Abstract
Introduction
In the USA, up to 95% of individuals harbouring cancer-predisposing germline pathogenic variants have not been identified despite recommendations for screening at the primary care level.
Methods and analysis
Our primary objective is to use a two-arm, single-institution randomised controlled trial to compare the proportion of eligible patients that are recommended genetic testing for hereditary cancer syndromes using a digital tool versus clinician interview for genetic cancer risk assessment in an urban academic gynaecology clinic. New gynaecology patients will be consented and randomised 1:1 to either the intervention arm, in which a digital tool is used for genetic cancer risk assessment, or usual care, in which the clinician performs genetic cancer risk assessment. Individuals will be considered eligible for hereditary cancer syndrome genetic testing if criteria set forth by the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology are met. Eligible patients are 18 years or older, speak and read English, have not yet undergone hereditary cancer genetic testing and have access to a smartphone. The study aims to enrol 50 patients in each arm to allow for 80% power with two-tailed alpha of 5% to detect a 20% difference in proportion of eligible patients recommended for genetic testing. The primary outcome is the proportion of eligible individuals recommended genetic testing in the digital tool arm versus usual care arm, analysed using the χ2 or Fisher’s exact test as appropriate for sample size. The secondary outcome is completion of genetic testing, as well as exploration of patient factors, particularly social determinants of health, which may affect the receipt, utilisation and experience with genetic services.
Ethics and dissemination
This study has been approved by the Weill Cornell Institutional Review Board (Protocol No. 21-11024123). Participants will be informed of the benefits and risks of participation prior to consent. Dissemination of data will be deidentified and conducted through academic conferences and journals. Patients identified to be eligible for genetic testing who did not receive counselling from their providers will be contacted; participants will not receive direct notification of trial results.
Registration details
This trial is registered at clinicaltrials.gov (NCT05562778) in September 2022.
Protocol version
This is protocol version 1, as of 22 May 2024.
Countries of recruitment and recruitment status
USA, currently recruiting.
Health conditions/problems studied
Genetic predisposition to cancers such as breast, ovarian, uterine and pancreatic.
Deidentified individual clinical trial participant-level data (IDP) sharing statement
IDP will not be shared.
Trial registration number
Keywords: GENETICS, Gynaecological oncology, Cancer genetics, GYNAECOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Randomised controlled design and comparison to usual care allows for evaluation of the impact a digital risk stratification tool may have on increasing counselling for patients eligible for genetic testing.
Study site at a racially and ethnically diverse, Medicaid-predominant clinic with a goal of capturing populations that have been historically underserved regarding genetic care.
Broad inclusion criteria were used to optimise generalisability. However, inclusion criteria requiring English-speaking and reading patients and single study site limits generalisability of the tool in certain populations.
The focus of the study is to increase the number of patients receiving genetic cancer risk assessment, which is endorsed by national guidelines.
Introduction
Hereditary cancer syndromes, or the genetic predisposition to specific cancers caused by inherited germline pathogenic variants, cause an estimated 13% of cancers.1 Among individuals with hereditary cancer syndromes, measures to reduce cancer risk have been shown to decrease cancer incidence, morbidity and mortality.2,4 However, in the USA, as many as 95% of individuals harbouring cancer-predisposing germline pathogenic variants have not been identified despite recommendations for screening at the primary care level and thus do not receive counselling regarding standard recommended risk reducing measures.5 Further, under-recognition of cancer-predisposing pathogenic variants and a lack of receipt of genetic services is more pronounced among individuals identifying as racial or ethnic minorities or with public insurances.6,9
Collection and interpretation of family cancer history is a cornerstone of genetic cancer risk assessment to determine national guideline-based eligibility for genetic testing for hereditary cancer syndromes. The use of digital tools has been demonstrated to be more effective than usual clinician interview for the collection and interpretation of personal and family history, with high patient acceptance and satisfaction.10,13
We hypothesise that implementation of a risk assessment tool in a gynaecology clinic will improve receipt of appropriate genetic services. The primary objective of this randomised controlled trial is to compare the rate of recommendation for genetic testing for hereditary cancer syndromes among eligible patients when genetic cancer risk assessment is performed via a digital tool versus usual clinician interview.
