that of our primary variables of interest, we include diagnosis and season [1, 46]. All models included diagnostic group and season as primary predictors and TT relative to midsleep as an approximation of any diurnal effects on PIPR magnitude. To test hypothesis 1 that iris pigmentation and diameter would interact to predict PIPR, we ran two models, models 1 and 2, as follows. Model 1 included covariates age, iris rating, pupil diameter during the stimulus, and their interaction. Model 2 did not include the interaction, which was done to evaluate whether the interaction predicted additional important variance over the iris and diameter alone.
To test hypothesis 2, that including the PLR could yield superior performance compared to models including multiple other covariates in predicting the PIPR, we compared models 1 and 2 to multiple other models, models 3–12, as follows. In models 3–10, the magnitude of the initial PLR in response to red or blue light was included. Model 3 added age to model 2 to test whether age effects beyond those related to retinal irradiance predicted additional variance in the PIPR. Subsequent models were added back in covariates to evaluate whether model fit could be improved. Models 11 and 12 included red and blue estimated retinal irradiance, respectively.
Photoperiod on the day of testing was not included as a covariate as it is redundant to season. Photoperiod is highly correlated with season (r = 0.81, p < .001) and multicollinear (VIF = 3.98). A season must be included to model repeated measures within participants and to test the umbrella study seasonal comparison.
Bivariate correlations were calculated among all variables prior to tests of our hypotheses. After collecting the PIPR data, participants were recontacted and invited to attend an eye exam screening for retinal diseases to rule out such explanations for our findings. Thirty-nine participants attended the exam, and 26 declined or were unavailable (i.e. moved out of the area). Eye exam was dummy coded as 1 = passed and 0 = missing, as no individuals failed the eye exam. The dummy coded variable was then included as a covariate in select models. Significance was set to 0.05 across all models. Correction for multiple tests is not indicated because each model tests the effect of group and season, covarying for nonspecific effects, on the same set of PIPR results. However, specific p values are reported for consideration of the p-value threshold when interpreting results. Unstandardized coefficients and their standard error, plus 95% Confidence Intervals are reported to evaluate the magnitude of the effect of each predictor in the unstandardized units of the predictor by units of the outcome (e.g. years of age per millimeter).
Measuring relative model fit and performance of covariate sets
Each model was evaluated for relative quality compared to other models using Akaike’s Information Criterion (AIC) [80]. AIC calculates the likelihood estimate of the model and considers the number of independent variables. Other metrics like Hurvich and Tsai’s Criterion (AICC), and Schwartz’s Bayesian Criterion (BIC) are corrected for small samples and can accommodate large heterogeneity, respectively. However, AIC minimizes predictive error in data-limited, small samples, and penalizes complex models less, making it the ideal metric for the present study. Each of the alternatives to AIC (not reported) gave consistent results in terms of which model was a better fit, so only AIC is reported. Lower AIC values reflect better fit even when values are negative. The difference in AIC between models (Δi) is considered no different from the original model when under 2, when over 2 the model should “not be disregarded,” and sufficiently better when over 10 [81, 82].
Results
Participant characteristics
Iris photos were taken for 47 participants, 25 with SAD and 22 controls. Self-reported race and ethnicity are reported in Table 2, and additional demographics and descriptive variables are reported in Table 3 by diagnostic group and season. See Supplementary Table 1 for bivariate correlations between variables. The SAD and control groups did not differ in the distribution of self-reported race (X2 = 3.78, p = .15) or ethnicity (Fisher’s Exact Test = 2.36, p = .22). As predicted, self-reported racial identity was associated with iris ratings (F(2,47) = 11.97, p < .001). Table 2 reports the mean and standard deviation for iris rating by race and ethnicity. Only two individuals in the study self-identified as Hispanic or Latinx. Iris ratings were not associated with ethnic identity in this sample. Table 3 includes demographic and descriptive variables by group. Our sample included 5 (10.6%) individuals in the SAD group currently taking antidepressants, but antidepressant use was not associated with the PIPR in our sample, likely due to low frequency. Antidepressant use was not a statistically significant predictor in each of the 10 models, and in each case, including antidepressant use resulted in worsened model fit (data not shown). Only one person in our sample reported taking stimulants, so we were unable to analyze any potential stimulant effects on the PIPR.
Table 2.
Iris Ratings by Race and Ethnicity
| N | M | SD | Range | F | P | ||
|---|---|---|---|---|---|---|---|
| Race | White | 38 | 12.5 | 6.4 | 2–23 | 11.97 | <.001 |
| Black | 8 | 21.6 | 2.1 | 17–24 | |||
| Asian | 3 | 23.3 | 0.6 | 23–24 | |||
| Ethnicity | Hispanic/Latinx | 2 | 14.3 | 7.0 | 2–24 | 2.08 | .156 |
| Not Hispanic/Latinx | 47 | 21.5 | 0.7 | 21–22 | |||
White—Endorsed Caucasian or white race. Black—Endorsed black or African American race. Asian—Endorsed Asian American race. Hispanic/Latinx—Endorsed being of Hispanic ethnicity, or Latino or Latina.
Table 3.
