Abstract
Objective/Background
Sleep problems challenge overall wellbeing. Magnesium has been implicated to benefit sleep, although the clinical evidences varied based on the magnesium source used. Magnesium L-threonate (MgT) is a promising intervention due to its brain bioavailability and effects on cognition, memory and mood. We investigated MgT supplementation on sleep quality and daily function.
Patients/methods
Eighty 35–55-year-olds with self-assessed sleep problems participated in a randomized, double-blind, placebo-controlled, parallel-arm study, taking 1 g/day of MgT or placebo for 21 days. Sleep and daily behaviors were measured subjectively using standardized questionnaires including the Insomnia Severity Index, Leeds Sleep Evaluation Questionnaire, and Restorative Sleep Questionnaire, and objectively using an Oura ring. The Profile of Mood States questionnaire and a daily diary were used to evaluate mood, energy and productivity, and record any safety concerns.
Results
The MgT group maintained good sleep quality and daytime functioning, while placebo declined. From objective Oura ring measurements, MgT significantly (p < 0.05) improved vs placebo deep sleep score, REM sleep score, light sleep time, and activity and readiness parameters activity score, activity daily movement score, readiness score, readiness activity balance, and readiness sleep balance. From subjective questionnaires, MgT significantly (p < 0.05) improved vs placebo behavior upon awakening, energy and daytime productivity, grouchiness, mood and mental alertness. MgT was safe and well tolerated.
Conclusions
This showed MgT improved sleep quality, especially deep/REM sleep stages, improved mood, energy, alertness, and daily activity and productivity. These are consistent with how MgT works in neuron cells and animal models, suggesting broader positive impacts on overall brain health.
Keywords: Magnesium, Threonate, Magnesium L-threonate, Sleep, Mood, Energy, Mental alertness
Highlights
-
•
Magnesium L-threonate is brain bioavailable and improves neuronal function.
-
•
We assessed 3 weeks magnesium L-threonate for sleep quality and daytime functioning.
-
•
Taking magnesium L-threonate improved both subjective and objective sleep scores.
-
•
Magnesium L-threonate improved both subjective and objective daytime functioning.
-
•
Magnesium L-threonate is safe and well-tolerated.
1. Introduction
Globally, 62 % of adults report poor sleep quality according to a commercial poll [1]. Recent sleep quality literature suggests insomnia within a Scandinavian population of over 21,000 respondents ranges as much as 8.5 %–23.6 %, depending on the diagnostic criteria [2]. Other populations report similar ranges, for example 3.9 %–22.1 % in the US [3] and 4.7 %–22.1 % in Hong Kong [4], again depending on diagnostic criteria. In older people, the prevalence of sleep disorders ranges from 9.1 % to 69 % [5]. Types of sleep disturbances include insomnia, sleep disordered breathing, circadian rhythm sleep disorder, and others [6]. Irrespective of the source and criteria, clearly these substantial prevalences of sleep disturbance are concerning since poor sleep quality is associated with increased risk for physical, cognitive, and mental health issues such as obesity, diabetes, high blood pressure, coronary heart disease, depression, dementia, and stroke. Furthermore, poor sleep quality commonly leads to negative activity states such as poor mood, greater anxiety, and reduced energy and productivity.
Magnesium, a key cofactor in numerous enzymatic reactions, is proposed to enhance neuroplasticity and synaptic function [7]. This would be expected to enhance the brain's capacity to adapt to emotional challenges and mitigate stress responses. Furthermore, magnesium may regulate neurotransmitters such as glutamate and gamma-aminobutyric acid (GABA) [8]. Regulating GABA, an inhibitory neurotransmitter that promotes relaxation and reduces neural excitability [9], may have a calming effect that benefits sleep. Additionally, magnesium is important for the synthesis of N-acetyltransferase, which converts 5-hydroxytryptamine (5-HT) into N-acetyl-5-hydroxytryptamine, which can then be converted to melatonin. Increased melatonin secretion leads to improved sleep onset [10]. It was reported that plasma magnesium levels are lower in sleep deprived people (e.g., sleep less than 7 h per night [11]). Magnesium has previously been associated with improved sleep length and quality [12]. Observational studies found magnesium helps in maintaining a normal circadian rhythm, reducing daytime sleepiness, and improving sleep quality [13,14]. However, there is limited randomized placebo-controlled clinical trial evidence to support the benefits of magnesium in sleep [13,15,16], potentially due to the poor brain bioavailability of the magnesium compounds used in those studies.
Magnesium-L-threonate (MgT), is a novel magnesium salt with superior brain bioavailability [7]. MgT has been shown in animal studies to effectively deliver magnesium through the blood-brain barrier into the neuron cells leading to increased neural plasticity, improved memory and cognition, and reduced anxiety and stress [7,[17], [18], [19], [20], [21], [22], [23], [24]]. These benefits have also been demonstrated in humans [[25], [26], [27], [28], [29]]. Magnesium in the form of a salt with L-threonate, an endogenous compound found in cerebrospinal fluid [24], appears to be an essential form for this brain delivery of magnesium [7,24].
Given our growing understanding of brain bioavailable MgT, we hypothesize that MgT would also have benefits for sleep. Our objectives were to investigate the impact of MgT supplementation on sleep parameters, as well as on mood, daytime energy, and other related measurements. With this randomized placebo-controlled double-blind trial, we examined the effectiveness of magnesium supplementation on sleep quality and daytime activity, using MgT in healthy adults with non-clinical insomnia symptoms (i.e., self-assessed sleep problems). Participants underwent MgT supplementation for 21 days, with sleep and daytime mood and activity outcomes assessed through standardized psychometric questionnaire accompanied by objective Oura ring measurements, with the aim of discerning the effects of MgT on sleep, daily functioning, and emotional states.
Primary outcomes measures were sleep quality measured weekly with the Insomnia Severity Index, Leeds Sleep Evaluation Questionnaire and Restorative Sleep Questionnaire, and the Oura ring nightly. Secondary outcome measures were a weekly mood questionnaire, daily activity measured by Oura ring, daily diary and study perceived effectiveness using a post-study questionnaire at day 21.
2. Materials and methods
2.1. Study design, recruitment and intervention
A randomized double-blind placebo-controlled, parallel-arm (1:1 allocation ratio) trial design was employed, with 80 participants. We structured and reported it according to CONSORT guidelines [30]. The clinical trial was conducted by Wellness Discovery Labs, Jacksonville, FL, USA from March 10, 2022, to October 18, 2022, under the supervision of a qualified investigator (QI). This study was reviewed by the research ethics board of Sterling IRB (Protocol Number 9806, Approval Date: March 10, 2022). Recruitment was for twelve weeks after that date (until June 2022). The study was closed upon receipt of the final participant's data twelve weeks after that (September 2022). The study is registered with the ISRCTN registry with study number ISRCTN14728094: https://www.isrctn.com/ISRCTN14728094.
Participants were recruited via social media announcements and from a participant recruitment list possessed by Wellness Discovery Labs. Interested participants completed a prescreen survey to determine eligibility (i.e., Insomnia Severity Index, inclusion/exclusion criteria and demographic information, e.g., age, health status, and health behaviors). Participants who met the selection criteria (based on responses to the prescreen survey) were contacted to discuss the intervention and provide institutional-review board-approved informed consent. The inclusion and exclusion criteria are described below.
Individuals included were those aged between 35 and 55 who self-reported poor sleep quality, determined by a score of between 8 and 21 on the insomnia severity index [31]; weight between 50 and 100 kg (i.e., 110–220 lbs); and not meeting any of the exclusion criteria.
Individuals excluded were those meeting any of the following criteria: a history of sleep-affecting disorders; recent highly stressful events within 2 weeks of baseline; use of sleep supplements or medications; usage of sleep-pattern-influencing medications within 1 month of baseline; use of calcium channel blockers, anxiolytics or SSRIs, no more than 5 times per month, and not within seven days of baseline; current hormone therapy; unstable use of other medication; excessive alcohol consumption; smoking; elevated caffeine intake; irregular sleep-inducing work schedules; recent travel to different time zones within 1 month of study; pregnancy, attempts at conception, or breastfeeding; refusal to abstain from other magnesium products for two weeks before and during the trial; and individuals incompatible with the study protocol.