Methods and analysis
Trial design
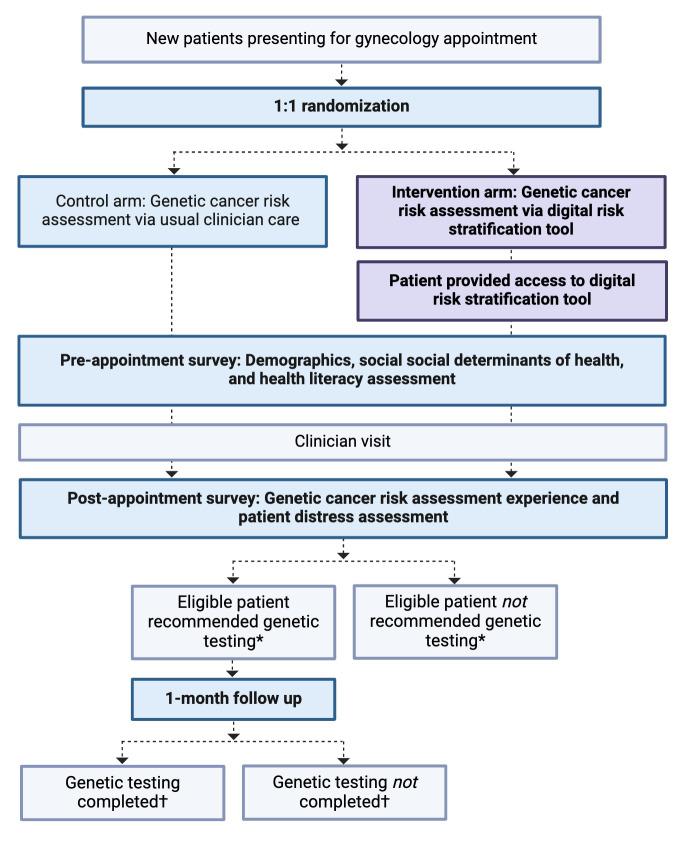
This single institution randomised controlled trial will compare the rates of recommendation for genetic testing among eligible patients to the standard of care (figure 1). The study will be conducted in an urban, academic gynaecology clinic in New York City, New York, which serves patients with Medicaid and other government-based insurance plans. A quality improvement initiative at this clinic site previously demonstrated a racially and ethnically diverse population.11 Patients will be screened and approached by study personnel for consent in the clinic waiting room prior to scheduled appointments. All patients scheduled for new appointments during periods of study personnel availability will be screened. Enrolled patients will be randomly assigned to either the intervention arm or control arm. Enrolment is anticipated to be conducted between January 2023 and December 2024. The trial is registered at clinicaltrials.gov (NCT05562778) in September 2022.
Figure 1. HeRITAGE (Health Risk Information Technology-Assisted Genetic Evaluation) study outline. Bolded lettering indicates areas of study intervention. *Primary outcome. †Secondary outcome.
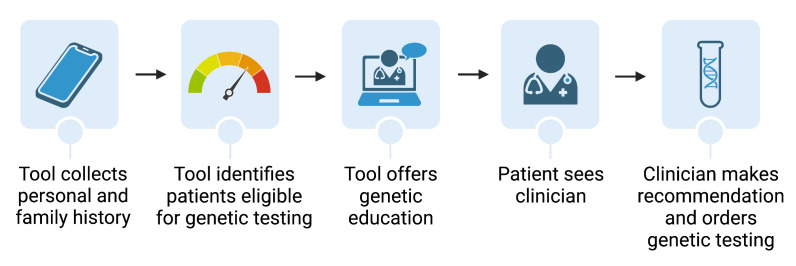
In the intervention arm, patients will be prompted to complete the digital tool, Ambry Genetics Comprehensive, Assessment, Risk, and Education (CARE) Program, in the waiting area prior to appointment (figure 2). CARE is a digital, patient-facing, risk stratification tool with complex, rule-based flow logic based on patient responses to pre-programmed input options designed to collect relevant personal and family history. Patient responses are evaluated against the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) for hereditary cancer testing.14 Patients who are unable to complete the tool in the waiting area prior to being called back to the examination room will be permitted to continue completion of the tool in the exam room. Completion of the tool is not mandated. Patients who complete the tool will be notified via the tool whether they met criteria for hereditary cancer testing, and clinicians will receive a CARE-generated clinical summary report denoting eligibility for genetic testing. CARE also provides optional educational videos on genetic testing to eligible patients.
