Abstract
Objective
The aim of this study was to report the interim 5-year safety and effectiveness of abatacept in patients with JIA in the PRINTO/PRCSG registry.
Methods
The Abatacept JIA Registry (NCT01357668) is an ongoing observational study of children with JIA receiving abatacept; enrolment started in January 2013. Clinical sites enrolled patients with JIA starting or currently receiving abatacept. Eligible patients were assessed for safety (primary end point) and effectiveness over 10 years. Effectiveness was measured by clinical 10-joint Juvenile Arthritis Disease Activity Score (cJADAS10) in patients with JIA over 5 years. As-observed analysis is presented according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Results
As of 31 March 2020, 587 patients were enrolled; 569 are included in this analysis (including 134 new users) with 1214.6 patient-years of safety data available. Over 5 years, the incidence rate (IR) per 100 patient-years of follow-up of serious adverse events was 5.52 (95% CI: 4.27, 7.01) and of events of special interest was 3.62 (95% CI: 2.63, 4.86), with 18 serious infections [IR 1.48 (95% CI: 0.88, 2.34)]. As early as month 3, 55.9% of patients achieved cJADAS10 low disease activity and inactive disease (20.3%, 72/354 and 35.6%, 126/354, respectively), sustained over 5 years. Disease activity measures improvement over 5 years across JIA categories.
Conclusion
Abatacept was well tolerated in patients with JIA, with no new safety signals identified and with well-controlled disease activity, including some patients achieving inactive disease or remission.
Trial registration
Clinicaltrials.gov, NCT01357668.
Keywords: adolescent rheumatology, biologic therapies, DMARDs, juvenile idiopathic arthritis, paediatric/juvenile rheumatology
Rheumatology key messages.
In this registry of patients with JIA, abatacept was well tolerated.
Abatacept treatment resulted in rapid, clinically important, and sustained effectiveness across JIA categories.
New onset of other autoimmune diseases was rare in patients receiving/having been exposed to abatacept.
Introduction
JIA describes a heterogeneous group of paediatric rheumatic diseases of unknown aetiology presenting in children <16 years of age [1]. JIA is a major cause of acquired disability in children [2], with reported prevalence in developed countries ranging from 16 to 150 cases per 100 000 people [1].
For active, polyarticular-course JIA (pcJIA; any JIA category with ≥5 affected joints, except systemic JIA) [1], the ACR recommends initial treatment with a conventional synthetic DMARD, such as MTX. For patients with an inadequate response/intolerance to MTX, treatment with a biologic DMARD, such as a TNF inhibitor (TNFi), anti–IL-6, or T cell costimulation modulator, is recommended [3–5].
Abatacept is a selective T cell costimulation modulator with a unique mechanism of action that works upstream of other currently available treatments for rheumatic diseases [6]. Abatacept is available in i.v. and s.c. formulations and is effective for the treatment of moderate-to-severe active pcJIA [6–13]. The efficacy and safety of i.v. and s.c. abatacept in patients with JIA with an inadequate response to DMARDs (including TNFi) has been demonstrated in two phase 3 studies [7, 9]. A post-marketing registry of patients receiving i.v. or s.c. abatacept treatment for JIA (Abatacept JIA Registry) has been established by the Paediatric Rheumatology International Trial Organisation (PRINTO) [14] and the Pediatric Rheumatology Collaborative Study Group (PRCSG) [15] to monitor the long-term safety and effectiveness of abatacept in the paediatric population [7–11, 13, 15].
Here, we report the longitudinal 5-year safety and effectiveness of abatacept in patients with JIA in a routine clinical setting, using data from the PRINTO and PRCSG Abatacept JIA Registry.
Methods
Study design
The Abatacept JIA Registry is an ongoing, multicentre, observational study of children with JIA receiving or initiating abatacept, regardless of its formulation (ClinicalTrials.gov identifier: NCT01357668). The primary objective of the registry is to describe the long-term safety of abatacept for the treatment of JIA in routine clinical practice by quantifying the incidence rates (IRs) of serious infections, autoimmune disorders, and malignancies.
Using a standardized protocol, 70 clinical sites across 23 countries in the PRINTO and PRCSG networks enrol patients with JIA into this longitudinal registry. Planned follow-up visits are scheduled every 3 months for year 1, every 6 months for years 2–5, and annually for years 6–10. After >4 months of receiving abatacept, patients remain in the registry even if abatacept is discontinued. Patients >18 years old continue to be followed in the registry. Enrolment in the registry started in January 2013; here, we report the data collected up to 31 March 2020 (up to 5 years of follow-up).
PRINTO, PRCSG, and sponsor roles
The Abatacept JIA Registry is a post-marketing requirement of Bristol Myers Squibb by the United States Food and Drug Administration and is part of a post-marketing commitment with the European Medicines Agency. The registry has been conducted by the PRINTO and PRCSG in an ethical and scientific manner independent of Bristol Myers Squibb, to whom annual reports are provided.
The registry was designed by PRINTO and PRCSG officers (D.J.L., H.I.B., A.M. and N.R.) in collaboration with Bristol Myers Squibb. Data were collected by the PRINTO and PRCSG networks, who have full responsibility for the data analysis and submission of the manuscript. Bristol Myers Squibb was provided with annual aggregated data in table, figure and text format; no individual patient data have been provided to the company except for anonymous data for pregnancies and serious adverse events (SAEs).
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki [16], the International Conference on Harmonisation Guidelines for Good Clinical Practice, and local regulations. The institutional review board of the Cincinnati Children’s Hospital Medical Center approved the study and the patient informed consent form prior to initiation of the study. At each site, an institutional review board or independent ethics committee approved the protocol. Written consent and, as per local requirements, assent was obtained from parents/legal guardians, and patients, respectively.
Patients and analysis population
At enrolment, eligible patients had a JIA diagnosis in any ILAR category [17], were aged <18 years and were taking or initiating abatacept as per the treating physician’s decision. Patients who participated in the i.v. or s.c. abatacept trials [7, 9] and patients from routine clinical care could be enrolled in the registry.
Data collection
Data were collected prospectively during routine clinic visits and entered into two secure online platforms (one for each network) with the same structure; data were then combined for this analysis. The PRINTO platform follows the same structure as Pharmachild [18, 19]. Previous use of DMARDs was recorded retrospectively. During registry visits, patients’ medical records were reviewed for safety events and complete drug exposure. Safety data were collected at each visit and after abatacept was discontinued, regardless of the reason. Effectiveness data were collected prospectively.
End points and assessments
Safety
The primary objective of the registry analysis is to describe the long-term safety of abatacept treatment in patients with JIA. SAEs and events of special interest [ESIs; defined as serious and targeted infections, autoimmune disorders (including new or worsening uveitis), IBD, malignancies, and pregnancies] were reported and coded using the latest release of the Medical Dictionary for Regulatory Activities (MedDRA; version 21.0). Targeted infections of interest included EBV, CMV, papilloma virus, primary and reactivation of herpes zoster, tuberculosis, and opportunistic infections. Exacerbations or new occurrences of autoimmune disorders were recorded; all malignancies were followed. The IRs per 100 patient-years of follow-up of SAEs, serious and targeted infections, autoimmune disorders, and malignancies were determined. Temporary and permanent discontinuations of abatacept due to adverse events (AEs) were analysed and the IRs were calculated. Patients were also tested for thyroid antibodies and anti-abatacept antibodies. Immunogenicity was evaluated based on the proportion of patients who developed anti-abatacept antibodies (to CTLA-4 and possibly Ig, or to Ig and/or junction region).
Effectiveness
Effectiveness was evaluated using the clinical 10-joint Juvenile Arthritis Disease Activity Score (cJADAS10); data were collected using both the patient and parent proxy ratings of patient well-being. Validated cJADAS10 cut-offs for oligoarticular JIA and pcJIA were used to evaluate high disease activity (>12 and >16, respectively), moderate disease activity (4.1–12 and 5.1–16, respectively), low disease activity (LDA; 1.2–4 and 2.6–5, respectively), inactive disease (ID; ≤1.1 and ≤2.5, respectively), and clinical remission (ID for ≥6 continuous months); all other JIA subtypes were evaluated using the pcJIA cJADAS cut-offs [20]. Disease activity using cJADAS10 was evaluated at baseline, every 3 months until year 1, and then every 6 months until year 5.
Another effectiveness measure was the Physician Global Assessment of Disease Activity (PGA; 21-circle visual analogue scale 0–10 cm) [21]. The degree of disease activity as measured by the PGA was categorized as follows: mild disease activity (<4), moderate disease activity (4 to <7), and severe disease activity (≥7). The numbers of swollen joints, painful joints, joints with limited range of movement, and those with active arthritis (defined as a joint with swelling or, in the absence of swelling, loss of motion with either pain on motion or tenderness) were also recorded in support of treatment effectiveness.
Statistical analysis
Data have been reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [22, 23]. Descriptive statistics for continuous variables included the number of observations and either mean (S.D.) or median [quartile (Q)1, Q3) values. Safety data are presented as IRs per 100 patient-years of follow-up. Patient follow-up duration was defined as the time between the registration date in the registry and the event, last follow-up, death, or data-lock for the report (whichever occurred first). IRs were calculated for SAEs, serious and targeted infections, autoimmune disorders, and malignancies and were estimated by dividing the number of incident cases (first occurrence of that outcome in each patient) by the patient-time at risk. Temporary and permanent discontinuations of abatacept due to AEs were analysed and the IRs were calculated. CIs, assuming Poisson distribution of the rates, were calculated for each IR estimate.