Post-illumination Pupil Response (PIPR) Values, Demographics, and Covariates Stratified by Season and Diagnostic Group (Mean ± Standard Deviation; SAD; Seasonal Affective Disorder)
| SAD | Control | Both groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Summer | Winter | Total | Summer | Winter | Total | Summer | Winter | Total | |
| N | 20 | 15 | 25 | 19 | 10 | 22 | 39 | 25 | 47 |
| Age | 36.0 ± 10.8 | 35.9 ± 9.7 | 35.9 ± 10.2 | 32.4 ± 11.0 | 36.0 ± 11.2 | 33.6 ± 11.0 | 36.0 ± 10.1 | 34.2 ± 10.9 | 34.9 ± 10.6 |
| Gender1 | — | — | 22(88%) | — | — | 15(68%) | — | — | 37(79%) |
| Testing time2 | 13.0 ± 2.0 | 15.0 ± 1.9 | 13.9 ± 2.2 | 12.0 ± 1.9 | 15.0 ± 2.2 | 13.0 ± 2.5 | 12.5 ± 2:0 | 15.0 ± 2.0 | 13.5 ± 2.4 |
| Eye exam3 | — | — | 15(60%) | — | — | 10(45%) | — | — | 25(53%) |
| PLRred(mm)4 | 2.8 ± 0.6 | 2.8 ± 0.6 | 2.8 ± 0.6 | 2.6 ± 0.5 | 2.8 ± 0.6 | 2.7 ± 0.5 | 2.7 ± 0.5 | 2.8 ± 0.6 | 2.7 ± 0.5 |
| PLRred(%) | 42.5 ± 8.5 | 39.0 ± 3.8 | 41.0 ± 7.0 | 43.0 ± 7.2 | 36.0 ± 6.0 | 40.6 ± 7.5 | 42.7 ± 7.8 | 37.8 ± 4.9 | 40.8 ± 7.2 |
| PLRblue(mm) | 2.8 ± 0.6 | 2.8 ± 0.6 | 2.8 ± 0.6 | 2.6 ± 0.6 | 2.9 ± 0.7 | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.8 ± 0.6 | 2.7 ± 0.6 |
| PLRblue(%) | 42.5 ± 8.1 | 39.8 ± 3.9 | 41.3 ± 6.7 | 42.5 ± 7.5 | 36.8 ± 8.5 | 40.6 ± 8.2 | 42.5 ± 7.7 | 38.6 ± 6.1 | 41.0 ± 7.3 |
| Iris ratings5 | — | — | 13.5 ± 7.5 | — | — | 16.0 ± 5.9 | — | — | 14.7 ± 6.8 |
| PIPRbluemm | 5.60 ± 1.32 | 6.23 ± 1.74 | 5.87 ± 1.52 | 5.31 ± 1.23 | 6.57 ± 1.24 | 5.74 ± 1.36 | 5.46 ± 1.27 | 6.37 ± 1.54 | 5.81 ± 1.44 |
| PIPRredmm | 6.43 ± 1.51 | 6.92 ± 1.69 | 6.64 ± 1.59 | 5.98 ± 1.31 | 7.63 ± 1.37 | 6.55 ± 1.53 | 6.21 ± 1.42 | 7.20 ± 1.58 | 6.60 ± 1.55 |
| NetPIPR(%)6 | 0.13 ± 0.04 | 0.11 ± 0.06 | 0.12 ± 0.05 | 0.12 ± 0.07 | 0.14 ± 0.06 | 0.13 ± 0.07 | 0.12 ± 0.06 | 0.13 ± 0.06 | 0.12 ± 0.06 |
| NetPIPR(mm)6 | 0.83 ± 0.44 | 0.69 ± 0.32 | 0.77 ± 0.40 | 0.68 ± 0.33 | 1.06 ± 0.31 | 0.80 ± 0.37 | 0.75 ± 0.39 | 0.83 ± 0.37 | 0.78 ± 0.38 |
| Baseline(mm) | 5.37 ± 1.41 | 6.49 ± 1.41 | 5.84 ± 1.49 | 5.32 ± 1.39 | 6.53 ± 1.23 | 5.96 ± 1.41 | 5.35 ± 1.37 | 6.51 ± 1.29 | 5.89 ± 1.44 |
| ret-iradblue7 | 15.33 ± 0.09 | 15.35 ± 0.09 | 15.34 ± 0.09 | 15.33 ± 0.09 | 15.39 ± 0.08 | 15.35 ± 0.09 | 15.33 ± 0.09 | 15.37 ± 0.09 | 15.34 ± 0.09 |
| ret-iradred7 | 15.30 ± 0.07 | 15.32 ± 0.07 | 15.31 ± 0.07 | 15.28 ± 0.07 | 15.35 ± 0.04 | 15.31 ± 0.07 | 15.29 ± 0.07 | 15.33 ± 0.06 | 15.31 ± 0.07 |
Values except gender, eye exams, and iris ratings are reported as mean plus or minus the standard deviation. Rows for a given season include individuals with data from either and both seasons, leading to N’s reflecting total number of visits rather than number of participants. Because some individuals attended two visits and some attended one visit, the total number of participants per group and in the total sample are not equal to the number of visits per group in each season (i.e. 64 total visits across 47 participants).
1Gender is reported as the number and percent of women per group.
2Testing time is decimal time of the PIPR assessment relative to midsleep by self-report.
3Eye exam is the number of individuals who underwent an Ophthalmological exam and were found to be free of retinal pathology.
4PLR is the pupil light reflex, after either red or blue stimulus, expressed as either millimeters (mm) or percent of baseline (%).
5Iris ratings are the consensus of two raters using the Franssen rating scale (Franssen et al., 2008).
6Values for post-illumination pupil response (PIPR) are first reported as Net percent of baseline (NetPIPR(%); e.g. 23%), which is the red response percent of baseline (e.g. 98%) minus the blue response percent of baseline (e.g. 75%; 98%red—75%blue = 23%net). Net values are highest when the pupil responses to red and blue stimulus differ the most, indicating a larger PIPR. PIPR is the PIPR from 10 to 40 seconds post-stimulus. When the PIPR is reported in millimeters (NetPIPR(mm)), it is the Net of red minus blue, and baseline corrected, meaning this is the difference in millimeters between the blue response minus baseline and the red response minus baseline.
7ret-iradblue and ret-iradred are calculated retinal irradiance values based on calculations in Roecklein et al. 2021 in units of log Photons/cm2/s.
Age
As expected, entrance pupil size (i.e. baseline calculated as the average diameter for 6.5 seconds prior to stimulus) and the PLR both correlated with age (Supplementary Table S1) [38, 83, 84]. Age was correlated with the PIPR when PIPR was expressed in millimeters, but not when the PIPR was expressed as a percent of baseline, possibly because baseline pupil diameter itself is so highly correlated with age. Age was significantly correlated with iris pigmentation ratings, such that younger individuals had higher pigmentation ratings and older individuals had lower ratings of iris pigmentation.
Hypothesis 1:
Iris-diameter interaction.
Contrary to Kardon et al. (2013), we did not observe an interaction between iris pigmentation ratings and baseline pupil diameter during the stimulus on the PIPR (see Supplementary Table 2; iris*baseline; model 1; p = .829) nor did we observe individual effects for iris pigmentation or pupil diameter (model 2; Supplementary Table S2). Therefore, we removed the nonsignificant iris*baseline interaction in subsequent models.