Figure 2. HeRITAGE (Health Risk Information Technology-Assisted Genetic Evaluation) patient and provider workflow for patients meeting eligibility criteria and proceeding with genetic testing on day of appointment: Intervention arm. Created with BioRender.com.
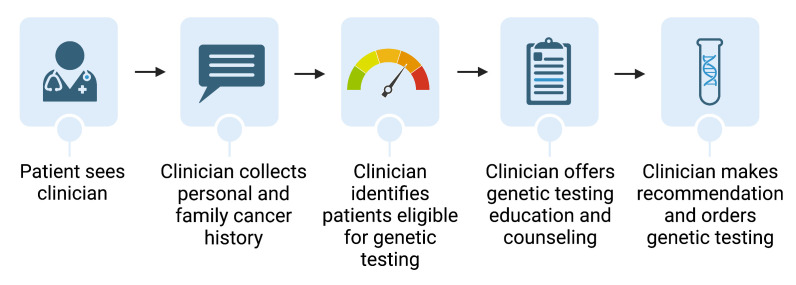
In the control arm, patients will undergo the usual clinic standard for new patients, in which genetic cancer risk assessment is performed via clinician-driven interview and assessment by their gynaecologic provider (figure 3).
Figure 3. HeRITAGE (Health Risk Information Technology-Assisted Genetic Evaluation) patient and provider workflow for patients meeting eligibility criteria and proceeding with genetic testing on day of appointment: Control arm. Created with BioRender.com.
At the clinicians’ discretion, patients in both arms can be offered multigene genetic testing for hereditary cancer syndromes at the time of their appointment. Genetic counselling and testing will be performed by the patients’ gynaecological providers, consistent with a ‘mainstreaming’ model that is the standard for the study site.
All patients’ personal and family history within the electronic medical records will be reviewed by study personnel to determine eligibility for genetic testing as per NCCN Guidelines. Patients determined by study personnel to meet NCCN Guidelines genetic testing criteria will be considered ‘eligible patients,’ which will serve as a denominator for the primary outcome (proportion of eligible patients recommended genetic testing) and the secondary outcome (proportion of eligible patients completing genetic testing).
All enrolled patients will be asked to complete paper pre-appointment and post-appointment surveys which are designed to facilitate understanding of patient characteristics and facilitate exploratory analysis. The pre-appointment survey will include questions regarding patient demographics, social determinants of health and health literacy. Social determinants of health will be assessed using several tools: the Health Leads Screening Toolkit,15 encompassing 10 yes/no questions designed to screen for items which contribute to adverse social determinants of health; the Healthcare Distrust Scale,16 a validated 9-item set that produces a numeric distrust score; and NCCN Guidelines Distress Management,17 in which patients answer yes/no questions regarding current stressors. Subjective health literacy will be assessed using the BRIEF Health Literacy Survey,18 a validated 4-item survey that produces a health literacy assessment of ‘inadequate,’ ‘marginal,’ or ‘adequate,’ as well as the Subjective Numeracy Scale,19 a validated 3-item survey that produces a numeric subjective numeracy score. The post-appointment survey will assess patients’ genetic cancer risk assessment experience and distress using the Hospital Anxiety and Depression Scale20 and NCCN Guidelines Distress Thermometer,17 in which patients provide a numeric 0–10 value correlating with subjective distress, as well as 5-point Likert scale items (strongly agree/agree/neither agree or disagree/disagree/strongly disagree) developed for the study to assess experience. Items include ‘I was satisfied with the genetic cancer assessment’ and ‘The genetic cancer assessment was a waste of time.’
Patients eligible for genetic testing will be contacted 1 month following their appointment to determine whether genetic testing for hereditary cancer syndromes was completed. All data will be entered into Research Electronic Data Capture (REDCap) by study personnel for the purpose of analysis.
Participants
Patients are eligible for enrolment if they meet the following criteria: (1) are scheduled for a new patient gynaecology appointment at the trial site clinic, (2) are 18 years or older, (3) speak and read English, (4) have not yet undergone genetic testing for predisposition to hereditary cancers and (5) have access to a phone with internet capability at the time of appointment. Patients not meeting the aforementioned criteria are excluded from the trial.
