Abstract
Due to concern regarding the consumption of high amount of sugar in diet and role of diet in combating overweigh and related disease, the aim of present study was to optimize a reduced calorie probiotic chocolate milk formula with suitable physicochemical properties. The formula comprising inulin, stevia (Stevia rebaudiana Bertoni), chia (Salvia hispanica L.) seed gum (CSG), and whey protein concentrate (WPC) which optimized using Box-Behnken design (BBD) and then enriched with an encapsulated probiotic strain Lactobacillus acidophilus (DSM1643). The independent variables included inulin (2–8%), CSG (0.1–0.5 %), stevia (50–100 % replacement of sugar), and WPC (1–3%). The dependent variables were selected as viscosity, average particle size, sedimentation percentage, and general acceptance. Optimization done toward achieving the highest viscosity and general acceptance and the lowest sedimentation percentage and average particle size. The optimal conditions were found to be 7.99 % inulin, 70 % stevia, 0.34 % CSG, and 1 % WPC. Under these conditions, the viscosity, sedimentation percentage, average particle size, and general acceptance of the product were equal to 40.69 mPa s, 2.2 %, 434.221 nm, and 5.1, respectively. Next, the chocolate milk was enriched with at 109 CFU/g probiotic bacteria and evaluated. The probiotic strain was resistant to simulated gastrointestinal conditions and under this condition the free bacterial cells count declined by 8 logCFU/g while the encapsulated cells decreased approximate 3 logCFU/g. The bacteria count did not undergo a significant change until the 5th day of storage. The results showed that the inulin, stevia, CSG, and WPC at optimal concentrations and encapsulated probiotic bacteria could be simultaneously applied to produce a product with good properties. This formula could be considered as a new product with health improving properties, low calorie which is suitable for people suffering from diabetes and obesity.
Keywords: Chocolate milk, Stevia, Viscosity, Optimization, Formulation, Functional
1. Introduction
Today, people prefer to consume low-calorie products to prevent obesity which is directly correlated with diseases like metabolic syndrome, cardiovascular diseases, and diabetes. Recently, low-calorie food products have gained the attention of consumers with emphasis on following a healthy diet using food with health improving properties [1]. Chocolate milk is the most well-known and accepted type of flavored milk. Chocolate dairy beverages are commonly prepared with milk, sugar, cocoa powder, and some hydrocolloids used to improve product consistency and physical stability [2]. Considering the popularity of chocolate milk, it is interesting to reformulate this product with healthy and functional ingredients with suitable nutritional and physicochemical properties.
The physical instability of chocolate milk is one of the biggest challenges of the dairy industry. The addition of stabilizers is a way of increasing the stability of chocolate milk during storage [3]. Chia (Salvia hispanica) is native to southern Mexico and is an edible seed which is good source of protein, dietary fiber, antioxidants and polyunsaturated fats. The seeds are covered by a mucilage with high water holding capacity and soluble fiber nature that can be used as fat replacer in foods [4]. Also, inulin can raise the nutritional value and enhance the technological properties of food products by improving the texture and substituting fat [5]. Inulin is used as a fat replacer and also reduces the synersis in non-fat yogurts while improving the taste and mouthfeel [6]. It is a water-soluble dietary fiber that is regarded as a functional ingredient, owing to its prebiotic and bifidogenic properties [5]. Whey proteins are known for their proper nutritional and functional properties [7]. They are considered a very important source of nutrition, due to their high content of essential amino acids. High solubility in a wide range of pH values, water binding capacity, emulsifying, thickening, gelling, and foaming properties, as well as viscosity and texture improvement, are included in the desirable properties of whey proteins [8]. Since most flavored milk available in the market has large amounts of sugar, and children consume such milk the most, there is an interest to replace its sugar with a natural sweetener. Stevia is a ‘natural’ calorie-free product with sweetening power of 200–300 times higher than sucrose [9].
Sugar replacement in chocolate milk by herbal sources has been examined in numerous studies. Abedini et al. [10] assessed the effect of rebaudioside A, as the sugar substitute, and maltodextrin, as the fat replacer, on the physicochemical and sensory properties of pasteurized chocolate milk during storage and declared that the sample comprising 50 % maltodextrin and 50 % rebaudioside A had desirable physicochemical and sensory properties and was similar to the control. In another research, Homayouni Rad et al. [3] produced chocolate milk using three polyols, namely maltitol, xylitol, and isomalt, accompanied by stevia as a strong sweetener. They concluded that the use of 11.16 % (w/w) maltitol, 8.9 % (w/w) xylitol, and 12.93 % (w/w) isomalt resulted in the production of chocolate milk with 100 % desirability and without any unfavorable changes in rheological and qualitative properties. Phuong Ta et al. [11] investigated the effect of various polysaccharides and their combination with prebiotic saccharides on the encapsulation of probiotic bacteria Lactobacillus casei and reported that encapsulation significantly improved the degree of gastric tolerance of probiotic cells even in the presence of pepsin. However, to best our knowledge there is not any study on simultaneous use of probiotics bacteria, prebiotic, fat and sugar replacer constitute in chocolate milk formulation. Therefore, this study aimed to optimize the formulation of probiotic-fortified chocolate milk consisting of inulin, stevia, CSG, and WPC to achieve the highest viscosity and general acceptance and the lowest sedimentation percentage and average particle size, as well as enriching it with encapsulated probiotic bacteria Lactobacillus acidophilus to develop a novel functional product.
2. Materials and methods
2.1. Materials
In this research, the following materials were used: stevia (SU A-397 contains 97 % Rebadioside A, SteviaPac, Singapore); inulin (Akbariyeh, Iran); cocoa powder (Kayseri, Turkey); WPC powder and skim milk powder (Damdaran, Iran); carrageenan (Sigma Aldrich, Germany), and Lactobacillus acidophilus (DSM 1643) (Iranian Research Organization for Science and Technology, IROST, Iran). Vanilla powder (Golestan Co., Iran) and salt (Golha Co., Iran) were purchased from the local market. All chemical reagents used in this study were supplied from Merck Co., Germany.
2.2. Formulation optimization of chocolate milk
2.2.1. CSG extraction
First, chia seeds and water were mixed at a ratio of 1:40 (w/v) in a 1L beaker. The mixture was then agitated at 1920 rpm and temperature 20 °C for 3 h. Next, it was centrifuged (Sigma 1–14, Germany) at 3200×g for 20 min. Following filtration using Whatman No.4 filter paper, the obtained mucilage was vacuum-dried at 0.5 bar, 50 °C for 4 h. Afterwards, the dried mucilage was powdered using a domestic grinder. Finally, the resulting powder was packed in polyethylene bags and stored in a dry cool place until use [12].
2.2.2. Preparation of WPC solution
The WPC powder was gradually combined with water at a ratio of 1:10 at temperature of 80–85 °C. The mixture was stirred so as to allow powder to disperse well and hydrate properly [13].
2.2.3. Formulation of fortified chocolate milk
Skim milk (1.5 % fat) was used to produce the chocolate-flavored milk. The independent variables included inulin (2–8%), CSG (0.1–0.5 %), stevia (50–100 % replacement of sugar considering the sugar concentration of 8 % in the control), and WPC (1–3%). The dependent variables were selected as viscosity, average particle size, sedimentation percentage, and general acceptance. The relationship between the independent and dependent variables was determined using Box-Behnken design (BBD). The other ingredients including cocoa powder (15 %), carrageenan (0.05 %), vanilla powder (0.01 %), and salt (0.025 %) were kept constant in all the samples.
