Abstract
The Lithium-chromium phosphate Li3Cr2(PO3)4 sample was synthesized via the solid-state reaction method. The morphological integrity and chemical homogeneity were verified by energy dispersive X-ray analysis (EDX) and scanning electron microscopy (SEM). Infrared and Raman patterns were also analyzed. Optical absorption spectrum analysis, conducted within the range of 10000 cm−1 to 30000 cm−1 at room temperature, yielded some optical parameters (Eg, Eu, , k, n). The Neuhauser model is used to interpret the interference dip which was on the absorption spectrum of Li3Cr2(PO3)4. The Fourier transform of the autocorrelation function leads to the Zero Phonon Lines of the observed absorption energies. The electronic structure of Cr3+ (3d (Huang et al., 2009) 33) ions in Li3Cr2(PO3)4 was calculated using Racah method, which allowed for precise calculations of Racah and crystal-field parameters. The results showed good agreement between the theoretical and experimental energy levels.
Keywords: Li3Cr2(PO4)3 material, Neuhauser model, Crystal structure, Optical parameters, Crystal field theory
1. Introduction
Lithium metal phosphate materials Li3M2(PO4)3 with M is a transition metal ion (V, Fe, Mn, Cr …) are the newest generation of active materials. Li3M2(PO4)3 are also an intriguing materials with potential applications in various fields, including energy storage and optoelectronics [[1], [2], [3]]. Beyond their use in energy storage, knowing how structural and optical characteristics interact within Li3M2(PO4)3 creates opportunities to improve their efficiency, safety, and performance. The presence of chromium (Cr) within the structure offers opportunities to enhance the optical properties, and consequently particularly interesting for optical applications [4,5]. In fact, chromium, can introduce unique optical characteristics to the material. One notable aspect is its ability to exhibit different oxidation states, leading to diverse optical properties [6,7]. In the context of Li3Cr2(PO4)3, chromium likely occupies octahedral coordination sites within the crystal lattice, influencing its electronic band structure and optical behavior [8,9].
Moreover, the octahedral environment surrounding chromium ions can induce interesting optical phenomena, such as crystal field effects and charge transfer transitions. These effects can impact the absorption and emission spectra of the material, making it potentially useful for applications such as phosphor materials, solid-state lighting, or optical sensors [[10], [11], [12], [13]]. However, it's important to note that chromium is typically present in smaller amounts compared to phosphorus in Li3Cr2(PO4)3. While this may limit the extent of its influence on the material's optical properties.
This paper explores the intriguing properties of Li3Cr2(PO4)3, with a particular focus on the investigation of their optical and structural properties. First, we will concentrate on the Li3Cr2(PO4)3 compound's preparation and structural analysis. Next, we intend to present some important optical parameters: the band gap energy (Eg), the Urbach energy (Eu), the skin depth (δ), the extinction coefficient (k) and the refractive index (n). Furthermore, we analyze the feature observed in the absorption band of Cr3+ ions using the Neuhauser model. The objective of this study is to identify the cause of interference dips. Finally, a crystal field theory study was conducted for the Cr3+(3d [3]) in an Oh site symmetry using Racah tensor algebraic methods. It's notable that a few investigations have used crystal field theory to analyze the compound's absorption spectrum.
2. Experimental details
The Li3Cr2(PO4)3 compound has been prepared by the conventional solid-state reaction. The starting reagents Li2CO3, Cr2O3, and NH4H2PO4 were calculated in the desired proportion according to the following reaction:
| 3Li2CO3 + 2Cr2O3 + 6(NH4)2HPO4 → 2Li3Cr2(PO4)3 +3CO2 +12NH3+ 9H2O |
The precursors are thoroughly mixed in equimolar proportions and ground in an agate mortar for 2h. The powder obtained is heated in a crucible at a temperature of 573K for 8h with intermediate regrinding to exhaust NH3, H2O, and CO2. Secondly, the calcined powders were uniaxial pressed into a cylindrical pellet using a hydraulic press and then the pellets were sintered at an optimized temperature 1100 K for 6h to acquire close packed grains.
A range of techniques was employed for the structural and optical characterization of phosphor. These techniques are included, scanning electron microscopy (SEM) coupled with energy dispersive X-ray (EDX) analysis, RAMAN, FTIR reflectance and absorption spectroscopy. Using a TESCAN VEGA3 SBH scanning electron microscope (SEM) equipped with an energy dispersive microscopy (EDS detector Bruker XFlagh 410 M), the morphology of the powder and its chemical composition were investigated. Using an image processing tool (Image J, version 1.50), at least 60 randomly chased grains were measured in order to collect size and size-distribution statistics from SEM pictures. The Fourier Transform Infrared Spectroscopy (FTIR) spectrum has covered a spectral range between 400 and 4000 cm⁻1, with the sample in solid form. The number of scans performed was 32, and the resolution was set at 4 cms⁻1, using a DTGS (Deuterated Triglycine Sulfate) detector. For the Raman spectrum, the laser wavelength used was 532 nm, covering a spectral range of 0–3600 cm⁻1. The Raman analysis also involved a solid sample, with 100 scans at a resolution of 30 accumulations, 0.5 s, detected by a CCD (Charge-Coupled Device)- black illuminate. For the evaluation of optical properties, we employed both reflectance and absorption spectroscopy techniques using the PerkinElmer Lambda 365 UV/Vis Spectrophotometer. This instrument, covering a wide wavelength range from 190 nm to 1100 nm, offers high resolution and sensitivity, crucial for detecting subtle spectral features.
3. Results and discussion
3.1. Morphological studies
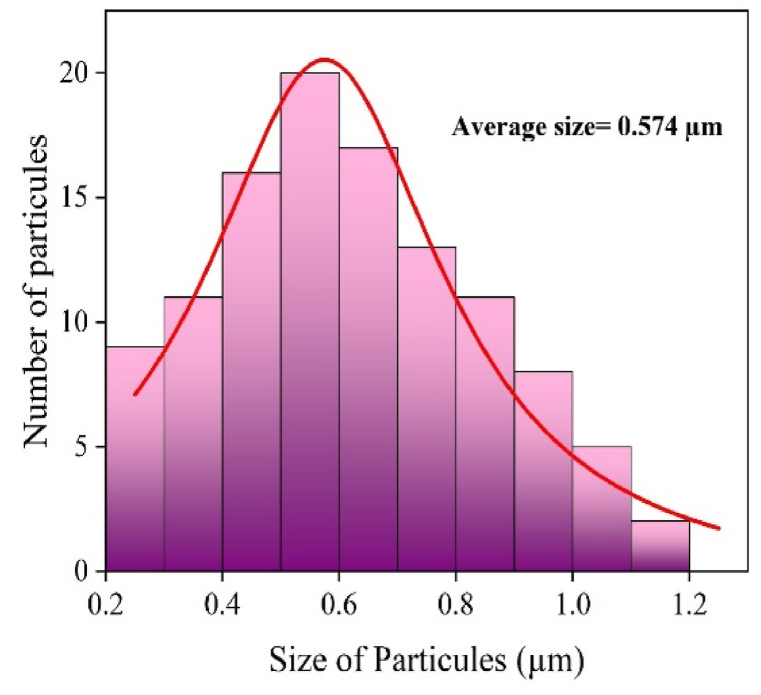
As it is mentioned previously, Li3Cr2(PO4)3's surface morphology, size distribution, and elemental composition were all thoroughly examined using an Energy Dispersive X-ray Spectroscopy (EDX) in conjunction with a Scanning Electron Microscope (SEM). To determine the size, shape, and morphological characteristics of the particles in the sample, SEM observations were used. The (SEM) micrograph of our compound is shown in Fig. 1 a. A detailed histogram of grain size was generated to analyze the particle size distribution (Fig. 1 b), providing quantitative insights into the dimensions of the particles comprising the material. The histogram of Fig. 1b shows a particle size that ranges from 0.2 to 1.2 μm, with an average size of 0.574 μm signifying the formation of micrometric particles. The fact that the particle is so small suggests that the synthesis of microparticles was efficient [14].
Fig. 1. a.
The SEM micrograph of Li3M2(PO4)3 around 5 μm.
Fig. 1. b.
Histogram of grain size distribution of Li3Cr2(PO4)3.
