Abstract
Background
Despite evidence showing that the intranasal and sublingual routes are safe and effective in providing analgesia, no data are available about their day-to-day use in the emergency department (ED). The aim of this study was to assess the frequency of the use of the intranasal and sublingual routes, and the clinical characteristics of the patients receiving analgesia through these routes.
Methods
A multicentre study was performed in the EDs participating in the Pain in Paediatric Emergency Room research group. It included a survey and a retrospective data collection in which the medical records of all patients who received analgesia from 1 April 2022 to 31 May 2022 were collected.
Results
48 centres (91%) answered the survey. The intranasal and sublingual routes were used in 25 centres (52%). 13 centres (27%) used both routes, 9 centres (19%) used only the sublingual and 3 centres (6%) used only the intranasal route.
12 centres (48%) participated in the retrospective study. Data about 3409 patients, median age 9 years (IQR 5–12), were collected. Among them, 337 patients (9.6%) received sublingual analgesia, and 87 patients (2.5%) received intranasal analgesia. The intranasal route was employed for injuries in 79 (90.8%) cases, and fentanyl was the drug delivered in 85 (97.7%) cases. The sublingual route was used mainly for injuries (57.3%), but also for abdominal pain (15.4%), musculoskeletal pain (14.5%) and headache (10.7%). Paracetamol, ketorolac and tramadol were administered through this route.
Conclusions
The use of the intranasal and sublingual routes for analgesia in the paediatric ED is still limited.
Keywords: Analgesia
What is already known on this topic.
What this study adds
The 52% of the EDs participating in this study use the intranasal or sublingual route for analgesia.
In these EDs, 9.6% and 2.5% of patients received sublingual and intranasal analgesia, respectively, in a 2-month period.
The intranasal route was employed mainly for injuries and for the administration of fentanyl.
The clinical characteristics of patients receiving analgesia through the oral and sublingual route were similar.
How this study might affect research, practice or policy
This large multicentre study showed that the use of the intranasal and sublingual routes for the administration of analgesia is still limited in the PEDs. This is the first study showing the actual use of these routes for analgesia in this setting. The results of this study may shed light on the reasons behind the limited use of these routes for the administration of analgesia, and support institutions and emergency physicians in implementing their use.
Introduction
Pain is the symptom most frequently reported by children and adolescents accessing the paediatric emergency department (PED).1
Appropriate pain management is considered one of the most important goals in PED care. Pharmacological analgesia is the most commonly used analgesic strategy for children and adolescents with acute pain in the PED. Many drugs are available, including paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), opioids and ketamine, which are mainly administered through the oral and intravenous routes.
In the last two decades, new routes of administration have been explored with fascinating results in children and adolescents. Transmucosal routes, including the intranasal and sublingual routes, are increasingly popular in the PED setting due to their easy and fast administration. Moreover, they can be used in patients without vascular access.2 The international literature shows the effectiveness of the intranasal and sublingual administration of drugs in children with both traumatic and non-traumatic pain, including severe pain. Contraindications to these routes are mainly related to local issues. Adverse events are less common compared with the intravenous route.3,6
Even though the transmucosal administration of analgesia seems a valuable tool for emergency physicians, no data are available about its use in the day-to-day practice in the PEDs.
Pain in Paediatric Emergency Room (PIPER) is an Italian research network including members from 52 Italian EDs. Participants are paediatricians, emergency physicians and anaesthesiologists who work together to share knowledge and research in pain recognition, assessment and management.7 8
This study aimed to describe the use of transmucosal routes for analgesia among the PIPER centres through a survey and a retrospective data collection.
Methods
This was a multicentre observational study that involved the EDs part of the PIPER research network. It was divided into two steps.
A survey was developed to establish the number of EDs employing the intranasal and sublingual routes for the administration of analgesia.
Subsequently, the frequency of analgesia administration through the intranasal and sublingual routes in the centres that had reported to use them in the survey, was retrospectively assessed.
The tertiary level, university teaching, children’s hospital Institute for Maternal and Child Health IRCCS Burlo Garofolo of Trieste, Italy, was the coordinator centre for this study.