2.3. Methods for evaluation of chocolate milk
2.3.1. Determination of sedimentation percentage
After being stirred, 20 g of the chocolate milk sample was poured into a 25-mL tube and subsequently centrifuged at 5500×g and temperature 20 °C for 15 min. After that, the supernatant was separated and weighed. The sedimentation percentage was quantified by subtracting the supernatant weight from the sample weight. The results were expressed as g per 100 g of chocolate milk [14].
2.3.2. Particle size measurement
The size of each sample particle was determined through the laser diffraction method. First, each sample was diluted 100-fold by using distilled water. The average particle size was measured by a dynamic light scattering (DLS) system (NanoSizer 3000, Malvern Instruments, Malvern, UK) at a 90° angle and 25 °C in a cell specified to the system with a 1 cm width [15].
2.3.3. Sensory evaluation
The sensory attributes of the samples were evaluated by 15 trained panelists (including 8 men and 7 women) between 20 and 45 years old on 5-point hedonic scale (1 = very bad; 2 = bad; 3 = moderate; 4 = good; and 5 = very good). The sensory attributes included flavor and taste, odor, consistency and texture, color or appearance, and general acceptance [16]. In addition, the same panelists were used for the study of general acceptance of the product during the optimization of the chocolate milk formula.
2.3.4. Determination of viscosity
The apparent viscosity of the samples was measured at temperature 24 °C using the DV-II viscometer (Brookfild, U.S.A) equipped with spindle No. 16 at 100 rpm. Samples, approximately 8 mL, were poured directly into a cup pre-heated to 24 °C. Samples were equilibrated again for 120 s and pre-sheared at a constant shear rate (0.1 s−1, 30 s) before each test. Viscosity measurements were performed with an increasing shear rate of 0.1–100 s −1. The results were expressed in mPa [13]. Ultimately, the optimal formula was selected by the software based on the results of the tests.
2.4. Enrichment of chocolate milk with encapsulated probiotic strain
2.4.1. Preparation of microorganisms
The L. acidophilus (DSM 1643) was activated in 20 mL of MRS broth at 37 °C for 24 h. The resulting sample was then inoculated into 95 mL of MRS broth and proliferated under the above-mentioned conditions to achieve 108–109 CFU/mL [17]. Then the cells were collected from the medium using centrifugation at 4600×g and temperature 4 °C for 5 min.
2.4.2. Encapsulation of probiotic bacteria with sodium alginate
In brief, 1 mL of bacterial cell suspension (108–109 CFU/mL) was mixed with 9 mL of sterile sodium alginate solution (2 % W/V). After gentle mixing, 1 mL of the cell mixture was added into 9 mL sterilized 0.15 M calcium chloride aqueous solution, and stirring was continued for 20 min till the alginate capsules formed. The alginate capsules were separated by centrifugation at 500×g and 9 °C for 2 min. The beads were rinsed with peptone water of 9.2 % through centrifugation [11]. Finally, the encapsulated samples were freeze-dried (LD PLUS Alpha 2–4, Germany) at −8 °C for 24 h. The viability of entrapped bacteria was evaluated using Moumita et al. [18] method. Accordingly, 1 g microcapsule was added into 9 mL sodium citrate (3 g/L) and slightly shaken for 10 min. Then, serially 10-fold diluted released bacteria were surface plated on de Man, Rogosa and Sharpe (MRS; Merck, Darmstadt, Hesse, Germany) agar. The cell count was enumerated after incubation at 37 °C for 24 h.
2.4.3. Probiotic fortified chocolate milk
The encapsulated bacteria were inoculated into the optimal formula of the chocolate milk at 109 CFU/g under sterile conditions, and the samples were stored at 4–8 °C.
2.5. Study on encapsulated probiotics and probiotic chocolate milk
2.5.1. Morphology of encapsulated probiotics
The morphological features of the samples were studied using scanning electron microscopy (SEM) (Lnu, Nira2, Tescan, Czech Republic) at a voltage of 10 kV. The encapsulated sample after freeze-drying were coated with gold (Au) and then analyzed [19].
2.5.2. Encapsulation yield (EY)
The EY of the encapsulated probiotic bacteria was calculated by the following equation [20]:
N: number of bacterial cells after drying (Log CFU.g−1).
N0: number of bacterial cells before drying (Log CFU.g−1).
EY: encapsulation yield of probiotic bacteria.
2.5.3. Number of bacteria entrapped in capsules
At first, 1 g of the prepared capsules was combined with 9 mL of sterile phosphate buffer 0.1 N (pH = 6) and stirred with a magnetic stirrer for 15 min. The bacteria released into the buffer were cultured onto MRS agar and incubated at 37 °C for 48 h, and the microbial population on the plates was monitored [21,22].
2.5.4. Resistance to simulated gastrointestinal conditions
The method Rao et al. [23] was used to study the resistance of free and encapsulated bacteria to gastrointestinal conditions. To simulate the gastric juice, pepsin was dissolved in saline 0.5 % (w/v) at 0.5 g/L, and the pH of the solution was set at 2 using HCl. To prepare the simulated intestinal juice, pancreatin, and bile salts were dissolved in saline 0.5 % (w/v) at 0.1 % and 0.8 %, respectively. To compare the resistance of the free and encapsulated bacteria to the simulated gastrointestinal conditions, 1 g of the dried capsules was blended with distilled water and set aside for 15 min to ensure complete hydration. After swelling, the capsules were added to 5 mL of the simulated gastric juice, and the solution was incubated in a shaking incubator at 37 °C for 2 h. After that, 1 mL of the solution was mixed with 9 mL of phosphate buffer solution to release the bacteria from the capsules, and incubation was continued at room temperature for 15 min. To investigate the amount of damaged bacteria, serial dilutions were prepared, and the samples were cultured on MRS agar and incubated at 37 °C for 24–48 h. The 4 mL remaining from the gastric test, was mixed with the simulated intestinal juice. Then, the mixture pH was elevated to 7.5 with NaOH 0.1 N, and its final volume was made to 10 mL with distilled water, and incubated in a shaking incubator. After 2 and 4 h, 1 mL of the sample was combined with 9 mL of phosphate buffer to release the encapsulated bacteria, afterwards, the samples were cultured on MRS agar and incubated for 24–48 h, and the colonies formed were counted to determine the survivability of the encapsulated bacteria.
2.5.5. Heat resistance
1 g of the sample was blended with 5 mL of distilled water and allowed to swell completely. Next, the mixture volume was made to 10 mL with distilled water. Subsequently, it was heated at 50, 55, and 60 °C for 0, 5, 10, 20, 30, 60, and 120 min. Following each heat treatment, 1 mL of the sample was mixed with 9 mL of phosphate buffer to release the bacteria from the capsules. After that, the serial dilutions were prepared, which were cultured on MRS agar and incubated at 37 °C for 24–48 h [24].
2.5.6. Survivability of probiotics during storage
The probiotic count was performed on the 1st, 3rd, and 5th days of storage using the streak plate method. The samples were cultured on MRS bile agar and incubated at 37 °C for 72 h [25]. The chocolate milk sample containing the free probiotics was used as the control.
2.5.7. Color of chocolate milk
The color of the samples was evaluated using a Hunter Lab device (Colorflex, USA). The sample was poured into the apparatus chamber, so the bottom of the chamber would be completely covered. The color parameters of L*, a*, and b* were recorded.
2.5.8. Microbial tests
Total plate count was evaluated using ISO 16297:2020 method [26], Escherichia coli by ISO 11866-1: 2005 [27], Fungi count using ISO 21527-1: 2008 [28], and Entrobacteriaceae count by ISO 21528-2: 2004 method [29].