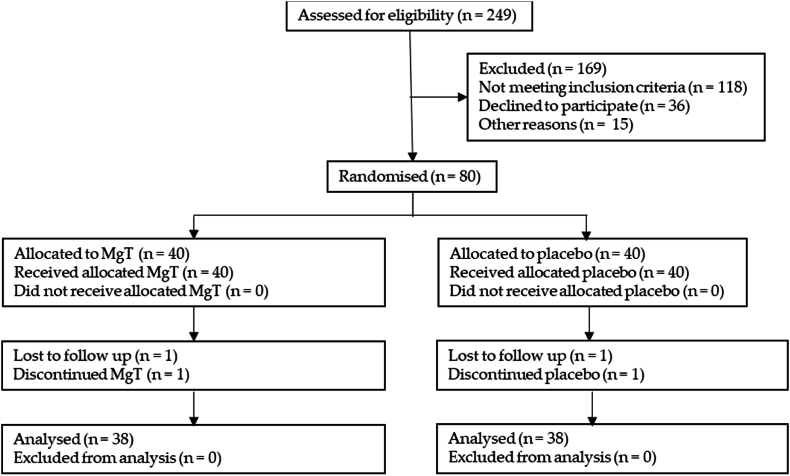
Consenting participants were randomly assigned participant ID numbers (using the random number function in Microsoft Excel) which assigned them into two equal groups, a 1:1 allocation ratio). These groups were either the MgT group or placebo group. Participants were sent investigational product or placebo, with usage instructions. The placebo was rice protein powder. MgT and placebo were identical in taste and appearance, in identical capsules, in identical bottles separated by lot numbers. Participants were blind to treatment. Investigators remained blind to treatment: lot number contents were only revealed after statistical analysis. As a decentralized trial, participants did not visit a clinic, all recruitments, contact, screening, consenting, and assessments were performed online. Participant flow is shown in Fig. 1.
Fig. 1.
Participant flow through recruitment, consenting, intervention and outcome measurement process.
The MgT supplement used, Magtein®, is a brain-bioavailable magnesium L-threonate, produced and supplied by Threotech LLC (NV, USA). Magtein contains about 75 mg/g of elemental magnesium. Participants were directed to consume 1 g/d of the testing products: 2 capsules each containing either 500 mg MgT or identically appearing placebo (rice protein powder, AIDP Inc., CA, USA) 2 h before bedtime.
2.2. Outcome measures
Primary outcomes measures were sleep quality measured weekly with the Insomnia Severity Index, Leeds Sleep Evaluation Questionnaire and Restorative Sleep Questionnaire, and the Oura ring nightly. Secondary outcome measures were a weekly mood questionnaire, daily activity measured by Oura ring, daily diary and study perceived effectiveness using a post-study questionnaire at day 21.
Participants were to complete psychometric self-report questionnaires on day 0 (baseline), day 7, day 14, and day 21. In addition, participants were to maintain a daily diary to document subjective sleep aspects, adherence, and adverse events. Participants also wore an Oura Ring to objectively determine sleep and daytime activity. Participants maintained their current lifestyle behaviors and did not engage in any new forms of structured exercise or begin a new diet or health intervention during the trial. At the end of the study, participants completed a post-study questionnaire.
Participants completed the Insomnia Severity Index, a self-report measure assessing symptoms of recent insomnia [31]. This index assesses sleep onset, sleep maintenance, early morning awakening problems; sleep dissatisfaction; interference of sleep difficulties with daytime functioning; whether sleep problems are noticed by others; and distress caused by sleep difficulties. A 5-point Likert scale is used to rate each item (e.g., 0 = no problem; 4 = very severe problem), yielding a total score ranging from 0 to 28. The total score is interpreted as follows: absence of insomnia (0–7); sub-threshold insomnia (8–14); moderate insomnia symptoms (15–21); and severe insomnia symptoms (22–28). The Insomnia Severity Index has good internal consistency (Cronbach alpha = 0.91; [31]). This was assessed at recruitment, and weekly throughout the study.
Participants completed the Leeds Sleep Evaluation Questionnaire (LSEQ), comprising self-rating questions concerned with aspects of sleep and early morning behavior [32]. The questionnaire has been used to monitor subjectively perceived changes in sleep in a variety of populations. The questionnaire was reworded from “medication” to “supplementation” in this study. This scale has good psychometric properties. Scoring categories reported here are behavior following awakening, awake following sleep, quality of sleep and getting to sleep. Higher scores indicate an improvement in sleep quality. This was assessed weekly.
Participants completed the Restorative Sleep Questionnaire (RSQ), which measures refreshing quality of sleep [33]. Nonrestorative sleep is one of the cardinal symptoms of insomnia and can occur independent of other components of insomnia. This questionnaire used nine questions with answers scaled from 1 to 5: grouchy, in a good mood, mentally alert, sleepy, tired, rested, refreshed and restored, ready to start the day, and energetic. The total score is an average score based on all nine items. This questionnaire has good psychometric properties [33]. Higher scores indicate an improvement in sleep quality, except where noted otherwise. This was assessed weekly.
Participants completed the Profile of Mood States (POMS) short form to assess mood states: it includes scores for tension, anger, vigor, fatigue, depression, esteem, and confusion [37]. A composite total score was computed by summing each of the individual scores for tension, depression, anxiety, fatigue, and confusion, with vigor and esteem scores subtracted to indicate participants’ total mood disturbance. Each item of the POMS short form was scored on a 5-point Likert scale ranging from 0 (not at all) to 4 (extremely). This scale has good-to-excellent reliability and validity [37]. This was assessed weekly.
Participants completed daily diaries encompassing aspects of daytime energy/productivity, events affecting sleep quality, recorded adverse events and adherence.
To objectively measure sleep quality and daytime functioning we employed the Oura ring, a waterproof wearable multisensory device that quantifies daily physical activity, night-time sleep duration, and estimates sleep stages (https://ouraring.com/). The ring also measures motion and body temperature. According to the manufacturer, the ring is worn on the index finger and uses physiological signals (a combination of motion, heart rate, heart rate variability, and pulse wave variability amplitude) in combination with machine learning-based methods to calculate deep, light and REM sleep in addition to sleep/wake states. The Oura ring has high validity in the assessment of nocturnal heart rate, heart rate variability, movement, and sleep outcomes in healthy adults in natural environment [[34], [35], [36]].
The Oura ring measurements come in three main categories: Sleep, Activity and Readiness. In the Sleep category, there are 15 measurements: (1) sleep duration, (2) time awake, (3) light sleep, (4) sleep latency (how quickly one fell asleep), (5) REM sleep, (6) deep sleep, (7) total sleep; (8) sleep efficiency (how much time in bed was actually spent sleeping) (these scores 1–8 measured in minutes); (9) overall sleep score, (10) deep sleep score, (11) sleep disturbance score, (12) sleep efficiency score, (13) latency sleep score, (14) REM sleep score, (15) total sleep score (these scores 9–15 calculated using manufacturer proprietary algorithms by combining the resting heart rate, body temperature, movement, and time spent in specific sleep stages); In the Activity category, there are three measurements: (1) activity score, (2) activity met daily movement goals, (3) activity daily movement score; In the Readiness category, there are three measurements: (1) readiness score, (2) readiness activity balance, and (3) readiness sleep balance. Activity scores are measured through daily outputs (e.g., step counts, training frequencies etc.) using a built-in accelerometer. Readiness scores comprises eight contributors (body temperature, sleep, heart rate variability, sleep balance, previous day activity, activity balance, resting heart rate, and recovery index) over 14-day weighted averages (with the most recent 2–5 days weighted more highly) that provide an indication of daily activity. Readiness activity balance compares recent with longer term average activity to estimate acclimatization to the demands of activity. These scores are calculated using manufacturer proprietary algorithms. Data from weekly averages were used.
Participants completed post-study questionnaires at the end of the study. These were yes/no queries regarding whether taking the product had a positive effect on their perceived stress, anxiety, or mood, as follows:
Please circle Yes or No to the below questions based on your experience over the past month. After taking the supplement:
-
•
Has the quality of your sleep improved? Yes/No
-
•
Is your sleep more refreshing/restoring? Yes/No
-
•
Do you feel more rested upon awakening? Yes/No
-
•
Do you feel that your physical health has improved? Yes/No
-
•
Do you feel that your mental health has improved? Yes/No
-
•
Do you feel calmer? Yes/No
-
•
Do you feel that your memory during the daytime improved? Yes/No
-
•
Do you feel that your concentration during the daytime improved? Yes/No
-
•
Do you feel like you have more energy during the daytime? Yes/No
-
•
Do you feel more alert during the daytime? Yes/No
In addition, the participants were also asked to complete the following two questions using a Likert scale anchored with 1 (strongly agree) to 5 (strongly disagree):
-
•
The supplement had a positive effect on my perceived stress and anxiety.
-
•
The supplement had a positive effect on my mood.
2.3. Statistical analysis
Determination of Sample Size. A sample size of 80 subjects (40 per arm) was considered sufficient to detect a significant difference between groups with 80 % power and a 5 % level of significance, allowing for 20 % drop out rate.