The EDX spectrum is displayed in Fig. 2. We notice that oxygen (O) is the predominant component, with phosphorus (P) and chromium (Cr) present in smaller quantities. Lithium (Li) is not detected in the EDX spectrum because it is a volatile element that tends to evaporate or sublimate when exposed to the electron beam used in EDX analysis. A non-negligible intensity peak labeled C is displayed in the spectrum. This peak corresponds to carbon emissions possibly coming from a carbon tape used during the measurement prosses. Fig. 2 shows also the presence of Na and Al, though in small quantities. These elements are considered impurities and may have originated due to contamination during sample preparation. The quantitative analysis of the compound's elemental composition as determined by EDX spectrum is summarized in Table 1.
Fig. 2.
The quantitative analysis of the compositional elements presents in Li3Cr2(PO4)3 using EDX.
Table 1.
The elements present in Li3Cr2(PO4)3and their percentage of occupation.
| Spectrum label | EDX Spectrum (%) |
|---|---|
| C | 16.25 |
| P | 18.51 |
| O | 42.89 |
| Cr | 22.34 |
| Total | 100.00 |
3.2. Vibrational studies: FTIR and Raman
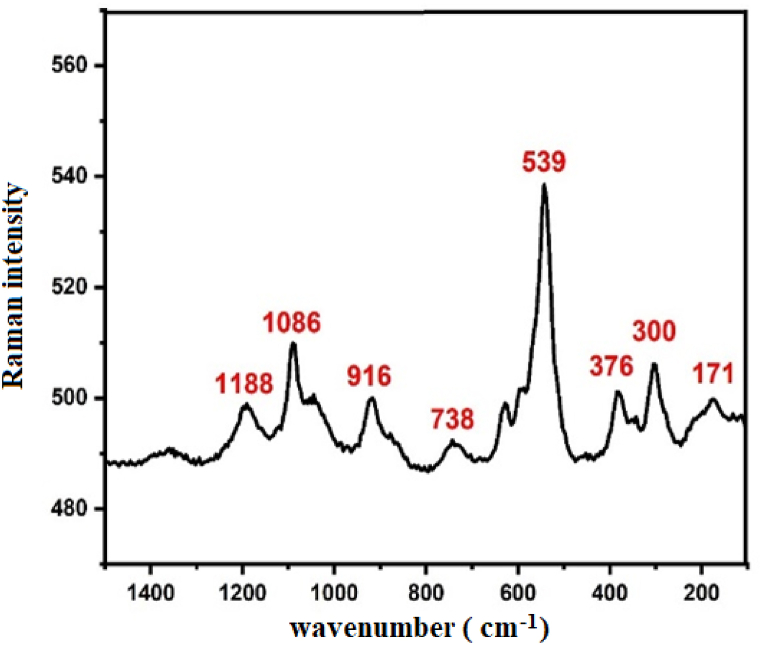
As described on the literature [[15], [16], [17]] Li3Cr2(PO4)3 crystallizes in a monoclinic structure, specifically in the space group P21/n. The structure of our sample indeed consists of CrO6 octahedra that share corners with six PO4 tetrahedra. This creates a three-dimensional framework where the CrO6 octahedra are interconnected by the PO4 tetrahedra, contributing to the stability of the structure. This structural arrangement is common in various phosphate compounds [[15], [16], [17]]. The FTIR pattern for the compound under investigation is displayed in Fig. 3a. The vibrational features in the lower wavenumber region (400 –1000 cm−1) are like that of PO43− in Ref. [18]. The PO43−group has a singlet (A1) at a frequency ν1 = 967 cm−1; a doublet (E) at ν2 = 447 cm−1 and two triply degenerate (T2) modes, ν3 at 1029 cm−1 and ν4 at 574 cm−1. The ν1 and ν3 modes involve the symmetric and anti-symmetric stretching vibrations of the P–O bonds, whereas ν2 and ν4 involve mainly O–P–O symmetric and antisymmetric bending mode [[19], [20], [21]]. The band at 1166 cm−1 region is owing to combined symmetric and antisymmetric mode [22]. Fig. 3b shows the Raman spectrum of our sample collected using a green laser line at 532 nm. The 300-376 cm−1 Raman components of Li3Cr2(PO4)3 are associated to Cr–O stretching modes of vibrations and are similar to those observed for Cr3+-O stretching modes in Na3Cr2(PO4)3 [23]. The band at 539 cm−1 in Raman spectrum corresponds to the attempts frequency of Lithium ions in the superionic [24]. Moreover, in the frequency region 900-1200 cm−1 (at 916–1086 – 1188 cm−1), The Raman bands are ascribed to the P–O stretching [18].
Fig. 3.a.
The FTIR spectrum of Li3Cr2(PO4)3 compound.
Fig. 3.b.
The Raman spectrum of Li3Cr2(PO4)3compound.
This part of our study is very interesting, especially in relation to the CrO6 octahedron. For complexes having an inversion symmetry center, vibronic origin is found to be a particularly useful method of increasing electron-dipole intensity during d-d transitions. Table 2 recapitulates the selection rules outlined for the Oh point group.
Table 2.
Selection rules for Cr3+ d-d electronic transitions in Oh site symmetry for in Li3Cr2(PO4)3.
| Electronic transition Oh | Electric dipole allowed with Γu vibration |
|---|---|
| 4A2g → 4T2g | a1u, t1u, t2u, eu |
| 4A2g → 4T1g | a2u, t1u, t2u, eu |
Concerning the octahedron CrO6 octahedron, by eliminating the translation and rotation modes we have 15 internal vibration modes [4]:
| Γvib = a1g + eg + t2g+ 2t1u + t2u | (1) |
As shown in Equation (1), in our context, the t1u mode is detected at 1166 cm−1 in Infrared spectroscopy, while Raman spectroscopy identifies activation of the a1g, eg, and t2g modes [25]. Notably, the t2u mode remains inactive in both spectroscopic techniques [25]. The observed d-d transitions are elucidated by vibronic coupling with the t1u symmetry mode, which exhibits activity in Infrared spectroscopy. Notably, the vibronic frequency of the Raman-active a1g mode is determined to be 376 cm−1, consistent with observations reported in previous studies.
3.3. Determination of some optical parameters
3.3.1. Optical band gap energy Eg
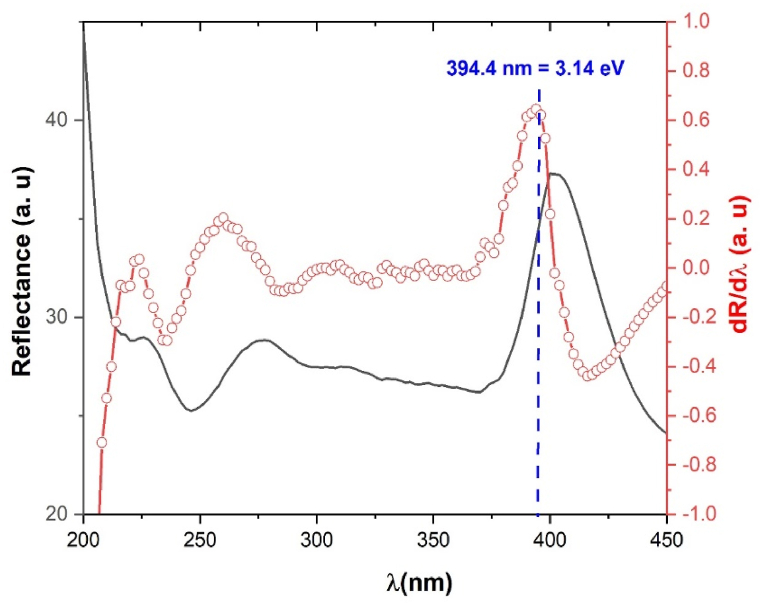
In this section we are going to determine the optical band gap Eg. We start our calculation by the Marotti method [26]. In this method the Eg parameter is determined directly from the maximum detected from the evolution of the spectrum dR/dλbeing R the reflectance and λ the wavelength. Fig. 4 shows the reflectance and the evolution dR/dλ spectra versus the wavelength λ. As it is clear we found the band gap Eg at 3.14 eV.
Fig. 4.
The spectrum of reflectance (dark solid line) and the evolution of dR/dλ(red circle) versus the wavelengthλ.