For efficacy, we measured changes and frequencies of effectiveness outcomes over time. The potential differences in effectiveness among JIA categories were also explored. The patient cohort enrolled in the registry ≤1 month after initiating abatacept treatment was categorized as ‘new users‘. Data were analysed as observed and per intention-to-treat (ITT), and included all patients (the overall registry population and the new user cohort) [24] enrolled in the registry, irrespective of study participation duration. The ITT analysis used non-responder imputation, in which patients who discontinued from the study for any reason were considered non-responders from that point onward. The last-observation-carried-forward method was used for missing data on patients still followed in the study.
Results
Patient population
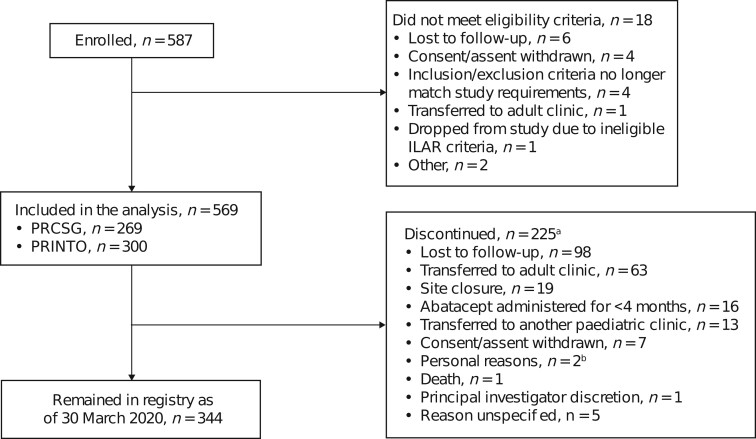
This 5-year interim analysis included data collected up to 31 March 2020, with a median follow-up time of 1.9 (Q1, Q3: 0.8, 3.3) years (Table 1) and an enrolment of 587 patients with JIA. We excluded 18 patients who did not meet the eligibility criteria (Fig. 1). Of the 569 patients included in the analysis, 409 (71.9%) and 158 (27.8%) patients received or continued to receive i.v. or s.c. abatacept, respectively.
Table 1.
Overall populationa, baseline demographic data and disease characteristics in the overall population
| Parameter | Overall patients (N = 569) | New abatacept users (n = 134) | Continuous abatacept users (n = 435) | P values (new vs continuing users) |
|---|---|---|---|---|
| Duration of abatacept therapy at enrolment, years, median (Q1, Q3) | 0.5 (0.1, 1.5) | 0.0 (0.0, 0.0) | 0.9 (0.4, 2.0) | <0.0001h |
| Patients with prior abatacept useb, n (%) | 493 (86.6)c | 58 (43.3) | 435 (100.0) | |
| Patients already receiving abatacept at baselined, n (%) | 567 (99.6)e | 134 (100.0) | 433 (99.5) | |
| Categories of overall observation time, years, n (%) | 0.0002i | |||
| ≤1 | 172 (30.2) | 57 (42.5) | 115 (26.4) | |
| >1 to ≤2 | 137 (24.1) | 36 (26.9) | 101 (23.2) | |
| >2 to ≤7 | 259 (45.5) | 41 (30.6) | 218 (50.1) | |
| >7 to 10 | 1 (0.2) | 0 0 | 1 (0.2) | |
| Overall observation, years, median (Q1, Q3) | 1.9 (0.8, 3.3) | 1.2 (0.5, 2.1) | 2.0 (1.0, 3.7) | <0.0001h |
| Patient-years of observation in study | 1214.6 | 204.7 | 1009.9 | |
| Baseline characteristics | ||||
| Age at enrolment, years, median (Q1, Q3) | 13.6 (10.7, 16.0) | 13.1 (10.2, 16.3) | 13.6 (10.9, 15.9) | 0.5818h |
| Age group at enrolment, years, n (%) | 0.0135i | |||
| 2–5f | 21 (3.7) | 9 (6.7) | 12 (2.8) | |
| 6–12 | 223 (39.2) | 57 (42.5) | 166 (38.2) | |
| 13–18 | 313 (55.0) | 68 (50.7) | 245 (56.3) | |
| >18 | 12 (2.1) | 0 (0.0) | 12 (2.8) | |
| Female, n (%) | 454 (79.8) | 110 (82.1) | 344 (79.1) | |
| Disease duration at enrolment, years, mean (S.D.) | 5.5 (3.8) | 5.0 (4.0) | 5.7 (3.8) | 0.0334h |
| Active joints (ACR definition), median (Q1, Q3) | 1.0 (0.0, 3.0) | 3.0 (1.0, 8.0) | 0.0 (0.0, 2.0) | <0.0001h |
| Joints with a limited range of movement, median (Q1, Q3) | 1.0 (0.0, 4.0) | 2.0 (1.0, 10.0) | 0.0 (0.0, 2.0) | <0.0001h |
| Physician Global Assessment of Disease Activity, VAS 0–10, median (Q1, Q3) | 1.0 (0.0, 3.0) | 3.8 (2.0, 5.0) | 1.0 (0.0, 2.0) | <0.0001h |
| Uveitis, n (%) | ||||
| History of uveitis (past and present) | 77 (13.5) | 24 (17.9) | 53 (12.2) | |
| Active uveitis at time of enrolment | 29 (5.1) | 14 (10.5) | 15 (3.5) | |
| JIA categories, n (%) | 0.0008i | |||
| Polyarticular RF− | 289 (50.8) | 62 (46.3) | 227 (52.2) | |
| Oligoarticular | 134 (23.6) | 40 (29.9) | 94 (21.6) | |
| Polyarticular RF+ | 54 (9.5) | 12 (9.0) | 42 (9.7) | |
| Undifferentiated | 33 (5.8) | 3 (2.2) | 30 (6.9) | |
| PsA | 26 (4.6) | 4 (3.0) | 22 (5.1) | |
| Enthesitis-related | 20 (3.5) | 12 (9.0) | 8 (1.8) | |
| Systemic | 13 (2.3) | 1 (0.8) | 12 (2.8) | |
| JIA-related medication (prior to and/or at baseline), n (%) | 523/558 (93.7) | 122/131 (93.1) | 401/427 (93.9) | |
| MTX | 523/558 (93.7) | 122/131 (93.1) | 401/427 (93.9) | |
| NSAIDs | 249/558 (44.6) | 46/131 (35.1) | 203/427 (47.5) | |
| Systemic steroids | 41/558 (7.4) | 6/131 (4.6) | 35/427 (8.2) | |
| LEF | 57/558 (10.2) | 10/131 (7.6) | 47/427 (11.0) | |
| SSZ | 37/558 (6.6) | 8/131 (6.1) | 29/427 (6.8) | |
| Adalimumabg | 224/558 (40.1) | 77/131 (58.8) | 147/427 (34.4) | |
| Etanercept | 267/558 (47.9) | 67/131 (51.2) | 200/427 (46.8) |
Patient-time of follow-up stratified by duration of abatacept treatment at registry entry.
For prior abatacept use, any patient whose first abatacept dose was on or after baseline was included.
Two patients took abatacept prior to baseline visit but were not on abatacept at baseline.
For patients already receiving abatacept at baseline, any patient taking abatacept at baseline where it was not the first dose was included.
All 569 patients had used abatacept prior to baseline, but two patients were not taking abatacept at the baseline visit.
Data are from real-world settings, as treatment was administered at the physician’s discretion.
One patient with PsA was receiving abatacept and adalimumab simultaneously.
Mann–Whitney U test.
Fisher’s exact test.
Q1: first quartile; Q3: third quartile; VAS: visual analogue scale.
Figure 1.
Patient disposition flow chart. aCategories are mutually exclusive. bPersonal reasons include married without notification and moved out of state and site closure (n = 1) and daughter going to college (n = 1). PRCSG: Pediatric Rheumatology Collaborative Study Group
At the baseline visit, 567/569 (99.6%) were already being treated with abatacept for a median (Q1, Q3) of 6.5 (1.3, 17.8) months, and 134/569 (23.6%) had been treated with abatacept for ≤1 month; the cJADAS10 (parent) at baseline was 5.5 (484/569). The total patient time on abatacept treatment at baseline was 622.0 patient-years, and the total observation time was 1214.6 patient-years (Table 1). Overall, 225/587 (38.3%) patients discontinued the study (Fig. 1), with the most frequent reason being loss to follow-up (98/225, 43.6%) or transfer to adult/other paediatric clinics (76/225, 33.8%).
As summarized in Table 1, most patients were female (79.8%) and had polyarticular RF-negative (RF−; 50.8%) or oligoarticular JIA (23.6%) at baseline. Uveitis (past or present history) at baseline was reported in 77 (13.5%) patients, with 29 (5.1%) patients having active uveitis at baseline. Concomitant medications related to JIA were taken by 93.7% (523/558) of patients at baseline, including MTX (523/558, 93.7%), LEF (57/558, 10.2%), systemic CSs (41/558, 7.4%) and NSAIDs (249/558, 44.6%). A total of 561 patients took prior medication before the baseline time-point; prior use of the biologic DMARDs etanercept, adalimumab, infliximab and golimumab was reported for 47.6% (267/561), 39.9% (224/561), 7.0% (39/561) and 1.3% (7/561) of patients, respectively.