The intent to treat (ITT) population were analyzed. Data were analyzed for normality using Shapiro-Wilk test and Q-Q plot. Outliers were characterized as data points that exceeded three interquartile ranges beyond 25th and 75th percentiles. However, no extreme outliers were observed. Continuous data are presented as Mean and standard deviation (SD) and analyzed using linear mixed model with condition, and time as fixed factors and subject as random factor. Multiple comparisons were corrected using Sidak Adjustment. Categorical variables were analyzed using Chi-square test and expressed as counts/percentage where appropriate. Statistical analyses were performed using Statistical Product and Service Solutions (SPSS) version 27. Post hoc analyses (T-tests) were performed using R (version 4.2.2).
3. Results
As detailed in Fig. 1, from 249 people assessed for eligibility, 169 were excluded from the study. Those not meeting inclusion criteria were 118 with 36 declined to further participate, and 15 withdrew for other reasons. Of the remaining 80 who enrolled and consented, 40 were randomized into each treatment group, MgT and placebo. Of the MgT group, all 40 received allocation of MgT, one was lost to follow up, and one discontinued for unknown reasons. All remaining 38 were analyzed. Of the placebo group, all 40 received allocation of placebo, one was lost to follow up, and one discontinued for unknown reasons. All remaining 38 were analyzed. All data was collected from completing participants, including Oura ring data.
Participants (Table 1) had an average age of 45.49 years (SD = 6.00; age range = 35–55 years). All participants exhibited nonclinical insomnia symptoms, as indicated by their scores on the Insomnia Severity Index (ISI), with a mean score of 12.46 (SD = 3.37), according to the assessment by Bastien and coworkers [31].
Table 1.
Baseline sociodemographics and clinical characteristics. Data shows mean and standard deviation.
| Parameter | MgT (n = 40) | Placebo (n = 40) | P value (Paired Sample T-test) |
|---|---|---|---|
| Age BMI |
46.99 ± 6.08 24.51 ± 3.84 |
43.63 ± 5.68 25.19 ± 4.19 |
0.012 0.489 |
| Sex | n = 7 (17.5 %) males n = 33 (82.5 %) females |
n = 10 (25 %) males n = 30 (75 %) females |
|
| Ethnicity | n = 37 (92.5 %) White/Caucasian n = 1 (2.5 %) Hispanic n = 1 (2.5 %) Hispanic n = 1 (2.5 %) Black & Hispanic n = 1 (2.5 %) Asian/Asian American |
n = 34 (85 %) White/Caucasian n = 3 (7.5 %) White/Caucasian & Asian/Asian American n = 2 (5 %) Asian/Asian American n = 1 2.5 %) Hispanic |
|
| Insomnia Severity Index | 12.46 ± 3.37 | 12.41 ± 3.71 | 0.977 |
3.1. Outcome measures
For the ISI, as demonstrated in Table 2, there was a significance improvement for condition (p < 0.001) and time (p < 0.01). MgT group significantly improved total ISI score from baseline to day 7, 14 and 21, while placebo also significantly improved total ISI score from baseline to day 7, 14 and 21, but there was no statistically significant interaction (condition x time) (p = 0.39). Interestingly, despite the high effect in the placebo group, only the MgT group appeared to continue to improve by days 14 and 21, while placebo group plateaus after the first week. There is a significant difference between the changes from week 1 to week 2 (p = 0.023) and week 1 to week 3 (p = 0.0001) of the ISI between MgT and placebo groups.
Table 2.
Insomnia Severity Index, Leeds Sleep Evaluation Questionnaire (LSEQ), Restorative Sleep Questionnaire (RSQ) and daily diary results (mean and standard deviation, n = 38 per group).
| MgT |
Placebo |
Condition |
Time |
Interaction (Condition x Time) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 7 | Day 14 | Day 21 | Baseline | Day 7 | Day 14 | Day 21 | P value | P value | P valuea | |
| Insomnia Severity Index |
12.32 (4.25) |
8.90 (3.09) |
8.16 (4.49) |
7.86 (4.79) |
12.57 (3.63) |
9.22 (4.15) |
9.34 (4.43) |
9.39 (4.04) |
<0.001 |
<0.01 |
0.390 |
| LSEQ Behavior following awakening | 142.37 (58.43) | 194.33 (47.02) | 185.85 (54.07) | 200.82 (46.83) | 130.71 (46.76) | 133.49 (49.21) | 159.67 (54.1) | 163.25 (57.58) | <0.001 | <0.010 | 0.004 |
| LSEQ Awake following sleep | 94.42 (38.91) | 124.11 (33.09) | 121.94 (31.79) | 124.75 (38.48) | 83.57 (36.68) | 105.56 (34.66) | 105.62 (24.64) | 100.91 (36.39) | <0.001 | <0.001 | 0.650 |
| LSEQ Quality of sleep | 73.36 (40.65) | 107.46 (28.21) | 114.45 (27.75) | 113.86 (34.77) | 77.16 (33.21) | 105.1 (27.70) | 108.87 (39.89) | 109.9 (32.98) | <0.001 | <0.001 | 0.810 |
| LSEQ Getting to sleep |
159.11 (43.00) |
172.86 (25.47) |
164.87 (39.95) |
183.46 (37.90) |
144.38 (51.49) |
177.36 (42.16) |
173.46 (51.63) |
176.62 (40.32) |
<0.001 |
<0.001 |
0.250 |
| RSQ Total Score | 24.16 (3.18) | 25.16 (2.82) | 26.13 (3.18) | 25.68 (3.57) | 22.23 (2.67) | 23.41 (3.51) | 23.25 (3.34) | 23.45 (3.52) | <0.001 | <0.010 | 0.134 |
| RSQ Grouchy (R) | 2.92 (0.59) | 2.78 (0.62) | 2.53 (0.72) | 2.4 (0.85) | 3.02 (0.72) | 2.88 (0.65) | 2.83 (0.75) | 2.88 (0.76) | <0.001 | <0.010 | 0.044 |
| RSQ In a good mood | 3.08 (0.59) | 3.22 (0.62) | 3.47 (0.72) | 3.60 (0.85) | 2.98 (0.72) | 3.12 (0.65) | 3.17 (0.75) | 3.12 (0.76) | <0.001 | <0.010 | 0.044 |
| RSQ Mentally alert | 2.87 (0.65) | 3.11 (0.65) | 3.45 (0.71) | 3.58 (0.71) | 2.64 (0.7) | 2.97 (0.75) | 2.79 (0.81) | 3 (0.77) | <0.001 | <0.010 | 0.003 |
| RSQ Sleepy (R) | 2.76 (0.79) | 3.65 (0.94) | 3.66 (0.87) | 3.8 (0.86) | 3.10 (0.95) | 3.57 (0.86) | 3.55 (0.89) | 3.68 (0.90) | <0.001 | <0.010 | 0.085 |
| RSQ Tired (R) | 2.49 (0.47) | 3.10 (0.65) | 3.36 (0.84) | 3.49 (0.85) | 2.62 (0.94) | 3.24 (0.82) | 3.19 (0.76) | 3.41 (0.91) | <0.001 | <0.010 | 0.238 |
| RSQ Rested | 2.11 (0.73) | 2.71 (0.93) | 2.95 (1.09) | 3.02 (1.03) | 1.99 (0.72) | 2.46 (0.92) | 2.47 (0.95) | 2.5 (0.86) | <0.001 | <0.010 | 0.144 |
| RSQ Refreshed and restored | 2.06 (0.77) | 2.74 (0.92) | 2.78 (0.96) | 2.75 (0.82) | 1.79 (0.78) | 2.36 (0.94) | 2.31 (0.84) | 2.47 (0.95) | <0.001 | <0.010 | 0.702 |
| RSQ Ready to start the day | 2.61 (0.89) | 3.05 (1.14) | 3.39 (0.78) | 3.3 (0.95) | 2.27 (0.89) | 2.63 (1.05) | 2.66 (0.99) | 2.68 (0.99) | <0.001 | <0.010 | 0.267 |
| RSQ Energetic | 2.58 (0.83) | 3.04 (0.52) | 3.14 (0.62) | 3.18 (0.8) | 2.04 (0.76) | 2.39 (1.05) | 2.43 (0.97) | 2.5 (1.08) | <0.001 | <0.010 | 0.829 |
| Daily diary Energy (1–10 low-high) | 5.87 (1.88) | 6.41 (1.86) | 6.78 (1.67) | 6.92 (1.63) | 6.54 (1.54) | 6.45 (1.64) | 6.73 (1.55) | 6.71 (1.79) | <0.001 | <0.010 | 0.004 |
| Daily diary Productivity (1–10 low-high) | 6.29 (2.05) | 6.54 (1.88) | 6.99 (1.75) | 6.99 (1.79) | 6.77 (1.85) | 6.75 (1.81) | 6.81 (1.62) | 6.86 (1.77) | <0.001 | <0.010 | 0.004 |
(R): reverse scoring (lower number indicates improvement).
p < 0.05 indicates significance between groups.