An online survey was performed through a brief questionnaire addressed to every member of the PIPER research network representing an ED centre. The questionnaire included 11 items. Two items were about the type of ED, paediatric or general, and the annual census in 2021. Five items addressed the use of the intranasal route. It was asked if this route was used for analgesia or for other purposes, if a mucosal atomisation device (MAD) was available for intranasal administrations, which analgesics were administered, and if the administration was allowed to emergency physicians or only to anaesthesiologists. Four similar items addressed the use of the sublingual route of administration.
The centres that reported to use the intranasal or sublingual routes for the administration of analgesia were invited to participate in a retrospective cross-sectional study.
The aim was to determine the number of patients receiving intranasal and sublingual analgesia, from 1 April 2022 to 31 May 2022, and to describe the main characteristics of the patients receiving analgesia through these routes.
The inclusion criteria for the data collection were:
Children and adolescents, from 0 to 17 years of age, accessing the EDs part of the PIPER research network.
Subjects who received pharmacological analgesia at the ED through any route.
The exclusion criteria were:
Adult patients accessing EDs included in the PIPER research network.
Patients receiving drugs through the transmucosal routes for purposes different from analgesia (eg, sedation).
Patients receiving only topical or inhalatory analgesia.
The medical records of every subject, who fulfilled the inclusion and exclusion criteria, were reviewed.
For each enrolled patient, the following characteristics were collected: age, gender, type of pain, type of analgesic received and route of administration.
The above data were anonymised, collected and stored in an electronic database developed explicitly for the study.
For comparisons:
Patients’ ages were grouped as follows: newborns (0–30 days), infants (1–11 months), toddlers (1–2 years), preschool children (3–5 years), school children (6–12 years) and adolescents (13–17 years).
The type of pain was characterised as: injury-related pain (caused by trauma, burns and wounds), abdominal pain, headache, non-traumatic musculoskeletal pain, ear pain, throat pain and others.
The types of analgesic drugs included: paracetamol, NSAIDs, opioids, ketamine and others.
The routes of administration included: intranasal, sublingual/buccal, oral, rectal, intravenous and intramuscular.
The primary study outcome was the frequency of intranasal and sublingual analgesia among the EDs included in the PIPER research network, assessed through the survey.
The secondary outcomes were:
The number of centres using transmucosal administrations for purposes different from analgesia.
The prevalence of children who received transmucosal analgesia.
The type of pain in which transmucosal analgesia was more used.
Statistical analysis
Categorical variables were described as frequency and percentage. Continuous variables were described by mean and SD if their distribution tested as normal, or else by median and IQR. To assess normality in the continuous variables’ distribution, the Kolmogorov-Smirnov test was used. The prevalence of the use of transmucosal analgesia was calculated as the ratio between the number of centres that reported to use it and the total number of centres participating in the study. The prevalence of subjects receiving transmucosal analgesia in a 2-month period in each ED was estimated as the ratio between the number of patients receiving transmucosal analgesia and the total number of patients receiving analgesics in the same period. This prevalence was stratified for age groups as described. To identify possible associations between two categorical variables, the χ2 or the Fisher exact test was applied, while the non-parametric Wilcoxon Mann-Whitney test was used to assess the different distribution of a continuous variable between categories of a qualitative variable. A p value <0.05 was considered statistically significant. Analyses were conducted using SAS software, V.9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Survey
Among the 52 centres invited to the survey, 48 centres (91%) filled in the questionnaire.
Among these, 37 centres (77%) were PEDs and 11 centres (23%) were general EDs. The average annual census of the centres in 2021 was 20 000 attendances per year.
The intranasal and sublingual routes were employed in 25 centres (52%). In particular, 13 centres (27%) reported to use both the intranasal and sublingual route for analgesia administration, 9 centres (19%) only the sublingual route and 3 centres (6%) only the intranasal route. 23 centres (48%) reported to not use the intranasal or sublingual routes for analgesia.
30 centres (63%) reported using the intranasal route to administer drugs, including drugs without analgesic effects and 15 centres (31%) using the sublingual route.
Among the 16 centres that used the intranasal route for analgesia, 15 centres (94%) reported to administer only fentanyl and 1 centre (6%) fentanyl and ketamine. 15 centres (94%) reported to use the MAD to administer intranasal drugs. The administration was performed by both emergency physicians and paediatricians in 13 centres (81%), and only by anaesthesiologists, in 3 centres (19%).