2.6. Statistical analysis
Design Expert 11 was employed for the formulation optimization. Analysis of variance (ANOVA) and Duncan's multiple range test was conducted at 95 % confidence level using SPSS 22. All the measurements were done in triplicate. In order to optimize the formulation of the chocolate milk, response surface methodology (RSM) and Design Expert version 11 were used. A BBD was created with five replications for the central point and three levels (−1, 0, and +1) for the independent variables to achieve the highest viscosity and general acceptance and the lowest sedimentation percentage and average particle size.
3. Results and discussion
3.1. Formulation optimization of fortified chocolate milk
3.1.1. Model fitting
The BBD matrix, composed of inulin (A), stevia (B), CSG (C), and WPC (D), as well as the responses, including viscosity, sedimentation percentage, average particle size, and general acceptance are summarized in Table 1.
Table 1.
Box-Behnken design matrix and responses.
| Run | Independent Variables |
Responses |
||||||
|---|---|---|---|---|---|---|---|---|
| Inulin (%) | Stevia (%) | Chia seed gum (%) | Whey protein concentrate (%) | Viscosity (mPa.s) | Sedimentation (%) | Average particle size (μm) | general acceptance | |
| 1 | 8 | 75 | 0.5 | 2 | 47.5 | 4 | 568 | 5 |
| 2 | 5 | 50 | 0.3 | 3 | 41.6 | 3.6 | 529.7 | 4 |
| 3 | 5 | 75 | 0.3 | 2 | 40.5 | 3 | 432 | 4 |
| 4 | 8 | 50 | 0.3 | 2 | 44.5 | 2.6 | 498.6 | 5 |
| 5 | 8 | 75 | 0.3 | 1 | 38.6 | 2.1 | 423.6 | 5 |
| 6 | 5 | 50 | 0.3 | 1 | 37.8 | 2.5 | 379.5 | 4 |
| 7 | 5 | 50 | 0.5 | 2 | 43.5 | 4.6 | 572.4 | 4 |
| 8 | 5 | 100 | 0.1 | 2 | 31.8 | 4.7 | 410.6 | 3 |
| 9 | 8 | 75 | 0.3 | 3 | 43.8 | 2.6 | 549.9 | 5 |
| 10 | 5 | 75 | 0.5 | 1 | 39.7 | 4.3 | 420.3 | 4 |
| 11 | 5 | 75 | 0.5 | 3 | 45.7 | 4.5 | 572.1 | 4 |
| 12 | 2 | 75 | 0.3 | 3 | 37.9 | 3.7 | 552.2 | 3 |
| 13 | 5 | 75 | 0.1 | 3 | 36.4 | 5.3 | 500.8 | 4 |
| 14 | 5 | 100 | 0.3 | 1 | 35 | 2.1 | 360.1 | 3 |
| 15 | 5 | 50 | 0.1 | 2 | 34.1 | 5.3 | 413 | 4 |
| 16 | 5 | 75 | 0.3 | 2 | 40.7 | 2.9 | 470 | 4 |
| 17 | 2 | 75 | 0.3 | 1 | 34.6 | 3.2 | 372.6 | 3 |
| 18 | 2 | 75 | 0.5 | 2 | 40.1 | 5.2 | 511 | 3 |
| 19 | 5 | 75 | 0.3 | 2 | 40.8 | 3 | 468.7 | 4 |
| 20 | 2 | 100 | 0.3 | 2 | 35.9 | 3.1 | 417.3 | 1 |
| 21 | 2 | 50 | 0.3 | 2 | 37 | 3.7 | 485.6 | 2 |
| 22 | 5 | 100 | 0.5 | 2 | 41.6 | 3.9 | 516.3 | 3 |
| 23 | 5 | 75 | 0.3 | 2 | 40.81 | 3.2 | 468.7 | 4 |
| 24 | 8 | 100 | 0.3 | 2 | 42.1 | 2.3 | 441.2 | 4 |
| 25 | 5 | 75 | 0.1 | 1 | 31.7 | 5 | 354.3 | 4 |
| 26 | 8 | 75 | 0.1 | 2 | 36.2 | 5.4 | 421.9 | 5 |
| 27 | 2 | 75 | 0.1 | 2 | 33.2 | 4.4 | 408.7 | 3 |
| 28 | 5 | 75 | 0.3 | 2 | 40.82 | 3.3 | 468.9 | 4 |
| 29 | 5 | 100 | 0.3 | 3 | 40 | 3 | 500.5 | 3 |
* The lowest and highest scores of general acceptance were equal to 1 and 5, respectively.
The model validity was confirmed by comparing the empirical and predicted results of the responses. The effects of the four factors on the responses were examined by the linear, 2FI, and quadratic models. The model with the most significant sum of squares (SS) and most non-significant lack of fit was opted as the best one. The ANOVA revealed that the quadratic model best fitted the experimental data of all the four responses (Table 2), and the regression models are as follows:
Table 2.
Response model fitting results.
| Response | viscosity | Sedimentation | Average particle size | General acceptance |
|---|---|---|---|---|
| Model | Quadratic | Quadratic | Quadratic | Quadratic |
| p-value | 0.0001 > | 0.0001 > | 0.0001 > | 0.0001 > |
| R-Squared | 0.985 | 0.9601 | 0.9505 | 0.9580 |
| Adj R-Squared | 0. 969 | 0.9203 | 0.9010 | 0.9159 |
| Pred R-Squared | 0.914 | 0.7857 | 0.7536 | 0.7579 |
| Adeg-Precision | 31.84 | 14.8659 | 17.4603 | 18.6425 |
| Lack-of-fit | N. S | N. S | N. S | N. S |
| C.V. % | 1.80 | 7.88 | 4.41 | 7.18 |
Viscosity = +15.44650 + 0.625556A + 0.194453B + 30.44500C + 5.25617D-0.004333AB + 1.83333AC+ 0.158333AD+ 0.020000BCE+ 0.012000BD+ 1.62500CD- 0.022278 A2- 0.001621 B2- 35.95000C2-1.27550 D2
Sedimentation = +4.79442 + 0.134259A + 0.028433B- 22.37917C + 0.872500D+0.001000AB-0.916667AC- 1.53899E-16AD- 0.005000BC- 0.002000BD- 0.125000CD- 0.005370 A2-0.000257 B2+ 43.47917C2- 0.098333D2
Average particle size = +163.47426–5.71481A + 1.50103B + 187.99167C + 131.02417D+ 0.036333AB+ 18.25000AC- 4.44167AD- 2.68500BC- 0.098000BD+ 6.62500CD+ 1.07259 A2-0.009715 B2+ 300.08333C2- 7.22167D2
General acceptance = −2.38657 + 0.574074A + 0.140000B-1.25000C- 0.333333D+ 6.27077E-17AB+ 2.28130E- 15AC+ 5.45510E- 16AD+ 1.71991E- 16BCE+ 3.71876E- 17BD+ 6.18147E- 15CD- 0.018519 A2- 0.0010670 B2+ 2.08333C2+ 0.083333 D2Which amount of inulin (A), stevia (B), CSG (C), and WPC (D) are presented as independent variable.