Another tool to determine the optical band gap is the Tauc method [27]. This method is a helpful technique for determining a material's optical bandgap (Eg) from its absorption spectrum. This technique is also used especially for materials with a distinct absorption edge that matches the bandgap, such as semiconductors and insulators. The Tauc method uses the absorption spectrum which is represented in Fig. 5 a. The band gap energy (Eg) of Li3Cr2(PO4)3 was calculated using the widely recognized quadratic Tauc equation [27]:
| (2) |
Where α is the absorption coefficient, h is the Planck constant, ν is the photon's frequency, Eg is the band gap energy and A is a constant. The exponent value n depends on the nature of the band gap which is 1/2 for an allowed direct band gap and has the value 2 for an allowed indirect bandgap [28]. Fig. 5 b displays graphs of (αhν)2 and (αhν)1/2 plotted against photon energy (hν). Based on this analysis, we determined the optical energy band gaps for direct transition Egd to be 2.95 eV and for indirect transition Egi to be 2.46 eV.
Fig. 5. a.
The absorption spectrum of Li3Cr2(PO4)3compound.
Fig. 5.b.
The evolution of (αhν)1/2and (αhν)2against (hν).
We reorganize Equation (2) to confirm the type of optical band gap (whether direct or indirect), and we obtain the following relationship:
| (3) |
as shown in Equation (3), the plot of Ln(αhv) against Ln(hν-2.95) for Li3Cr2(PO4)3 is shown in Fig. 6. The power factor (n = 0.51), which is near to 0.5, is represented by the slope of the line in this plot, which depicts a linear relationship. According to this value, our sample coincides with wide optical band gap semiconductors that exhibit a direct band gap transition. The obtained value (2.95 eV) is in close agreement with the Marotti method result.
Fig. 6.
Evolution of Ln(αhν) against Ln(hν-2.95) for Li3Cr2(PO4)3.
3.3.2. Urbach energy Eu
The Urbach energy is a fundamental parameter in solid-state physics that characterizes the exponential tail of the absorption spectrum in a semiconductor or insulator. It is typically determined by the following relation [29]:
| (4) |
The Urbach energy can be calculated based on Equation (4) by plotting (Ln(α)) against photon energy (hv) as illustrated in Fig. 7. The calculation of the Urbach energy is done by taking the reciprocal of the slope of the linear portion of this curve. Based on our analysis, we found that the band gap energy is around 18.3 % of the Urbach energy (Eu), which is 0.54 eV. This value indicates the density of localized states that exist within the compound, so verifying the existence of imperfections and structural disorder.
Fig. 7.
Curve of Ln(α) against hν to determine the Urbach energy for the Li3Cr2(PO4)3 compound.
3.3.3. The skin depth δ, the extinction coefficient k and the refractive index n of Li3Cr2(PO4)3
Penetration depth or alternatively optical depth or optical skin depth is a measure of how deep light can penetrate into a material before being significantly absorbed or attenuated. This parameter depends of the frequency of the light and it is from absorption coefficient (α) according to the formula [[30], [31], [32]]:
| (5) |
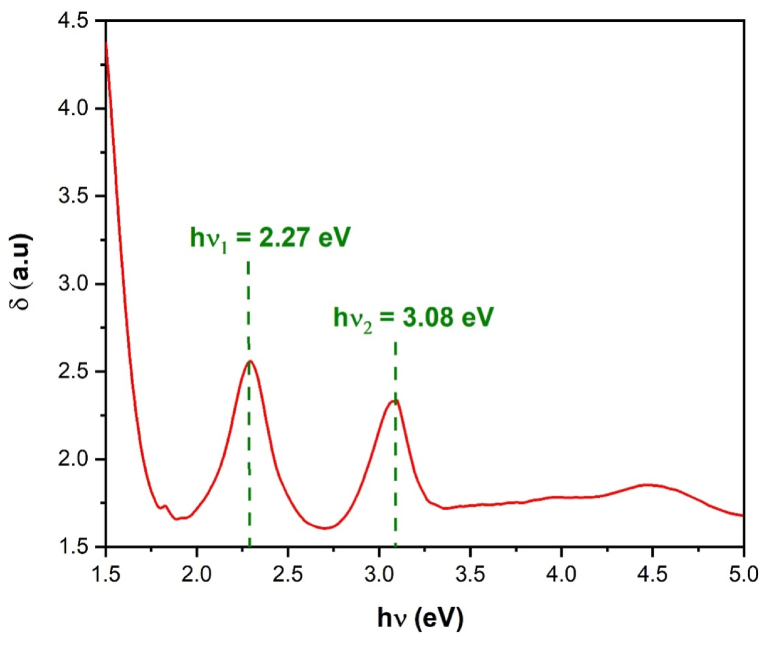
As shown in Equation (5), Fig. 8 shows the plot of skin depth δ versus (hν) for Li3Cr2(PO4)3. The spectrum exhibits the detection of two important penetration depths at specific energy levels within the visible region: hν1 = 2.27 eV and hν2 = 3.08 eV, signifies significant characteristics of materials' interactions with light in this spectral range. These particular penetration depths impact their optical behavior.
Fig. 8.
Skin depth (δ) against photon energy (hν) for Li3Cr2(PO4)3.
The absorption and scattering of light by a substance at a particular wavelength are quantitatively represented by the extinction coefficient k which is crucial in the design and characterization of optical materials and devices. The evolution of k parameter is determined by the following equation [26]:
| (6) |
According to Equation (6), Fig. 9 illustrates the evolution of k parameter versus hν. This figure demonstrates the presence of at almost three peaks in the visible region. These variations in the extinction coefficient are caused by variation in absorbance and the dark blue colour of the crystal.
Fig. 9.
The evolution of the extinction coefficient (k) as a function of the wavelength hν.
Understanding the refractive index (n) of materials is important for designing and optimizing optical components and devices [[33], [34], [35]]. For reflectance and extinction, the coefficient n is defined by the following Equation (7) [36]:
| (7) |
Fig. 10 shows the curve of refractive index against wavelength λ. This curve displays two important peaks at almost 402 nm (3.1 eV) and 541 nm (2.3 eV) which are very close to the maxima of the optical depth depicted in Fig. 8 and the minima of extinction displayed in Fig. 9.
Fig. 10.
Refractive index (n) versus wavelength λ forLi3Cr2(PO4)3.
4. The analysis of the absorption spectrum of Li3Cr2(PO4)3
4.1. Identification of interference dip in 4T2g(4F) exited state
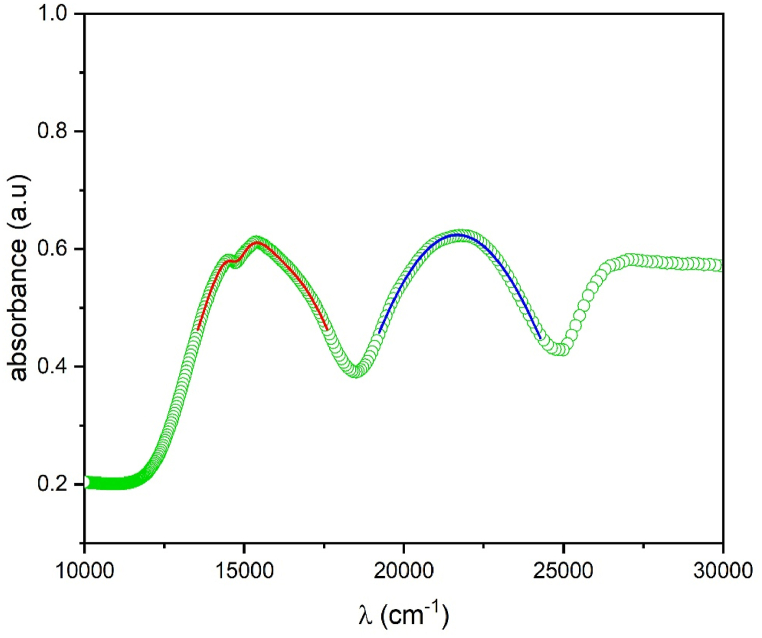
In this part we will focus on the absorption spectrum of Fig. 11 recorded at ambient temperature. So, by examining the absorption features in this spectrum, we can elucidate the energy levels involved in the transitions and gain a deeper understanding of the electronic structure of the material. In this spectrum, two broad absorption bands are observed, corresponding to spin-allowed transitions from the ground state 4A2g(4F) to the excited states 4T2g(4F) and 4T1g(4F). Notably, an interference dip is observed within the broad band associated with the 4T2g(4F) transition. This interference dip is caused by the overlap of spin-forbidden transitions from the ground state 4A2g(4F) to the excited state 2Eg(2G). Our group is interested in understanding the likely features observed in absorption spectra [[37], [38], [39]] (see Fig. 12).