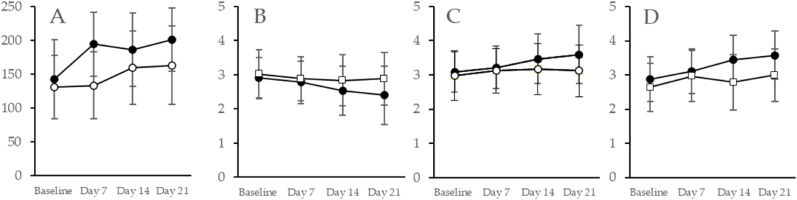
For the LSEQ, as demonstrated in Table 2 and Fig. 2A, the behavior following awakening subscale showed a significant interaction (condition x time) (p = 0.004). However, the awake following sleep; sleep quality (i.e., calmer sleep with less wakeful periods); and getting to sleep (i.e., ease and time to fall asleep) subscales each showed significant effects for condition (p < 0.001) and time (p < 0.01) but no significant interaction (awake following sleep, p = 0.65; sleep quality, p = 0.81; and getting to sleep, p = 0.25), due to a strong response from the placebo group. For the behavior following awakening subscale showing a significant interaction, we conducted a post hoc analysis (T-test) comparing MgT to placebo. The MgT group showed a significant improvement on day 7 (p < 0.001), day 14 (p = 0.023), and day 21 (p = 0.003) over the placebo.
Fig. 2.
Magnesium L-threonate (MgT, black symbols) showed significant (p < 0.05) improvements over placebo (white symbols) for Leeds Sleep Questionnaire (LSEQ) subcategory behavior following awakening (A); and Restorative Sleep Questionnaire (RSQ) subcategories grouchy (B), in a good mood (C), and mental alertness (D). Data shown are mean and standard deviation (n = 38 per group).
These data showed that participants all felt sleep quality improvements, but the MgT group demonstrated significantly better results over the placebo in the behavior upon awakening subcategory, which includes alertness, coordination and balance following awakening.
For the RSQ, as demonstrated in Table 2 and Fig. 2B–D, the grouchy (Fig. 2B), in a good mood (Fig. 2C) and mentally alert (Fig. 2D) subscales showed a significant condition × time interaction (grouchy, p = 0.044; good mood, p = 0.044; and mentally alert, p = 0.003). Significant main effects for condition, (p < 0.001) and time, (p < 0.01) for all three subscales were also demonstrated. For the values showing a significant interaction, we conducted post hoc analyses (T-tests). For grouchy, the MgT group showed significant reduction at day 14 (p = 0.084) and day 21 (p = 0.020) as compared to placebo. For in a good mood, MgT improved significantly at day 14 (p = 0.083) and day 21 (p = 0.012) versus placebo. For mentally alert, MgT again significantly improved at day 7 (p = 0.051), day 14 (p < 0.001), and day 21 (p < 0.001). As demonstrated in Table 1, there were also significant main effects for condition (p < 0.001) and time (p < 0.010), but not interaction (p > 0.05) for the subscales tired, sleepy, rested, refreshed and restored, ready to start the day and energetic, due to high responses from the placebo group.
From the daily diary assessments, as shown in Table 2, the MgT group showed significantly greater improvements to energy and daytime productivity than the placebo group, both with significant condition (p < 0.001), time (p < 0.010) and condition × time interaction (p = 0.004).
For the POMS short form, as shown in Table 3, POMS subscales anger and esteem showed significant condition × time interactions (both p = 0.020). Significant main effects for condition (both p < 0.001) and time (both p < 0.001) were also recorded. Post hoc analyses (T-tests) showed that MgT was significantly improved at day 21 (p = 0.026) for the esteem subscale as compared to the placebo.
Table 3.
Profile of Mood States (POMS) questionnaire results (mean and standard deviation, n = 38 per group).
| MgT |
Placebo |
Condition |
Time |
Interaction (Condition x Time) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 7 | Day 14 | Day 21 | Baseline | Day 7 | Day 14 | Day 21 | P value | P value | P valuea | |
| POMS: Total | 164.03 (14.22) | 157.87 (12.57) | 154.63 (11.44) | 153.32 (12.99) | 159.46 (12.32) | 150.26 (10.83) | 148.91 (10.73) | 148.84 (10.12) | <0.001 | <0.001 | 0.480 |
| Anger | 6.14 (3.64) | 4.87 (3.04) | 4.96 (3.03) | 3.46 (2.24) | 7.14 (4.33) | 4.82 (3.7) | 4.03 (2.91) | 3.98 (3.12) | <0.001 | <0.001 | 0.020 |
| Esteem | 14.69 (2.57) | 15.94 (3.25) | 16.96 (2.70) | 17.84 (2.67) | 14.76 (3.60) | 15.59 (3.40) | 15.75 (2.93) | 16.18 (3.60) | <0.001 | <0.001 | 0.020 |
| Tension | 10.42 (4.32) | 7.55 (3.40) | 6.70 (3.33) | 6.35 (3.60) | 9.78 (4.42) | 7.26 (3.56) | 6.25 (3.44) | 5.55 (2.66) | <0.001 | <0.001 | 0.900 |
| Fatigue | 9.5 (3.35) | 7.74 (4.07) | 6.59 (3.75) | 6.22 (4.16) | 9.07 (3.89) | 7.27 (3.58) | 6.60 (3.45) | 7.01 (3.32) | <0.001 | <0.001 | 0.220 |
| Depression | 6.17 (4.29) | 5.53 (4.94) | 3.77 (3.17) | 2.90 (2.44) | 5.76 (4.48) | 3.91 (3.29) | 2.80 (2.65) | 2.73 (2.85) | <0.001 | <0.001 | 0.250 |
| Vigor | 9.01 (2.78) | 10.08 (2.93) | 11.20 (2.91) | 11.22 (3.84) | 7.10 (3.84) | 7.74 (4.14) | 8.74 (4.67) | 8.66 (4.52) | <0.001 | <0.001 | 0.790 |
| Confusion | 6.55 (3.30) | 5.06 (2.78) | 4.26 (2.68) | 3.87 (2.77) | 6.43 (3.15) | 4.29 (2.71) | 3.61 (2.22) | 3.71 (2.22) | <0.001 | <0.001 | 0.550 |
p < 0.05 indicates significance between groups.
Using objective Oura ring measurements, as demonstrated in Table 4, the sleep parameters that showed a significant interaction (condition x time) included deep sleep score (p < 0.001), REM sleep score (p = 0.020), light sleep time (p = 0.006). For the activity and readiness parameters that showed a significant interaction (condition x time) included activity score (p = 0.010), activity daily movement score (p < 0.001), readiness score (p = 0.010), readiness activity balance (p = 0.003), and readiness sleep balance (p < 0.001). For the values showing a significant interaction, we conducted post hoc analyses (T-tests). MgT was significantly better than the placebo for deep sleep score at day 14 (p = 0.040). Comparing the activity score, MgT was significantly different than placebo at day 7 (p = 0.044) and day 21 (p = 0.028). Comparing activity daily movement score, MgT approached significance at day 7 (p = 0.059) and was significantly improved at day 14 (p = 0.035) and day 21 (p = 0.044) over placebo. Comparing readiness activity balance, MgT approached significance for day 7 (p = 0.086), as compared to placebo.
Table 4.
Oura Ring sleep results (mean and standard deviation, n = 38 per group).