3.1.2. 2.1.3viscosity
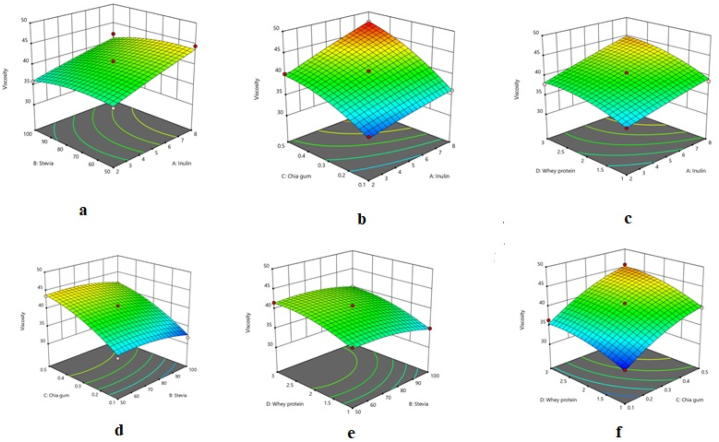
As can be observed in Table 2, the quadratic model was statistically significant for viscosity, with its lack of fit not being significant, indicating the adequacy of the model. The high determination coefficient (R-square 0.985) and adjusted determination coefficient (Adj R-square 0.969) of the model demonstrated that the model was able to fit the empirical data properly. The impacts of the factors on the viscosity of the chocolate milk are illustrated in Fig. 1a-f. It can be observed that the response increased with a rise in all four factors.
Fig. 1.
Response surface plots illustrating the interactive effects of a) inulin and stevia, b) inulin and chia seed gum, c) inulin and whey protein concentrate, d) stevia and chia seed gum, e) stevia and whey protein concentrate, and f) chia seed gum and whey protein concentrate on the viscosity of chocolate milk.
As the concentrations of inulin, stevia, CSG, and WPC were elevated, the chocolate milk viscosity significantly increased (Fig. 1a–f). The most profound effect belonged to CSG percentage, and as its concentration increased from 0.1 % to 0.5 %, the response increased by about 10 mPa s. It is reported that the viscosity of chocolate milk was raised with an increase in gum concentration, which is consistent with the findings of the present study [14,30]. Zhu et al. [13] claimed that there was a direct correlation between the viscosity and whey protein content of chocolate milk due to the presence of more casein in the drink, which gave rise to the interaction between the proteins during thermal processing, leading to the formation of a stronger network and, consequently, an increase in viscosity. It can be stated that the main reason behind the viscosity increase was the presence of proteins and polysaccharides in the chocolate milk samples, as these high-molecular-weight compounds bind with water and form a gel network, thus raising the viscosity.
3.1.3. 3.1.3. sedimentation percentage
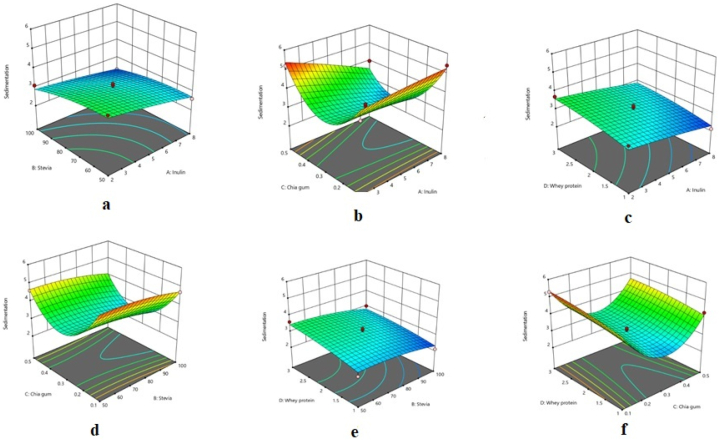
As shown in Table 2, the quadratic model was statistically significant for this response; moreover, its lack of fit was not significant, both showing that the model was appropriate to describe the variation in the experimental data. The high R-square (0.960) and Adj R-square (0.920) of the model indicated that the model could adequately fit the obtained data. Fig. 2(a–f) depicts the effects of the different factors on the sedimentation percentage of the chocolate milk. As CSG concentration increased, the response first declined and then increased. Furthermore, stevia and inulin percentages did not significantly affect this response.
Fig. 2.
Response surface plots illustrating the interactive effects of a) inulin and stevia, b) inulin and chia seed gum, c) inulin and whey protein concentrate, d) stevia and chia seed gum, e) stevia and whey protein concentrate, and f) chia seed gum and whey protein concentrate on the sedimentation percentage of chocolate milk.
As CSG percentage increased from 0.1 to 0.3, the sedimentation percentage of the chocolate milk significantly decreased. However, it considerably increased with an increase in CSG from 0.3 % to 0.5 %. Based on the theory developed by Syrbe et al. [31] this could be ascribed to depletion flocculation which occurs when the concentration of free hydrocolloid exceeds the required amount, the hydrocolloid cannot absorb water anymore, and thus precipitates. The reduction in the sedimentation percentage of chocolate milk by increasing the concentration of WPC, inulin, and stevia could be owing to the hydrophilicity of these compounds and the formation of a stable colloidal system [3]. It was stated that caseins are essential for structure formation and whey proteins are found to support this structure which can be attributed to more casein–whey proteins and whey proteins–whey proteins interactions [32].
3.1.4. 4.1.3. average particle size
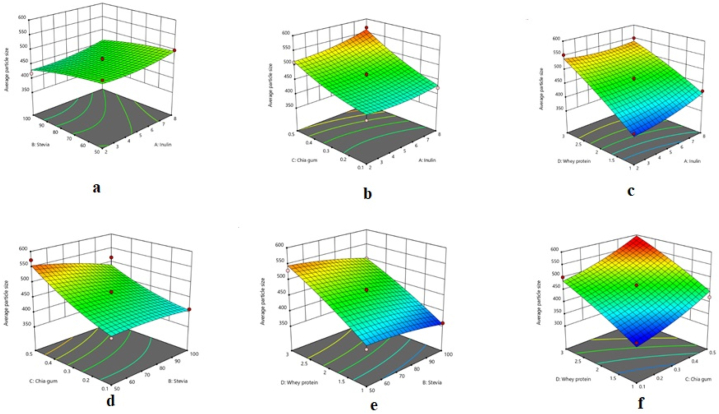
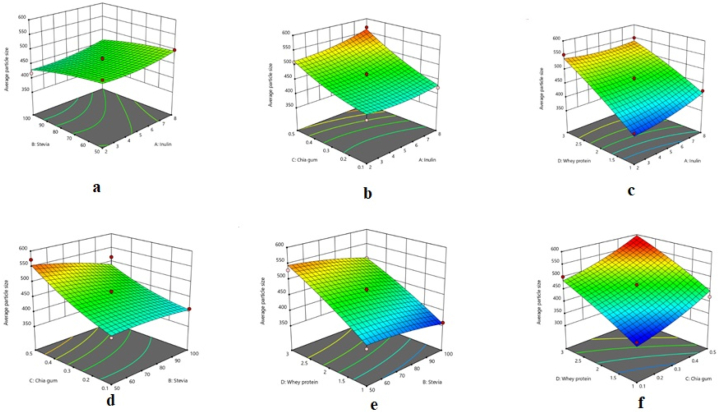
The quadratic model was statistically significant for the average particle size of the chocolate milk; furthermore, the lack of fit of the model was not significant, which indicates the model's adequacy for explaining the variation in this response (Table 2). The high R-square (0.950) and Adj R-square (0.901) of the model demonstrated that the model was able to properly fit the empirical data. The interactive effects of the factors on the average particle size of the samples are presented in Fig. 3(a–f). It can be seen that the response increased with a rise in the concentrations of inulin, CSG, and WPC.
Fig. 3.
Response surface plots illustrating the interactive effects of a) inulin and stevia, b) inulin and chia seed gum, c) inulin and whey protein concentrate, d) stevia and chia seed gum, e) stevia and whey protein concentrate, and f) chia seed gum and whey protein concentrate on the average particle size of chocolate milk.
