Abstract
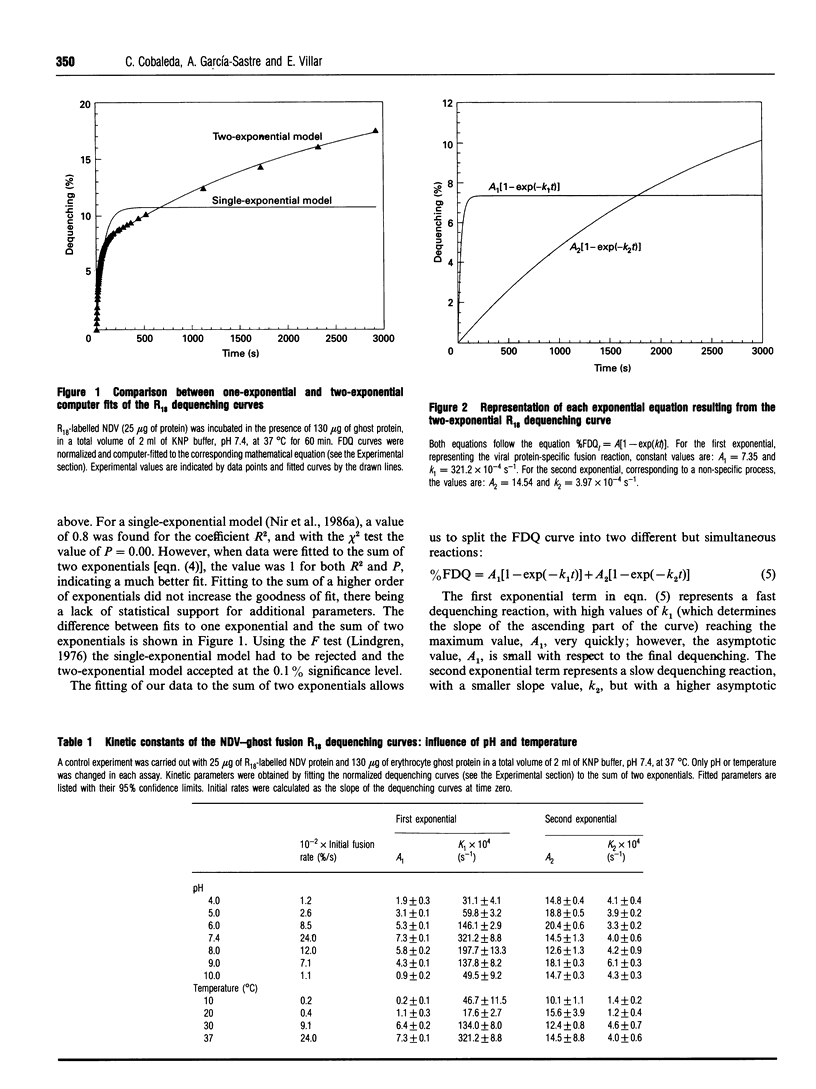
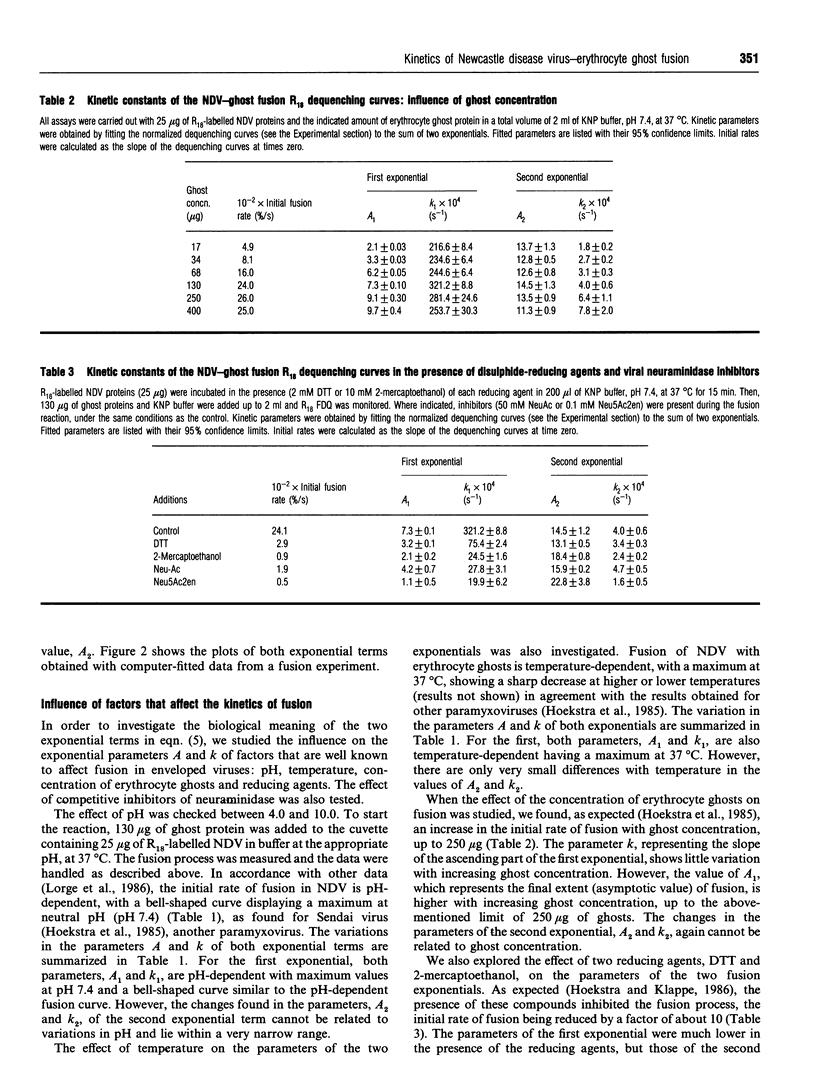
The kinetics of fusion between Newcastle disease virus and erythrocyte ghosts has been investigated with the octadecyl Rhodamine B chloride assay [Hoekstra, De Boer, Klappe, and Wilschut (1984) Biochemistry 23, 5675-5681], and the data from the dequenching curves were fitted by non-linear regression to currently used kinetic models. We used direct computer-assisted fitting of the dequenching curves to the mathematical equations. Discrimination between models was performed by statistical analysis of different fits. The experimental data fit the exponential model previously published [Nir, Klappe, and Hoekstra (1986) Biochemistry 25, 2155-2161] but we describe for the first time that the best fit was achieved for the sum of two exponential terms: A1[1-exp(-k1t)]+A2[1-exp(-k2t)]. The first exponential term represents a fast reaction and the second a slow dequenching reaction. These findings reveal the existence of two independent, but simultaneous, processes during the fusion assay. In order to challenge the model and to understand the meaning of both equation, fusion experiments were carried out under different conditions well known to affect viral fusion (changes in pH, temperature and ghost concentration, and the presence of disulphide-reducing agents or inhibitors of viral neuraminidase activity), and the same computer fitting scheme was followed. The first exponential equation represents the viral protein-dependent fusion process itself, because it is affected by the assay conditions. The second exponential equation accounts for a nonspecific reaction, because it is completely independent of the assay conditions and hence of the viral proteins. An interpretation of this second process is discussed in terms of probe transfer between vesicles.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R., Bali-Puri A., Walter A., Covell D., Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J Biol Chem. 1987 Oct 5;262(28):13614–13619. [PubMed] [Google Scholar]
- Cabezas J. A., Calvo P., Eid P., Martin J., Perez N., Reglero A., Rodrigo M., Hannoun C. Studies on neuraminidase from influenza virus A(H3N2) obtained by two procedures. Int J Biochem. 1982;14(4):311–319. doi: 10.1016/0020-711x(82)90092-1. [DOI] [PubMed] [Google Scholar]
- Chejanovsky N., Loyter A. Fusion between Sendai virus envelopes and biological membranes. The use of fluorescent probes for quantitative estimation of virus-membrane fusion. J Biol Chem. 1985 Jul 5;260(13):7911–7918. [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Loyter A. Fusion of Sendai virions or reconstituted Sendai virus envelopes with liposomes or erythrocyte membranes lacking virus receptors. J Biol Chem. 1985 Oct 5;260(22):12072–12077. [PubMed] [Google Scholar]