Safety
There were 67 (11.8%) patients who had at least one SAE; the IR (95% CI) was 5.52 (4.27, 7.01) (Table 2 and Supplementary Table S1, available at Rheumatology online). Of the 67 reported SAEs, uveitis, migraine, nephrolithiasis, infusion-related reaction, diabetic ketoacidosis, arthralgia, arthritis, depression, and major depression were most commonly reported (≥2 events each). There were 16 (2.8%) patients with ≥1 treatment-related SAE [IR 1.32 (95% CI: 0.75, 2.14)], the most common of which was infusion-related reaction (n = 3). One patient had an AE resulting in death [IR 0.08 (95% CI: 0.00, 0.46)], due to a cardiac arrest related to pre-existing disease (cardiovascular event); the treating physician deemed it unrelated to abatacept treatment.
Table 2.
Safety summary (SAEs and ESIs) over a 5-year follow-up period in the overall population
| System organ class MedDRA preferred term | Patients, n (N = 569) | Incidence rate a | 95% CI |
|---|---|---|---|
| Patients with ≥1 SAE, n (%) | 67 (11.8) | 5.52 | 4.27, 7.01 |
| Patients with ≥1 treatment-related SAE, n (%) | 16 (2.8) | 1.32 | 0.75, 2.14 |
| Blood and lymphatic system disorders | 1 | 0.08 | 0.00, 0.46 |
| Neutropenia | 1 | 0.08 | 0.00, 0.46 |
| Immune system disorders | 1 | 0.08 | 0.00, 0.46 |
| Anaphylactic shock | 1 | 0.08 | 0.00, 0.46 |
| Infections and infestations | 2 | 0.16 | 0.02, 0.59 |
| Respiratory tract infection | 1 | 0.08 | 0.00, 0.46 |
| Sinusitis | 1 | 0.08 | 0.00, 0.46 |
| Injury, poisoning, and procedural complications | 5 | 0.41 | 0.13, 0.96 |
| Cartilage injury | 1 | 0.08 | 0.00, 0.46 |
| Infusion-related reaction | 3 | 0.25 | 0.05, 0.72 |
| Ligament injury | 1 | 0.08 | 0.00, 0.46 |
| Musculoskeletal and CTDs | 1 | 0.08 | 0.00, 0.46 |
| Arthralgia | 1 | 0.08 | 0.00, 0.46 |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 | 0.08 | 0.00, 0.46 |
| Medulloblastoma | 1 | 0.08 | 0.00, 0.46 |
| Nervous system disorders | 3 | 0.25 | 0.05, 0.72 |
| Demyelination | 1 | 0.08 | 0.00, 0.46 |
| Migraine | 1 | 0.08 | 0.00, 0.46 |
| Optic neuritis | 1 | 0.08 | 0.00, 0.46 |
| Seizure | 1 | 0.08 | 0.00, 0.46 |
| Reproductive system and breast disorders | 1 | 0.08 | 0.00, 0.46 |
| Perineal ulceration | 1 | 0.08 | 0.00, 0.46 |
| Skin and s.c. tissue disorders | 1 | 0.08 | 0.00, 0.46 |
| Psoriasis | 1 | 0.08 | 0.00,0.46 |
| Patients with ≥1 SAE causing permanent discontinuation, n (%) | 9 (1.6) | 0.74 | 0.34, 1.41 |
| Gastrointestinal disorders | 1 | 0.08 | 0.00, 0.46 |
| IBD | 1 | 0.08 | 0.00, 0.46 |
| General disorders and administration-site conditions | 1 | 0.08 | 0.00, 0.46 |
| Malaise | 1 | 0.08 | 0.00, 0.46 |
| Immune system disorders | 1 | 0.08 | 0.00, 0.46 |
| Anaphylactic shock | 1 | 0.08 | 0.00, 0.46 |
| Infections and infestations | 2 | 0.16 | 0.02, 0.59 |
| Respiratory tract infection | 1 | 0.08 | 0.00, 0.46 |
| Sinusitis | 1 | 0.08 | 0.00, 0.46 |
| Injury, poisoning, and procedural complications | 2 | 0.16 | 0.02, 0.59 |
| Infusion-related reaction | 2 | 0.16 | 0.02, 0.59 |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 | 0.08 | 0.00, 0.46 |
| Medulloblastoma | 1 | 0.08 | 0.00, 0.46 |
| Skin and s.c. tissue disorders | 1 | 0.08 | 0.00, 0.46 |
| Psoriasis | 1 | 0.08 | 0.00, 0.46 |
| Patients with SAE resulting in death b , n (%) | 1 (0.2) | 0.08 | 0.00, 0.46 |
| Patients with at least 1 ESI, n (%) | 44 (7.7) | 3.62 | 2.63, 4.86 |
| Serious/targeted infections | 18 | 1.48 | 0.88, 2.34 |
| Skin and s.c. tissue disorders | 8 | 0.66 | 0.28, 1.30 |
| Eye disorders | 3 | 0.25 | 0.05, 0.72 |
| Infusion-related reaction | 2 | 0.16 | 0.02, 0.59 |
| Demyelination | 1 | 0.08 | 0.00, 0.46 |
| IBD | 1 | 0.08 | 0.00, 0.46 |
| Investigations | 1 | 0.08 | 0.00, 0.46 |
| Neoplasm (medulloblastoma) | 1 | 0.08 | 0.00, 0.46 |
| Neoplasm (ovarian adenoma) | 1 | 0.08 | 0.00, 0.46 |
| Neutropenia | 1 | 0.08 | 0.00, 0.46 |
| Optic neuritis | 1 | 0.08 | 0.00, 0.46 |
| Other autoimmune diseasesc | 8 | 0.66 | 0.28, 1.30 |
| Eye disorders | 6 | 0.49 | 0.18, 1.08 |
| Musculoskeletal and CTDs | 1 | 0.08 | 0.00, 0.46 |
| Skin and s.c. tissue disorders | 1 | 0.08 | 0.00, 0.46 |
Patients with >1 SAE in a category are counted only once in each preferred term category, and only once in each system organ class category.
Incidence rate reported per 100 person-years of follow-up. MedDRA was used to code SAEs.
Due to a cardiovascular event related to pre-existing disease and assessed as unrelated to abatacept treatment.
Repetition of some SAEs as other autoimmune diseases occurred as a result of clinician data input.
ESI: event of special interest; MedDRA: Medical Dictionary for Regulatory Activities; SAE: serious adverse event.
There were 44 (7.7%) patients with ≥1 ESI [IR 3.62 (95% CI: 2.63, 4.86)] during the 1214.61 person-years of study observation (Table 2) and an IR of 4.60 (95% CI: 3.34, 6.17) during the 956.95 person-years of abatacept treatment. Of these, 18 serious infections (3.2%) were reported [IR 1.48 (95% CI: 0.88, 2.34)]; 8 (1.4%) patients developed ≥1 other autoimmune disease [eye disorders: IR 0.49 (95% CI: 0.18, 1.08), musculoskeletal and CTDs: IR 0.08 (95% CI: 0.00, 0.46), and skin and s.c. tissue disorders: IR 0.08 (95% CI: 0.00, 0.46)]. There were two cases of new or worsening uveitis during the study period [IR 0.16 (95% CI: 0.02, 0.59); Supplementary Table S1, available at Rheumatology online]. One SAE of IBD was reported [IR 0.08 (95% CI 0.00, 0.46)], resulting in permanent abatacept discontinuation (Table 2). No active tuberculosis cases were reported in the study over 5 years; 1 malignancy occurred (medulloblastoma). Permanent discontinuation of abatacept was reported in 9 (1.6%) patients [IR 0.74 (95% CI 0.34, 1.41)] due to IBD, malaise, anaphylactic shock, respiratory tract infection, sinusitis, medulloblastoma, psoriasis (all n = 1), and infusion-related reaction (n = 2).
A total of 200 patients were tested for anti-abatacept antibodies (35.2% of 569 patients enrolled); 11 (5.5%) patients were positive. The patients testing positive for anti-abatacept antibodies did not develop new-onset autoimmune events. Further, 244 patients were tested for anti-thyroid antibodies (42.9% of 569 patients enrolled), of which 25 (10.2%) patients had abnormal laboratory results, including 10 with known thyroid-related comorbid conditions.
Effectiveness
Overall population
The cJADAS10s for up to 5 years of follow-up are shown in Fig. 2. The median cJADAS10s (parent) were 5.5 at baseline, 2.0 at month 24, and 3.3 at month 60 (Table 3). As early as month 3, cJADAS10 LDA and ID were achieved by 20.3% (72/354) and 35.6% (126/354) of patients, respectively, and increased or were sustained overall over time in the as-observed population (Fig. 3). In the ITT analysis, changes in the median cJADAS10 scores (Fig. 2) were very similar to the as-observed changes. Although the proportion of patients demonstrating LDA, ID, and remission were lower for the ITT analysis, the overall patterns of response were similar to the as-observed results (Fig. 3).
Figure 2.