| MgT |
Placebo |
Condition |
Time |
Interaction (Condition x Time) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep parameters | Baseline | Day 7 | Day 14 | Day 21 | Baseline | Day 7 | Day 14 | Day 21 | P value | P value | P valuea |
| Time awake (Minutes) | 55.33 (27.28) | 54.71 (26.44) | 56.83 (27.39) | 57.39 (28.21) | 51.75 (21.28) | 53.29 (23.85) | 54.34 (24.43) | 55.82 (26.75) | <0.001 | 0.110 | 0.950 |
| Sleep Latency (Minutes) | 9.07 (6.00) | 9.64 (6.57) | 10.39 (6.20) | 9.96 (6.34) | 10.07 (6.28) | 11.06 (6.77) | 9.86 (6.44) | 9.99 (6.05) | <0.001 | 0.280 | 0.050 |
| Sleep duration (Minutes) | 476.52 (66.2) | 485.41 (73.89) | 479.57 (68.5) | 481.07 (66.58) | 497.29 (66.31) | 497.85 (65.39) | 493.38 (66.80) | 498.17 (74.04) | <0.001 | 0.420 | 0.580 |
| Light Sleep (Minutes) | 218.89 (46.68) | 228.00 (45.69) | 223.70 (44.69) | 218.18 (44.72) | 227.53 (57.2) | 222.53 (50.87) | 229.79 (53.77) | 236.21 (50.59) | <0.001 | 0.530 | 0.006 |
| REM Sleep (minutes) | 97.75 (33.51) | 99.86 (34.09) | 97.10 (33.7) | 95.76 (32.65) | 107.37 (37.55) | 101.81 (37.05) | 100.93 (37.91) | 104.10 (38.77) | <0.001 | 0.570 | 0.190 |
| Deep Sleep (minutes) | 96.3 (33.01) | 92.73 (35.04) | 94.75 (33.52) | 97.75 (36.2) | 100.52 (34.7) | 99.55 (35.85) | 99.53 (41.09) | 93.65 (37.12) | <0.001 | 0.490 | 0.070 |
| Total Sleep (Minutes) | 417.88 (52.31) | 425.13 (62.06) | 420.57 (56.68) | 418.91 (58.31) | 439.54 (60.9) | 438.94 (62.22) | 437.5 (61.03) | 440.43 (66.8) | <0.001 | 0.550 | 0.590 |
| Sleep Efficiency | 88.24 (5.2) | 88.73 (4.86) | 88.17 (4.94) | 87.58 (5.82) | 89.44 (4.12) | 89.19 (4.43) | 89.03 (4.83) | 88.59 (4.95) | <0.001 | 0.010 | 0.590 |
| Sleep Disturbance Score | 75.44 (8.64) | 75.78 (8.84) | 75.24 (8.40) | 74.62 (8.41) | 76.77 (8.67) | 76.41 (8.79) | 75.50 (10.40) | 74.97 (9.95) | <0.001 | 0.050 | 0.760 |
| Sleep Efficiency Score | 89.41 (9.85) | 90.04 (9.61) | 89.07 (9.9) | 86.2 (15.15) | 92.14 (6.99) | 91.07 (8.63) | 90.54 (8.19) | 89.4 (9.69) | <0.001 | <0.001 | 0.210 |
| Latency Sleep Score | 78.92 (10.83) | 79.65 (10.86) | 81.46 (10.98) | 79.6 (10.31) | 80.18 (10.11) | 80.81 (10.62) | 79.73 (10.37) | 80.49 (10.36) | <0.001 | 0.540 | 0.100 |
| REM Sleep Score | 84.65 (17.37) | 87.43 (14.77) | 86.42 (15.26) | 84.82 (16.84) | 88.31 (13.88) | 85.96 (15.15) | 84.55 (17.87) | 85.61 (16.52) | <0.001 | 0.190 | 0.020 |
| Deep Sleep Score | 93.84 (8.33) | 92.65 (10.31) | 93.58 (8.63) | 94.2 (8.54) | 93.58 (9.18) | 94.05 (8.51) | 88.03 (12.73) | 90.7 (12.99) | <0.001 | <0.001 | < 0.001 |
| Total Sleep Score | 80.74 (12.43) | 81.9 (12.83) | 80.72 (12.58) | 81.16 (12.76) | 84.26 (12.87) | 83.5 (12.59) | 82.58 (13.12) | 83.64 (13.6) | <0.001 | 0.310 | 0.420 |
| Overall Sleep Score |
82.33 (7.25) |
83.4 (7.3) |
82.62 (7.05) |
82.44 (7.68) |
84.6 (6.07) |
84.19 (6.94) |
82.96 (7.18) |
83.13 (7.64) |
<0.001 |
0.070 |
0.130 |
| Activity and readiness parameters | |||||||||||
| Activity Score | 88.53 (8.38) | 85.19 (11.34) | 85.84 (10.58) | 87.82 (9.02) | 86.17 (11.16) | 78.61 (16.18) | 80.83 (15.67) | 80.87 (16.77) | <0.001 | <0.001 | 0.010 |
| Activity Met Daily Movement Goals | 81.33 (13.94) | 81.90 (13.6) | 81.89 (13.34) | 81.97 (13.49) | 84.69 (12.94) | 84.74 (12.38) | 84.44 (13.1) | 84.93 (12.52) | <0.001 | 0.880 | 0.810 |
| Activity Daily Movement Score | 79.72 (20.26) | 79.59 (22.31) | 80.71 (24.55) | 79.81 (22.93) | 76.52 (25.47) | 65.62 (38.85) | 64.74 (38.45) | 64.6 (39.62) | <0.001 | 0.010 | < 0.001 |
| Readiness Score | 79.75 (7.56) | 82.05 (6.36) | 80.01 (6.41) | 80.16 (7.24) | 82.21 (6.69) | 81.52 (6.97) | 80.73 (6.27) | 79.95 (7.02) | <0.001 | <0.001 | 0.010 |
| Readiness Activity Balance | 82.69 (7.25) | 81.84 (9.91) | 82.85 (8.77) | 84.75 (7.17) | 83.69 (13.19) | 85.39 (7.73) | 85.54 (8.11) | 84.9 (8.97) | <0.001 | 0.007 | 0.003 |
| Readiness Sleep Balance | 78.53 (9.51) | 81.97 (9.85) | 80.17 (11.06) | 78.25 (12.2) | 83.24 (7.52) | 80.85 (11.06) | 81.09 (10.07) | 82.74 (11.46) | <0.001 | 0.710 | < 0.001 |
p < 0.05 indicates significance between groups.
Overall, the Oura ring data shows MgT maintained good sleep qualities and daytime functioning, significantly better than the placebo group.
For the post-study questionnaires, as shown in Table 5, at the end of the intervention period, significantly (p < 0.05) more of the MgT group as compared to the placebo group answered YES to the following questions: feeling their sleep was more refreshing/restoring, feeling more rested upon awakening, feeling their physical health had improved, and feeling their mental health had improved, feeling their concentration during the daytime improved, feeling they had more energy during the daytime, and feeling more alert during the daytime.
Table 5.
Post-study self-report assessments (percentage answering “yes”, n = 38 per group).
| Question | MgT | Placebo | Chi-square |
|---|---|---|---|
| Has the quality of your sleep improved? | 57 % | 42 % | χ2 = 0.15 |
| Is your sleep more refreshing/restoring? | 57 % | 29 % | χ2 = 0.01a |
| Do you feel more rested upon awakening? | 62 % | 29 % | χ2 = 0.004a |
| Do you feel that your physical health has improved? | 49 % | 24 % | χ2 = 0.022a |
| Do you feel that your mental health has improved? | 59 % | 29 % | χ2 = 0.007a |
| Do you feel calmer? | 57 % | 42 % | χ2 = 0.15 |
| Do you feel that your memory during the daytime improved? | 41 % | 24 % | χ2 = 0.09 |
| Do you feel that your concentration during the daytime improved? | 46 % | 24 % | χ2 = 0.04a |
| Do you feel like you have more energy during the daytime? | 51 % | 29 % | χ2 = 0.04a |
| Do you feel more alert during the daytime? |
59 % |
26 % |
χ2 = 0.004a |
| Taking the supplement had a positive effect on my perceived stress and anxiety. | 46 % | 26 % | χ2 = 0.06 |
| Taking the supplement had a positive effect on my mood. | 33 % | 23 % | χ2 = 0.24 |
p < 0.05 indicates significance between groups.
3.2. Adverse event recording
Participant comments from daily diaries were assessed for possible adverse events. Four AEs were recorded from the MgT group, and 13 from the placebo group. None were regarded as “probable” or higher by MedDRA severity classifications. For the MgT group AEs recorded using MedDRA terminology were nocturnal awakening (1), cramps legs (2) and sunken eyes (1). From placebo group AEs recorded were nocturnal awakening (2), sleepiness (6), feeling anxious (1), restlessness (3), pulse increased (1). MgT appeared to be safe and well-tolerated.
4. Discussion
MgT has previously been clinically demonstrated to improve cognitive scores reflecting individual's executive function, working memory, episodic memory, and attention by six weeks [26]. Similarly, it has previously been demonstrated to significantly improve memory, associational learning, figure recognition, recall and character-face association [25]. Additionally, it was shown to reduce self-reported stress and anxiety after twelve weeks consumption [27] while also reducing clinically administered fear scores after six- and twelve-weeks consumption [27]. Furthermore, it was reported in an eight-week open-label study with 15 dementia patients to improve overall cognitive functioning [28] and in a twelve-week open-label study with ADHD participants to improve self-reported ADHD symptoms, executive function, and visual scanning measures [29]. These are consistent with the animal data. Magnesium has always been implicated to benefit sleep, but the clinical evidence is not always conclusive [13,15,16,38,39], most likely because most of the magnesium compounds used in the studies are not brain bioavailable. MgT is able to cross the blood brain barrier and increase magnesium levels in the brain and neuron cells. Thus, it is of great interest to examine the effect of MgT on sleep. Here we report the effects of 1.0 g daily MgT (about 75 mg of bioavailable elemental magnesium) intake over three weeks. The results of this study demonstrated that supplementation with MgT alone is safe, well-tolerated and resulted in statistically significant improvements in sleep quality, daytime activity, and mood outcomes, as compared to placebo.