- Citovsky V., Yanai P., Loyter A. The use of circular dichroism to study conformational changes induced in Sendai virus envelope glycoproteins. A correlation with the viral fusogenic activity. J Biol Chem. 1986 Feb 15;261(5):2235–2239. [PubMed] [Google Scholar]
- Clague M. J., Schoch C., Zech L., Blumenthal R. Gating kinetics of pH-activated membrane fusion of vesicular stomatitis virus with cells: stopped-flow measurements by dequenching of octadecylrhodamine fluorescence. Biochemistry. 1990 Feb 6;29(5):1303–1308. doi: 10.1021/bi00457a028. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Pedroso de Lima M. C., Stamatatos L., Flasher D., Alford D., Friend D. S., Nir S. Fusion activity and inactivation of influenza virus: kinetics of low pH-induced fusion with cultured cells. J Gen Virol. 1992 Jan;73(Pt 1):27–37. doi: 10.1099/0022-1317-73-1-27. [DOI] [PubMed] [Google Scholar]
- García Sastre A., Cobaleda C., Cabezas J. A., Villar E. On the inhibition mechanism of the sialidase activity from Newcastle disease virus. Biol Chem Hoppe Seyler. 1991 Oct;372(10):923–927. doi: 10.1515/bchm3.1991.372.2.923. [DOI] [PubMed] [Google Scholar]
- García-Sastre A., Cabezas J. A., Villar E. Proteins of Newcastle disease virus envelope: interaction between the outer hemagglutinin-neuraminidase glycoprotein and the inner non-glycosylated matrix protein. Biochim Biophys Acta. 1989 Nov 30;999(2):171–175. doi: 10.1016/0167-4838(89)90214-8. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K., Hoff H., Nir S. Mechanism of fusion of Sendai virus: role of hydrophobic interactions and mobility constraints of viral membrane proteins. Effects of polyethylene glycol. J Biol Chem. 1989 Apr 25;264(12):6786–6792. [PubMed] [Google Scholar]
- Hoekstra D., Klappe K. Sendai virus-erythrocyte membrane interaction: quantitative and kinetic analysis of viral binding, dissociation, and fusion. J Virol. 1986 Apr;58(1):87–95. doi: 10.1128/jvi.58.1.87-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K., de Boer T., Wilschut J. Characterization of the fusogenic properties of Sendai virus: kinetics of fusion with erythrocyte membranes. Biochemistry. 1985 Aug 27;24(18):4739–4745. doi: 10.1021/bi00339a005. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Iorio R. M., Glickman R. L., Sheehan J. P. Inhibition of fusion by neutralizing monoclonal antibodies to the haemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. J Gen Virol. 1992 May;73(Pt 5):1167–1176. doi: 10.1099/0022-1317-73-5-1167. [DOI] [PubMed] [Google Scholar]
- Kim J., Okada Y. Difference in capacities for virion-to-virion fusion of young and aged HVJ (Sendai virus): a model of membrane fusion. J Membr Biol. 1987;97(3):241–249. doi: 10.1007/BF01869226. [DOI] [PubMed] [Google Scholar]
- Klappe K., Wilschut J., Nir S., Hoekstra D. Parameters affecting fusion between Sendai virus and liposomes. Role of viral proteins, liposome composition, and pH. Biochemistry. 1986 Dec 16;25(25):8252–8260. doi: 10.1021/bi00373a019. [DOI] [PubMed] [Google Scholar]