The size of chocolate milk particles substantially influences the physical and sensory properties of the product. The sandiness and coarse particles of the product are sensed when it is drunk [33]. In the present study, as the percentages of CSG, inulin, and WPC were elevated, the average particle size of the chocolate milk significantly increased (Fig. 3a–f). On the other hand, the response decreased with an increase in stevia, caused by the decrease in the sugar content of the chocolate milk. WPC and inulin brought about the rise in the chocolate milk particles by interacting and creating complexes with the polysaccharides present in the drink [34].
3.1.5. general acceptance
It can be seen in Table 2 that the quadratic model was statistically significant for the general acceptance of the chocolate milk, and its lack of fit was not significant, both revealing the competence of the model. The high R-square (0.958) and Adj R-square (0.916) of the model demonstrated that the model could adequately fit the experimental data. As it can be seen in Fig. 4(a–f), the general acceptance of chocolate milk gradually increased as the levels of inulin, CSG, and WPC were elevated. However, it firstly increased and then declined with a rise in the stevia level.
Fig. 4.
Response surface plots illustrating the interactive effects of a) inulin and stevia, b) inulin and chia seed gum, c) inulin and whey protein concentrate, d) stevia and chia seed gum, e) stevia and whey protein concentrate, and f) chia seed gum and whey protein concentrate on the general acceptance of chocolate milk.
Paixao et al. [35] suggested that the general acceptance of chocolate milk highly depended on its mouthfeel profile and sweetness. Mouthfeel itself is dependent on the different ingredients of chocolate milk, including carbohydrates, fat, protein, stabilizers such as carrageenan, and other thickening agents [36]. In the case of stevia, the response experienced a rise with an increase in its concentration, followed by a reduction which was because of its bitter and metal taste. Ferreira et al. [37] cited that inulin-containing milk had a better flavor.
3.1.6. optimization
The process variables were optimized to acquire the lowest sedimentation percentage and average particle size, as well as the highest viscosity and general acceptance. The formulation was optimized based on the selected models and presented in Table 3. Under the optimum conditions, the predicted values were found to be 40.69 mPa s, 2.2 %, 434.221 nm,5.1, and experimental data 43. 1 mPa s, 2.28, 442 nm and 5.0 for viscosity, sedimentation percentage, average particle size, and general acceptance of the product, respectively with a desirability of 0.769 which confirmed the obtained results.
Table 3.
Optimal percentages of independent variables.
| Percentage of inulin | Percentage of stevia replacement | Percentage of chia seed gum | Percentage of whey protein concentrate |
|---|---|---|---|
| 7.99 | 70 | 0.346 | 1 |
3.2. Encapsulation of probiotic strain
3.2.1. morphological properties and size
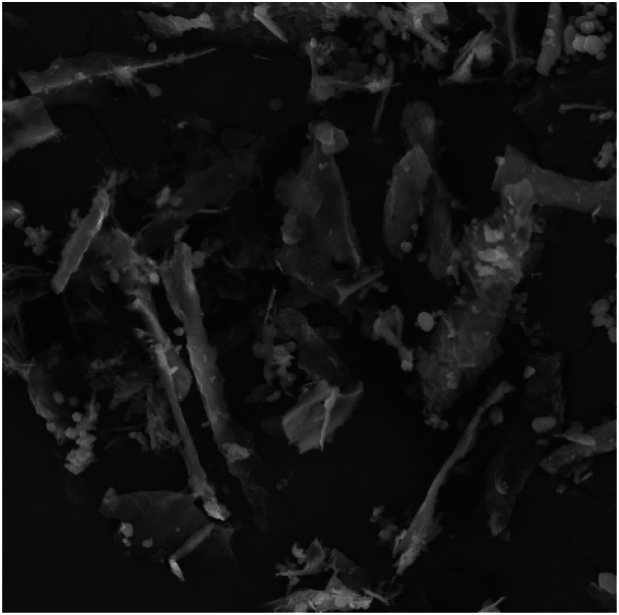
As demonstrated in Fig. 5, the microcapsules had an irregular structure with no specific shape. The wrinkles on the surface of the capsules are associated with water evaporation from the freeze-dried capsules. This could be owing to the high rate of frozen water sublimation from the wall matrix and the creation of a hole in the ice crystal surfaces without sufficient time for the development of wrinkles [20].
Fig. 5.
Micrograph of sodium alginate capsules contained probiotic bacteria.
Dynamic light scattering (DLS) determined the average particle size of the capsules to be 80.5 ± 0.56 μm. In the study of Qi et al. [38] Saccharomyces boulardii and Enterococcus faecium were microencapsulated by a method based on emulsion and internal gelation and it was reported that the average capsule was between 300 and 500 μm. Very large capsules cause the unfavorable texture of food products, however, capsules with a size below 100 μm are suitable for food products [20].
3.2.2. Encapsulation yield
The EY of the encapsulated bacteria was found to be 86.7 ± 2.1 %. the low percentage of the bacterial loss revealed the successful encapsulation of the cells. Whilst, in the research conducted by Phuong Ta et al. [11] the EY of the probiotic strain, Lactobacillus casei, encapsulated with alginate, was reported 64.4 %, whereas Sharifi et al. [20] recorded an EY of 93.80 % for the probiotic strain of Lactobacillus plantarum encapsulated with whey protein.
3.2.3. Resistance to simulated gastrointestinal conditions
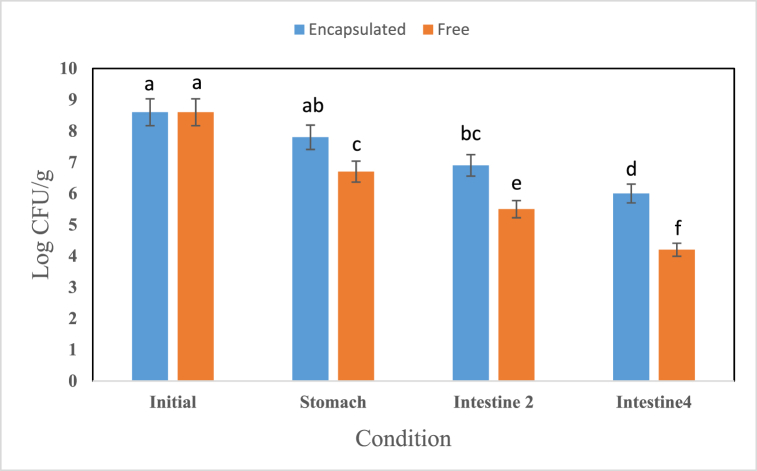
Our results showed an intense reduction in the number of active non-encapsulated (free) bacteria (Fig. 6). The influence of encapsulation was significant on the survivability of the bacterial cells in the alginate capsules. Based on the results, encapsulation could raise the survivability of the bacteria under simulated gastrointestinal conditions. The count of the free bacterial cells declined by 8 logCFU/g after passing through these conditions, while the encapsulated cells showed an approximate decrease of 3 logCFU/g. By comparing the free and encapsulated bacteria, it can be concluded that encapsulation could protect the bacteria to a great extent against unfavorable factors such as simulated gastrointestinal conditions and enzymes. These findings conformed to those previously reported by others [17]. Bosnea and Moschakis [39] also showed that the number of Lactobacillus paracasei and Lactobacillus plantarum encapsulated in coacervates consisting of whey protein isolate and gum Arabic in two conditions of the stomach and intestine decreased by 5.2 and 5 logCFU/g, respectively.
Fig. 6.
Survivability of free and encapsulated bacteria under simulated gastrointestinal conditions Similar letters represent non-significant differences at p < 0.05.