Endpoints
The primary objective is to evaluate the proportion of eligible patients recommended for hereditary cancer syndrome genetic testing when genetic cancer risk assessment is performed via a digital tool versus usual care with clinician interview. To determine the denominator of patients eligible for genetic testing, study personnel will review electronic medical records for personal and family history to determine eligibility per NCCN Guidelines. To determine the numerator of patients recommended for genetic testing, study personnel will review electronic medical record visit documentation.
The secondary objective is to compare the rates of completion of genetic testing within 1 month of the appointment among participants for whom genetic testing is recommended. Additionally, associations between patient factors, with focus on social determinants of health, and the receipt of genetic services, such as counselling and testing, will be explored. Assessment of patient experience with genetic cancer risk assessment in the intervention arm versus the control arm will also be conducted.
Sample size
Based on prior institutional experience and quality improvement investigations, we estimate less than 5% of patients would be eligible for genetic testing and recommended for hereditary cancer testing in our control group.21 An increase of at least 20% more eligible patients being recommended for genetic testing within the intervention arm would be considered clinically meaningful. Thus, enrolment is planned for a total of 100 participants, with 50 participants in each study arm, allowing for 80% power with two-tailed alpha of 5% to detect a difference in proportion of eligible patients recommended for genetic testing.
Randomisation and blinding
Participants will be randomised 1:1 to either the digital tool arm or usual care arm via a preset computer-generated randomisation scheme accessed through REDCap. After enrolment, patients are randomised and informed of their study arm. Clinicians are not blinded to enrolment arm as a tool-generated risk assessment summary is received for participants randomised to the intervention arm.
Statistical methods
The primary aim, evaluating the proportion of eligible individuals recommended genetic testing in the digital tool arm versus usual care arm, will be analysed using the χ2 test or Fisher’s exact test, as appropriate for sample size. The secondary aim, comparing the rates of uptake of genetic testing among participants for whom testing is recommended in the digital tool arm vs the usual care arm, will also be analysed with the χ2 test or Fisher’s exact test, as appropriate for sample size. Associations between risk assessment experience and utilisation of genetic services and participant characteristics will be explored with univariate tests as appropriate based on variable type and distribution (i.e., t-test, analysis of variance, Mann-Whitney U test, Kruskal-Wallis test). For all analyses, statistical significance will be evaluated at an alpha value of 0.05.
Patient and public involvement
Neither patients nor the public have been involved in the design or implementation of this study.
Ethics and dissemination
This study has been approved by the Weill Cornell Institutional Review Board (Institutional Review Board Protocol No. 21-11024123). Participants will be informed verbally and in writing of the benefits and risks of participation prior to consent. Risks include psychological risks as a result of cancer risk assessment and/or social risks such as possible invasion of privacy, breach of confidentiality and loss of community standing. Participants will be provided the opportunity to approve or deny whether their data are retained by the study team for use in future research as part of the consent process. All data will be deposited in REDCap. Dissemination of data will be deidentified and conducted through academic conferences and journals. Participants identified to be eligible for genetic testing who did not receive counselling from their providers will be contacted at the conclusion of their participation in the study. Participants will not receive direct notification of trial results. The authors report no conflicts of interest.
Discussion
The results of this study will provide data regarding the use of a digital tool for genetic cancer screening compared with usual clinician care. The primary outcome will be proportion of eligible patients recommended for genetic testing for cancer-predisposing pathogenic variants. We hypothesise that the use of the digital tool will be associated with a higher proportion of eligible patients being recommended for testing.
Similar tools have been integrated into clinical practices with the publication of observational data. In a previous work, Loving et al reported on the implementation of screening via CARE in women undergoing breast cancer imaging.22 A total of 3345 patients were screened, and 1080 (32.3%) met the genetic testing criteria. Patients who met the genetic testing criteria received counselling by a pre-recorded video, and consent and sample collection was performed by medical assistants. Among those eligible for genetic testing, 416 (38.5%) proceeded with genetic testing, which identified 38 individuals with cancer-predisposing pathogenic variants. While the findings in Loving et al support the feasibility of tool implementation, the study population was primarily non-Hispanic White (78.3%), which differs from the anticipated study population of the HeRITAGE study. Further, the observational design and study setting at an imaging centre limit understanding of the effectiveness of the tool compared with usual care in office practice.