Among the 22 centres (46%) which reported to use the sublingual route for analgesia, the drugs administered were ketorolac in 17 centres (77%), tramadol in 14 centres (29%), morphine in 4 centres (8%) and paracetamol in 1 centre (5%). In all these centres, the sublingual administration of analgesic drugs was performed by emergency physicians and paediatricians.
Retrospective study
The 25 centres that reported to use the intranasal or sublingual route for analgesia were invited to participate in the retrospective study.
Among these, 12 centres (48%) participated in the study. All of these were PEDs. The mean annual census in 2021 was 20 000 visits.
During the study period, data about 3525 patients were collected for a total of 3623 administrations of analgesia. 116 subjects (3.3%) were excluded due to the lack of specification of the route of administration in the database. Finally, 3409 patients with 3507 administrations were analysed; 1954 (57.3%) were males. The median age was 9 years (IQR 5–12). In particular, 32 (0.9%) were infants (0–11 months), 258 (7.6%) were toddlers (1–2 years), 595 (17.5%) were preschool children (3–5 years), 1679 (49.2%) were school children (6–12 years) and 845 (24.8%) were adolescents (13–17 years).
Overall, 2581 (73.6%) analgesics were administered through the oral route, 453 (12.9%) through the intravenous route, 337 (9.6%) through the sublingual route, 87 (2.5%) through the intranasal route, 40 (1.1%) through the rectal route and 9 (0.3%) through the intramuscular route.
3323 patients (97.5%) received analgesia through a single route of administration. 86 patients (2.5%) received analgesia through a combination of routes.
Table 1 shows the analgesics administered in relation to the route of administration.
Table 1. Distribution of the analgesics administered in relationship with the route of administration.
| Routes of administration | ||||
| Intranasal (n=87) | Sublingual (n=337) | Oral (n=2581) | Intravenous (n=453) | |
| Analgesics, n (%) | ||||
| Paracetamol | – | 272 (80.7) | 1762 (68.3) | 298 (65.8) |
| Ibuprofen | – | – | 759 (29.4) | 8 (1.8) |
| Ketorolac | 1 (1.2) | 63 (18.7) | 5 (0.2) | 52 (11.5) |
| Fentanyl | 85 (97.7) | – | – | 13 (2.9) |
| Ketamine | – | – | – | 38 (8.3) |
| Tramadol | – | 1 (0.3) | 6 (0.2) | 25 (5.5) |
| Paracetamol/codeine | – | – | 13 (0.5) | – |
| Morphine | – | – | – | 5 (1.1) |
| Others | 1 (1.2) | 1 (0.3) | 36 (1.4) | 14 (3.1) |
Paracetamol was used in 2377 cases (67.8%), ibuprofen in 785 cases (22.4%), ketorolac in 124 cases (3.5%) and fentanyl in 115 cases (3.3%). In general, 170 patients (4.9%) received an opioid.
The intranasal route was used significantly more frequently in males (p=0.0002).
Patients treated through the intranasal route were significantly younger compared with patients receiving analgesia through other routes, with a median age of 7 (IQR 5–10) and 9 (IQR 5–13), respectively (p=0.001).
No significant difference was found in the gender of patients receiving analgesia through the sublingual route (p=0.96).
Patients treated through the sublingual route were significantly older compared with patients receiving analgesia through other routes; the median age was 11 years (IQR 8–13) and 9 years (IQR 5–13), respectively (p<0.0001).
Figure 1 describes the distribution of analgesics administered through the intranasal, sublingual, oral and intravenous routes in relation to the cause of pain.
Figure 1. Distribution of pain’s causes by route of administration. Percentage is shown in the figure.
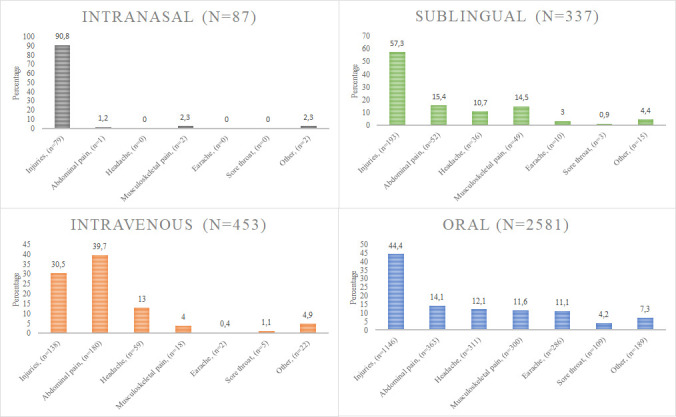
The intranasal route was used in 79 cases (90.8%) for injuries. In 85 cases (97.7%), patients received fentanyl.