Hypothesis 2
Capturing retinal irradiance. Including PLR after red light was a significantly better fit to the data (model 3; i.e., greater negative number; AIC = −161.63; Table 5) than the original model that included iris ratings, entrance pupil diameter, and age (AIC = −144.98; Table 5). In every model, we found significant associations for the diagnostic group (SAD vs. controls) and season (winter vs. summer) as well as the group*season interaction. Including the PLR did not reduce group and season differences on the PIPR, in fact, coefficients for group and season did not significantly vary from model to model (unstandardized coefficient ranges from low to high: group = 0.09–0.11mm, season = 0.09–0.11mm, and group* season = −0.20–−0.23, see Table S2. However, model fit varied significantly depending on the covariates included, as detailed below. Above all, the models with the best model fit indices included only the PLR to red or blue light and TT (Table 4). The difference in AIC between models (Δi) is considered no different from the original model when under 2, and sufficiently better when over 10 (†; Richards et al., 2010; Burnham et al., 2010). In Table 5 we report the difference between a given model and the best-performing model. Each model was superior to model 1, with Δi AIC’s > 10 (Δi range: 19.2–39.2).
Table 5.
Mixed-Effects Models Comparing PIPR by Diagnostic Group, Season, and Group*Season Interaction With Differing Covariate Sets Rank Ordered by Aikaike’s Information Criterion Measure of Model Fit (Best Model at Bottom With Lowest AIC Value)
| Model | Covariates | AIC | Δi decline from model 12 |
|---|---|---|---|
| Model 1 | TT, iris, baseline, baseline*iris, age | −122.43 | 44.37† |
| Model 10 | TT, PLRblue(%), age | −141.59 | 25.21† |
| Model 2 | TT, iris, baseline, age | −144.98 | 21.82† |
| Model 6 | TT, PLRred(%), age | −145.86 | 20.94† |
| Model 9 | TT, PLRblue (mm), age | −146.99 | 19.81† |
| Model 5 | TT, PLRred(mm), age | −151.08 | 15.72† |
| Model 8 | TT, PLRblue(%) | −151.52 | 15.28† |
| Model 4 | TT, PLRred(%) | −156.00 | 10.8† |
| Model 7 | TT, PLRblue(mm) | −157.57 | 9.23 |
| Model 3 | TT, PLRred(mm) | −161.63 | 5.17 |
| Model 11 | TT, ret-iradred | −165.86 | 0.94 |
| Model 12 | TT, ret-iradblue | −166.80 | best model |
Coefficient estimates represent unstandardized beta coefficients. SE—standard error. PLRred and PLRblue are the pupil light reflex after the red and blue stimuli, respectively. TT—testing time, and iris—iris pigmentation ratings were log transformed prior to inclusion in models. PLRred(mm) is expressed in millimeters and PLRred(%) is percent of baseline. Baseline is the entrance pupil diameter at the onset of the stimulus. ret-iradblue and ret-iradred are calculated retinal irradiance values based on calculations reported in text in units of log Photons/cm2/s. AIC—Aikaike’s Information Criterion (lower values reflect better model fit, even when negative). The difference in AIC between models (Δi) is considered no different from the original model when under 2, and sufficiently better when over 10 (
†; Richards et al., 2010; Burnham et al., 2010). In the table above we report the difference between a given model and the best performing model, model 3. Each model was superior to model 1, with all Δi AIC’s > 10 (Δi range: 19.2–39.2).
Table 4.
Best Fit Mixed-Effects Models Comparing PIPR by Diagnostic Group and Season Including PLR and Estimated Retinal Irradiance in Response to Red and Blue Stimuli
| Coefficient | SE | 95% CI | P | AIC | |
|---|---|---|---|---|---|
| Model 3 | −161.63 | ||||
| Intercept | 0.022 | 0.071 | [−0.120 −0.164] | .757 | |
| Group | 0.094 | 0.038 | [0.018 −.169] | .016* | |
| Season | 0.096 | 0.040 | [0.016 −.176] | .020* | |
| Group*season | −0.206 | 0.075 | [−0.357 to −0.055] | .008** | |
| −0.084 | 0.039 | [−0.163 to -.005] | .037* | ||
| TT | 0.129 | 0.072 | [−0.016 to 0.273] | .079 | |
| PLRred(mm) | −0.013 | 0.012 | [−0.037 to 0.011] | .283 | |
| Model 7 | −157.57 | ||||
| Intercept | 0.013 | 0.069 | [−0.127 to 0.152] | .856 | |
| Group | 0.091 | 0.038 | [0.015 to 0.168] | .020* | |
| Season | 0.093 | 0.040 | [0.012 to 0.174] | .025* | |
| Group*season | −0.200 | 0.076 | [−0.354 to −0.047] | .011* | |
| −0.081 | 0.040 | [−0.161 to −0.001] | .049* | ||
| TT | 0.137 | 0.074 | [−0.012 to 0.286] | .071 | |
| PLRblue(mm) | −0.013 | 0.011 | [−0.035 to 0.010] | .266 | |
| Model 11 | −165.86 | ||||
| Intercept | 3.581 | 1.56 | [0.448 6.713] | .026* | |
| Group | 0.079 | 0.037 | [0.006.153] | .036* | |
| Season | 0.089 | 0.038 | [0.012.166] | .024* | |
| Group*season | −0.182 | 0.073 | [−0.329 to 0.035] | .016* | |
| −0.069 | 0.039 | [−0.146.009] | .082 | ||
| TT | 0.161 | 0.072 | [0.016.305] | .030* | |
| ret-iradred | −0.237 | 0.103 | [−0.444 to 0.030] | .025* | |
| Model 12 | −166.80 | ||||
| Intercept | 1.797 | 1.079 | [−0.365 to 3.960] | .101 | |
| Group | 0.092 | 0.037 | [0.018 to 0.167] | .016* | |
| Season | 0.101 | 0.039 | [0.023 to 0.179] | .013* | |
| Group*season | −0.209 | 0.074 | [−0.357 to 0.060] | .007** | |
| −0.084 | 0.039 | [−0.162 to 0.007] | .034* | ||
| TT | 0.126 | 0.071 | [−0.017 to .270] | .083 | |
| ret-iradblue | −0.118 | 0.070 | [−0.259 to 0.023] | .100 |
These four models were less than 10 AIC points different from one another, and significantly improved compared to the next best models. Coefficient estimates represent unstandardized beta coefficients. SE, standard error. AIC, Aikaike’s Information Criterion (lower values reflect better model fit (larger negative values), and improvement is significant when a given model is 2 or more units lower). 95% CI is the 95% Confidence Interval for the coefficient. *p < .05, **p < .01, ***p < .001. PLRred is the pupil light reflex after the red stimuli, respectively. TT, testing time, PLRred(mm) is expressed in millimeters. ret-iradblue and ret-iradred are calculated retinal irradiance values based on calculations reported in text in units of log Photons/cm2/s. See supplementary materials for all mixed-effects model results.