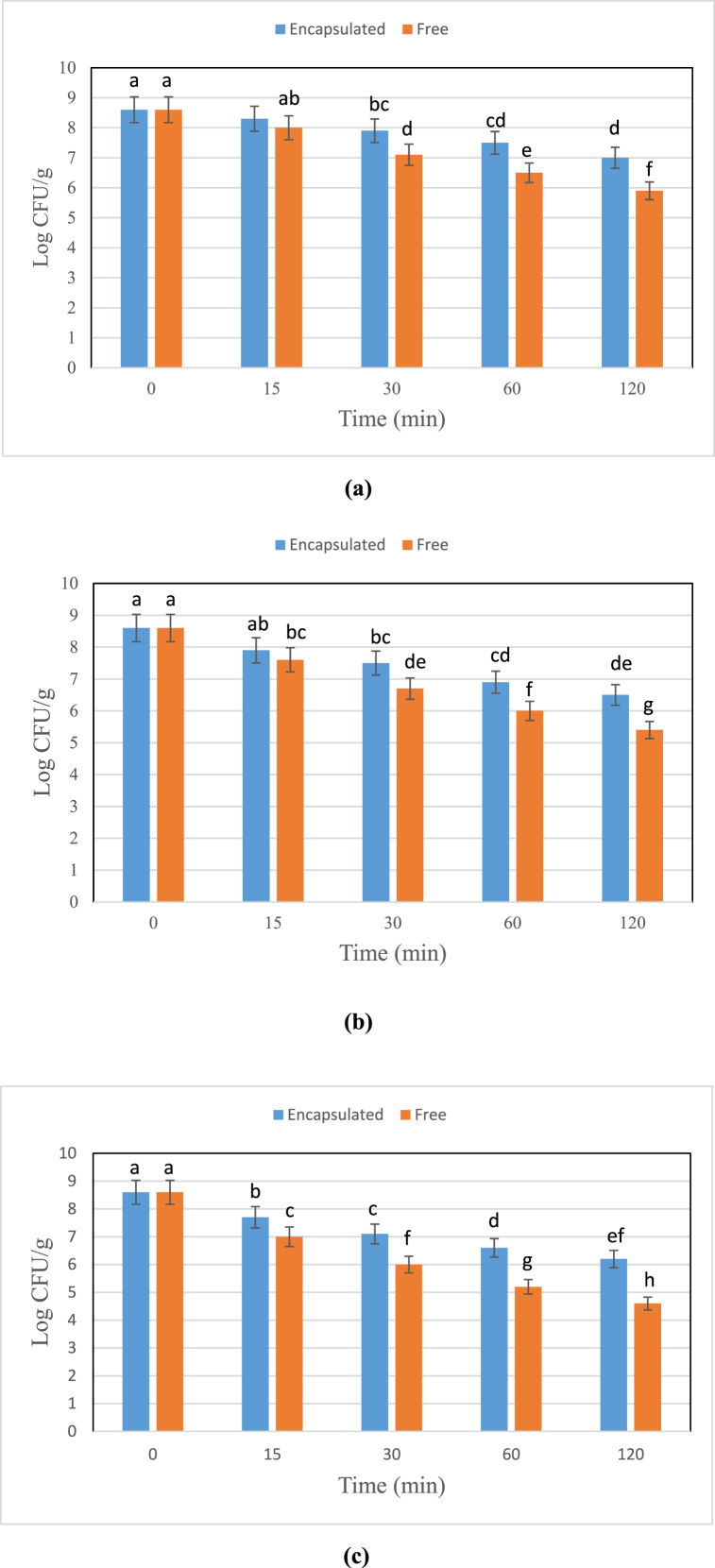
3.2.4. Heat resistance
As can be observed in Fig. 7 (a, b, C), the heat treatment at a temperature of 50 °C had a substantially less destructive effect on the bacteria than treatment at temperatures 55 °C and 60 °C. After 120 min of heating at 50 °C, the number of the encapsulated cells declined by 1.6 Log CFU/g, and the number of the free bacteria lowered by 2.7 Log CFU/g. The numbers of the free and encapsulated bacteria after 120 min heating at 55 °C reduced by 3.2 and 2.1 Log CFU/g, and at 60 °C reduced by 4 and 2.4 Log CFU/g, respectively.
Fig. 7.
Survivability of free and encapsulated L. acidophilus at a) 50 °C, b) 55 °C, and c) 60°C
Similar letters represent non-significant differences at p < 0.05.
As results show, the sodium alginate capsules could preserve the core material against heat to some extent compared to free cells. As heating proceeded in all temperatures, the protective effect of the capsules was lost owing to the transfer of heat to their internal layers [40]. In the study carried out by Muzaffar et al. [41], 5 logCFU/ml of active cells were reduced after heating the Lactobacillus acidophilus cells encapsulated in alginate, inulin, and resistant corn starch at 60 °C for 1 h. Nakkarach and Withayagiat [17] also maintained that encapsulation of Lactobacillus casei with alginate increased the bacteria heat resistance by 20 min at 55 °C.
3.3. Study on probiotic fortified chocolate milk
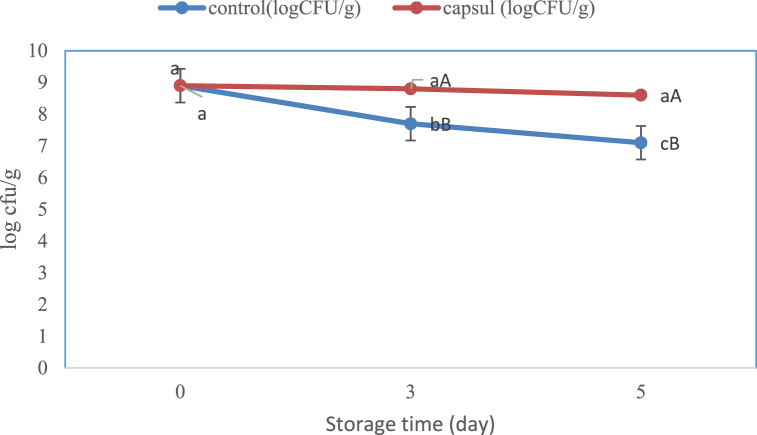
3.3.1. Survivability
As illustrated in Fig. 8, the number of encapsulated L. acidophilus cells did not significantly change until the 5th day of the chocolate milk storage and was higher than the recommended limit for health promotion. However, the population of the free (non-encapsulated) bacteria significantly decreased after 3 days of storage (p < 0.05). Furthermore, a significant difference was observed between the number of encapsulated and free probiotics (p < 0.05).
Fig. 8.
Changes in survivability of free and encapsulated bacteria in functional chocolate milk during storage
Similar letters represent non-significant differences at p < 0.05.
Previous studies on the protective roles of capsules on the survival of encapsulated L. rhamnosus GG was greater than 7 log CFU/ml during storage for 21 days in mocha milk [42], L. acidophilus encapsulated with sodium alginate-whey protein at 9 log CFU/ml [43], Bifidobacterium longum encapsulated with sodium alginate-whey protein at 7 log CFU/ml [44] and B. lactis encapsulated with sodium alginate-inulin at 8 log CFU/ml in goat milk [45] reported similar results.
Zakirul et al. [25] revealed that storage time and temperature affected the survivability of probiotics, as L. acidophilus count gradually decreased during refrigerated storage, whereas it dramatically declined at ambient temperature such that the bacteria disappeared after 30 days.