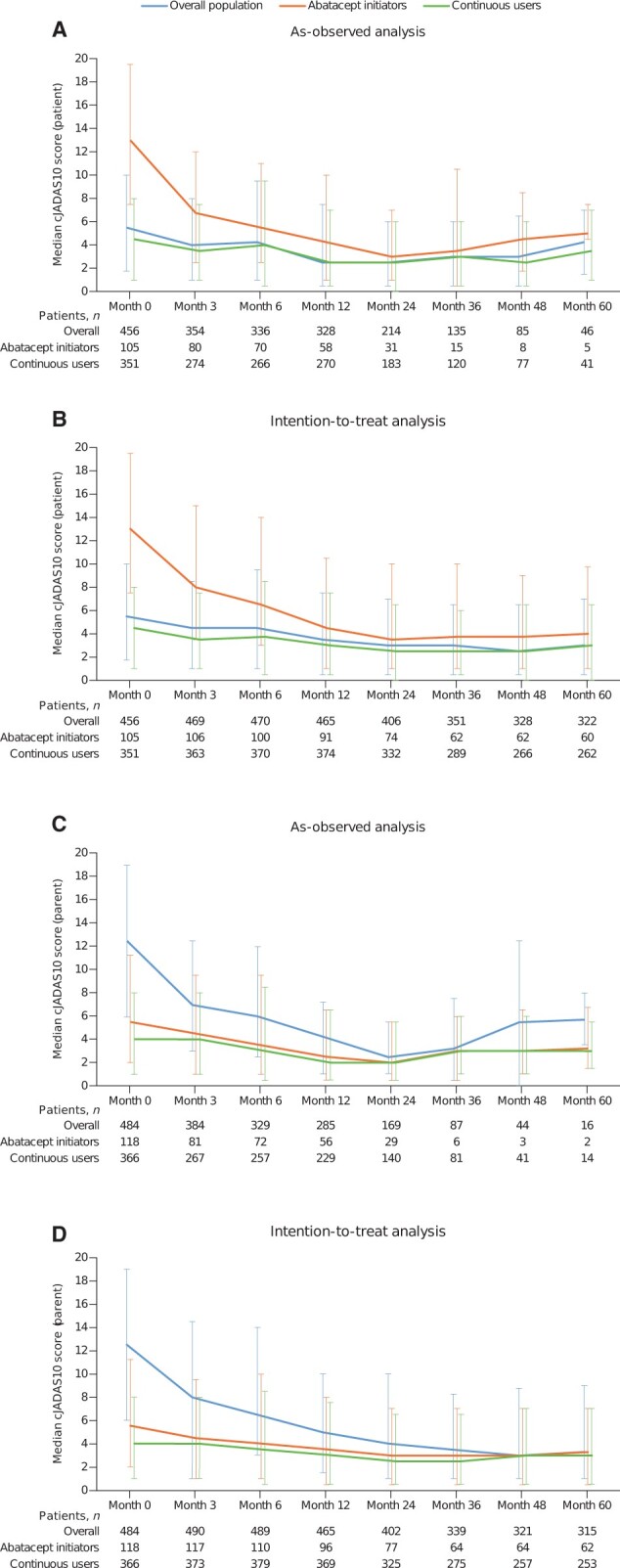
Patient-reported (A, B) and parent-reported (C, D) cJADAS10 scores over 5-year follow-up. For oligoarticular arthritis, the following cut-offs were used: ID: ≤1.1, LDA: 1.2–4, MDA: 4.1–12, and HDA: >12. For polyarticular arthritis and other JIA subtypes, the following cut-offs were used: ID: ≤2.5, LDA: 2.6–5, MDA: 5.1–16, and HDA: >16. cJADAS10: clinical 10-joint Juvenile Arthritis Disease Activity Score; continuous users: cohort of patients who enrolled in the registry >1 month after initiating abatacept treatment; HDA: high disease activity; ID: inactive disease; LDA: low disease activity; MDA: moderate disease activity; new users/initiators: cohort of patients who enrolled in the registry ≤1 month after initiating abatacept treatment
Table 3.
Effectiveness over 5 years in patients enrolled in the registry by JIA category (as-observed analysis)
| Parameter | Visit |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | 24 months | 36 months | 48 months | 60 months | |
| Overall patients | ||||||||
| n | 569 | 448 (78.7%) | 421 (73.9%) | 387 (68.0%) | 274 (48.2%) | 154 (27.1%) | 99 (17.4%) | 50 (8.8%) |
| PGA (cm) | 1.0 (0.0, 3.0) | 1.0 (0.0, 2.5) | 1.0 (0.0, 2.5) | 0.5 (0.0, 2.0) | 0.5 (0.0, 2.0) | 0.5 (0.0, 1.0) | 0.0 (0.0, 1.5) | 1.0 (0.0, 2.0) |
| cJADAS10 (parent) | n = 484 | n = 348 | n = 329 | n = 285 | n = 169 | n = 87 | n = 44 | n = 16 |
| 5.5 (2.0, 11.3) | 4.5 (1.0, 9.5) | 3.5 (1.0, 9.5) | 2.5 (0.5, 6.5) | 2.0 (0.5, 5.5) | 3.0 (0.5, 6.0) | 3.0 (1.0, 6.5) | 3.3 (1.5, 6.8) | |
| No. of active joints (0–71) | 1.0 (0.0, 3.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) |
| No. of joints with LOM | 1.0 (0.0, 4.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 4.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) |
| Categories of polyarticular disease courses in patients with JIA | ||||||||
| Polyarticular RF− JIA | ||||||||
| n | 289 | 235 | 222 | 206 | 152 | 86 | 54 | 34 |
| PGA (cm) | 1.5 (0.5, 3.5) | 1.0 (0.0, 2.5) | 1.0 (0.0, 3.0) | 0.5 (0.0, 2.0) | 0.5 (0.0, 1.8) | 0.5 (0.0, 1.0) | 0.5 (0.0, 1.5) | 1.0 (0.0, 2.0) |
| cJADAS10 (parent) | n = 247 | n = 186 | n = 178 | n = 149 | n = 95 | n = 46 | n = 24 | n = 10 |
| 6.0 (2.0, 13.0) | 5.0 (1.0, 10.5) | 4.0 (1.5, 10.5) | 3.0 (0.5, 6.5) | 2.5 (0.5, 5.5) | 3.3 (1.0, 6.5) | 3.8 (1.0, 6.5) | 4.8 (1.5, 9.0) | |
| No. of active joints (0–71) | 1.0 (0.0, 4.0) | 0.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 2.0) |
| No. of joints with LOM | 1.0 (0.0, 6.0) | 0.0 (0.0, 4.0) | 1.0 (0.0, 5.0) | 0.0 (0.0, 4.0) | 0.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | 0.0 (0.0, 3.0) | 0.5 (0.0, 2.0) |
| Oligoarticular JIA | ||||||||
| n | 134 | 111 | 102 | 87 | 63 | 35 | 22 | 9 |
| PGA (cm) | 1.0 (0.0, 3.0) | 0.5 (0.0, 2.0) | 0.8 (0.0, 2.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.5 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.5) |
| cJADAS10 (parent) | n = 116 | n = 87 | n = 80 | n = 74 | n = 43 | n = 24 | n = 14 | n = 5 |
| 4.0 (1.0, 8.5) | 3.0 (0.5, 7.5) | 3.0 (0.5, 7.5) | 1.3 (0.0, 5.0) | 2.0 (0.0, 5.0) | 3.0 (0.1, 5.3) | 2.3 (0.0, 7.0) | 3.0 (2.5, 3.0) | |
| No. of active joints (0–71) | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) |
| No. of joints with LOM | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.0) |
| Polyarticular RF+ JIA | ||||||||
| n | 54 | 36 | 37 | 37 | 24 | 15 | 9 | 2 |
| PGA (cm) | 1.0 (0.0, 3.0) | 0.5 (0.0, 2.0) | 1.0 (0.0, 2.0) | 0.5 (0.0, 2.0) | 0.8 (0.0, 2.0) | 1.0 (0.0, 2.5) | 0.0 (0.0, 1.5) | 0.3 (0.0, 0.5) |
| cJADAS10 (parent) | n = 43 | n = 25 | n = 31 | n = 22 | n = 9 | n = 6 | n = 1 | n = 0 |
| 5.0 (1.0, 9.0) | 2.0 (0.5, 5.5) | 3.0 (0.5, 11.0) | 1.5 (0.5, 6.0) | 5.5 (1.0, 7.0) | 0.8 (0.0, 3.0) | 0.0 (0.0, 0.0) | – | |
| No. of active joints (0–71) | 0.0 (0.0, 3.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 3.5) | 1.0 (0.0, 5.0) | 0.0 (0.0, 1.0) | 0.5 (0.0, 1.0) |
| No. of joints with LOM | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 3.5) | 0.0 (0.0, 6.0) | 0.0 (0.0, 2.0) | 15.0 (0.0, 30.0) |
| Enthesitis-related JIA | ||||||||
| n | 20 | 15 | 15 | 13 | 6 | 3 | 3 | 0 |
| PGA (cm) | 2.8 (1.0, 3.8) | 1.0 (0.5, 2.5) | 1.5 (0.0, 2.5) | 1.0 (0.0, 1.5) | 0.5 (0.0, 1.0) | 0.0 (0.0, 0.5) | 0.0 (0.0, 0.5) | – |
| cJADAS10 (parent) | n = 15 | n = 10 | n = 11 | n = 9 | n = 5 | n = 2 | n = 1 | n = 0 |
| 9.0 (5.0, 17.0) | 5.3 (3.0, 10.0) | 6.0 (3.0, 10.5) | 5.0 (1.0, 9.0) | 2.5 (1.5, 4.0) | 3.8 (3.5, 4.0) | 2.0 (2.0, 2.0) | – | |
| No. of active joints (0–71) | 1.5 (0.5, 3.5) | 1.0 (0.0, 2.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 0.0) | – |
| No. of joints with LOM | 1.0 (0.0, 4.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 4.0) | 0.0 (0.0, 6.0) | 0.0 (0.0, 5.0) | – |