We tested whether age improved AIC by accounting for variance due to age that is not captured by the PLR such as age effects on the neural pathway from the retina to the pupil (Table 5, models 5, 6, 9, and 10 add age to models 3, 4, 7, and 8). We also tested whether the PLRblue (after the blue stimulus) might be a better covariate than the PLRred (after the red stimulus) because the lens and cornea filter blue light preferentially with increasing age-related lens opacity (Table 5; compare models with PLRred to Models with PLRblue). We included calculations estimating retinal irradiance for the red and blue stimuli separately in models along with the circadian time of testing. Finally, we examined models based on whether the PLR was expressed in millimeters or as a percent of the baseline. We also tested whether adding self-reported gender as a covariate to each of the models affected model fit, and while the main results did not change, the model fit worsened. All models are reported in Table 5 beginning with the worst model fit and ending with the best model fit (i.e. lowest negative AIC value) at the bottom of the table. Three models had indistinguishable AICs and included either retinal irradiance or the PLRred in millimeters as well as TT relative to midsleep (models 3, 11, and 12; Table 5). The next best model was that including the PLRblue (mm) and TT (model 7; Table 5).
Discussion
This study identified two covariates of non-melanopsin-specific contributions to the PIPR with similar quantitative performance: (1) the PLR and (2) our calculations of estimated retinal irradiance. These findings are specific to our PIPR collection methods (e.g. presenting stimuli without mydriasis in Newtonian view) which prioritized minimizing participant and experimenter impact over tighter controls of individual differences in retinal irradiance (e.g. mydriasis and Maxwellian view). Our reanalysis indicates that the PIPR significantly predicts SAD diagnosis, while considering season, and that this association is robust to statistical adjustment for retinal irradiance [31]. The PLR and estimated retinal irradiance model fits were superior to other covariates based on AIC criteria, although this is specific to our PIPR methods and our sample of SAD and healthy controls without inner or outer eye diseases. Iris pigmentation ratings and entrance pupil diameter did not individually predict the PIPR, nor did they interact in this sample as they did in Kardon et al. (2013). This is likely due to unequal distributions of iris pigmentation across ages in the sample, indicating that future samples should be ascertained with an equal distribution of iris pigmentation across age groups. The models including only the PLR or estimated retinal irradiance and TT were superior to models including age, iris pigmentation ratings, entrance pupil diameter, and TT. Based on these findings, we recommend that iris pigmentation ratings and entrance pupil diameter be replaced with the PLR or estimated retinal irradiance to model nonspecific effects on pupil responses when calculating the PIPR in some studies. Specifically, such controls are necessitated when other methods to avoid differences in nonspecific effects are not used. Also, these approaches are appropriate for studies aiming to isolate the melanopsin-driven component of the PIPR in samples of SAD or healthy individuals.
Adding age to models did not substantially improve model fit. This could be the case if the PLR and estimated retinal irradiance account for variance in the PIPR due to age. Mechanistically, age likely impacts retinohypothalamic tract signaling to any retinorecipient areas including the central clock, as well as lens opacity, baseline pupil diameter, age-related decreases in noradrenergic neurons in locus coeruleus, and possibly others [85, 86]. Consistent with other reports, we found that age is inversely associated with the PLR (Table S1) indicating that older individuals received lower retinal illumination than younger individuals. This has implications for PIPR studies since age-related increases in lens opacity are evident as early as the second decade, before the average age of onset of SAD ranging from 20 to 30 years [87–89]. Middle-aged and older adult samples are important to study because multiple health consequences of circadian misalignment emerge in adulthood including mood disorders and cardiometabolic disorders.
Objective measures of the degree of optical media, including the lens, filter light capture reflections of stimuli from the eye and compare them to standards to measure individual ocular media filtering [90–92]. While these are advantageous in that they are objective and individually specific, the drawback is that the Purkinje image equipment, technology, and expertise needed may not be available in research settings outside of Ophthalmology. Other approaches that are similar to ours use historical data to generate normative values as a function of age which would then be used to statistically adjust data [66, 90]. The drawback of estimates based on age norms is that individual differences in the rate of aging lead to differences in ocular media filtering relative to others of the same age [91]. Both approaches may not capture individual differences in signaling downstream of the retina. Collecting an individually specific and objective measure of retinal optical media filtering and testing its relationship to the PIPR would be valuable.
Strengths and limitations
The current study used a binocular pupillometer with Newtonian view stimulus presentation to measure the ipsilateral response with natural pupils and did not use dilation medications. As a result, age-related pupillary miosis and lens density cause retinal irradiance to vary across participants. Our calculated range of retinal irradiance for the blue stimulus was 15.1 to 15.5 log Photons/cm2/s, and similar for red (Table 3). There are more precise methods to match individuals on retinal irradiance, however. For example, participants could be pharmacologically dilated [47], an artificial pupil could be added [93], or the light could be presented in Maxwellian view to negate differences in baseline or fluctuating pupil size during the stimulus [94]. Age-related lenticular changes could instead be quantified using lens grading, calibration, or an analytical model of human ocular media. Stimuli as short as 180 ms would conclude before the pupil light response can begin, negating change in pupil diameter during the stimulus. Short stimuli combined with an artificial pupil would standardize pupil diameter across participants during the stimulus without a Maxwellian view system and dilation and should be considered in future studies. However, neither accounts for individual differences in light filtering by ocular media (e.g. lens) prior to the retina. Though dilation and Maxwellian view systems may be preferable from a methodological standpoint, they may present a barrier to the broad use of the PIPR in clinical research settings without such technology (for a more detailed methodological overview see Kelbsch et al., 2019). Each of these methodological choices was made with participant impact (i.e. dilation), and available resources in mind (e.g. Maxwellian view systems are custom-built). Our efforts to minimize participant impact may explain high participation rates resulting in a total of 64 visits across 47 participants which is one of the largest PIPR samples and only the third to include repeated visits [95, 96]. Due to these methodological choices, our results are only generalizable to studies using the PIPR collection methods and metrics used here. This is because the PIPR varies as a function of the specific spectral distribution and irradiance of the stimulus, the stimulus duration, the size of the stimulus on the visual field, whether or not the pupil is freely reactive, prior light history, and time of day. Our measure of TT was based on wake time rather than the gold standard measure of circadian timing, dim light melatonin onset. Our TT window (10:00 am–6:48 pm) may not be sufficient to account for decreases in the PIPR in the later portion of the circadian day because participants will vary in circadian alignment. While our PIPRs ranged from 10:00 am to 6:48 pm (M = 1:30 pm; SD = 144 minutes)., that is still before the evening drop in PIPR observed by Zele et al., (2011). Additionally, 75% of our sample was tested before 2:45 pm. This variation, plus individual variation in circadian time, would contribute to measurement error given the diurnal rhythm of the PIPR. TT is therefore a problematic proxy for circadian time, making our use of circadian time of testing (i.e. Pittsburgh Sleep Quality Index midsleep–TT) preferable. However, calculating the circadian time of testing using actigraphic measures of midsleep or dim-light melatonin onset time would better ensure that the PIPR was not confounded by individual differences in the circadian time of collection. In addition, because ipRGCs sum light input over a much larger time frame than the classical photoreceptors [17], the PLR is unlikely to account for many effects of light history on the PIPR, although light history for 2 hours prior to the PIPR was standardized in our studies. Because ipRGCs sum light input over a longer timeframe than classical photoreceptors, prior light history should be studied systematically to determine the preceding timeframe, intensity, and duration of light that is relevant for the PIPR. Information criteria vary in their ability to identify the best model fit [97], but in the present case, each of the four other criteria considered was consistent with AIC. The previously reported group*season interaction was statistically significant in all models tested, indicating that this is a robust result in the current sample. Although the PLR was used by Kardon et al. in 2009, this is the first application of the PLR as a covariate in PIPR analyses to control for non-melanopsin effects on the PIPR [98].