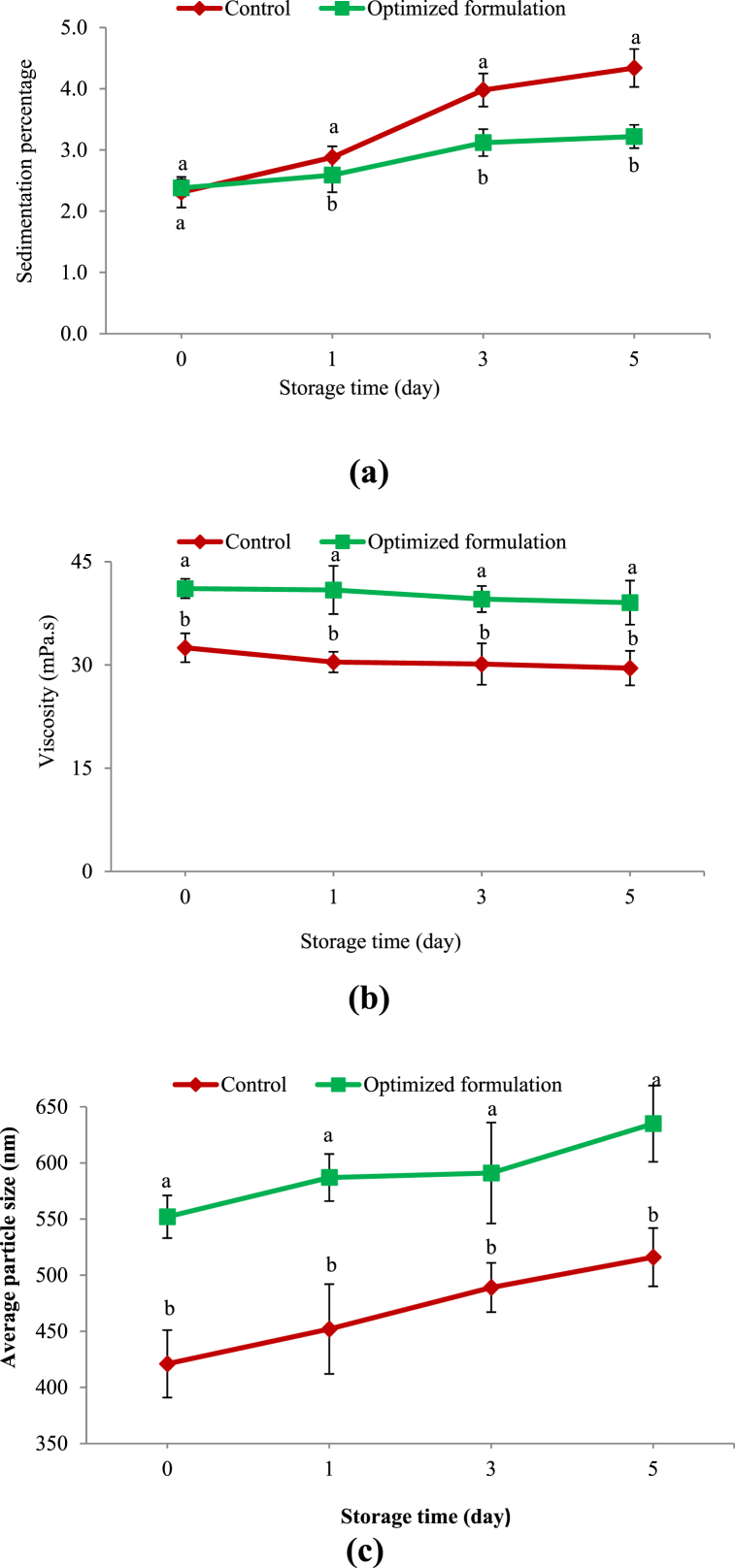
3.3.2. sedimentation percentage
Sedimentation occurs in chocolate milk as a result of the aggregation and precipitation of cocoa particles during storage. It is attempted to be prevented by using stabilizers which decrease phase separation to a large extent. It can be seen in Fig. 9a that the sedimentation percentages of the fortified probiotic milk and control samples did not significantly differ on the 0th and 1st days of storage (p > 0.05). As time proceeded, the response increased in both samples. On the 5th day, the response reached 14.4 and 3.42 % in the control and probiotic fortified samples, respectively. Significant differences were observed between the sedimentation percentages of the samples on the 3rd and 5th days (p < 0.05). The lower sedimentation percentage of the fortified probiotic chocolate milk compared to the control sample can be attributed to the high hydrophilicity of CSG and inulin which raised the system stability by increasing the viscosity [46]. The reducing effects of gums on the sedimentation percentage of chocolate milk have been reported by other researchers [14].
Fig. 9.
Changes in a) sedimentation percentage, b) viscosity, and c) average particle size of functional and control chocolate milks during storage
Similar letters represent non-significant differences at p < 0.05.
3.3.3. Viscosity
As denoted in Fig. 9b, the fortified probiotic sample had a significantly higher viscosity than the control during the whole storage period. The viscosity of both samples slightly decreased during storage. The higher viscosity of the probiotic fortified sample can be due to the presence of inulin, CSG, WPC, and stevia in this sample. Whey protein is capable of holding water after denaturation. The ability of WPC to form gel during heat processing is so effective in improving the consistency of food products. These results conform to most of those already reported. Guggisberg et al. [47] and Lisak et al. [48] reported a rise in the viscosity of yogurt and strawberry yogurt with the addition of stevia, respectively. Prakash et al. [14] expressed that chocolate milk viscosity was elevated by adding carrageenan. Schmidt and Smith [49] showed that guar, xanthan, and carrageenan at different concentrations brought about increases in the viscosity of flavored milk.
3.3.4. Average particle size
Particle size distribution is widely used to evaluate the formation and growth of the electrostatic complexes between proteins and polysaccharides. The results indicated that the average particle sizes of the control and fortified probiotic chocolate milk were respectively found to be 421 and 552 nm immediately after production (Fig. 9c). During the 5-day storage of the samples, the particles enlarged considerably, as the average sizes of the control and fortified probiotic samples reached 516 and 635 nm, respectively. During the whole storage, the particles of the control were significantly smaller than those of the fortified probiotic sample, probably due to the presence of CSG and WPC in the latter. In this respect, Zarabadipour et al. [50] maintained that the particle sizes of chocolate milk significantly increased after the addition of carrageenan, agreeing with the results of the present study.
3.3.5. Color
Table 4 presents the color indices (L*, a*, and b*) of the fortified probiotic and control samples immediately after production and on the 1st, 3rd, and 5th days of storage. There was a significant difference between the control and fortified probiotic samples in terms of L*; the latter one was darker than the control during the entire storage. Sugar replacement can highly affect the L* of chocolate milk probably because of adding new ingredients with lower brightness. The L* values of both samples did not significantly change during the 5-day storage, implying that time did not have any effect on the color of the chocolate milk. Moreover, the other ingredients incorporated into the chocolate milk, including CSG, WPC, and inulin, could have impacted the color indices. b* (yellowness) was significantly lower in the control than in the fortified probiotic sample, which was the result of adding WPC and inulin to the latter. a* (redness) did not differ between the samples.
Table 4.