The sublingual route was used in 193 cases (57.3%) for injuries, 52 cases (15.4%) for abdominal pain, 49 cases (14.5%) for musculoskeletal pain and 36 cases (10.7%) for headache. The drugs administered through this route consisted of paracetamol in 272 cases (80.7%), ketorolac in 63 cases (18.7%) and tramadol in 1 cases (0.3%).
In general, for every cause of pain, paracetamol and ibuprofen were the analgesics most commonly employed.
Discussion
This multicentre study showed that the use of the intranasal and sublingual routes for the administration of analgesia is still limited among EDs in Italy, with only 52% of EDs using these routes. Moreover, among the centres that reported to use the intranasal or sublingual routes for analgesia, only 12.1% of patients received drugs through these routes.
The intranasal and sublingual administration of drugs is fascinating because it employs mucosal regions with an extensive vascular supply and a high permeable epithelium. The direct absorption of drugs into the systemic circulation through these routes bypasses the first-pass metabolism in the liver, increasing medications’ bioavailability and shortening the onset of action.
From the early 2000s, several studies were published about the usefulness of the intranasal route of administration for analgesia. Intranasal fentanyl provided similar analgesia to intravenous morphine in children with bone fractures.9 Moreover, it was successfully employed for other causes of acute pain10,12 and to control procedural pain.13 14 Recently published trials showed a similar efficacy of intranasal ketamine compared with intranasal fentanyl.15 16 A meta-analysis suggested that intranasal analgesics are an excellent alternative to intramuscular analgesics in children with acute moderate to severe pain, and they may be an alternative to intravenous administration.11
Nevertheless, apart from trials and case series, to our knowledge, no studies have assessed the use of the intranasal route of administration of analgesia in the common PED practice.
Likewise, several studies proved the usefulness of sublingual analgesia for different causes of acute pain, such as traumatic pain, abdominal pain and sickle cell crisis.3 4 17 However, no previous data were available about the actual use of the sublingual route of administration in the PED setting.
This study demonstrated that the intranasal route was strictly limited to the administration of fentanyl, and this could be different from other settings in which ketamine and ketorolac may be administered more frequently through this route. Moreover, the administration of fentanyl was limited in almost all cases to the treatment of injuries.
Similarly, the sublingual route of administration was substantially limited to paracetamol and ketorolac, resembling an Italian practice, which could differ substantially from other countries where opioids are more widely used and more formulations are available.17
Regarding opioids, this study showed that in the Italian PEDs, their use is consistent with that reported in the PEDs of the USA.18 Considering the formulation of opioid used in the present study, consisted of fentanyl in the 67.4% of patients, tramadol in the 21.2%, paracetamol/codeine in the 7.6% and morphine in the 3.5%. This distribution was substantially different from that reported in a previous multicentre PIPER study performed in 2014 and 2015, in which patients received paracetamol/codeine in 52% of cases, morphine in 27% and tramadol in 21%.19 This difference was related to the current limitation of the use of codeine for children and mounting use of intranasal fentanyl.20
Considering that some participating centres reported to use the intranasal routes for drug administration for purposes different from analgesia, we can argue that, in some of them, a limitation of the use of this route may be related to the limited use of some analgesic agents, rather than to the route itself. In Italy, in many ED settings, the administration of fentanyl is still only allowed to anaesthesiologists.
The present study was not primarily designed to investigate possible causes limiting the use of transmucosal analgesia in clinical practice. Nevertheless, several issues can be hypothesised. Evidence regarding sublingual analgesia still lacks clear comparisons between the efficacy of this route and the oral or intravenous one. Moreover, drugs frequently administered via the sublingual route, such as ketorolac and tramadol, are off-label in children younger than 16 and 12 years of age, respectively. In general, the transmucosal routes of administration for analgesia are still off-label in Italy, thus limiting their use in clinical practice. On the contrary, we cannot exclude that there is still little awareness of the data in support of their effectiveness, which could explain a possible limited availability of MADs.