Implications
Critically for the design and sample acquisition of PIPR studies, the degree of iris pigmentation has been shown to vary with age and race/ethnicity. In our sample, self-identified black or African American and Asian American race was associated with higher iris pigmentation ratings (Table 2) as expected [99]. Due to race being a social construct rather than a biological construct, race and ethnicity are insufficient proxies for biological variables like iris pigmentation [100, 101]. The ideal iris pigmentation rating scale would be psychometrically reliable and valid, as well as feasible in clinical research settings. The Franssen et al. (2008) scale is comprised of photographic examples of irises for each rating, while other scales like Mackey et al. (2011) consist of verbal descriptions of iris color for each of the nine ratings, making the Franssen scale easier to use [102]. We recommend that PIPR studies aiming to ensure iris pigmentation is evenly distributed across age groups at the time of enrollment employ a rating scale like the one used here by Franssen et al. (2008). However, in analyses, the PLR and estimated retinal irradiance account for more variance in the PIPR than iris pigmentation rankings. While many studies have estimated retinal irradiance and calculated individual values, adding the PLR as a covariate represents a change in study design and quantitative method, and the advantage of doing so will differ by study aims. For studies that aim to include all individual differences in non-image-forming responses to light as a naturalistic measure of photic input, fewer covariates are indicated. However, studies such as ours that aim to isolate the effects of melanopsin from nonspecific effects on pupil responses could calculate estimated irradiance. Alternatively, studies could instead use the PLR from pupil data collected immediately prior to a PIPR as an individually specific, objective, and contemporaneous measure of non-melanopsin influences on the PIPR. Including the PLR is a novel quantitative approach that could improve the scientific rigor of the PIPR measure of retinal responsivity while minimizing participant and experimenter impact. Such methodological refinements will foster the PIPR’s emergence as an indispensable method to measure sensitivity to light.
Supplementary Material
Supplementary material is available at SLEEP online.
Acknowledgments
We dedicate this manuscript to the memory of Dr. Michael Menaker for his pioneering work on photoentrainment.
Contributor Information
Alison M Klevens, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Maddison L Taylor, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Delainey L Wescott, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Paul D Gamlin, Department of Ophthalmology and Visual Sciences, University of Alabama at Birmingham, Birmingham, AL, USA.
Peter L Franzen, Department of Psychiatry, University of Pittsburgh School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Brant P Hasler, Department of Psychiatry, University of Pittsburgh School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Greg Siegle, Department of Psychiatry, University of Pittsburgh School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Kathryn A Roecklein, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA; Department of Psychiatry, University of Pittsburgh School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Funding
Support for this project has been provided by NIH grants MH096199, MH103313, and UL1TR001857.
Disclosure Statement
Financial disclosure: none. Nonfinancial disclosure: none.
Data Availability
The data that supports this study is available upon request from the corresponding author, AMK. The data are not publicly available.
References
- 1. Paul KN, Saafir TB, Tosini G.. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev Endocr Metab Disord. 2010;10(4):271–278. doi: 10.1007/s11154-009-9120-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM.. Human and macaque pupil responses driven by melanopsin- containing retinal ganglion cells. Vis Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelbsch C, Strasser T, Chen Y, et al. Standards in pupillography. Front Neurol. 2019;10:129. doi: 10.3389/fneur.2019.00129. https://www.frontiersin.org/articles/10.3389/fneur.2019.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klein DC, Weller JL.. Rapid light-induced decrease in pineal serotonin N-acetyltransferase activity. Science. 1972;177:532–533. doi: 10.1126/science.177.4048.532 [DOI] [PubMed] [Google Scholar]
- 5. Phillips AJK, Vidafar P, Burns AC, et al. (2019). High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci USA. 1201;116(24):9–12024. doi: 10.1073/pnas.1901824116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berson DM, Dunn FA, Takao M.. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- 7. Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB.. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768 [DOI] [PubMed] [Google Scholar]
- 10. Lucas RJ, Freedman MS, Muñoz M, Garcia-Fernández JM, Foster RG.. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505 [DOI] [PubMed] [Google Scholar]
- 11. Hannibal J, Hindersson P, Ostergaard J, et al. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004;45:4202–4209. doi: 10.1167/iovs.04-0313 [DOI] [PubMed] [Google Scholar]
- 12. Panda S, Provencio I, Tu DC, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179 [DOI] [PubMed] [Google Scholar]
- 13. LeGates TA, Altimus CM, Wang H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi: 10.1038/nature11673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hannibal J, Kankipati L, Strang CE, Peterson BB, Dacey D, Gamlin PD.. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol. 2014;522:2231–2248. doi: 10.1002/cne.23588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–349. doi: 10.1002/cne.20970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Do MTH. Melanopsin and the intrinsically photosensitive retinal ganglion cells: Biophysics to behavior. Neuron. 2019;104(2):205–226. doi: 10.1016/j.neuron.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aranda ML, Schmidt TM.. Diversity of intrinsically photosensitive retinal ganglion cells: Circuits and functions. Cell Mol Life Sci. 2021;78(3):889–907. doi: 10.1007/s00018-020-03641-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zele AJ, Dey A, Adhikari P, Feigl B.. Melanopsin hypersensitivity dominates interictal photophobia in migraine. Cephalalgia. 2021;41(2):217–226. doi: 10.1177/0333102420963850 [DOI] [PubMed] [Google Scholar]