Fig. 11.
Absorption spectrum of Li3Cr2(PO4)3 phosphate and the results of the Neuhauser fit (Red colour), the Gaussian fit (blue colour).
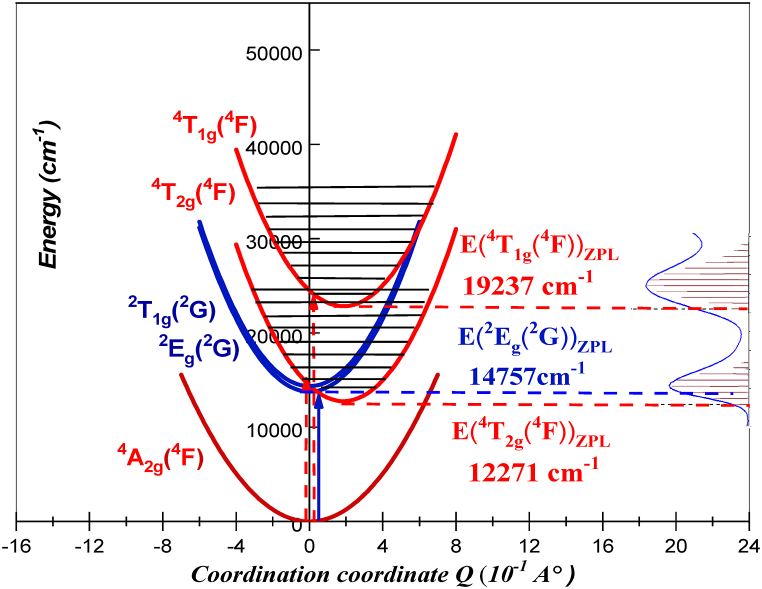
Fig. 12.
Modeling of the absorption spectrum for the of Li3Cr2(PO4)3 phosphate. The vertical arrows indicate the positions of the excited-state ZPL energies, E(4T2g(4F))ZPL, E(2Eg(2G))ZPL and E(4T1g(4F))ZPL.
The interaction between states with distinct spin multiplicities is the source of the interference dip seen in this situation. In this work, we utilize the Neuhauser method for a main objective which is to accurately determine the levels of 2Eg(2G) and the position of 4T2g(4F) state. All the details of the methodology outlined by Neuhauser et al. [40] and Bussière et al. [41] are presented in the following references [5,38,39].
In the case of Li3Cr2(PO4)3 the analytical expression describing the profile of the absorption spectrum is written as:
| (8) |
As given by Equation (8), γ1 is the coupling constant of the forbidden state αwith the allowed one. ω is the frequency, β is the spectrum without coupling. The α and β equations are:
| (9) |
| (10) |
The width of individual vibronic lines is determined by Γ, a phenomenological damping factor, where Δ is the maximum of the absorption band without coupling and εF is the energies of the forbidden state. The width of the allowed band in Equation (9) is denoted by , where λe = Δ - εA and ω0 is the metal-ligand band's stretching frequency.
The absorption spectrum is fitted by:
| (11) |
Equation (10), which is specifically programmed in Origin software, was used in the study to examine a single interference dip that was seen on the 4T2g(4F) band, as depicted in Fig. 11. The location of the dip is determined by this equation by calculating the energies associated with the 4T2g(4F) and 2Eg(2G) states. After the software's calculation of these energies, the coupling (γ) and damping (Γ) parameters are important for the fitting process. The optimal values for γ and Γ, with other fitting parameters, are found in Table 3 and lead to the best fit.
Table 3.
Fitting parameters in cm−1 for the calculation of the absorption spectra of Cr3+ in Li3Cr2(PO4)3.
| Transitions | Δ(εA(4T2g(4F))) | εF(2Eg(2G)) | Г | γ | |
|---|---|---|---|---|---|
|
4A2g(4F) → 4T2g(4F) (Neuhauserfit Eq (11)) |
15367 | 3038 | 14757 | 388 | 258 |
|
4A2g(4F) → 4T1g(4F) (Gaussian fit) |
21365 | – | – | – | – |
ωo = 376 cm−1 (totally symmetric Cr–O stretching mode of CrO63−).
By applying the analytical Equation (10), the analysis successfully reproduces the observed dip by showing a strong correspondence between the computed results and the absorption spectrum. It is noteworthy to emphasize that the energy corresponding to the doublet state (εF = 2Eg(2G)) is located at the depth of the dip instead of at the maximum of the peak. The parameter Δ = E(4T2g(4F)) represents the energy at the maximum peak of the wider band, roughly at the halfway point. The depth of these dips depends on the parameter Γ. γ = 258 cm⁻1 is the coupling constant between 2E(2G) and 4T2(4F). Furthermore, γ and the spin-orbit (S–O) coupling constant are related as γ = [39], which results in ξ = 235 cm⁻1. For the band 4T1g(4F), the maximum of the peak is obtained by Gaussian fit and is represented in Table 3.
The Neuhauser method primarily allows for a precise reading of the spin-forbidden transition 4A2g(4F)→2Eg(2G) due to the overlap between the broad band 4T2g(4F) and the narrow peak 2Eg(2G) wich lead to interference deep. It's important to note that for the broad bands 4T2g(4F) and 4T1g(4F), the Neuhauser method fits a Gaussian band, and the obtained energies corresponds to the maximum of these bands. Additionally, it is worth noting that when considering the zero-phonon line, the 4T2g(4F) level appears before the 2Eg(2G) level, which is the opposite of what is obtained with the Neuhauser method. For this reason we determine the zero-phonon line ZPL energies (E(4T2g(4F))ZPL and E(4T1g(4F))ZPL energies) of the Cr3+ ions from the absorption spectrum (Fig. 11). The E(2Eg(2G))ZPL energy is already determined from the Neuhauser method (Table 3). For determining the ZPL energies, we model the absorption spectrum (Fig. 11) by using the autocorrelation function. This function illustrates the dynamic evolution of the wave function from its initial state to its final state within a potential energy well. The Fourier transform of the autocorrelation function, which is the overlap of φ(t) with φ(t = 0), results in the absorption spectrum [4]:
The evaluation of the autocorrelation function is simplified by assuming (i) the potential energy wells are harmonic with identical force constants for the initial and final states, (ii) a constant transition dipole moment, and (iii) identical normal coordinates for both states. Under these assumptions, the autocorrelation function can be expressed analytically as follows [4]:
EZPL is the energy at the origin of the broad band (Zero Phonon Line), the frequency of the absorption spectrum is ω, and the breadth of each line in the spectrum is determined by the phenomenological damping factor Γ. For all amounts, wavenumber units are used. The terms kj (frequency of 376 cm−1 is used) and represent the vibrational frequencies of each mode and the displacement along the normal coordination Qj of the potential surfaces, respectively. The experimental absorption spectrum is fitted by adjusting the parameters E(4T2g(4F))ZPL and E(4T1g(4F))ZPL, Γ, and . Table 4 lists the values that were obtained.
Table 4.