Nazareth et al reported retrospectively on the implementation of a similar patient-facing digital health chatbot to perform genetic cancer risk assessment across 180 outpatient sites, including primary care clinics.23 A total of 95 166 patients were invited to complete the chatbot, with 54 547 (89.4%) completing the chatbot risk assessment, and 14 850 (27.2%) meeting NCCN Guidelines for genetic testing for cancer-predisposing pathogenic variants. In the study design, risk assessments were disclosed to patients by the clinician. Downstream data on the impact of the tool, such as number of patients who were appropriately recommended for genetic testing or received genetic testing, were not included in the publication, except for a subset of 5594 eligible patients, among whom 1622 (29.0%) had genetic testing ordered. Lack of comprehensive outcomes regarding counselling or utilisation of genetic services after tool use limits the ability to make conclusions about the utility of the chatbot tool. In the HeRITAGE study, recommendation for genetic testing of eligible individuals was chosen as the primary outcome as this was felt to represent a clinically relevant milestone in which the genetic risk has been communicated to the patient. Further downstream data, such as the rate of genetic testing, will also be reported.
While data regarding digital screening tools have generally supported feasibility and acceptance, the impact of the tool on disparities in genetic services warrants exploration as health systems begin to incorporate such tools into routine practice and smartphones become increasingly widespread.24 An urban, academic, Medicaid-predominant clinic was chosen for the site of the HeRITAGE study due to the high proportion of patients that are historically under-represented in genetics research. Data regarding the association of demographics, health literacy and other social determinants of health on the experience of genetic risk assessment and receipt of genetic counselling may allow for insight into the impact of a smartphone-based tool on equitable care.
The inclusion criteria of the HeRITAGE study are broad, and thus, the results of the study have potential to be generalisable to other practices. However, the exclusion of non-English-speaking and reading patients, those without access to phones with internet capability and the location of the study at a single site may limit generalisability. While a smartphone-based tool may address common genetic cancer risk assessment barriers such as limited appointment time and provider knowledge, HeRITAGE is not designed to address language barriers which have also been shown to affect access to genetic services.6 At this time, additional efforts are underway to explore the effect of the smartphone-based tool in non-English-speaking populations through translation of the tool to other languages and recruitment of non-English-speaking patient cohorts at multiple sites. Additionally, HeRITAGE requires in-person appointment attendance for recruitment and does not address physical access to clinic spaces as a barrier to genetic testing.
Given the randomised study design and the urban Medicaid-predominant clinic setting, the results of HeRITAGE will provide informative data regarding the influence of screening technology in genetic cancer risk assessment on clinically relevant outcomes.
Footnotes
Funding: MKF was supported by the following grant: American Board of Obstetrics and Gynecology / American Association of Obstetricians and Gynecologists Foundation (ABOG/AAOGF) Scholar Award. RNS was supported by the following grants: National Cancer Institute Grant # K07CA216326 and R01CA211723 and Patient Centered Outcomes Research Institute Grant # IHS-2017C3-9211. PC was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (UL1TR002384). The funding sources had no role in study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-082658).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Contributor Information
Leslie E Bull, Email: leb4009@med.cornell.edu.
Emily M Webster, Email: ew2485@cumc.columbia.edu.
Auja McDougale, Email: aum9022@med.cornell.edu.
Denise Howard, Email: deh3002@med.cornell.edu.
Muhammad Danyal Ahsan, Email: mua2023@qatar-med.cornell.edu.
Sarah Levi, Email: sal7024@med.cornell.edu.
Benjamin Grant, Email: bjg4001@med.cornell.edu.
Isabelle Chandler, Email: irc4005@med.cornell.edu.
Paul Christos, Email: pac2001@med.cornell.edu.
Ravi N Sharaf, Email: ras9030@med.cornell.edu.
Melissa K Frey, Email: mkf2002@med.cornell.edu.