- 19. Najjar RP, Chiquet C, Teikari P, et al. Aging of non-visual spectral sensitivity to light in humans: compensatory mechanisms? PLoS One. 2014;9(1):e85837. doi: 10.1371/journal.pone.0085837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M.. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. 2010;36(2):308–312. doi: 10.1016/j.jcrs.2009.08.035 [DOI] [PubMed] [Google Scholar]
- 21. Kessel L, Siganos G, Jørgensen T, Larsen M.. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep. 2011;34(9):1215–1219. doi: 10.5665/SLEEP.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winn B, Whitaker D, Elliott DB, Phillips NJ.. Factors affecting light-adapted pupil size in normal human subjects. Invest Ophthalmol Vis Sci. 1994;35(3):1132–1137. [PubMed] [Google Scholar]
- 23. Guillon M, Dumbleton K, Theodoratos P, Gobbe M, Wooley CB, Moody K.. The effects of age, refractive status, and luminance on pupil size. Optom Vis Sci. 2016;93(9):1093–1100. doi: 10.1097/OPX.0000000000000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kardon RH, Hong S, Kawasaki A.. Entrance pupil size predicts retinal illumination in darkly pigmented eyes, but not lightly pigmented eyes. Invest Ophthalmol Vis Sci. 2013;54(8):5559–5567. doi: 10.1167/iovs.13-12319 [DOI] [PubMed] [Google Scholar]
- 25. Davey PG, Lievens C, Amonoo-Monney S.. (2020). Differences in macular pigment optical density across four ethnicities: a comparative study. Ther Adv Ophthalmol. 2515;1:841420924167. doi: 10.1177/2515841420924167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feigl B, Zele AJ, Fader SM, et al. The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmologica. 2012;90(3):e230–e234. doi: 10.1111/j.1755-3768.2011.02226.x [DOI] [PubMed] [Google Scholar]
- 27. Feigl B, Ojha G, Hides L, Zele AJ.. Melanopsin-driven pupil response and light exposure in non-seasonal major depressive disorder. Front Neurol. 2018;9:764. doi: 10.3389/fneur.2018.00764. https://www.frontiersin.org/articles/10.3389/fneur.2018.00764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kankipati L, Girkin CA, Gamlin PD.. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011;52(5):2287–2292. doi: 10.1167/iovs.10-6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A.. Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology. 2011;118(2):376–381. doi: 10.1016/j.ophtha.2010.06.033 [DOI] [PubMed] [Google Scholar]
- 30. Kawasaki A, Crippa SV, Kardon R, Leon L, Hamel C.. Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Invest Ophthalmol Vis Sci. 2012;53(9):5562–5569. doi: 10.1167/iovs.12-10230 [DOI] [PubMed] [Google Scholar]
- 31. Roecklein KA, Franzen PL, Wescott DL, et al. Melanopsin-driven pupil response in summer and winter in unipolar seasonal affective disorder. J Affect Disord. 2021;291:93–101. doi: 10.1016/j.jad.2021.04.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Meijden WP, Van Someren JL, te Lindert BHW, et al. Individual differences in sleep timing relate to melanopsin-based phototransduction in healthy adolescents and young adults. Sleep. 2016;39(6):1305–1310. doi: 10.5665/sleep.5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abbott KS, Queener HM, Ostrin LA.. The ipRGC Driven pupil response with light exposure, refractive error, and sleep. Optom Vis Sci. 2018;95:323–331. doi: 10.1097/OPX.0000000000001198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burgess HJ, Rizvydeen M, Kikyo F, et al. Sleep and circadian differences between light and heavy adult alcohol drinkers. Alcohol Clin Exp Res. 2022;46(7):1181–1191. doi: 10.1111/acer.14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laurenzo SA, Kardon R, Ledolter J, et al. Pupillary response abnormalities in depressive disorders. Psychiatry Res. 2016;246:492–499. doi: 10.1016/j.psychres.2016.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bullock B, McGlashan EM, Burns AC, Lu BS, Cain SW.. Traits related to bipolar disorder are associated with an increased post-illumination pupil response. Psychiatry Res. 2019;278:35–41. doi: 10.1016/j.psychres.2019.05.025 [DOI] [PubMed] [Google Scholar]
- 37. Madsen H, Ba-Ali S, Heegaard S, et al. Melanopsin-mediated pupillary responses in bipolar disorder—A cross-sectional pupillometric investigation. Int J Bipolar Disord. 2021;9(1):7. doi: 10.1186/s40345-020-00211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Birren JE, Casperson RC, Botwinick J.. Age changes in pupil size. J Gerontol. 1950;5(3):216–221. doi: 10.1093/geronj/5.3.216 [DOI] [PubMed] [Google Scholar]
- 39. Bradley JC, Bentley KC, Mughal AI, Bodhireddy H, Brown SM.. Dark-adapted pupil diameter as a function of age measured with the NeurOptics pupillometer. J Refract Surg. 2011;27:202–207. doi: 10.3928/1081597X-20100511-01 [DOI] [PubMed] [Google Scholar]
- 40. Telek HH, Erdol H, Turk A.. The effects of age on pupil diameter at different light amplitudes. Beyoglu Eye J. 2018;3(2):80–85. [Google Scholar]
- 41. Chen Y, Pinto AA, Paulsen AJ, et al. The post-illumination pupil response (PIPR) is associated with cognitive function in an epidemiologic cohort study. Front Neurol. 2019;10:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan SS, Wang W.. The effect of lens aging and cataract surgery on circadian rhythm. Int J Ophthalmol. 2016;9(7):1066–1074. doi: 10.18240/ijo.2016.07.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Broendsted AE, Hansen MS, Lund-Andersen H, Sander B, Kessel L.. Human lens transmission of blue light: a comparison of autofluorescence-based and direct spectral transmission determination. Ophthalmic Res. 2011;46(3):118–124. doi: 10.1159/000323576 [DOI] [PubMed] [Google Scholar]