Parameters used to calculate the absorption spectrum shown in Fig. 11.
| parameters | values |
|---|---|
| EZPL(4T2g(4F)) (cm−1) | 12756 |
| EZPL(2Eg(2G)) (cm−1) | 14757 |
| Γ (cm−1) | 18 |
| k (cm−1), (dim,less) | 376, 1.86 |
| EZPL(4T1g(4F)) (cm−1) | 19237 |
| Γ (cm−1) | 20 |
| k (cm−1), (dim,less) | 376, 2.21 |
4.2. Crystal field study of the Cr3+ in Li3Cr2(PO4)3
Understanding the electronic structure and behavior of transition metal complexes, especially coordination compounds, is possible through the application of Crystal Field Theory (CFT) framework. These complexes have ligands, or atoms or molecules that coordinate with the metal, all around a central metal ion. According to CFT, there is only electrostatic interaction between the metal ion and ligands. Ligands, which are usually negatively charged, surround the metal ion with a crystal field that affects the distribution and energy levels of its d-electrons. The following general Hamiltonian (Equation (12)) is utilized to ascertain the energy states of Cr3+ in Li3Cr2(PO4)3 compound [[42], [43], [44]]:
| H = H0+ Hee(B,C) + HTrees(α) + HCF(Dq) + HSO(ζ) | (12) |
-
•
H0 the Hamiltonian configuration
-
•
Hee the electron-electron repulsion term given as function of the Racah parameters (B and C) [[45], [46], [47]].In our case Cr3+ (3d [3]),the Russell-Saunders terms, representing the 2S+1L states, comprise 4F,4P,2H, 2G, 2F, and 2P terms.
-
•
The HTrees Hamiltonian relies on the fine structure parameter αTree and incorporates the Trees correction [48,49].
-
•
HCF is the crystal field Hamiltonian. In fact, in the case of Li3Cr2(PO4)3, The Cr3+ (3 d3) occupy an octahedral site symmetry and is given by the following equation [50,51]:
| (13) |
The ligand field splitting in the crystal field Hamiltonian HCF is denoted by the parameter Dq, which is frequently used to describe the structure of transition metal ions. The matrix elements of the operators are numerically calculated using the Racah tensor algebraic methods [52]. The crystal field Hamiltonian HCF is developed as a function of the Racah tensor operators defined as follows:
are the spherical harmonics.
For transition ions of the first series with electronic configuration 3dN, the crystal-field is of the intermediate strength [5,8,9], the basis functions in the LS-coupling scheme have been adopted for the theoretical calculation.
By using the Racah tensor algebraic methods, the matrix elements of Cq(k) Racah tensor operators are calculated numerically [27]:
| (14) |
According to Equation (14), the 3j-symbols carry all the dependence on the labels ML, q and ML’ while the reduced matrix elements is independent of these labels, but depends on the normalization of the tensor operators.
HSOisthe spin–orbit coupling Hamiltonian and is function of the ζ parameter which is the strength of the interaction between the orbital motion of an electron and its spin. The αTree and ζ parameters are calculated from the equations [26]:
| αTrees = N4α0 and ζ = N2ζ0 | (15-a) |
where the parameter N describes the average reduction factor due to covalency [26]is defined as follows:
| (15-b) |
The free ion Cr3+ parameters B0, C0, α0 and ζ0are illustrated in Table 5 [8,9].
Table 5.
Crystal field, Racah, trees, spin-orbit coupling and nephelauxetic effect parameters values for Cr3+ in Li3Cr2(PO4)3.
| Dq (cm−1) | 1275 |
|---|---|
| B (cm−1) | 730 |
| C (cm−1) | 3190 |
| α (cm−1) | 23.504 |
| ζ (cm−1) | 243.414 |
| Dq/B | 1.75 |
| C/B | 4.37 |
| βB | 0.795 |
| βC | 0.772 |
| B0 (cm−1)[8–9] | 918 |
| C0(cm−1)[8–9] | 4133 |
| α0 [8–9] | 30 |
| ζ0 [8–9] | 275 |
The theoretical computation of energy levels for Cr3+ ions in Li3Cr2(PO4)3 involves diagonalizing the Hamiltonian Equation (13), which consists of a (120x120) matrix representing free ion eigenstates within quartet (4F ground state and 4P excited state) and doublet excited terms (2H, 2G, 2F, and 2P). A specialized code developed by our team, based on Maple software, is employed for diagonalization. The computed energies align closely with Yeung et al.'s findings [53], validating our computational approach. These energies, expressed as analytical equations, depend on parameters B, C, Dq, α, and ζ. Dq is determined from quartet excited state absorption energy, while B and C are derived from observed quartet and doublet excited states, respectively. Parameters α and ζ are calculated from Equation (15-a), (15-b). Inputting these parameters into our software package allows simulation of Cr3+ ion electronic structure and energy levels in Li3Cr2(PO4)3. The resulting parameters, detailed in Table 5, enable deduction of theoretical energy levels for Cr3+(3 d3) in Oh site symmetry (Table 6). Our computational approach, simplified by the developed software package, shows remarkable consistency between predicted Stark energy levels and actual experimental results.
Table 6.
Experimental and calculated energies (cm−1) of Cr3+ in Li3Cr2(PO4)3.
| Oh | Eobs | Ecal* [this work] | Eacal* [this work] |
|---|---|---|---|
| 4A2g(4F) | 0 | 0 | 0 |
| 4T2g(4F) | 12756 | 12750 | 12656 (2) |
| 12706 (4) | |||
| 12772 (4) | |||
| 12809 (2) | |||
| 2Eg(2G) | 14757 | 14755 | 14964 (4) |
| 2T1g(2G) | 15578 (2) | ||
| 15678 (2) | |||
| 4T1g(4F) | 19237 | 19239 | 19063 (2) |
| 19088 (4) | |||
| 19239 (4) | |||
| 19286 (2) | |||
| 2T2g(2G) | – | 21420 | 21520 (4) |
| 21669 (2) | |||
| 2A1g(2G) | – | 25240 | 25447 (2) |
| 2T1g(2P) | – | 27421 | 27665 (2) |
| 27718 (4) | |||
| 2T1g(2H) | – | 27992 | 28058 (2) |
| 28242 (4) | |||
| 2Eg(2H) | – | 29738 | 29653 (4) |
| 4T1g(4P) | – | 29960 | 29818 (2) |
| 29836 (2) | |||
| 29837 (4) | |||
| 30082 (4) | |||
| 2T1g(2H) | – | 32633 | 32692 (2) |
| 32786 (4) | |||
| 2T2g(2H) | – | 38006 | 37970 (4) |
| 38004 (2) | |||
| 2A2g(2F) | – | 39840 | 39853 (2) |
| 2T2g() | – | 40360 | 40663 (2) |
| 40772 (4) | |||
| 2T2g(2F) | – | 42741 | 42708 (2) |
| 42920 (4) | |||
| 2Eg() | – | 46052 | 46139 (4) |
| 2T1g(2F) | – | 48074 | 47988 (2) |
| 48099 (4) | |||
| 2Eg() | – | 62933 | 62909 (4) |
| 63591 (4) | |||
| 2T2g() | – | 63730 | 63748 (2) |
An intriguing approach for comprehending the absorption spectrum is the coupled potential energy surface model. This approach allows for the examination of electronic transitions, particularly intra-configuration d-d excitations. A single normal coordinate, Q, is employed in the model of coupled potential energy surfaces. Notably, the doublet 2Eg(2G) state and the ground 4A2g(4F) state share identical vibrational frequencies and positions of the potential energy minimum at Q = 0 Å [43]. However, a shift ΔQ causes the 4T2g(4F) state transitioning to longer metal-ligand bond distances. This change occurs because of the way certain molecular orbitals, specifically metal-ligand antibonding ones, are populated through a process called d-d excitation [43]. The potentials for the excited states 2Eg(2G), 4T2g(4F) and 4T1g(4F) are as follows:
| (16-a) |
| (16-b) |
| (16-c) |
in the absorption spectrum, the breadth of the 4T2g(4F) and 4T1g(4F) bands corresponds to the magnitude of the displacements ΔQ. The EZPL(2Eg(2G)), EZPL(4T2g(4F)) and EZPL(4T1g(4F)) are the energies of the potential minimum for the 2Eg(2G), 4T2g(4F) and 4T1g(4F) states, respectively. The autocorrelation function's modelling of the absorption spectra yielded the values of the quantities ΔQ, EZPL(2Eg(2G)), EZPL(4T2g(4F)) and EZPL(4T1g(4F)) (Table 4). In equation (15-a), (15-b), k is the frequency observed on the Raman spectrum of Li3Cr2(PO4)3 assigned to Cr–O symmetric stretching mode (a1g) denoted as ω0 (376 cm-1) in section 4-1. The potentials state surfaces, obtained from Equation (15-a), (15-b) and from the spectroscopic parameters shown in Table 4, are depicted in Fig. 13.