References
- 1.Mandelker D, Zhang L, Kemel Y, et al. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. JAMA . 2017;318:825–35. doi: 10.1001/jama.2017.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong AE, Hendriks YMC, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology . 2006;130:665–71. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 3.Evans DG, Harkness EF, Howell A, et al. Intensive breast screening in BRCA2 mutation carriers is associated with reduced breast cancer specific and all cause mortality. Hered Cancer Clin Pract. 2016;14:8. doi: 10.1186/s13053-016-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg. 2016;212:660–9. doi: 10.1016/j.amjsurg.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Force U, Owens DK, Davidson KW, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652–65. doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- 6.Cragun D, Weidner A, Lewis C, et al. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer . 2017;123:2497–505. doi: 10.1002/cncr.30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JJ, Fikre T, Fischman A, et al. The Role of Race and Insurance Status in Access to Genetic Counseling and Testing Among High-Risk Breast Cancer Patients. Oncologist . 2022;27:832–8. doi: 10.1093/oncolo/oyac132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delikurt T, Williamson GR, Anastasiadou V, et al. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet . 2015;23:739–45. doi: 10.1038/ejhg.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman-Davis E, Zhou ZN, Fields JC, et al. Racial and Ethnic Disparities in Genetic Testing at a Hereditary Breast and Ovarian Cancer Center. J Gen Intern Med . 2021;36:35–42. doi: 10.1007/s11606-020-06064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Kahn RM, Wing N, et al. Leveraging Health Information Technology to Collect Family Cancer History: A Systematic Review and Meta-Analysis. JCO Clin Cancer Inform. 2021;5:775–88. doi: 10.1200/CCI.21.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster EM, Perez L, Ahsan MD, et al. Integration and usability of a digital cancer risk stratification tool to optimize identification of patients at risk for hereditary cancers: A pilot study. Gynecol Oncol. 2024;183:1–6. doi: 10.1016/j.ygyno.2024.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Webster EM, Ahsan MD, McDougale A, et al. Implementation of a smartphone survey and mainstreaming for genetic cancer risk assessment in a diverse, urban, Medicaid-predominant gynecology clinic: a step toward health equity. Am J Obstet Gynecol. 2024;230:e108–9. doi: 10.1016/j.ajog.2024.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Frey MK, Ahsan MD, Webster E, et al. Web-based tool for cancer family history collection: A prospective randomized controlled trial. Gynecol Oncol. 2023;173:22–30. doi: 10.1016/j.ygyno.2023.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NCCN . NCCN; [15-Mar-2023]. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic.https://www.nccn.org/guidelines/category_2 Available. Accessed. [Google Scholar]
- 15.Health Leads . Health Leads; [15-Mar-2023]. The health leads screening toolkit.https://healthleadsusa.org/news-resources/the-health-leads-screening-toolkit/ Available. Accessed. [Google Scholar]
- 16.Shea JA, Micco E, Dean LT, et al. Development of a revised Health Care System Distrust scale. J Gen Intern Med . 2008;23:727–32. doi: 10.1007/s11606-008-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCCN NCCN guidelines version 2.2021 distress management nccn distress thermometer. 2024. [15-Mar-2023]. https://www.nccn.org/docs/default-source/patient-resources/nccn_distress_thermometer.pdf?sfvrsn=ef1df1a2_4 Available. Accessed.
- 18.Huan J, Noland-Dodd V, Varnes J, et al. Federal Practitioner; 2009. [6-May-2024]. Testing the brief health literacy screening tool.https://cdn.mdedge.com/files/s3fs-public/Document/September-2017/026120024.pdf Available. Accessed. [Google Scholar]
- 19.McNaughton CD, Cavanaugh KL, Kripalani S, et al. Validation of a Short, 3-Item Version of the Subjective Numeracy Scale. Med Decis Making . 2015;35:932–6. doi: 10.1177/0272989X15581800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand . 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Wolfe I, Ahsan MD, et al. Room for improvement in capturing cancer family history in a gynecologic oncology outpatient setting. Gynecol Oncol Rep . 2022;40:100941. doi: 10.1016/j.gore.2022.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loving VA, Luiten RC, Siettmann JM, et al. A Breast Radiology Department-operated, Proactive Same-day Program Identifies Pathogenic Breast Cancer Mutations in Unaffected Women. Acad Radiol. 2022;29 Suppl 1:S239–45. doi: 10.1016/j.acra.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Nazareth S, Hayward L, Simmons E, et al. Hereditary Cancer Risk Using a Genetic Chatbot Before Routine Care Visits. Obstet Gynecol . 2021;138:860–70. doi: 10.1097/AOG.0000000000004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnbull S, Cabral C, Hay A, et al. Health Equity in the Effectiveness of Web-Based Health Interventions for the Self-Care of People With Chronic Health Conditions: Systematic Review. J Med Internet Res . 2020;22:e17849. doi: 10.2196/17849. [DOI] [PMC free article] [PubMed] [Google Scholar]