MgT improves sleep outcomes. For self-reported sleep quality, the MgT-consuming group had significantly improved behavior following awakening LSEQ subscale, mental alertness, good mood and were less grouchy in the RSQ measurements compared to the placebo group. Nonsignificant improvements (i.e., trending in the right direction) were evidenced for the MgT compared to the placebo for most other LSEQ and RSQ parameters; they did not reach statistical significance, apparently due to the strong placebo effects that is often associated with subjective measures. However, it is interesting that most of the improvements observed in the placebo happened in the first week of product intake, and the effects plateaued afterwards, while in the MgT group, the effects continued to improve for the rest of the study. Other magnesium sleep studies also showed strong placebo responses [37,38].
With this study, measures recorded from a wearable device (Oura ring) were more objective and thus statistically more definitive than the participant-assessed sleep outcomes. MgT significantly outperformed the placebo for deep sleep score (p < 0.001), activity score (p = 0.010), activity daily movement score (p < 0.001), readiness score (p = 0.010), and readiness activity balance (p = 0.003), light sleep (p = 0.006), REM sleep score (p = 0.020) and readiness sleep balance (p < 0.001). Deep sleep is the most restorative and rejuvenating sleep stage. During deep sleep, breathing is slow, heartbeat is regular, and muscles are relaxed; and it is the period where the body heals itself (e.g., replaces cells, builds muscle tissue, and heals wounds). Deep sleep encompasses between 0 and 35 % of total sleep, and the average adult spends 15–20 % of their total sleep time in deep sleep. In terms of cognition, deep sleep contributes to thinking, creativity, and memory consolidation and restoration, specifically procedural and declarative memory. This deep sleep stage allows the brain to rest and recover, which assists with improving cognitive function, concentration, and alertness. Plus during deep sleep the brain processes and integrates new information, which can improve memory retention and learning. We found that MgT supplementation improved deep sleep score, which provides an explanation for previous findings that MgT improved cognitive abilities compared to placebo [[25], [26], [27]]. It is plausible that some of the cognitive enhancing effects of MgT may, in part, be due to its ability to improve deep sleep, although the causal/effect connection still needs to be investigated. Relatedly, magnesium improves synapse configuration associated with learning and new memory formation [40], those functions perhaps occurring during deep sleep.
Insufficient sleep is linked to reduced energy, productivity, and physical activity as well as increased feeling of fatigue [45,46]. With MgT supplementation, individuals demonstrated an improved sleep quality, and an improved daytime mental clarity and physical activity. The MgT results support previous research findings of a bidirectional relationship between improved sleep quality and increased daytime activity [[41], [42], [43], [44]]. It is also interesting to notice that MgT slightly increased sleep latency as compared to the placebo (p < 0.05) in this study, suggesting that MgT's benefits on sleep is more on the improved deep sleep quality and refreshed awakening, improved daily activity and mental alertness, rather than helping people to fall sleep faster.
Finally, we find it interesting that in vitro work shows increasing magnesium by one third from 0.8 mM to 1.2 mM, within biologically plausible concentrations, improves neuronal cell energy in terms of increased mitochondrial function by 68 % within 4 h, doubling neuronal ATP production [47]. It occurs to us that improved neuronal energy in response to MgT may be a source of the improved daytime functioning observed by the participants in this study.
MgT improves mood. Here we report that 1 g daily MgT consumption improved good mood (Table 2), reduced grouchiness (Table 2) and reduced anger (Table 3). This highlights the role of MgT in improving behavior (and mood) upon awakening. Magnesium consumption has been linked to improved mood previously, albeit weakly, perhaps due to the lower brain bioavailability of other types of magnesium. We find the mood improvements reported here to be statistically significant as compared to the placebo. This observation is consistent with the improved quality of sleep, suggesting that the benefits of MgT is multi-functional, which can be expected from the multi-functional benefits of magnesium in overall brain health.
MgT starts working within 14 days. We also observed that, the effects of MgT intake reached significance as compared to the placebo by 7–14 days into this study, namely LSEQ behavior following awakening and Oura ring activity score (days 7–21); RSQ mentally alert, Oura ring deep sleep and activity daily movement (days 14–21). This is the first human study which has demonstrated the response time of MgT as within 7–14 days (Table 1, Table 2, Table 3).
MgT outperforms considerable placebo effects. From the self-assessed outcomes we reported here, it was evident that almost all participants reported their sleep quality and their behavior improved over the three weeks, irrespective of treatment. This highlighted a particularly strong placebo effect with these self-assessed scoring questionnaires. Placebo effects are often observed in various clinical studies using these subjective questionnaires. These effects often exhibit temporal dynamics that can be influenced by factors such as the duration of the intervention, the nature of the outcome measure, and individual expectations.
In terms of duration, placebo effects on mood and anxiety often manifests as short-term improvements in self-reported measures. However, the sustainability of these effects over longer periods is often limited. The transient nature of placebo effects in non-diagnosed participants underscores the importance of distinguishing between short-term and lasting changes. A large part of this difference is, obviously, actual treatment efficacy versus participant expectation.
Expectation plays a significant role in the temporal aspects of the placebo effect. When participants believe they are receiving an active intervention, their expectations often lead to rapid improvements. These effects are often particularly pronounced in the early stages of an intervention but diminish as participants become more aware, thus lacking long-term effect. This placebo effect is also observed in this study. In most of the measurements using questionnaires, both MgT group and placebo group showed a statistical significance over the baseline (as evidenced by the significant p values of condition and time separately) but failed to reach significance when compared between MgT and placebo (as evidenced by the p value of the interaction). In these measurements, the MgT group appeared to continue to improve after the first week, while placebo group showed most effect in the first week and plateaued afterwards.
Neurobiological mechanisms do underlie placebo effects, even in non-diagnosed participants. In terms of mood and anxiety, neuroimaging studies have shown that placebo interventions can lead to changes in brain activity patterns associated with emotional processing and mood regulation, as well as motor control, endocrine and immune effects [[48], [49], [50]]. These mechanisms can contribute to the temporal dynamics of placebo responses in mood and anxiety. These effects may be driven by neurotransmitter activity or brain activation patterns: Short-term placebo effects might involve the release of neurotransmitters like endorphins, dopamine, and serotonin, which are associated with sleep and mood regulation. These neurotransmitters can lead to immediate improvements, but sustained changes may require more comprehensive neural adaptations.
As mentioned previously, MgT has been shown in animals to result in neural adaptations which improved memory outcomes [7,[17], [18], [19], [20], [21], [22], [23], [24],40,47], and similar outcome benefits in terms of memory and cognition (as well as mood) have been observed in humans [[25], [26], [27], [28], [29]]. It may be safe to conclude that such adaptations have occurred here.
Collectively, these data showing improved sleep quality and daytime functioning from daily consumption of magnesium L-threonate suggest they may play a role in clinical practice as an adjunct therapy for healthy individuals experiencing sleeplessness or the consequences thereof. Both magnesium and threonic acid ligand are endogenously present in our body [24]) and possess a good safety profile. Together with the demonstrated benefits in sleep, cognition, mood and many other areas of brain health, magnesium L threonate would be the choice of brain magnesium in clinical practices. Research into the role magnesium L-threonate plays in areas such as restoring or ameliorating memory deficit, neuroinflammation and pain, ADHD, stress and anxiety have occurred and are ongoing [7,[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29],40,47].
Study strengths, limitations and direction for further research. The primary strength of this study includes the use of both objective and subjective outcome measures to assess the sleep-enhancing effect of MgT on healthy adults experiencing self-assessed, non-clinical sleep disturbance. Despite positive outcomes there were limitations to this study and suggested directions for further research. Subjective measures had a strong placebo effect, as discussed earlier. As this effect had a strong temporal component, future research should involve a longer study duration to further differentiate the longer-term positive benefits of MgT.
5. Conclusions
Collectively, in this randomized placebo-controlled double-blind trial, we examined the effectiveness of MgT supplementation on sleep parameters and daytime activity in adults with nonclinical insomnia symptoms. Using objective oura ring measurement, sleep and daytime functioning were all significantly improved as compared to the placebo. Additionally, using subjective questionairres, MgT significantly improved over the placebo on behavior upon awakening, energy and daytime productivity. MgT intake also significantly reduced grouchiness, led to significantly improved mood and mental alertness, as compared to the placebo. MgT was well tolerated with no report of major side effects. The results are consistent with the action of magnesium on the brain reported elsewhere, and suggest a broader positive impact of MgT beyond simply sleep, and highlighted MgT's potential to induce sustained, progressive benefits to overall brain functioning.
Funding
This research was funded by AIDP Inc., CA, USA.