| Psoriatic JIA | ||||||||
| n | 26 | 21 | 18 | 16 | 10 | 7 | 6 | 2 |
| PGA (cm) | 0.5 (0.0, 2.0) | 0.5 (0.0, 1.5) | 0.5 (0.0, 1.0) | 0.8 (0.0, 1.5) | 0.0 (0.0, 0.5) | 0.0 (0.0, 1.0) | 0.0 (0.0, 2.5) | 0.3 (0.0, 0.5) |
| cJADAS10 (parent) | n = 23 | n = 16 | n = 13 | n = 13 | n = 5 | n = 4 | n = 4 | n = 1 |
| 4.0 (2.0, 8.0) | 4.8 (2.3, 5.8) | 3.5 (1.5, 5.5) | 2.5 (1.0, 6.0) | 1.0 (1.0, 1.0) | 2.8 (2.0, 3.8) | 2.8 (1.8, 5.5) | 1.5 (1.5, 1.5) | |
| No. of active joints (0–71) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 1.5) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) |
| No. of joints with LOM | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.5 (0.0, 1.0) | 0.5 (0.0, 1.0) |
| Systemic JIA | ||||||||
| n | 13 | 9 | 10 | 7 | 6 | 3 | 1 | 1 |
| PGA (cm) | 2.5 (1.0, 4.0) | 3.0 (2.0, 3.0) | 3.0 (1.5, 4.0) | 2.0 (2.0, 3.0) | 1.0 (0.5, 3.0) | 3.0 (0.0, 6.0) | 0.0 (0.0, 0.0) | 4.0 (4.0, 4.0) |
| cJADAS10 (parent) | n = 11 | n = 7 | n = 5 | n = 6 | n = 4 | n = 2 | n = 0 | n = 0 |
| 10.0 (2.0, 18.0) | 13.0 (6.5, 18.0) | 9.5 (8.5, 11.0) | 8.0 (3.5, 20.5) | 5.3 (0.8, 10.8) | 12.5 (0.0, 25.0) | – | – | |
| No. of active joints (0–71) | 2.0 (0.0, 6.0) | 10.0 (1.0, 10.0) | 3.5 (1.0, 11.0) | 2.0 (0.0, 11.0) | 0.0 (0.0, 2.0) | 10.0 (0.0, 26.0) | 0.0 (0.0, 0.0) | 11.0 (11.0, 11.0) |
| No. of joints with LOM | 2.0 (0.0, 23.0) | 8.0 (1.0, 12.0) | 2.5 (1.0, 17.0) | 3.0 (0.0, 23.0) | 11.5 (1.0, 19.0) | 2.0 (0.0, 8.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Undifferentiated JIA | ||||||||
| n | 33 | 21 | 17 | 21 | 13 | 5 | 4 | 2 |
| PGA (cm) | 1.0 (0.0, 5.5) | 1.5 (0.5, 3.5) | 1.0 (0.0, 5.0) | 0.5 (0.0, 2.0) | 0.5 (0.0, 4.0) | 0.0 (0.0, 1.0) | 0.5 (0.0, 3.0) | 1.3 (0.0, 2.5) |
| cJADAS10 (parent) | n = 29 | n = 17 | n = 11 | n = 12 | n = 8 | n = 3 | n = 0 | n = 0 |
| 7.0 (2.0, 16.0) | 7.5 (4.5, 10.5) | 10.0 (0.5, 15.5) | 4.5 (0.3, 14.8) | 4.8 (1.5, 14.0) | 0.0 (0.0, 7.0) | – | – | |
| No. of active joints (0–71) | 0.0 (0.0, 7.0) | 1.0 (0.0, 2.0) | 0.0 (0.0, 5.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 3.5) | 1.0 (1.0, 1.0) |
| No. of joints with LOM | 1.0 (0.0, 7.0) | 1.0 (0.0, 3.0) | 4.0 (1.0, 8.0) | 2.0 (0.0, 5.0) | 1.0 (0.0, 4.0) | 0.0 (0.0, 0.0) | 1.5 (0.0, 6.0) | 1.0 (1.0, 1.0) |
Data are median (Q1, Q3) unless otherwise stated.
cJADAS10: clinical 10-joint Juvenile Arthritis Disease Activity Score; LOM: loss of motion; PGA: Physician Global Assessment of Disease Activity (0–10 VAS); Q1: first quartile; Q3: third quartile; VAS: visual analogue scale.
Figure 3.
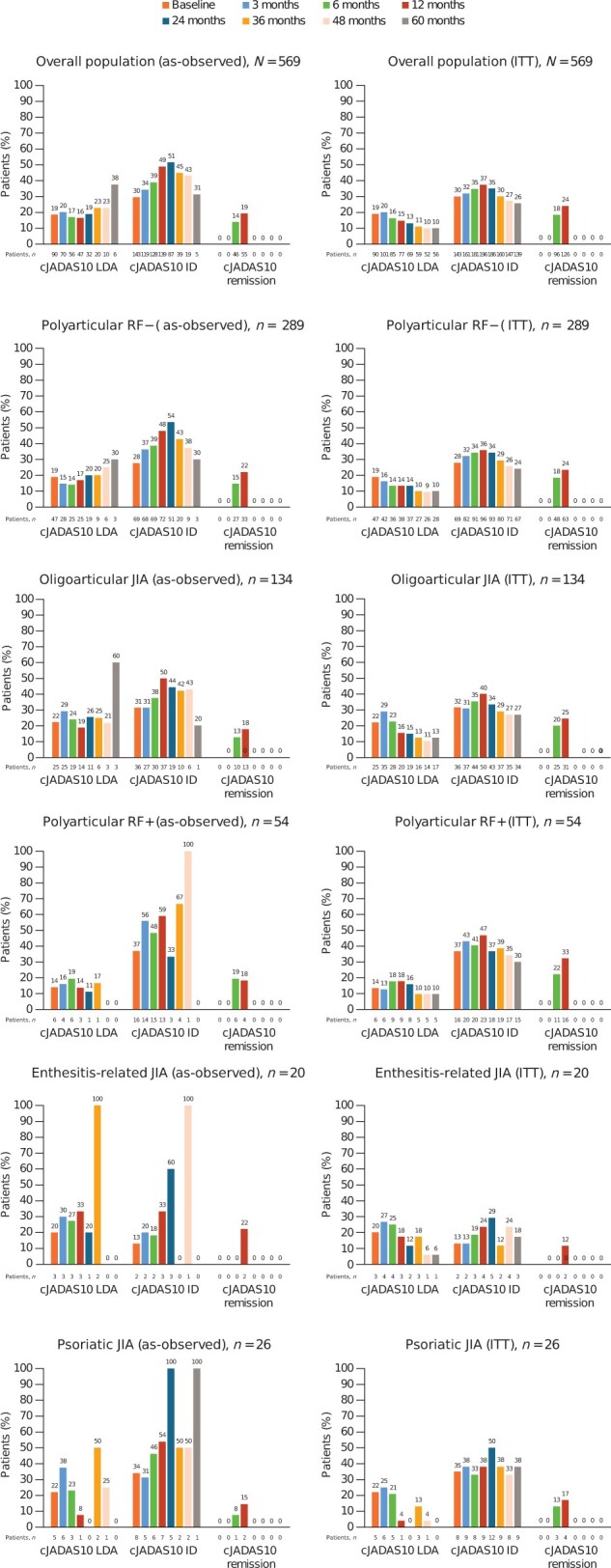
Proportions of patients enrolled in the registry who achieved cJADAS10 (parent) LDA, ID and remission over 5 years by JIA category (as-observed and ITT analyses). cJADAS10: clinical 10-joint Juvenile Arthritis Disease Activity Score; ID: inactive disease; ITT: intention-to-treat; LDA: low disease activity
Median disease activity as rated by the PGA was sustained over time (Table 3). Over the course of the study, an increasing proportion of patients achieved a mild disease state as per PGA (79.4% at baseline, 92.3% at year 2, and 96.0% at year 5); a decreasing proportion had moderate (15.3% at baseline, 5.1% at year 2, and 4.0% at year 5) and severe disease (5.3% at baseline, 2.6% at year 2, and 0% at year 5). The median number of active joints and joints with limited range of movement improved numerically (decreased) over time (Table 3).
New users and continuous users
In the observed analysis of new abatacept users (abatacept use for ≤1 month at time of enrolment), the median cJADAS10 (parent) decreased markedly, from 12.5 at baseline to 2.5 at month 24 and to 5.8 at month 60 (Supplementary Table S2, available at Rheumatology online). Across all JIA categories, PGA-rated disease activity improved over time, with the overall median value of 3.8 at baseline (n = 134), 0.3 at year 2 (n = 44), and 1.5 at year 5 (n = 5), as shown in Supplementary Tables S2 and S3, available at Rheumatology online; substantial improvements (differences of >2.5 and 3 points) were particularly noted in the pJIA and oligoarthritis JIA categories during the follow-up, respectively. Similar to the overall population, the proportion of patients with mild disease activity per PGA increased (50% at baseline to 90.9% at year 2; 100% at year 5); the proportion of patients with moderate disease decreased (36.6% at baseline to 4.6% at year 2 and 0% at year 5) and with severe disease decreased (13.4% at baseline to 4.6% at year 2, and 0% at year 5) during the follow-up across JIA categories. The median number of active joints and joints with limited range of movement generally decreased in the registry cohort and in all JIA categories over time (Supplementary Tables S2 and S3).