- 44. Herbst K, Sander B, Lund-Anderson H, et al. Intrinsically photosensitive retinal ganglion cell function in relation to age: a pupillometric study in humans with special reference to the age-related optic properties of the lens. BMC Ophthalmol. 2012;12(4):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Esquiva G, Lax P, Perez-Santonja JJ, Garcia-Fernandez JM, Cuenca N.. Loss of melanopsin-expressing ganglion cell subtypes and dendritic degeneration in the aging human retina. Front Aging Neurosci. 2017;9:79. doi: 10.3389/fnagi.2017.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roecklein KA, Wong PM, Ernecoff NC, et al. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 2013;210(1):150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kankipati L, Girkin CA, Gamlin PD.. Post-Illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010;51:2764–2769. doi: 10.1167/iovs.09-4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adhikari P, Pearson CA, Anderson AM, Zele AJ, Feigl B.. (2015). Effect of age and refractive error on the melanopsin mediated Post-Illumination Pupil Response (PIPR). Sci Rep. 1761;5:0. doi: 10.1038/srep17610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maynard ML, Zele AJ, Feigl B.. Melanopsin-mediated post-illumination pupil response in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56(11):6906–6913. doi: doi: 10.1167/iovs.15-17357 [DOI] [PubMed] [Google Scholar]
- 50. Van Den Berg TJ, Van Rijn LJ, Michael R, et al. Straylight effects with aging and lens extraction. Am J Ophthalmol. 2007;144(3):358–363. doi: 10.1016/j.ajo.2007.05.037 [DOI] [PubMed] [Google Scholar]
- 51. Doragaleleh S, Naghipoor K, Barahouie A, Dastaviz F, Oladnabi M.. Molecular and biochemical mechanisms of human iris color. J Cell Physiol. 2020;235(12):8972–8982. [DOI] [PubMed] [Google Scholar]
- 52. Wielgus AR, Sarna T.. Melanin in human irides of different color and age of donors. Pigment Cell Res. 2005;18(6):454–464. doi: 10.1111/j.1600-0749.2005.00268.x [DOI] [PubMed] [Google Scholar]
- 53. Frank RN, Puklin JE, Stock C, Canter LA.. Race, iris color, and age-related macular degeneration. Trans Am Ophthalmol Soc. 2000;98:109–15; discussion 115. [PMC free article] [PubMed] [Google Scholar]
- 54. Vajdic CM, Kricker A, Giblin M, et al. Eye color and cutaneous nevi predict risk of ocular melanoma in Australia. Int J Cancer. 2001;92:906–912. doi: 10.1002/ijc.1281 [DOI] [PubMed] [Google Scholar]
- 55. Weis E, Shah CP, Lajous M, Shields JA, Shields CL.. The association between host susceptibility factors and uveal melanoma: a meta-analysis. Arch Ophthalmol. 2006;124:54–60. doi: 10.1001/archopht.124.1.54 [DOI] [PubMed] [Google Scholar]
- 56. Davey PG, Lievens C, Amonoo-Monney S.. (2020). Differences in macular pigment optical density across four ethnicities: a comparative study. Ther Adv Ophthalmol. 2515;1:841420924167. doi: 10.1177/2515841420924167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klevens AM, Wescott DL, Drexler SP, Gamlin PD, Roecklein KA.. Iris color predicts melanopsin-driven retinal responses in older but not younger individuals. Sleep. 2022;45(suppl_1). doi: 10.1093/sleep/zsac079.197 [DOI] [Google Scholar]
- 58. Steinhauer SR, Bradley MM, Siegle GJ, Roecklein KA, Dix A.. Publication guidelines and recommendations for pupillary measurement in psychophysiological studies. Psychophysiology. 2022;59(4):e14035. doi: 10.1111/psyp.14035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ellis CJ. The pupillary light reflex in normal subjects. Br J Ophthalmol. 1981;65(11):754–759. doi: 10.1136/bjo.65.11.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sun FC, Liu HK, Liu YM.. Dynamic pupillary response to positive differential of light stimulus. Scientia Sin. 1981;24(6):872–884. [PubMed] [Google Scholar]
- 61. Loewenfeld IE. Otto Lowenstein: Neurologic and ophthalmologic testing methods during his lifetime. Doc Ophthalmol. 1999;98(1):3–20. doi: 10.1023/a:1002134106425 [DOI] [PubMed] [Google Scholar]
- 62. Münch M, Léon L, Crippa SV, Kawasaki A.. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Invest Ophthalmol Vis Sci. 2012;53(8):4546–4555. doi: 10.1167/iovs.12-9494 [DOI] [PubMed] [Google Scholar]
- 63. Zele AJ, Feigl B, Smith SS, Markwell EL.. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One. 2011;6(3):e17860. doi: 10.1371/journal.pone.0017860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Clark JH. The Ishihara test for color blindness. Am J Physiol Opt. 1924;5:269–276. [Google Scholar]
- 65. First, M. B., Spitzer, R. L., Gibbon, M., Williams, J. B. (2002). Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition with psychotic screen (SCID-I/P W/PSY SCREEN). Biometrics Research, New York State Psychiatric Institute, NY. [Google Scholar]
- 66. Pokorny J, Smith VC, Lutze M.. Aging of the human lens. Appl Opt. 1987;26(8):1437–1440. doi: 10.1364/AO.26.001437 [DOI] [PubMed] [Google Scholar]
- 67. Van Norren D, Voss JJ.. Spectral transmission of the human ocular media. Vision Res. 1974;14(11):1237–1244. doi: 10.1016/0042-6989(74)90222-3 [DOI] [PubMed] [Google Scholar]
- 68. Boettner EA, Wolter R.. Transmission of the ocular media. Invest Opthalmol Vis Sci. 1962;1(6):776–783. [Google Scholar]
- 69. Coren S, Girgus JS.. Density of human lens pigmentation: in vivo measures over an extended age range. Vis Res. 1972;12(2):343–346. doi: 10.1016/0042-6989(72)90124-1 [DOI] [PubMed] [Google Scholar]
- 70. Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Spitschan M, Kervezee L, Lok R, et al. ENLIGHT: A consensus checklist for reporting laboratory-based studies on the non-visual effects of light in humans. EBioMedicine. 2023;98:104889. doi: 10.1016/j.ebiom.2023.104889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Steinhauer SR, Condray R, Kasparek A.. Cognitive modulation of midbrain function: task-induced reduction of the pupillary light reflex. Int J Psychophysiol. 2000;39(1):21–30. doi: 10.1016/s0167-8760(00)00119-7 [DOI] [PubMed] [Google Scholar]
- 73. Adhikari P, Zele AJ, Feigl B.. The Post-Illumination Pupil Response (PIPR). Invest Ophthalmol Vis Sci. 2015;56:3838–3849. doi: 10.1167/iovs.14-16233 [DOI] [PubMed] [Google Scholar]