Institutional review board statement
This study was reviewed by a research ethics board of Sterling IRB (Protocol Number 9806, Approval Date: March 10, 2022). This study was conducted in accordance with the ethical principles that originate in the Declaration of Helsinki and its subsequent amendments, and in compliance with International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice Current Step 4 Version dated November 9, 2016, including the archiving of essential documents.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
Data is available upon request.
CRediT authorship contribution statement
Heather A. Hausenblas: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Tarah Lynch: Resources, Methodology, Investigation. Stephanie Hooper: Validation, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Aahana Shrestha: Writing – review & editing, Visualization, Validation, Software, Formal analysis. Doug Rosendale: Writing – original draft, Visualization. Jennifer Gu: Writing – review & editing, Visualization, Funding acquisition, Conceptualization.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used ChatGPT v3.5 in order to structure regions of the introduction and discussion sections. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgments
We thank the anonymous participants.
Contributor Information
Heather A. Hausenblas, Email: hhausen@ju.edu.
Tarah Lynch, Email: tlynch3@ju.edu.
Stephanie Hooper, Email: s.hooper@unf.edu.
Aahana Shrestha, Email: a.shrestha@aidp.com.
Doug Rosendale, Email: d.rosendale@aidp.com.
Jennifer Gu, Email: jennifer@aidp.com.
References
- 1.Global philips sleep survey. 2019. [Google Scholar]
- 2.Porcheret K., Hopstock L.A., Nilsen K.B. Prevalence of insomnia in a general adult population cohort using different diagnostic criteria: the seventh survey of the Tromsø study 2015-2016. Sleep Med. 2024 May 3;119:289–295. doi: 10.1016/j.sleep.2024.05.002. Epub ahead of print. PMID: 38718598. [DOI] [PubMed] [Google Scholar]
- 3.Roth T., Coulouvrat C., Hajak G., Lakoma M.D., Sampson N.A., Shahly V., Shillington A.C., Stephenson J.J., Walsh J.K., Kessler R.C. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, criteria: results from the America insomnia survey. Biol Psychiatr. 2011 Mar 15;69(6):592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Chung K.F., Yeung W.F., Ho F.Y., Yung K.P., Yu Y.M., Kwok C.W. Cross-cultural and comparative epidemiology of insomnia: the Diagnostic and statistical manual (DSM), International classification of diseases (ICD) and International classification of sleep disorders (ICSD) Sleep Med. 2015 Apr 1;16(4):477–482. doi: 10.1016/j.sleep.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Foley D., Ancoli-Israel S., Britz P., Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004 May;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. PMID: 15172205. [DOI] [PubMed] [Google Scholar]
- 6.Shi L., Chen S.J., Ma M.Y., Bao Y.P., Han Y., Wang Y.M., Shi J., Vitiello M.V., Lu L. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018 Aug;40:4–16. doi: 10.1016/j.smrv.2017.06.010. Epub 2017 Jul 6. PMID: 28890168. [DOI] [PubMed] [Google Scholar]
- 7.Slutsky I., Abumaria N., Wu L.J., Huang C., Zhang L., Li B., Zhao X., Govindarajan A., Zhao M.G., Zhuo M., Tonegawa S., Liu G. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010 Jan 28;65(2):165–177. doi: 10.1016/j.neuron.2009.12.026. PMID: 20152124. [DOI] [PubMed] [Google Scholar]
- 8.Górska N., Słupski J., Szałach Ł.P., Włodarczyk A., Szarmach J., Jakuszkowiak-Wojten K., Gałuszko-Węgielnik M., Wilkowska A., Wiglusz M.S., Cubała W.J. Magnesium and ketamine in the treatment of depression. Psychiatr Danub. 2019 Sep;31(Suppl 3):549–553. PMID: 31488789. [PubMed] [Google Scholar]
- 9.Kalueff A.V., Nutt D.J. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24(7):495–517. doi: 10.1002/da.20262. PMID: 17117412. [DOI] [PubMed] [Google Scholar]
- 10.Peuhkuri K., Sihvola N., Korpela R. Diet promotes sleep duration and quality. Nutr Res. 2012 May;32(5):309–319. doi: 10.1016/j.nutres.2012.03.009. Epub 2012 Apr 25. PMID: 22652369. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon V.S., Deo P., Thomas P., Fenech M. Low magnesium in conjunction with high homocysteine and less sleep accelerates telomere attrition in healthy elderly Australian. Int J Mol Sci. 2023 Jan 4;24(2):982. doi: 10.3390/ijms24020982. PMID: 36674498; PMCID: PMC9866301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Chen C., Lu L., Knutson K.L., Carnethon M.R., Fly A.D., Luo J., Haas D.M., Shikany J.M., Kahe K. Association of magnesium intake with sleep duration and sleep quality: findings from the CARDIA study. Sleep. 2022 Apr 11;45(4) doi: 10.1093/sleep/zsab276. PMID: 34883514; PMCID: PMC8996025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arab A., Rafie N., Amani R., Shirani F. The role of magnesium in sleep health: a systematic review of available literature. Biol Trace Elem Res. 2023 Jan;201(1):121–128. doi: 10.1007/s12011-022-03162-1. Epub 2022 Feb 19. PMID: 35184264. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y., Zhen S., Taylor A.W., Appleton S., Atlantis E., Shi Z. Magnesium intake and sleep disorder symptoms: findings from the Jiangsu nutrition study of Chinese adults at five-year follow-up. Nutrients. 2018 Sep 21;10(10):1354. doi: 10.3390/nu10101354. PMID: 30248967; PMCID: PMC6212970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan V., Lo K. Efficacy of dietary supplements on improving sleep quality: a systematic review and meta-analysis. Postgrad Med. 2022 Apr;98(1158):285–293. doi: 10.1136/postgradmedj-2020-139319. Epub 2021 Jan 13. PMID: 33441476. [DOI] [PubMed] [Google Scholar]
- 16.Mah J., Pitre T. Oral magnesium supplementation for insomnia in older adults: a Systematic Review & Meta-Analysis. BMC Complement Med Ther. 2021 Apr 17;21(1):125. doi: 10.1186/s12906-021-03297-z. PMID: 33865376; PMCID: PMC8053283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Liu Y., Zhou L.J., Wu Y., Li F., Shen K.F., Pang R.P., Wei X.H., Li Y.Y., Liu X.G. Magnesium L-threonate prevents and restores memory deficits associated with neuropathic pain by inhibition of TNF-α. Pain Physician. 2013 Sep-Oct;16(5):E563–E575. PMID: 24077207. [PubMed] [Google Scholar]
- 18.Zhou X., Huang Z., Zhang J., Chen J.L., Yao P.W., Mai C.L., Mai J.Z., Zhang H., Liu X.G. Chronic oral administration of magnesium-L-threonate prevents oxaliplatin-induced memory and emotional deficits by normalization of TNF-α/NF-κB signaling in rats. Neurosci Bull. 2021 Jan;37(1):55–69. doi: 10.1007/s12264-020-00563-x. Epub 2020 Aug 28. PMID: 32857294; PMCID: PMC7811972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu T., Li D., Zhou X., Ouyang H.D., Zhou L.J., Zhou H., Zhang H.M., Wei X.H., Liu G., Liu X.G. Oral application of magnesium-L-threonate attenuates vincristine-induced allodynia and hyperalgesia by normalization of tumor necrosis factor-α/nuclear factor-κb signaling. Anesthesiology. 2017 Jun;126(6):1151–1168. doi: 10.1097/ALN.0000000000001601. PMID: 28306698. [DOI] [PubMed] [Google Scholar]