This study has several limitations. Due to the retrospective design, some cases may have been missed or mislabelled. Moreover, we decided to limit the collection of data to 2 months to encourage participation. Therefore, we cannot exclude that the use of analgesics could be different in other periods of the year, considering the usual different distribution of attendances across the year. We decided not to collect data about analgesic efficacy because, in our opinion, the latter can be rigorously measured only through specifically designed randomised controlled trials. Providing data about analgesic efficacy in a study with the present design would have exposed us to several biases, limiting the strength of our findings.
The data collection was limited to the PEDs, so it was not possible to determine whether children accessing general EDs were treated differently.
The study results reflected the Italian practice, and no similar international data are available for comparisons, thus limiting generalisability to other PED settings. On the contrary, the multicentre nature and the considerable sample size strengthened the results and provided a reliable picture of the current practice in the PED setting in Italy.
In conclusion, this large multicentre study demonstrated that the use of the intranasal and sublingual routes to treat acute pain in the PEDs is still limited in Italy. Future studies are needed to provide data from other PED settings.
Acknowledgements
The authors thank Martina Bradaschia for the English revision of the manuscript.
Footnotes
Funding: This work was supported by the Ministry of Health, Rome, Italy, in collaboration with the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy.
Data availability free text: Data are available after reasonable request to GC writing at giorgio.cozzi@burlo.trieste.it.
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: The Institutional Review Board of the Institute for Maternal and Child Health IRCCS Burlo Garofolo of Trieste, Italy, provided ethical approval for the study protocol (RC 45/22).
Contributor Information
Giorgio Cozzi, Email: giorgio.cozzi@burlo.trieste.it.
Sara Chiappa, Email: sarachiappa1982@gmail.com.
Giovanna La Fauci, Email: giovanna.lafauci@aovr.veneto.it.
Matteo Calvi, Email: mcalvi@asst-pg23.it.
Emanuele Castagno, Email: ecastagno@cittadellasalute.to.it.
Eleonora Tappi, Email: tappi.e@ospedale.cuneo.it.
Giovanna Villa, Email: giovannavilla@gaslini.org.
Paola Tommasi, Email: paola.tommasi@asst-fbf-sacco.it.
Gregorio Paolo Milani, Email: milani.gregoriop@gmail.com.
Marta Cellai Rustici, Email: marta.cellairustici@meyer.it.
Maria Luisa Casciana, Email: marialuisa.casciana@asst-mantova.it.
Nicola Tovaglieri, Email: nicola.tovaglieri@ospedaleniguarda.it.
Stefano Masi, Email: stefano.masi@meyer.it.
Cesare Vezzoli, Email: vezzolice@gmail.com.
Sofia Zeuditù Tilatti, Email: SOFIAZEUDITU.TILATTI@studenti.units.it.
Manuela Giangreco, Email: manuela.giangreco@burlo.trieste.it.
Egidio Barbi, Email: egidio.barbi@burlo.trieste.it.
Franca Benini, Email: franca.benini@unipd.it.
Data availability statement
Data are available upon reasonable request.