Fig. 13.
The diabatic potentials energy curves for 2Eg(2G), 4T2g(4F) and 4T1g(4F).
This diagram describes the electronic transitions at the zero-phonon line (ZPL) between the ground state 4A2g(4F) and the excited states 2Eg(2G), 4T2g(4F) and 4T1g(4F).
Assuming that for Cr3+, the value of k is 0.21 [54], the bonding nature of Cr3+ is evaluated using the following expression [55]:
| (17) |
Based on Equation (17), the calculation of h gives a value of 0.975. This value indicates a d-electron delocalization, indicating predominant ionic bonding between Cr3+ and its ligands. Utilizing the Racah parameters B and C from Table 3 (with C/B = 4.37), we construct the Tanabe-Sugano diagram for Cr3+ in an octahedral site symmetry (Fig. 14). This diagram illustrates the energy levels of Cr3+ ions concerning Dq/B as influenced by the local field strength. The specific case of Cr3+ in Li3Cr2(PO4)3 phosphate is represented by a vertical line corresponding to the Dq/B value obtained from our theoretical calculations.
Fig. 14.
Tanabe-Sugano diagram for Cr3+ in an octahedral site symmetry. The vertical red line corresponds to the Dq/B = 1.75 obtained from our theoretical calculations.
An additional method to elucidate the origin of interference dips involves examining the exchange of characteristics between the 2Eg(2G) and 4T2g(4F) states, which exhibit proximal behavior. These two states may combine and exchange some of their respective characters. As illustrated in Fig. 15 through the process of spin-orbit coupling, the excited states 2Eg (2G) and 4T2g (4F) can be divides into distinct E1, E2, and G states. Remarkably, these closely positioned states maintain an identical G character, facilitating a confluence of their attributes.
Fig. 15.
Splitting of Stark levels 4T2g (4F) and 2Eg (2G) under spin-orbit coupling of Cr3+ in Li3Cr2(PO4)3. Spin-allowed transition is indicated by solid arrows. Under spin-orbit coupling, pairs of interacting levels are connected by dot-point arrows.
Consequently, the typically forbidden transition 4A2g(4F)→2Eg(2G) assumes some features of the permitted transition 4A2g(4F)→ 4T2g(4F), resulting a gain in intensity which is lower than that of the allowed transition.
4.3. The diabatic/adiabatic approach for the interpretation of the forbidden transition
As we have mentioned in previous, the existence of a dip in the 4T2g(4F) band, which is a forbidden transition. This feature can be caused by the interaction between states that are close to one another but have different spin multiplicities. As shown by Fano in atomic spectroscopy, these bands are not just additive but also closely connected, leading to interference phenomena [[37], [38], [39]]. To investigate the coupling between states of different spin multiplicities, we use again the model of coupled potential energy surfaces with a single normal coordinate Q. The potentials for the excited states 2Eg(2G) and 4T2g(4F) in the absence of spin-orbit coupling are given by equation (15-a), (15-b). In this diabatic approach, the two potential wells of the excited states are considered independently of each other. So, Rapid nuclear motion prevents transitions between these wells, effectively sealing off any exchange of characteristics. The uncoupled (diabatic) potentials, resulting in the absence of spin-orbit coupling are illustrated in Fig. 16a. For the sake of simplification, only the derivative states that have G irreducible representations are displayed in Fig. 16b. The curves obtained from Equation (15a) and b cannot describe the spin forbidden transition 4A2g(4F) → 2Eg(2G) detected in the absorption spectrum (Fig. 11).
Fig. 16.
a) The uncoupled (diabatic) potentials for 4T2g(4F) and 2E2g(2G) subdivided into E1, E2, and G states b) we represent only the derivative states that have G irreducible representations. c) the coupled (adiabatic) excited state potential energy curves V1 and V2, (spin-orbit coupling between 4T2g(4F) and 2Eg(2G) is taken into account).
Doublet excited state G(2Eg) can interact with the G(4T2g) states. According to this analysis, the doublet states receive most of their intensities from the allowed quartet transitions and the spin forbidden transition 4T2g(4F)→2Eg(2G) can arise from the interaction of the 3d [3] electrons of Cr3+ with the Li3Cr2(PO4)3 host matrix. The observed spin-forbidden transitions between the 4A2g(4F) ground state and the 2Eg(2G) excited state are caused by the interaction between the same symmetry levels (G) derived from electronic states of different multiplicities [4]. Effectively, a constant with a magnitude proportional to the spin-orbit coupling λ() [55] are considered to mix the characters of the 4T2g(4F), and 2Eg(2G) diabatic potentials. In this situation, the coupled potentials' matrix (adiabatic potentials) 2x2 is expressed in the following:
| (18) |
The diagonals Terms in Equation (18) represent the equations of the diabatic potential energy wells and are therefore identical to those of Equation (16-c), (16-a), (16-b). The diabatic/adiabatic approach will considerably modify the appearance of the theoretical absorption spectrum and the appearance of observed forbidden transitions experimentally, such as the band corresponding to states 4T2g(4F) and 2Eg of Fig. 11, will appear in the calculation. The adiabatic potentials state surfaces, obtained from Equation (18) and from the spectroscopic parameters shown in Table 4, are depicted in Fig. 16c. The spin-forbidden 4A2g(4F) → 2Eg(2G) transitions are caused by the adiabatic excited state potential energy curves V1 and V2, which are produced when the spin-orbit coupling between 4T2g(4F) and 2Eg(2G) is taken into account.
5. Conclusion
This study focused on the synthesis and analysis of Li3Cr2(PO4)3 phosphate. Scanning electron microscopy (SEM) imaging exhibited particle dimensions spanning from 0.2 to 1.2 μm, with an average size of 0.574 μm, indicating the emergence of micrometer-scale particles. UV/Vis absorption spectra, assessed through Tauc's law and Urbach energy calculations, revealed direct transition properties, with increased disorder and defect density attributed to the presence of Cr3+ ions. Significantly, absorption spectra at room temperature displayed an interference dip within the extensive 4T2g(4F) band, ascribed to the overlapping of spin-forbidden transitions 4A2g(4F) → 2Eg(2G). This phenomenon was clarified using the Neuhauser model, which relies on coupled potential energy surfaces. This model gives the ZPL energy of the 2Eg(2G) level. The Fourier transform of the autocorrelation function leads to the Zero Phonon Lines of the observed absorption energies 4T2g(4F) and 4T1g(4F). From these ZPL energies, we determine a deeper understanding of the electronic configuration of Cr3+ (3 d3) ions within Li3Cr2(PO4)3 phosphate by using the tensoriel Racah theory. It aided in establishing Racah and crystal-field parameters, ensuring a good agreement between theoretical predictions and observed energy levels. The diabatic/adiabatic approach was an important model to demonstrate the origin of a dip in the 4T2g(4F). The obtained results hold significance for numerous optical applications.
CRediT authorship contribution statement
Hajer Souissi: Writing – original draft. Souha Kammoun: Software. Essebti Dhahri: Visualization. E. López-Lago: Data curation. B.F.O. Costa: Supervision.
Declaration of competing interest
This work includes an experimental study Lithium-chromium phosphate Li3Cr2(PO3)4 using the solid-state reaction method. The morphological integrity and chemical homogeneity were verified by energy dispersive X-ray analysis (EDX) and scanning electron microscopy (SEM). Infrared and Raman patterns were also analyzed. The optical properties have been study using the absorption characterization. An interference dip was found on the 4T2g(4F) band in the absorption spectrum of Li3Cr2(PO3)4, which was explained by spin-orbit coupling between the 2Eg(2G) and 4T2g(4F) states. The electronic structure of Cr3+ (3d [3]) ions in Li3Cr2(PO3)4 was calculated using the Neuhauser model. The theoretical part is also carried out using software that we have developed ourselves in our laboratory and it is rarely that we find this type of calculation because this software is based on Racah method (the most powerful method that describe the electronic structure of transition ions) and it is not easy and requires specialists. The programming lasted about 6 years and each time we add more precision. This software uses Maple 20 as software is as well tested and gives the same result as another software using the Fortran software and we can prove that. We have published several articles and in well-indexed newspapers.