- 20.Chen J.L., Zhou X., Liu B.L., Wei X.H., Ding H.L., Lin Z.J., Zhan H.L., Yang F., Li W.B., Xie J.C., Su M.Z., Liu X.G., Zhou X.F. Normalization of magnesium deficiency attenuated mechanical allodynia, depressive-like behaviors, and memory deficits associated with cyclophosphamide-induced cystitis by inhibiting TNF-α/NF-κB signaling in female rats. J Neuroinflammation. 2020 Apr 2;17(1):99. doi: 10.1186/s12974-020-01786-5. Erratum in: J Neuroinflammation. 2021 Nov 14;18(1):269. PMID: 32241292; PMCID: PMC7118907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J., Mai C.L., Xiong Y., Lin Z.J., Jie Y.T., Mai J.Z., Liu C., Xie M.X., Zhou X., Liu X.G. The causal role of magnesium deficiency in the neuroinflammation, pain hypersensitivity and memory/emotional deficits in ovariectomized and aged female mice. J Inflamm Res. 2021 Dec 7;14:6633–6656. doi: 10.2147/JIR.S330894. PMID: 34908863; PMCID: PMC8665878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia S., Liu Y., Shi Y., Ma Y., Hu Y., Wang M., Li X. Elevation of brain magnesium potentiates neural stem cell proliferation in the Hippocampus of young and aged mice. J Cell Physiol. 2016 Sep;231(9):1903–1912. doi: 10.1002/jcp.25306. Epub 2016 Feb 2. PMID: 26754806. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y., Dai L., Tian H., Xu R., Li F., Li Z., Zhou J., Wang L., Dong J., Sun L. Treatment of magnesium-L-threonate elevates the magnesium level in the cerebrospinal fluid and attenuates motor deficits and dopamine neuron loss in A mouse model of Parkinson's disease. Neuropsychiatric Dis Treat. 2019 Nov 11;15:3143–3153. doi: 10.2147/NDT.S230688. PMID: 31806980; PMCID: PMC6857673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q., Weinger J.G., Mao F., Liu G. Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. Neuropharmacology. 2016 Sep;108:426–439. doi: 10.1016/j.neuropharm.2016.05.006. Epub 2016 May 10. PMID: 27178134. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C., Hu Q., Li S., Dai F., Qian W., Hewlings S., Yan T., Wang Y. A Magtein®, magnesium L-threonate, -based formula improves brain cognitive functions in healthy Chinese adults. Nutrients. 2022 Dec 8;14(24):5235. doi: 10.3390/nu14245235. PMID: 36558392; PMCID: PMC9786204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G., Weinger J.G., Lu Z.L., Xue F., Sadeghpour S. Efficacy and safety of MMFS-01, a synapse density enhancer, for treating cognitive impairment in older adults: a randomized, double-blind, placebo-controlled trial. J Alzheimers Dis. 2016;49(4):971–990. doi: 10.3233/JAD-150538. PMID: 26519439; PMCID: PMC4927823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewlings S.J., Kalman D. A randomized, double-blind, placebo-controlled, comparator trial evaluating Magtein® magnesium supplement on quality of life as related to levels of stress, anxiety, fear and other indicators. EC Nutrition. 2022;17(3):7–14. [Google Scholar]
- 28.Wroolie T.E., Chen K., Watson K.T., Iagaru A., Sonni I., Snyder N., Lee W., Reiman E.M., Rasgon N.L. An 8-week open label trial of l-Threonic Acid Magnesium Salt in patients with mild to moderate dementia. Personalized medicine in psychiatry. 2017;4:7–12. [Google Scholar]
- 29.Surman C., Vaudreuil C., Boland H., Rhodewalt L., DiSalvo M., Biederman J. L-threonic acid magnesium salt supplementation in ADHD: an open-label pilot study. J Diet Suppl. 2021;18(2):119–131. doi: 10.1080/19390211.2020.1731044. Epub 2020 Mar 12. PMID: 32162987. [DOI] [PubMed] [Google Scholar]
- 30.Schulz K.F., Altman D.G., Moher D., for the CONSORT Group . 2010. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. [DOI] [PubMed] [Google Scholar]
- 31.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001 Jul;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. PMID: 11438246. [DOI] [PubMed] [Google Scholar]
- 32.Parrott A.C., Hindmarch I. The Leeds sleep evaluation questionnaire in psychopharmacological investigations - a review. Psychopharmacology (Berl) 1980;71(2):173–179. doi: 10.1007/BF00434408. PMID: 6777817. [DOI] [PubMed] [Google Scholar]
- 33.Drake C.L., Hays R.D., Morlock R., Wang F., Shikiar R., Frank L., Downey R., Roth T. Development and evaluation of a measure to assess restorative sleep. J Clin Sleep Med. 2014 Jul 15;10(7):733–741. doi: 10.5664/jcsm.3860. PMID: 25024650; PMCID: PMC4067436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Zambotti M., Rosas L., Colrain I.M., Baker F.C. The sleep of the ring: comparison of the ŌURA sleep tracker against polysomnography. Behav Sleep Med. 2019 Mar-Apr;17(2):124–136. doi: 10.1080/15402002.2017.1300587. Epub 2017 Mar 21. PMID: 28323455; PMCID: PMC6095823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinnunen H., Rantanen A., Kenttä T., Koskimäki H. Feasible assessment of recovery and cardiovascular health: accuracy of nocturnal HR and HRV assessed via ring PPG in comparison to medical grade ECG. Physiol Meas. 2020 May 7;41(4) doi: 10.1088/1361-6579/ab840a. PMID: 32217820. [DOI] [PubMed] [Google Scholar]
- 36.Asgari Mehrabadi M., Azimi I., Sarhaddi F., Axelin A., Niela-Vilén H., Myllyntausta S., Stenholm S., Dutt N., Liljeberg P., Rahmani A.M. Sleep tracking of a commercially available smart ring and smartwatch against medical-grade actigraphy in everyday settings: instrument validation study. JMIR Mhealth Uhealth. 2020 Nov 2;8(10) doi: 10.2196/20465. PMID: 33038869; PMCID: PMC7669442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNair D.M., Lorr M., Droppleman L.F. EdITS/Educational and Industrial Testing Service; San Diego: 1992. Manual of the profile of mood states; p. CA1992. [Google Scholar]
- 38.Roguin Maor N., Alperin M., Shturman E., et al. Effect of magnesium oxide supplementation on nocturnal leg cramps: a randomized clinical trial. JAMA Intern Med. 2017;177(5):617–623. doi: 10.1001/jamainternmed.2016.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen F.H., Johnson L.K., Zeng H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes Res. 2010;23(4):158–168. doi: 10.1684/mrh.2010.0220. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H., Bi G.Q., Liu G. Intracellular magnesium optimizes transmission efficiency and plasticity of hippocampal synapses by reconfiguring their connectivity. Nat Commun. 2024 Apr 22;15(1):3406. doi: 10.1038/s41467-024-47571-3. PMID: 38649706; PMCID: PMC11035601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzierzewski J.M., Buman M.P., Giacobbi P.R., Jr., Roberts B.L., Aiken-Morgan A.T., Marsiske M., McCrae C.S. Exercise and sleep in community-dwelling older adults: evidence for a reciprocal relationship. J Sleep Res. 2014 Feb;23(1):61–68. doi: 10.1111/jsr.12078. Epub 2013 Aug 24. PMID: 23980920; PMCID: PMC3844037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Master L., Nye R.T., Lee S., Nahmod N.G., Mariani S., Hale L., Buxton O.M. Bidirectional, daily temporal associations between sleep and physical activity in adolescents. Sci Rep. 2019 May 22;9(1):7732. doi: 10.1038/s41598-019-44059-9. PMID: 31118441; PMCID: PMC6531611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mead M.P., Baron K., Sorby M., Irish L.A. Daily associations between sleep and physical activity. Int J Behav Med. 2019 Oct;26(5):562–568. doi: 10.1007/s12529-019-09810-6. PMID: 31372835. [DOI] [PubMed] [Google Scholar]
- 44.Tracy E.L., Reid K.J., Baron K.G. The relationship between sleep and physical activity: the moderating role of daily alcohol consumption. Sleep. 2021 Oct 11;44(10) doi: 10.1093/sleep/zsab112. PMID: 34009345; PMCID: PMC8503823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boolani A., Schelly D., Reid J., Towler C., Smith M.L., Ohl A. Health differences in didactic versus clinical stage graduate allied health students. J Allied Health. 2018 Winter;47(4):282–288. PMID: 30508840. [PubMed] [Google Scholar]
- 46.Herring M.P., Monroe D.C., Kline C.E., O'Connor P.J., MacDonncha C. Sleep quality moderates the association between physical activity frequency and feelings of energy and fatigue in adolescents. Eur Child Adolesc Psychiatr. 2018 Nov;27(11):1425–1432. doi: 10.1007/s00787-018-1134-z. Epub 2018 Mar 5. PMID: 29508054; PMCID: PMC6410735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H., Liu G. Regulation of density of functional presynaptic terminals by local energy supply. Mol Brain. 2015 Jul 17;8:42. doi: 10.1186/s13041-015-0132-z. Erratum in: Mol Brain. 2015;8:45. PMID: 26184109; PMCID: PMC4504454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brietzke C., Cesario J.C.S., Hettinga F.J., Pires F.O. The reward for placebos: mechanisms underpinning placebo-induced effects on motor performance. Eur J Appl Physiol. 2022;122(11):2321–2329. doi: 10.1007/s00421-022-05029-8. [DOI] [PubMed] [Google Scholar]
- 49.Ortega Á., Salazar J., Galban N., et al. Psycho-neuro-endocrine-immunological basis of the placebo effect: potential applications beyond pain therapy. Int J Mol Sci. 2022;23(8):4196. doi: 10.3390/ijms23084196. Published 2022 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turi Z., Bjørkedal E., Gunkel L., Antal A., Paulus W., Mittner M. Evidence for cognitive placebo and nocebo effects in healthy individuals. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-35124-w. Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.