- 74. Kennaway KJ. The dim light melatonin onset across ages, methodologies, and sex and its relationship with morningness/eveningness. Sleep. 2023;45(6). doi: 10.1093/sleep/zsad033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ.. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 76. Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D.. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med. 2003;1(2):102–114. doi: 10.1207/S15402010BSM0102_3 [DOI] [PubMed] [Google Scholar]
- 77. Hasler BP, Smith LJ, Cousins JC, Bootzin RR.. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16(1):67–81. doi: 10.1016/j.smrv.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Franssen L, Coppens JE, van den Berg TJTP.. Grading of Iris color with an extended photographic reference set. J Optometry 2008;1(1):36–40. doi: 10.3921/joptom.2008.36 [DOI] [Google Scholar]
- 79. Koo TK, Li MY.. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Akaike, H. (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov, B.N. and Csaki, F., eds., International Symposium on Information Theory. New York, NY; 267–281. [Google Scholar]
- 81. Richards SA, Whittingham MJ, Stephens PA.. Model selection and model averaging in behavioural ecology. Behav Ecol Sociobiol. 2010;65:77–89. [Google Scholar]
- 82. Burham, K. C. & Anderson, D. R. (2002). Model Selection and Multimodal Inference. Second edition. Springer, NY. [Google Scholar]
- 83. Winn B, Whitaker D, Elliott DB, Phillips NJ.. Factors affecting light-adapted pupil size in normal human subjects. Invest Ophthalmol Vis Sci. 1994;35(3):1132–1137. [PubMed] [Google Scholar]
- 84. Bentley KC, Bradley JC, Mughal AI, Bodhireddy H, Young RSL, Brown S.. The Effect of age, gender, and iris color on the dark-adapted pupil diameter measured with the neuroptics pupillometer. Invest Ophthalmol Vis Sci. 2010;51(13):5661. [Google Scholar]
- 85. Bitsios P, Prettyman R, Szabadi E.. Changes in autonomic function with age: a study of pupillary kinetics in healthy young and old people. Age Ageing. 1996;25:432–438. doi: 10.1093/ageing/25.6.432 [DOI] [PubMed] [Google Scholar]
- 86. Samuels ER, Szabadi E.. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol. 2008;6(235):254–285. doi: 10.2174/157015908785777193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brainard GC, Gaddy JR, Ruberg FL, Barker FM.. Ocular mechanisms that regulate the human pineal gland. Adv Pineal Res. 1994;8:415–415. [Google Scholar]
- 88. Thompson C, Isaac G.. Seasonal affective disorder – a British sample. Syptamology in relation to mode of referral and diagnostic subtype. J Affect Disord. 1988;14:1–11. [DOI] [PubMed] [Google Scholar]
- 89. Faedda GL, Tondo L, Teicher MH, Baldessarini RJ, Gelbard HA, Floris GF.. Seasonal mood disorders: patterns of seasonal recurrence in mania and depression. Arch Gen Psychiatry. 1993;50(1):17–23. doi: 10.1001/archpsyc.1993.01820130019004 [DOI] [PubMed] [Google Scholar]
- 90. Van De Kraats J, Van Norren D.. Optical density of the aging human ocular media in the visible and the UV. J Opt Soc Am A. 2007;24(7):1842–1857. doi: 10.1364/josaa.24.001842 [DOI] [PubMed] [Google Scholar]
- 91. Savage GL, Johnson CA, Howard DL.. A comparison of noninvasive objective and subjective measurements of the optical density of human ocular media. Optom Vis Sci. 2001;78(6):386–395. doi: 10.1097/00006324-200106000-00010 [DOI] [PubMed] [Google Scholar]
- 92. Eto T, Teikari P, Najjar RP, et al. A Purkinje image-based system for an assessment of the density and transmittance spectra of the human crystalline lens in vivo. Sci Rep. 2020;10(1):16445. doi: 10.1038/s41598-020-73541-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zele AJ, Adhikari P, Cao D, Feigl B.. Melanopsin and cone photoreceptor inputs to the afferant pupil response. Front Neurol. 2019;10:529. doi: 10.3389/fneur.2019.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Westheimer G. The Maxwellian view. Vision Res. 1966;6(12):669–682. doi: 10.1016/0042-6989(66)90078-2 [DOI] [PubMed] [Google Scholar]
- 95. Herbst K, Sander B, Milea D, Lund-Andersen H, Kawasaki A.. Test–retest repeatability of the pupil light response to blue and red light stimuli in normal human eyes using a novel pupillometer. Front Neurol. 2011;2:10. doi: 10.3389/fneur.2011.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lei S, Goltz HC, Chandrakumar M, Wong AM.. Test-retest reliability of hemifield central-field and full-field chromatic pupillometry for assessing the function of melanopsin-containing retinal ganglion cells. Invest Ophthalmol Vis Sci. 2015;56:1267–1273. doi: 10.1167/iovs.14-15945 [DOI] [PubMed] [Google Scholar]
- 97. Barnett AG, Koper N, Dobson AJ, Schmiegelow F, Manseau M.. Using information criteria to select the correct variance–covariance structure for longitudinal data in ecology. Methods Ecol Evol. 2010;1(1):15–24. doi: 10.1111/j.2041-210x.2009.00009.x [DOI] [Google Scholar]
- 98. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A.. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009;116(8):1564–1573. doi: 10.1016/j.ophtha.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 99. Adetunji MO, Meer E, Whitehead G, et al. Self-identified black race as a risk factor for intraocular pressure elevation and iritis following prophylactic laser peripheral iridotomy. J Glaucoma. 2022;31(4):218–223. doi: 10.1097/IJG.0000000000001995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Umek W, Fischer B.. We Should Abandon “Race” as a biological category in biomedical research. Female Pelvic Med Reconstr Surg. 2020;26(12):719–720. doi: 10.1097/SPV.0000000000000979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rodney RA. The attainment of patient diversity in clinical trials: race, ethnicity, genetics. Am J Med. 2021;134(12):1440–1441. doi: 10.1016/j.amjmed.2021.06.050 [DOI] [PubMed] [Google Scholar]
- 102. Mackey DA, Wilkinson CH, Kearns LS, Hewitt AW.. Classification of iris colour: Review and refinement of a classification schema. Clin Exp Ophthalmol. 2011;39(5):462–471. doi: 10.1111/j.1442-9071.2010.02487.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports this study is available upon request from the corresponding author, AMK. The data are not publicly available.