References
- 1.Saïdi M.Y., Barker z J., Huang H., Swoyer J.L., Adamson G. Electrochemical properties of lithium vanadium phosphate as a cathode material for lithium-ion batteries. Journal of Electrochemical and Solid-State Letters. 2002;5:149–151. doi: 10.1016/S0378-7753(03)00245-3. [DOI] [Google Scholar]
- 2.Saïdi M.Y., Barker J., Huang H., Swoyer J.L., Adamson G. Performance characteristics of lithium vanadium phosphate as a cathode material for lithium-ion batteries. J. Power Sources. 2003:266–272. doi: 10.1016/s0378-7753(03)00245-3. [DOI] [Google Scholar]
- 3.Huang H., Faulkner T., Barker J., Saidi M.Y. Lithium metal phosphates, power and automotive applications. J. Power Sources. 2009;189:748–751. doi: 10.1016/j.jpowsour.2008.08.024. [DOI] [Google Scholar]
- 4.Souissi H., Kammoun S. Theoretical study of the electronic structure of a tetragonal chromium (III) complex. J. Lumin. 2011;131:2515–2520. doi: 10.1016/j.jlumin.2011.05.044. [DOI] [Google Scholar]
- 5.Taktak O., Souissi H., Kammoun S. Electronic structure and Fano antiresonance of chromium Cr(III) ions in alkali silicate glasses. J. Lumin. 2015;161:368–373. doi: 10.1016/j.jlumin.2015.01.047. [DOI] [Google Scholar]
- 6.Sassi M., Oueslati A., Moutia N., Khirouni K., Gargouri M. A study of optical absorption and dielectric properties in lithium chromium diphosphate compound. Ionics. 2016;23:847–855. doi: 10.1007/s11581-016-1903-y. [DOI] [Google Scholar]
- 7.Herklotz M., Scheibaa F., Glaumc R., Mosymowc E., Oswalda S., Eckert J., Ehrenberg H. Electrochemical oxidation of trivalent chromium in a phosphate matrix: Li3Cr2(PO4)3 as cathode material for lithium-ion batteries. J. Electrochimica Acta. 2014;139:356–364. doi: 10.1016/j.electacta.2014.06.170. [DOI] [Google Scholar]
- 8.Taktak O., Souissi H., Kammoun S. Optical properties of the phosphors Zn2SnO4:Cr3+ with near-infrared long-persistence phosphorescence for bio-imaging applications. J. Lumin. 2020;228 doi: 10.1016/j.jlumin.2020.117563. [DOI] [Google Scholar]
- 9.Souissi H., Taktak O., Kammoun S. Theoretical study by analytical equations of optical properties of the phosphors Zn2SnO4 codoped with Cr3+ and (Al3+ or Eu3+) via the crystal field control. Opt. Mater. 2021;121 doi: 10.1016/j.optmat.2021.111529. [DOI] [Google Scholar]
- 10.Ledoux I., Zyss J. Polar and non-polar organic molecules, crystals and Langmuir-blodgett films for quadratic nonlinear optics. Organic Molecules for Nonlinear Optics and Photonics. 1991:81–103. doi: 10.1007/978-94-011-3370-8_6. [DOI] [Google Scholar]
- 11.Zaafouri A., Megdiche M., Gargouri M. AC conductivity and dielectric behavior in lithium and sodium diphosphate LiNa3P2O7. J. Alloys Comp. 2014;584:152–158. doi: 10.1016/j.jallcom.2013.08.143. [DOI] [Google Scholar]
- 12.Krichen M., Megdiche M., Guidara K., Gargouri M. AC conductivity and mechanism of conduction study of lithium barium pyrophosphate Li2BaP2O7 using impedance spectroscopy. Ionics. 2015;21:935–948. doi: 10.1007/s11581-014-1261-6. [DOI] [Google Scholar]
- 13.Pang R., Li C., Jiang L., Su Q. Blue long-lasting phosphorescence of Tm3+ in zinc pyrophosphate phosphor. J. Alloys Compd. 2009;471:364–367. doi: 10.1016/j.jallcom.2008.03.104. [DOI] [Google Scholar]
- 14.Pu Y., Huang Y., Tsuboi T., Cheng H., Seo H.J. Intrinsic [VO4]3− emission of cesium vanadate Cs5V3O10. RSC Adv. 2015;5:73467–73473. doi: 10.1039/c5ra11823a. [DOI] [Google Scholar]
- 15.Huang H., Yin S.-C., Kerr T., Taylor N., Nazar L.F. Nanostructured composites: a high capacity, fast rate Li3V2(PO4)3/carbon cathode for rechargeable lithium batteries. Adv. Mater. 2002;14:1525. doi: 10.1002/1521-4095(20021104)14:21%3C1525::aidadma1525%3E3.0.co;2-3. [DOI] [Google Scholar]
- 16.Bykov A.B., Chirkin A.P., Demyanets L.N., Doronin S.N., Genkina E.A., Ivanov-Shits A.K., Kondratyul I.P., Maksimov B.A., Mel'nikov O.K., Muradyan L.N., Simonov V.I., Timofeeva V.A. Synthesis and electrophysical properties of superionic conductors Li3M2(PO4)3 (M = Fe, Sc, Cr) Solid State Ionics. 1990;38:31. doi: 10.1007/978-1-4615-3662-8_4. [DOI] [Google Scholar]
- 17.Herklotz M., Scheiba F., Glaum R., Mosymow E., Oswald S., Eckert J. Electrochemical oxidation of trivalent chromium in a phosphate matrix: Li3Cr2(PO4)3 as cathode material for lithium ion batteries. Electrochim. Acta. 2014;139:356. doi: 10.1016/j.electacta.2014.06.170. [DOI] [Google Scholar]
- 18.Bih H., Bih L., Manoun B., Azdouz M., Benmokhtar S., Lazor P. Raman spectroscopic study of the phase transitions sequence in Li3Fe2(PO4)3 and Na3Fe2(PO4)3 at high temperature. J. Mol. Struct. 2009;936:147–155. doi: 10.1016/j.molstruc.2009.07.035. [DOI] [Google Scholar]
- 19.Higazy A.A., Bridge B. Infrared spectra of the vitreous system Co3O4-P2O5 and their interpretation. J. Mater. Sci. 1985;20:2345. doi: 10.1007/bf00556064. [DOI] [Google Scholar]
- 20.Nakamoto K. Wiley; New York: 1997. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A. Theory and Applications in Inorganic Chemistry. [DOI] [Google Scholar]
- 21.Dayanand C., Bhikshaaiah G., Jayatyagaraju V., Salagram M., Krishnamurthy A.S.R. Structural investigations of phosphate glasses: a detailed infrared study of the x(PbO)-(1−x) P2O5 vitreous system. J. Mater. Sci. 1996;31:1945. doi: 10.1007/bf00356615. [DOI] [Google Scholar]
- 22.Rao K.J., Sobha K.C., Kumar S. Infrared and Raman spectroscopic studies of glasses with NASICON-type chemistry. J. Chem. Sci. Oct. 2001;113(5–6):497–514. doi: 10.1007/bf02708786. [DOI] [Google Scholar]
- 23.Barj M., Lucazeau G., Delmas C. Raman and infrared spectra of some chromium Nasicon-type materials: short-range disorder characterization. J. Solid State Chem. 1992;100:141–150. doi: 10.1016/0022-4596(92)90164-q. [DOI] [Google Scholar]
- 24.Sigaryov Sergei E. Fast-ion transport mechanism in Li3M2(PO4)3 crystals (M ≡ Sc, Cr, Fe, In)) Mater. Sci. Eng. 1992;B13:121–123. doi: 10.1016/0921-5107(92)90152-y. [DOI] [Google Scholar]
- 25.Mguedla R., Ben Jazia Kharrat A., Saadi M., Khirouni K., Chniba-Boudjada N., Boujelben W. electrical, dielectric and optical properties of PrCrO3 ortho-chromite. J. Alloys Compd. 2020;812 doi: 10.1016/j.jallcom.2019.152130. [DOI] [Google Scholar]
- 26.Taktak O., Souissi H., Elhamdi I., Oueslati A., Kammoun S., Gargouri M., Dhahri E. Optical investigations and theoretical simulation of organic-inorganic hybrid: TPA-CoCl4. J. Opt. Mater. 2024;150 doi: 10.1016/j.optmat.2024.115251. [DOI] [Google Scholar]