- Larsen C. E., Nir S., Alford D. R., Jennings M., Lee K. D., Düzgüneş N. Human immunodeficiency virus type 1 (HIV-1) fusion with model membranes: kinetic analysis and the role of lipid composition, pH and divalent cations. Biochim Biophys Acta. 1993 Apr 22;1147(2):223–236. doi: 10.1016/0005-2736(93)90007-m. [DOI] [PubMed] [Google Scholar]
- Lorge P., Cabiaux V., Long L., Ruysschaert J. M. Fusion of Newcastle disease virus with liposomes: role of the lipid composition of liposomes. Biochim Biophys Acta. 1986 Jun 26;858(2):312–316. doi: 10.1016/0005-2736(86)90337-8. [DOI] [PubMed] [Google Scholar]
- Lyles D. S. Glycoproteins of Sendai virus are transmembrane proteins. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5621–5625. doi: 10.1073/pnas.76.11.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., Landsberger F. R. Kinetics of Sendai virus envelope fusion with erythrocyte membranes and virus-induced hemolysis. Biochemistry. 1979 Nov 13;18(23):5088–5095. doi: 10.1021/bi00590a011. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miura N., Uchida T., Okada Y. HVJ (Sendai virus)-induced envelope fusion and cell fusion are blocked by monoclonal anti-HN protein antibody that does not inhibit hemagglutination activity of HVJ. Exp Cell Res. 1982 Oct;141(2):409–420. doi: 10.1016/0014-4827(82)90229-4. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Sarkar D. P., White J. M., Blumenthal R. Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. Measurement by dequenching of fluorescence. J Biol Chem. 1989 Mar 5;264(7):3972–3978. [PubMed] [Google Scholar]
- Morrison T. G. Structure, function, and intracellular processing of paramyxovirus membrane proteins. Virus Res. 1988 May;10(2-3):113–135. doi: 10.1016/0168-1702(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Morrison T., McQuain C., McGinnes L. Complementation between avirulent Newcastle disease virus and a fusion protein gene expressed from a retrovirus vector: requirements for membrane fusion. J Virol. 1991 Feb;65(2):813–822. doi: 10.1128/jvi.65.2.813-822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991 Jun;65(6):2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir S., Düzgünes N., de Lima M. C., Hoekstra D. Fusion of enveloped viruses with cells and liposomes. Activity and inactivation. Cell Biophys. 1990 Oct;17(2):181–201. doi: 10.1007/BF02990496. [DOI] [PubMed] [Google Scholar]
- Nir S., Klappe K., Hoekstra D. Kinetics and extent of fusion between Sendai virus and erythrocyte ghosts: application of a mass action kinetic model. Biochemistry. 1986 Apr 22;25(8):2155–2161. doi: 10.1021/bi00356a046. [DOI] [PubMed] [Google Scholar]