In the observed analysis of continuous users (those taking abatacept for >1 month at the time of enrolment), the median cJADAS10 scores (parent) were 4.0 at baseline, 2.0 at month 24, and 3.0 at month 60 (Supplementary Table S2, available at Rheumatology online). Disease activity (per PGA) was sustained or improved over time, particularly in the pcJIA and oligoarthritis JIA categories, with an overall median of 1.0 at baseline (n = 435), 0.5 at year 2 (n = 230), and 1.0 at year 5 (n = 45) (Supplementary Tables S2 and S4, available at Rheumatology online). The proportion of patients with mild disease activity per PGA increased (88.5% at baseline to 92.6% at year 2 and 95.6% at year 5); the proportion of patients with moderate disease decreased (8.7% at baseline to 5.2 at year 2 and 4.4% at year 5), and the proportion of patients with severe disease (2.8% at baseline to 2.2% at year 2 and 0% at year 5) decreased over 5 years across JIA categories.
Discussion
The analysis of up to 5 years of follow-up data from this phase 4 registry of abatacept in JIA showed that abatacept is well tolerated, with no new safety signals being identified, even with long-term exposure. Abatacept treatment resulted in sustained disease control, including ID and clinical remission in some patients, across pcJIA and oligoarticular JIA categories in the overall cohort. Rapid JIA improvement was observed in registry participants who entered the registry ≤1 month of commencing abatacept. Overall, these registry data support the safety profile of abatacept seen in clinical trials [7–10]. Results of the as-observed and ITT analyses were similar regarding the amount and rate of improvement in the cJADAS10 scores and patterns of response, although the response proportions were lower for the ITT analyses.
While in the registry, the rates of SAEs (5.52/100 patient-years) and ESIs (3.62/100 patient-years) were low. Treatment-related SAEs (1.32/100 patient-years) were rare; no new safety findings were observed since the last follow-up at 3.5 years, in which SAEs were reported in 23/354 (6%) patients [IR 4.48 (95% CI: 2.77, 6.85)] [25].
Data from this analysis are consistent with those from a long-term safety analysis of the German BIKER registry [26]. The BIKER registry covers the majority of patients with JIA treated with biologics in Germany since 2001 and includes 569 children treated with abatacept. In BIKER, the rate of SAEs for abatacept was 3.39/100 patient-years [26]. The highest rate of SAEs in the BIKER registry analysis was observed for infliximab, and the lowest for MTX. Our results were also consistent with Pharmachild, a large observational international study of combined registries published in 2018, wherein 5% of patients received abatacept with a median of 342 days of drug exposure; SAEs were reported in 7% of the entire registry patient population [19]. In the 7-year follow-up of the 10-year STRIVE registry of adalimumab in patients with pcJIA, 134 SAEs were reported with a rate of 7.2/100 patient-years [27]. A systematic review comparing the safety of biologics in JIA clinical trials concluded that abatacept seemed to have the most favourable safety profile compared with other biologic DMARDs [28].
In general, infections are of particular interest in children with JIA, as infections occur frequently in paediatric patients due to the population’s susceptibility. Infections are also associated with the use of synthetic and biologic DMARDs [19, 29–32]. In this analysis, infections and procedural complications were the most frequently reported classes of SAEs (Supplementary Table S1, available at Rheumatology online); however, serious and targeted infections were rare. Importantly, none of the infection-related SAEs were deemed treatment-related nor were a cause for discontinuation (Table 2). No cases of active tuberculosis infections were reported in this registry; notably, no such cases were reported in clinical trials of abatacept either [7–10]. We carefully monitored the occurrence of infections with abatacept use. Although direct comparisons between registries cannot be achieved due to differences in eligibility criteria and data collection, in other studies, numerically higher IRs for infection and serious infections have been reported for TNFis, compared with tocilizumab and abatacept [26].
As abatacept is a T cell co-stimulatory signal modulator, its safety in autoimmune diseases such as JIA is an important research question. In this study, autoimmune diseases, including, but not limited to, new or worsening uveitis, were reported infrequently (<1.5% of patients), similar to observations from other registries [26]. Rates for uveitis were low, suggesting a protective effect; for example, only two patients developed new or worsening uveitis as an ESI during the study. Recent reports from the BIKER registry reported that IRs of autoimmune diseases were higher with abatacept than with TNFi, with even lower rates reported for MTX [26]. Notably, the use of abatacept for the treatment of uveitis has not been extensively studied. Rates of IBD and malignancy reported in this registry population were similar to those reported in the BIKER analysis: while one case of IBD was reported in this 5-year follow-up analysis, no cases of IBD were reported in the long-term German BIKER registry [26]. Only one case of malignancy (medulloblastoma) was reported in this 5-year follow-up, whereas the BIKER registry reported no cases with abatacept and very few cases with TNFi [n = 5; IR per 100 patient-years 0.07 (95% CI 0.03, 0.17)] [26]. In the Pharmachild registry, overall for DMARDs, a neoplasm (either benign, malignant or unspecified) was reported in 16 (<1%) patients, with 10 additional cases of other neoplasms [19]. A notable finding of this study was the high proportion of patients with abnormal laboratory results for thyroid antibodies (10.2%). This proportion of patients with anti-thyroid antibody is higher than the 3% (15/499 patients) recently reported from the Inception Cohort of Newly diagnosed patients with JIA longitudinal and observational German study [33] and should be further examined in relation to abatacept treatment in future studies.
Although the primary objective of this study was the evaluation of safety in routine clinical use, the effectiveness of abatacept was also studied. In the clinical trial of abatacept (ClinicalTrials.gov identifier: NCT01357668), the largest improvement of JIA signs and symptoms occurred within 6 months of abatacept initiation [9]. Because the majority of participants in this study had received abatacept over a prolonged period before entering the registry, we did not expect further major improvement while they were in the registry. Indeed, in the overall cohort in the as-observed analysis, abatacept provided good disease control, with a trend towards decreased disease activity over time.
The cohort of patients who enrolled in the registry ≤1 month after initiating abatacept treatment (‘new users’) provides a more appropriate view of the real-time effectiveness of abatacept. Herein, we confirm, as found in the clinical trials [7–10], abatacept treatment results in rapid, clinically important, and sustained cJADAS10 responses across all JIA categories included in the registry. Lastly, we show both ITT and observed analyses and, as expected, effectiveness was numerically higher in the observed analyses. We would like to point out that ITT estimates shown herein are comparable with the results of the relevant clinical trials in JIA of shorter duration [7–10].
This analysis has important strengths and limitations that need to be considered. This is a global registry study with >500 participants comprising a heterogeneous population and a range of concomitant medications and comorbid diagnoses. This study also has a longitudinal observation period, enabling the comprehensive detection of safety signals. Further, data collection in the Abatacept JIA Registry was rigorous when compared with other registries such as BIKER, particularly as the JIA Registry included patients from 23 countries globally, and patients >18 years continued to be followed by PRINTO/PRCSG [26]. Overall, data from this registry complement those from previous registry reports and the clinical trials with more restricted populations and shorter periods of observation [9, 26]. Considering its registry nature, we also acknowledge several potential sources of bias in this study. Due to its registry design, there was no randomization in this study, which may have resulted in some degree of bias. An additional source of bias in reporting as-observed data in registries stems from the greater likelihood of patients who demonstrated a better response and tolerance to treatment to remain in the study, which would lead to an overly positive reporting of benefit and underestimation of the safety issues. To partially address this source of bias, we included an ITT analysis using non-responder imputation, in which patients who discontinued from the study for any reason were considered non-responders from that point onward. This ITT approach is overly conservative, as many patients who discontinued the study did so for reasons other than non-response, and thus the ITT analysis was biased in underrepresenting the actual response rates. Given the limited size of the registry, complete data on background rates of disease-related AEs and drug switching may not have been captured. The registry is thus far also limited by the duration of data collection (5 years), leading to difficulties in identifying rare safety signals. While results up to 3 years of follow-up in the registry provide robust estimates, only 569 patients in the overall population were followed-up for up to 5 years at the time of this interim analysis, but for a median of 22.3 months. Hence, effectiveness estimates after 5 years must be interpreted with caution due to the small number of patients available for evaluation at each follow-up time point. Finally, about 40% of patients included in the analysis discontinued the study mainly due to loss to follow-up (17.2%) and transfer to an adult clinic (11.1%).
In conclusion, in this longitudinal registry, abatacept was well tolerated, with no new safety risks being identified. The analysis population demonstrated well-controlled JIA with LDA throughout the course of the study. Abatacept treatment resulted in rapid, clinically important, and sustained effectiveness responses in all JIA categories with polyarticular or oligoarticular disease course, with some patients achieving ID and remission status.
Supplementary Material
Acknowledgements
We thank the patients and all the investigators who participated in the study. We are grateful to Lisa Clark, Jennifer O’Shaughnessy, and Najima Mwase from PRCSG and Chiara Pallotti, Silvia Scala, Luca Villa, and all research assistants from PRINTO and PRCSG who have overseen the collection of data, merger of databases, and analysis of the data. We would also like to thank the PRCSG and PRINTO staff at the coordinating centres for their dedication and support in managing the data and data analysis. We are indebted to them for their research regulatory support. A full list of PRCSG and PRINTO investigators can be found in Supplementary Data S1 and S2, available at Rheumatology online. We are also grateful to Robert Wong and Doug Kou, both of Bristol Myers Squibb, for helpful discussions. We thank Ngoc-Bao Duong from the Clinical Research Unit at the Necker Hospital, Paris, France. Professional medical writing and editorial assistance was provided by Katerina Kumpan, PhD, and Candice Dcosta, MSc, at Caudex, and was funded by Bristol Myers Squibb under the supervision of PRINTO and PRCSG officers (D.J.L., H.I.B., A.M. and N.R.).