- 27.Tauc J., Menth A. States in the gap. J. Non-Cryst. Solids. 1972;8:569–585. doi: 10.1016/0022-3093(72)90194-9. [DOI] [Google Scholar]
- 28.Gagandeep, Singh K., Lark B.S., Sahota H.S. Attenuation measurements in solutions of some carbohydrates. Nucl. Sci. Eng. 2000;134:208–217. doi: 10.13182/nse00-a2111. [DOI] [Google Scholar]
- 29.Kalyanaraman S., Shajinshinu P.M., Vijayalakshmi S. Determination of optical constants and polarizability studies on ferroic Tetramethylammonium Tetracholorozincate crystal. Phys. B Condens. Matter. 2016;482:38–42. doi: 10.1016/j.physb.2015.12.009. [DOI] [Google Scholar]
- 30.John Wiley & amp Sons, Inc. Analytical Chemistry. 1984;56:333. doi: 10.1021/ac00266a874. [DOI] [Google Scholar]
- 31.Chiad S.S. Int. Lett. Chem. Phys. Astron. 2015;6:50–54. [Google Scholar]
- 32.Hassanien A.S., Akl A.A. Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50−xSex thin films. J. Alloys Compd. 2015;648:280–290. doi: 10.1016/j.jallcom.2015.06.231. [DOI] [Google Scholar]
- 33.Hassanien A.S., Aly K.A., Akl A.A. Study of optical properties of thermally evaporated ZnSe thin films annealed at different pulsed laser powers. J. Alloys Compd. 2016;685:733–742. doi: 10.1016/j.jallcom.2016.06.180. [DOI] [Google Scholar]
- 34.Duerloo K.-A.N., Ong M.T., Reed E.J. Intrinsic piezoelectricity in two-dimensional materials. Journal of Phys Chem Letters. 2012;3:2871–2876. doi: 10.1021/jz3012436. [DOI] [Google Scholar]
- 35.Dhas C.R., Venkatesh R., Sivakumar R., Raj A.M.E., Sanjeeviraja C. Effect of solution molarity on optical dispersion energy parameters and electrochromic performance of Co3O4 films. Opt. Mater. 2017;72:717–729. doi: 10.1016/j.optmat.2017.07.026. [DOI] [Google Scholar]
- 36.Souissi H., Taktak O., Khalfa M., Oueslati A., Kammoun S., Gargouri M. Experimental and optical studies of the new organic inorganic bromide: [(C3H7)4N]2CoBr4. Opt. Mater. 2022;129 doi: 10.1016/j.optmat.2022.112513. [DOI] [Google Scholar]
- 37.Taktak O., Souissi H., Kammoun S. Optical absorption properties of ZnF2-RO-TeO2 (R = Pb, Cd and Zn) glasses doped with chromium (III): Neuhauser model and crystal field study. Opt. Mater. 2021;113 doi: 10.1016/j.optmat.2020.110682. [DOI] [Google Scholar]
- 38.Souissi H., Taktak O., Kammoun S. Crystal field study of Chromium(III) ions doped antimony phosphate glass: Fano's antiresonnance and Neuhauser models. Indian J. Phys. 2018;92:1153–1160. doi: 10.1007/s12648-018-1212-6. [DOI] [Google Scholar]
- 39.Maalej O., Taktak O., Boulard B., Kammoun S. Study with analytical equations of absorption spectra containing interference dips in fluoride glasses doped with Cr3+ J. Phys. Chem. B. 2016;120:7538–7545. doi: 10.1021/acs.jpcb.6b03230. [DOI] [PubMed] [Google Scholar]
- 40.Neuhauser D., Park T.-J., Zink J.I. Analytical derivation of interference dips in molecular absorption spectra: molecular properties and relationships to fano's antiresonance. Phys Rev Letters. 2000;85:5304–5307. doi: 10.1103/physrevlett.85.5304. [DOI] [PubMed] [Google Scholar]
- 41.Bussière G., Reber C., Neuhauser D., Walter D.A., Zink J.I. Molecular properties obtained by analysis of electronic spectra containing interference dips. Comparisons of analytical equations and exact models based on coupled potential energy surfaces. J. Phys Chem A. 2003;107:1258–1267. doi: 10.1021/jp0218490. [DOI] [Google Scholar]
- 42.Elhamdi I., Mselmi F., Kammoun S., Dhahri E., Carvalho A.J., Tavares B., Costa B.F.O. A far-red-emitting ZnAl1.95Cr0.05O4 phosphor for plant growth LED applications. Dalton Trans. 2023;52:9301–9314. doi: 10.1039/d3dt00969f. [DOI] [PubMed] [Google Scholar]
- 43.Souissi I. Elhamdi H., Kammoun S., Dhahri E., Brito A.L.B., Fausto R., Costa B.F.O. Experimental determination and modeling of structural, vibrational and optical properties of the ZnAl2−Cr O4 (x = 0 and 0.05) spinels. J. Lumin. 2023;263 doi: 10.1016/j.jlumin.2023.119968. [DOI] [Google Scholar]
- 44.Taktak O., Souissi H., Maalej O., Boulard B., Kammoun S. Fano׳s antiresonance and crystal-field study of Cr3+ in metaphosphate glasses. J. Lumin. 2016;180:183–189. doi: 10.1016/j.jlumin.2016.08.034. [DOI] [Google Scholar]
- 45.Sugano S., Tanabe Y., Kamimura H. Academic Press; New York: 1970. Multiplets of Transition-Metal Ions in Crystals. [DOI] [Google Scholar]
- 46.Griffith J.S. Cambridge University Press; 1961. The Theory of Transition-Metal Ions. [DOI] [Google Scholar]
- 47.Powell R.C. Al2O3: Cr3+ laser crystals. Physics of Solid-State Laser Materials. 1998:215–253. doi: 10.1007/978-1-4612-0643-9_6. [DOI] [Google Scholar]
- 48.Yang Z.-Y., Rudowicz C., Yeung Y.-Y. Microscopic spin-Hamiltonian parameters and crystal field energy levels for the low C3 symmetry Ni2+ centre in LiNbO3 crystals. Phys. B Condens. Matter. 2004;348:151–159. doi: 10.1016/j.physb.2003.11.085. [DOI] [Google Scholar]
- 49.Rudowicz C., Yang Z.-Y., Yeung Y., Qin J. Crystal field and microscopic spin Hamiltonians approach including spin–spin and spin–other-orbit interactions for d2 and d8 ions at low symmetry C3 symmetry sites: V3+ in Al2O3. J. Phys. Chem. Solid. 2003;64:1419–1428. doi: 10.1016/s0022-3697(03)00190-2. [DOI] [Google Scholar]
- 50.Newman D.J., Ng B. Crystal field splitting mechanisms. Crystal Field Handbook. 2000:6–25. doi: 10.1017/cbo9780511524295.003. [DOI] [Google Scholar]
- 51.Spector N. Vol. 148. Brian G. Wybourne Interscience (Wiley); New York: 1965. p. 1082. (Techniques and Interpretations: Spectroscopic Properties of Rare Earths). 1082. [DOI] [Google Scholar]
- 52.Elliott J.P., Judd B.R., Runciman W.A. Energy levels in rare-earth ions. Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. 1957;240:509–523. doi: 10.1098/rspa.1957.0104. [DOI] [Google Scholar]
- 53.Yeung Y.Y., Rudowicz C. Ligand field analysis of the 3dN ions at orthorhombic or higher symmetry sites. Computers & Chemistry. 1992;16:207–216. doi: 10.1016/0097-8485(92)80004-j. [DOI] [Google Scholar]
- 54.Seeber W., Ehrt D., Ebendorff-Heidepriem H. Spectroscopic and laser properties of Ce3+/Cr3+/Nd3+ co-doped fluoride phosphate and phosphate glasses. J. Non-Cryst. Solids. 1994;171:94–104. doi: 10.1016/0022-3093(94)90036-1. [DOI] [Google Scholar]
- 55.Jorgensen C.K., Hartmann H. Pergamon Press; Oxford (UK): 1963. Absorption Spectra and Chemical Bonding in Complex; p. 113. [DOI] [Google Scholar]