- Nir S., Klappe K., Hoekstra D. Mass action analysis of kinetics and extent of fusion between Sendai virus and phospholipid vesicles. Biochemistry. 1986 Dec 16;25(25):8261–8266. doi: 10.1021/bi00373a020. [DOI] [PubMed] [Google Scholar]
- Nir S., Stegmann T., Wilschut J. Fusion of influenza virus with cardiolipin liposomes at low pH: mass action analysis of kinetics and extent. Biochemistry. 1986 Jan 14;25(1):257–266. doi: 10.1021/bi00349a036. [DOI] [PubMed] [Google Scholar]
- Pedroso de Lima M. C., Nir S., Flasher D., Klappe K., Hoekstra D., Düzgüneş N. Fusion of Sendai virus with human HL-60 and CEM cells: different kinetics of fusion for two isolates. Biochim Biophys Acta. 1991 Dec 9;1070(2):446–454. doi: 10.1016/0005-2736(91)90085-m. [DOI] [PubMed] [Google Scholar]
- Pedroso de Lima M. C., Ramalho-Santos J., Martins M. F., Pato de Carvalho A., Bairos V., Nir S. Kinetic modeling of Sendai virus fusion with PC-12 cells. Effect of pH and temperature on fusion and viral inactivation. Eur J Biochem. 1992 Apr 1;205(1):181–186. doi: 10.1111/j.1432-1033.1992.tb16766.x. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos J., Nir S., Düzgünes N., de Carvalho A. P., de Lima M. da C. A common mechanism for influenza virus fusion activity and inactivation. Biochemistry. 1993 Mar 23;32(11):2771–2779. doi: 10.1021/bi00062a006. [DOI] [PubMed] [Google Scholar]
- Rott R. Molecular basis of infectivity and pathogenicity of myxovirus. Brief review. Arch Virol. 1979;59(4):285–298. doi: 10.1007/BF01317469. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Nir S., Wilschut J. Membrane fusion activity of influenza virus. Effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry. 1989 Feb 21;28(4):1698–1704. doi: 10.1021/bi00430a041. [DOI] [PubMed] [Google Scholar]
- Tsao Y. S., Huang L. Kinetic studies of Sendai virus-target membrane interactions: independent analysis of binding and fusion. Biochemistry. 1986 Jul 1;25(13):3971–3976. doi: 10.1021/bi00361a035. [DOI] [PubMed] [Google Scholar]
- Warner T. G., O'Brien J. S. Synthesis of 2'-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochemistry. 1979 Jun 26;18(13):2783–2787. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- Wunderli-Allenspach H., Günthert M., Ott S. Inactivation of PR8 influenza virus through the octadecylrhodamine B chloride membrane marker. Biochemistry. 1993 Jan 26;32(3):900–907. doi: 10.1021/bi00054a022. [DOI] [PubMed] [Google Scholar]
- Wunderli-Allenspach H., Günthert M., Ott S. Temperature-dependent kinetics of the activities of influenza virus. J Struct Biol. 1990 Jul-Sep;104(1-3):63–69. doi: 10.1016/1047-8477(90)90058-k. [DOI] [PubMed] [Google Scholar]
- Wunderli-Allenspach H., Ott S. Kinetics of fusion and lipid transfer between virus receptor containing liposomes and influenza viruses as measured with the octadecylrhodamine B chloride assay. Biochemistry. 1990 Feb 27;29(8):1990–1997. doi: 10.1021/bi00460a005. [DOI] [PubMed] [Google Scholar]