Contributor Information
Daniel J Lovell, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH, USA.
Nikolay Tzaribachev, PRI Research, Bad Bramstedt, Germany.
Michael Henrickson, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH, USA.
Gabriele Simonini, IRCCS Meyer Children’s Hospital, Rheumatology Unit, ERN-ReCONNECT Center, Florence, Italy.
Thomas A Griffin, Atrium Health, Levine Children’s Hospital, Charlotte, NC, USA.
Ekaterina Alexeeva, Department of Rheumatology, National Medical Research Center of Children’s Health, Moscow, Russia; Sechenov First Moscow State Medical University, Moscow, Russia.
John F Bohnsack, Division of Allergy, Immunology and Pediatric Rheumatology, University of Utah, Salt Lake City, UT, USA.
Andrew Zeft, Center for Pediatric Rheumatology and Immunology, Cleveland Clinic, Cleveland, OH, USA.
Gerd Horneff, Asklepios Clinic Sankt Augustin, Sankt Augustin, Germany; Department of Pediatric and Adolescent Medicine, Medical Faculty, University Hospital of Cologne, Cologne, Germany.
Richard K Vehe, Department of Pediatrics, Division of Pediatric Rheumatology, University of Minnesota, Minneapolis, MN, USA.
Valda Staņēviča, Riga Stradins University, Riga, Latvia.
Stacey Tarvin, Riley Hospital for Children at Indiana University, Indianapolis, IN, USA.
Maria Trachana, Aristotle University of Thessaloniki, Thessaloníki, Greece.
Ana Quintero del Río, University of Oklahoma Health Science Center, Oklahoma City, OK, USA.
Adam M Huber, IWK Health Centre and Dalhousie University, Halifax, NS, Canada.
Daniel Kietz, Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA.
Ilonka Orbán, National Institute of Locomotor Diseases and Disabilities, Budapest, Hungary.
Jason Dare, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Ivan Foeldvari, Hamburg Centre for Pediatric and Adolescent Rheumatology, Hamburg, Germany.
Pierre Quartier, Necker-Enfants Malades University Hospital, Assistance Publique-Hopitaux de Paris, Paris, France; Université Paris-Cité, Paris, France.
Alyssa Dominique, Bristol Myers Squibb, Princeton, NJ, USA.
Teresa A Simon, Bristol Myers Squibb, Princeton, NJ, USA.
Alberto Martini, Dipartimento di Neuroscienze, Riabilitazione, Oftalmologia, Genetica e Scienze Materno-Infantili (DiNOGMI), Università degli Studi di Genova, Genova, Italy.
Hermine I Brunner, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH, USA.
Nicolino Ruperto, IRCCS Istituto Giannina Gaslini, UOSID Centro Trial—PRINTO, Genova, Italy.
for PRINTO and the Pediatric Rheumatology Collaborative Study Group (PRCSG)§:
Jurgen Brunner, Taciana Fernandes, Simone Appenzeller, Sheila Oliveira, Maria Teresa Terreri, Nikolay Tzaribachev, Kirsten Minden, Mark Hufnagel, Ivan Foeldvari, Gerd Horneff, Astrid Helling-Bakki, Troels Herlin, Estefania Moreno, Jordi Anton, Pablo Mesa- del-Castillo, Clara Udaondo, Inmaculada Calvo Penades, Pierre Quartier, Karine Brochard, Athimalaipet Ramanan, Maria Trachana, Ilonka Orban, Philip (Pinchas) Hashkes, Nicolino Ruperto, Gabriele Simonini, Alma Nunzia Olivieri, Francesco Zulian, Davide Montin, Diego Peroni, Valda Stanevicha, Gabriel Vega Cornejo, Nico Wulffraat, Sylvia Kamphuis, Maria Eliana Paz Gastanaga, Tatiana Miraval, Filipa Oliveira-Ramos, Calin Lazar, Irina Nikishina, Ekaterina Alexeeva, Aleksej Sarychev, Vyacheslav Chasnyk, Lyudmila Grebenkina, Wafaa Mohammed Saad Suwairi, Elena Koskova, Mahmood Ally, Ingrid Louw, Johannes Breedt, Hermine Brunner, Tracy Ting, Janalee Taylor, Jennifer Huggins, Michael Henrickson, Esi Morgan DeWitt, Alexei Grom, Daniel Lovell, Grant Schulert, Jackeline Rodriguez-Smith, Jason Dare, Paula Morris, Sukesh Sukumarain, Marissa Klein Gitelman, Michael Miller, Megan Curran, Risa Alperin, Kaveh Ardalan, Deirdre De Ranieri, Megan Hiskey, Brian Nolan, Beth Chalom, Andy Zelf, Steven Spalding, Denise Costanzo, Robert Rennebohm, Brenda Waugaman, Elizabeth Brodus, Angela Robinson, Sirada Panupattanapong, Dan Kietz, Margalit Rosenkranz, Elaine Cassidy, Kathryn Torok, Dan Kingsbury, Victoria Cartwright, Andrew Lasky, Diane Brown, Andreas Reiff, Bracha Shaham, Katherine Marzan, Linda Wagner-Weiner, Karen Onel, Melissa Tesher, Cuoghi Edens, Terry Moore, Reema Syed, Peri Pepmueller, Paul Tuttle, Austin Dalrymple, Srikanth Barhula, Lance Feller, Mara Horwitz, Matt Justice, James Nocton, Judyann Olson, Calvin Williams, James Versbsy, Dominic Co, Elizabeth Roth-Wojcicki, Colleen Correll, Richard Vehe, Bryce Binstadt, Patricia Hobday, Danielle Brueck, Tom Griffin, Miriah Gillispie-Taylor, Sheetal Vora, Stacey Tarvin, Kathleen O'Neil, Susan Ballinger, Michael Blakley, Thomas Klausmeier, Melissa Oliver, Brandi Stevens, Martha Rodriguez, Ellen Go, John Bohnsack, Christi Inman, Aimee Hersh, Sara Stern, Amy Woodward, Debbie Durkee, Sylvie Fadrhonc Boulva, Karen James, Erin Treemarcki, Donald Goldsmith, Svetlana Lvovich, Dana Toib, Julisa Patel, Rita Jerath, Nirupma Sharma, Lauren Newhall, Ruy Carrasco, Nandini Moorthy, Alexis Boneparth, Ana Quintero, Thomas Graham, Stephanie Spence, Alaina Davis, Alisa Gotte, Jay Mehta, Heather Walters, Zanab Mian, Elizabeth Parkinson, Joyce Hui-Yen, Katherine Steigerwald, Marla Guzman, Beth Gottlieb, Ana Quintero, Connie Whitaker, Leslie Kelly, Ruy Carrasco, Rosie Succimarri, Elizabeth Hazel, Gaelle Chedeville, Sarah Compillo, Claire LeBlance, Lori Tucker, David Cabral, Kristin Houghton, Jamie Guzman, Kim Morishita, Adam Huber, Elizabeth Stringer, Suzanne Ramsey, Bianca Lang, Deborah Levy, Earl Silverman, Heinrike Schmeling, Nicole Johnson, Nadia Luca, and Muhammed Dhalla
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Request to use PRINTO data should be directed to the network at printo@gaslini.org and to use PRCSG data, at prcsg@cchmc.org. Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Contribution statement
The author contributions to the study are as follows: study design and conception: D.J.L., H.I.B., N.R., A.M.; data collection, analysis and interpretation: D.J.L., A.M.H., H.I.B., N.R., A.M., S.T., T.A.S.; manuscript drafting and revision: D.J.L., N.T., M.H., G.S., T.A.G., E.A., J.F.B., A.Z., G.H., R.K.V., V.S., S.T., M.T., A.Q.R., A.M.H., D.K., I.O., J.D., I.F., P.Q., A.D., T.A.S., A.M., H.I.B., N.R..
Funding
This study was supported by Bristol Myers Squibb, who provided support to all participating study sites, in addition to the PRINTO and PRCSG coordinating centers. Data were submitted by PRINTO and PRCSG. Data submitted by PRINTO are owned by PRINTO.