References
- 1.Krauss BS, Calligaris L, Green SM, et al. Current concepts in management of pain in children in the emergency department. Lancet. 2016;387:83–92. doi: 10.1016/S0140-6736(14)61686-X. [DOI] [PubMed] [Google Scholar]
- 2.Rech MA, Barbas B, Chaney W, et al. When to Pick the Nose: Out-of-Hospital and Emergency Department Intranasal Administration of Medications. Ann Emerg Med. 2017;70:203–11. doi: 10.1016/j.annemergmed.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Neri E, Maestro A, Minen F, et al. Sublingual ketorolac versus sublingual tramadol for moderate to severe post-traumatic bone pain in children: a double-blind, randomised, controlled trial. Arch Dis Child. 2013;98:721–4. doi: 10.1136/archdischild-2012-303527. [DOI] [PubMed] [Google Scholar]
- 4.Cozzi G, Zanchi C, Chiaretti A, et al. Administering analgesia sublingually is a suitable option for children with acute abdominal pain in the emergency department. Acta Paediatr. 2019;108:143–8. doi: 10.1111/apa.14514. [DOI] [PubMed] [Google Scholar]
- 5.Fantacci C, Fabrizio GC, Ferrara P, et al. Intranasal drug administration for procedural sedation in children admitted to pediatric Emergency Room. Eur Rev Med Pharmacol Sci. 2018;22:217–22. doi: 10.26355/eurrev_201801_14120. [DOI] [PubMed] [Google Scholar]
- 6.Pansini V, Curatola A, Gatto A, et al. Intranasal drugs for analgesia and sedation in children admitted to pediatric emergency department: a narrative review. Ann Transl Med. 2021;9:189. doi: 10.21037/atm-20-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benini F, Castagno E, Urbino AF, et al. Pain management in children has significantly improved in the Italian emergency departments. Acta Paediatr. 2020;109:1445–9. doi: 10.1111/apa.15137. [DOI] [PubMed] [Google Scholar]
- 8.Cozzi G, Lucarelli A, Borrometi F, et al. How to recognize and manage psychosomatic pain in the pediatric emergency department. Ital J Pediatr. 2021;47:74. doi: 10.1186/s13052-021-01029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borland M, Jacobs I, King B, et al. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med. 2007;49:335–40. doi: 10.1016/j.annemergmed.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Ruffin TB, Jr, Salinero E, Papa L, et al. Intranasal Fentanyl to Reduce Pain and Improve Oral Intake in the Management of Children With Painful Infectious Mouth Lesions. Pediatr Emerg Care. 2022;38:363–6. doi: 10.1097/PEC.0000000000002779. [DOI] [PubMed] [Google Scholar]
- 11.Prescott MG, Iakovleva E, Simpson MR, et al. Intranasal analgesia for acute moderate to severe pain in children - a systematic review and meta-analysis. BMC Pediatr. 2023;23:405. doi: 10.1186/s12887-023-04203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson T, Harrell C, Snider M, et al. The Safety of High-Dose Intranasal Fentanyl in the Pediatric Emergency Department. Pediatr Emerg Care. 2022;38:e447–50. doi: 10.1097/PEC.0000000000002627. [DOI] [PubMed] [Google Scholar]
- 13.Hoeffe J, Doyon Trottier E, Bailey B, et al. Intranasal fentanyl and inhaled nitrous oxide for fracture reduction: The FAN observational study. Am J Emerg Med. 2017;35:710–5. doi: 10.1016/j.ajem.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Roback MG, Carlson DW, Babl FE, et al. Update on pharmacological management of procedural sedation for children. Curr Opin Anaesthesiol. 2016;29 Suppl 1:S21–35. doi: 10.1097/ACO.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 15.Frey TM, Florin TA, Caruso M, et al. Effect of Intranasal Ketamine vs Fentanyl on Pain Reduction for Extremity Injuries in Children: The PRIME Randomized Clinical Trial. JAMA Pediatr. 2019;173:140–6. doi: 10.1001/jamapediatrics.2018.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds SL, Bryant KK, Studnek JR, et al. Randomized Controlled Feasibility Trial of Intranasal Ketamine Compared to Intranasal Fentanyl for Analgesia in Children with Suspected Extremity Fractures. Acad Emerg Med. 2017;24:1430–40. doi: 10.1111/acem.13313. [DOI] [PubMed] [Google Scholar]
- 17.Ojo AS, Odipe OG, Owoseni O. Improving the Emergency Department Management of Sickle Cell Vaso-Occlusive Pain Crisis: The Role and Options of Sublingual and Intranasally Administered Analgesia. J Clin Med Res. 2023;15:10–22. doi: 10.14740/jocmr4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menchine M, Lam CN, Arora S. Prescription Opioid Use in General and Pediatric Emergency Departments. Pediatrics. 2019;144:e20190302. doi: 10.1542/peds.2019-0302. [DOI] [PubMed] [Google Scholar]
- 19.Benini F, Castagno E, Barbi E, et al. Multicentre emergency department study found that paracetamol and ibuprofen were inappropriately used in 83% and 63% of paediatric cases. Acta Paediatr. 2018;107:1766–74. doi: 10.1111/apa.14306. [DOI] [PubMed] [Google Scholar]
- 20.Poonai N, Zhu R. Analgesia for Children in Acute Pain in the Post-codeine Era. Curr Pediatr Rev. 2018;14:34–40. doi: 10.2174/1573396313666170829115631. [DOI] [PubMed] [Google Scholar]