Disclosure statement: D.J.L.’s institution, the Cincinnati Children’s Hospital Medical Center, has received research grants from Bristol Myers Squibb, Janssen, Novartis, Pfizer, Roche, and UBC; has received consulting fees or other remuneration from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Roche, Takeda, and UBC for the work of D.J.L; D.J.L. has received speakers bureau fees from Novartis and Roche, and is a member of data safety and monitoring boards for the National Institutes of Health and the Canadian Arthritis Society. G.S. has received speaker fees/honoraria from AbbVie, Novartis, and SOBI. E.A.’s institution, the National Medical Research Center of Children’s Health, has received research grants from AbbVie, Amgen, Bristol Myers Squibb, Centocor, Eli Lilly, MSD, Novartis, Pfizer, Roche, and Sanofi; E.A. has received speakers bureau fees from AbbVie, Bristol Myers Squibb, MSD, Novartis, Pfizer, and Roche. J.F.B.’s institution, the University of Utah, has received research grants from AbbVie, Bristol Myers Squibb, HyQvia, Janssen, Pfizer, and Roche for research performed by J.F.B. A.Z. owns stock in Athersys, Lixte Biotherapeutics, Merck, and Teva. G.H. has received grant/research support from AbbVie, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, and Roche; and speakers bureau fees from AbbVie, Bayer, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. S.T. has received research grants/support from the Arthritis Foundation, Pfizer, Roche, and UBC; consulting fees from the American Academy of Pediatrics. J.D. has received grant/research support from AbbVie, Bristol Myers Squibb, Pfizer, Roche, and UBC; and is an employee of and shareholder in Centene Corporation. I.F. is on an advisory board for Lilly, MEDAC, Novartis, and Pfizer. P.Q. has received consulting/speaker fees from AbbVie, Bristol Myers Squibb, Chugai-Roche, Eli Lilly, Novartis, Novimmune, Pfizer, and Swedish Orphan Biovitrum; and has participated in a data safety monitoring board for Sanofi. A.D. is a shareholder in Bristol Myers Squibb. T.A.S. was an employee of Bristol Myers Squibb at the time of the analysis. A.M. has received consultancy fees from Amgen, Biogen, Boehringer Ingelheim, Cerecor, Idorsia, Janssen, Merck Serono, Novartis, and Pfizer. H.I.B. has received grant/research support from Bristol Myers Squibb, Genentech, and Pfizer; speakers bureau fees from Genentech, GlaxoSmithKline, Novartis, Pfizer, and Roche; paid instructor fees from Novartis, Pfizer (funds go to CCHMC/employer); consultant fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Merck, EMD Serono, Idorsia, Novartis, Eli Lilly, Pfizer, Roche/Genentech, UBC (funds go to CCHMC/employer), and Sanofi; and grant/research support from Bristol Myers Squibb, Pfizer (funds go to CCHMC/employer). N.R. has received honoraria for consultancy/speakers bureau from the following pharmaceutical companies in the past 3 years: Ablynx, Amgen, AstraZeneca-Medimmune, Aurinia, Bayer, Bristol Myers Squibb, Cambridge Healthcare Research (CHR), Celgene, Domain therapeutic, Eli Lilly, EMD Serono, GlaxoSmithKline, Idorsia, Janssen, Novartis, Pfizer, Sobi, and UBC. The remaining authors have declared no conflicts of interest.
References
- 1. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 2. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet 2011;377:2138–49. [DOI] [PubMed] [Google Scholar]
- 3. Ringold S, Angeles‐Han ST, Beukelman T et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non‐systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res (Hoboken) 2019;71:717–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lovell DJ, Ruperto N, Giannini EH, Martini A. Advances from clinical trials in juvenile idiopathic arthritis. Nat Rev Rheumatol 2013;9:557–63. [DOI] [PubMed] [Google Scholar]
- 5. Ruperto N, Martini A. Current and future perspectives in the management of juvenile idiopathic arthritis. Lancet Child Adolesc Health 2018;2:360–70. [DOI] [PubMed] [Google Scholar]
- 6. Brunner HI, Wong R, Nys M et al. ; Paediatric Rheumatology International Trials Organisation (PRINTO) and the Pediatric Rheumatology Collaborative Study Group (PRCSG). Abatacept: a Review of the Treatment of Polyarticular-Course Juvenile Idiopathic Arthritis. Paediatr Drugs 2020;22:653–72. [DOI] [PubMed] [Google Scholar]
- 7. Brunner HI, Tzaribachev N, Vega‐Cornejo G et al. ; Paediatric Rheumatology International Trials Organisation (PRINTO) and the Pediatric Rheumatology Collaborative Study Group (PRCSG). Subcutaneous abatacept in patients with polyarticular‐course juvenile idiopathic arthritis: results from a phase III open‐label study. Arthritis Rheumatol 2018;70:1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lovell D, Ruperto N, Mouy R et al. ; Pediatric Rheumatology Collaborative Study Group and the Paediatric Rheumatology International Trials Organisation. Long-term safety, efficacy, and quality of life in patients with juvenile idiopathic arthritis treated with intravenous abatacept for up to seven years. Arthritis Rheumatol 2015;67:2759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruperto N, Lovell DJ, Quartier P et al. ; Pediatric Rheumatology Collaborative Study Group. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet 2008;372:383–91. [DOI] [PubMed] [Google Scholar]
- 10. Ruperto N, Lovell DJ, Quartier P et al. ; Paediatric Rheumatology International Trials Organization and the Pediatric Rheumatology Collaborative Study Group. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum 2010;62:1792–802. [DOI] [PubMed] [Google Scholar]
- 11. Brunner HI, Tzaribachev N, Cornejo GV et al. ; Pediatric Rheumatology Collaborative Study Group and the Paediatric Rheumatology International Trials Organisation. Maintenance of antibody response to diphtheria/tetanus vaccine in patients aged 2-5 years with polyarticular-course juvenile idiopathic arthritis receiving subcutaneous abatacept. Pediatr Rheumatol Online J 2020;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruperto N, Brunner HI, Tzaribachev N et al. ; Pediatric Rheumatology Collaborative Study Group (PRCSG) and the Paediatric Rheumatology International Trials Organisation (PRINTO). Absence of association between abatacept exposure and initial infection in patients with juvenile idiopathic arthritis. J Rheumatol 2021;48:1073–81. [DOI] [PubMed] [Google Scholar]
- 13. Ruperto N, Lovell DJ, Li T et al. ; Pediatric Rheumatology Collaborative Study Group (PRCSG). Abatacept improves health-related quality of life, pain, sleep quality, and daily participation in subjects with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2010;62:1542–51. [DOI] [PubMed] [Google Scholar]
- 14. Ruperto N, Martini A. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child 2011;96:596–601. [DOI] [PubMed] [Google Scholar]
- 15. Brunner HI, Rider LG, Kingsbury DJ et al. ; PRCSG Advisory Council. Pediatric Rheumatology Collaborative Study Group—over four decades of pivotal clinical drug research in pediatric rheumatology. Pediatr Rheumatol Online J 2018;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–6. [PubMed] [Google Scholar]
- 17. Petty RE, Southwood TR, Manners P et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 18. Giancane G, Swart JF, Castagnola E et al. ; for the Paediatric Rheumatology International Trials Organisation (PRINTO). Opportunistic infections in immunosuppressed patients with juvenile idiopathic arthritis: analysis by the Pharmachild Safety Adjudication Committee. Arthritis Res Ther 2020;22:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swart J, Giancane G, Horneff G et al. ; Paediatric Rheumatology International Trials Organisation (PRINTO), BiKeR and the board of the Swedish Registry. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther 2018;20:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Consolaro A, Negro G, Chiara Gallo M et al. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three‐variable juvenile arthritis disease activity score. Arthritis Care Res 2014;66:1703–9. [DOI] [PubMed] [Google Scholar]
- 21. Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N; Paediatric Rheumatology International Trials Organisation American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:929–36. [DOI] [PubMed] [Google Scholar]
- 22. Vandenbroucke JP, von Elm E, Altman DG et al. ; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Elm E, Altman DG, Egger M et al. ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol 2015;11:437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lovell D, Ruperto N, Tzaribachev N et al. Long-term effectiveness and safety of abatacept in juvenile idiopathic arthritis: interim results from the abatacept in JIA registry [abstract]. Arthritis Rheumatol 2017;69(Suppl 10):2272. [Google Scholar]
- 26. Klein A, Becker I, Minden K et al. Biologic therapies in polyarticular juvenile idiopathic arthritis. Comparison of long-term safety data from the German BIKER registry. ACR Open Rheumatol 2020;2:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brunner HI, Nanda K, Toth M et al. Safety and effectiveness of adalimumab in patients with polyarticular course of juvenile idiopathic arthritis: STRIVE registry 7-year interim results. Arthritis Care Res (Hoboken) 2020;72:1420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diener C, Horneff G. Comparison of adverse events of biologicals for treatment of juvenile idiopathic arthritis: a systematic review. Expert Opin Drug Saf 2019;18:719–32. [DOI] [PubMed] [Google Scholar]
- 29. Woodrick RS, Ruderman EM. Safety of biologic therapy in rheumatoid arthritis. Nat Rev Rheumatol 2011;7:639–52. [DOI] [PubMed] [Google Scholar]
- 30. Tarkiainen M, Tynjala P, Vahasalo P, Lahdenne P. Occurrence of adverse events in patients with JIA receiving biologic agents: long-term follow-up in a real-life setting. Rheumatology 2015;54:1170–6. [DOI] [PubMed] [Google Scholar]
- 31. Kalden JR, Schattenkirchner M, Sörensen H et al. The efficacy and safety of leflunomide in patients with active rheumatoid arthritis: a five-year followup study. Arthritis Rheum 2003;48:1513–20. [DOI] [PubMed] [Google Scholar]
- 32. Silverman E, Spiegel L, Hawkins D et al. Long-term open-label preliminary study of the safety and efficacy of leflunomide in patients with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 2005;52:554–62. [DOI] [PubMed] [Google Scholar]
- 33. Schulz C, Fuehner S, Schluter B et al. Prevalence of autoantibodies in patients with juvenile idiopathic arthritis: results from the German inception cohort ICON-JIA. Pediatr Rheumatol Online J 2022;20:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Request to use PRINTO data should be directed to the network at printo@gaslini.org and to use PRCSG data, at prcsg@cchmc.org. Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.