Changes in color indices of control and fortified probiotic chocolate milk samples during storage.
| Sample | Storage time (days) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 5 | |
| L* | ||||
| Control | 71.23 ± 3.14a | 72.35 ± 2.46a | 70.89 ± 1.22a | 71.90 ± 1.1a |
| Fortified | 67.79 ± 2.6b | 68.15 ± 0.98b | 67.5 ± 1.76b | 68.31 ± 1.5b |
| b* | ||||
| Control | 14.77 ± 0.68a | 13.92 ± 1.23a | 14.48 ± 1.38a | 14.37 ± 1.42a |
| Fortified | 16.96 ± 0.79b | 17.09 ± 0.98b | 17.35 ± 2.08b | 17.25 ± 1.07b |
| a* | ||||
| Control | 3.75 ± 0.13a | 3.69 ± 0.09a | 3.11 ± 0.39a | 2.95 ± 0.12a |
| Fortified | 3.15 ± 0.07a | 3.27 ± 0.17a | 3.87 ± 0.11a | 3.41 ± 0.28a |
3.3.6. Sensory evaluation
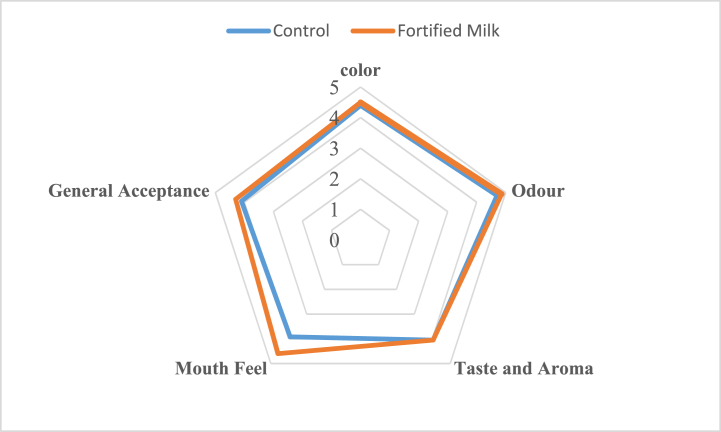
The results of the sensory evaluation of the chocolate milk samples are illustrated in Fig. 10. Despite the slight decline in the scores of colors, taste, and odor, no significant differences were observed between the control and fortified probiotic samples in terms of these attributes. The mouthfeel score of the fortified probiotic samples (4.6) was significantly higher than that of the control (3.9), showing the positive effects of the added ingredients on the textural properties of the chocolate milk. The results of viscosity measurements confirm the sensory evaluation results. There were no significant differences between the general acceptance scores of the two samples, revealing that sugar replacement did not have any adverse effects on the sensory properties of the product. Reductions in sensory scores have already been reported after the replacement of sugar with dietary sweeteners. Li et al. [36] substituted sucralose for sucrose up to 50 % and suggested that the strawberry-flavored fresh yogurt did not show any significant difference with the control in terms of sensory attributes. However, the general acceptance lowered at 60 and 80 % replacement levels, consistent with our results.
Fig. 10.
Sensory attributes of fortified probiotic and control chocolate milk samples immediately after production.
3.3.7. Microbial properties
The total plate count, fungi, Enterobacteriaceae, and E. coli counts of the control and fortified probiotic chocolate milk were measured during 5 days of storage at 4 °C (Table 5). The results demonstrated the efficiency of pasteurization, and no contamination was observed in either of the samples until the end of storage. No significant differences were observed between the samples (p > 0.05). All the microbial properties were in the standard range.
Table 5.
Microbial properties of control and fortified probiotic chocolate milk samples during storage.
| Storage Time (Days) | Microbial Test | Control | Fortified probiotic sample |
|---|---|---|---|
| 0 | Total Plate Count | 1.4 × 104aA | 1.4 × 104 aA |
| E.coli | ND | ND | |
| Entrobacteriaceae | ND | ND | |
| Fungi/Yeast | ND | ND | |
| 1 | Total Plate Count | 1.4 × 104 aA | 1.4 × 104 aA |
| E.coli | ND | ND | |
| Entrobacteriaceae | ND | ND | |
| Fungi/Yeast | ND | ND | |
| 3 | Total Plate Count | 1.44 × 104 aA | 1.41 × 104 aA |
| E.coli | ND | ND | |
| Entrobacteriaceae | ND | ND | |
| Fungi/Yeast | ND | ND | |
| 5 | Total Plate Count | 1.54 × 104 aA | 1.49 × 104 aA |
| E.coli | ND | ND | |
| Entrobacteriaceae | ND | ND | |
| Fungi/Yeast | ND | ND |
ND: Not Detected.
Different small and capital letters indicate significant differences within each row and column, respectively (p < 0.05).
4. Conclusions
The prevalence of obesity is esteemed to account 10–13 % of deaths all around the world. Especially high rate of childhood obesity is concerning. Many children and adults receive around 10 % of energy intake from added sugars. Therefore, reducing sugar in foods is of utmost importance. However, maintaining good diet quality is important in order to meet nutrient requirement at a lower energy intake. Considering the popularity of chocolate milk among children, it is interesting to reformulate this product with reduced sugar and healthy, functional ingredients. Based on results of this study incorporation of inulin, stevia, CSG, and WPC into chocolate milk improved its functional properties including viscosity, sedimentation percentage, average particle size, and general acceptance. The optimal formula found to be 7.99 % inulin, 70 % stevia replacement, 0.346 CSG, and 1 % WPC. The results revealed that the simultaneous use of inulin, stevia, CSG, and WPC at optimal concentrations could stabilize the chocolate milk without any undesirable effects on its sensory properties. The results of this study also showed that the encapsulation of L. acidophilus with sodium alginate enhanced the survivability of the bacteria by 4 logCFU/g after being subjected to the simulated gastrointestinal conditions. From an industrial point of view, the results of this study can help food companies overcome the technical challenges of reducing sugar in milk-based beverages, thus remarkably benefiting the health of people, in particular children. It also opens up new way to make low-calorie functional probiotic dairy beverages using different ingredients and possibility of fortification of them with other probiotic strains encapsulated by different methods.
Ethics and consent
The study did not require formal ethical approval by the Islamic Azad University, Noor Branch on Health Research Ethics. Consumers in this study participated on a voluntary basis and received no compensation. All participants provided written informed consent to participate in the study and for their data to be published.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
CRediT authorship contribution statement
Shahram Saedi: Writing – original draft, Investigation, Formal analysis, Data curation. Sara Jafarian: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Data curation, Conceptualization. Seyyed Hossein Hosseini Ghaboos: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Data curation, Conceptualization. Leila Rozbeh Nasiraei: Writing – review & editing, Supervision, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36430.
Contributor Information
Sara Jafarian, Email: drsjafarian@yahoo.com.
Seyyed Hossein Hosseini Ghaboos, Email: hosseinighaboos@yahoo.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Naseer B., Naik H.R., Hussain S.Z., Zargar I., Beenish Bhat T.A., Nazir N. Effect of carboxymethyl cellulose and baking conditions on in-vitro starch digestibility and physico-textural characteristics of low glycemic index gluten-free rice cookies. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2021;141 [Google Scholar]
- 2.Eduardo M.F., Correa De Mello K.G.P., PolakieWicz B., Da Silva Lannes S.C. Evaluation of chocolate milk beverage formulated with modified chitosan. J. Agric. Sci. Technol. 2014;16:1301–1312. [Google Scholar]
- 3.Homayouni Rad A., Rasouli Pirouzian H., Toker O., Konar N. Application of simplex lattice mixture design for optimization of sucrose free milk chocolate produced in a ball mill. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;115 [Google Scholar]
- 4.Narayani S., Subba Rao D. Extraction of Mucilage from Chia Seeds and its Application as Fat Replacer in Biscuits International Journal of Engineering Research & Technology (IJERT) 2020;9:922–927. [Google Scholar]
- 5.Meyer D., Bayarri S., Tarrega A., Costtel E. Inulin as texture modifier in dairy products. Food Hydrocolloids. 2011;25:1881–1890. [Google Scholar]
- 6.Allgeyer L., Miller M., Lee S.Y. Drivers of liking for yogurt drinks with prebiotics and probiotics. J. Food Sci. 2010;75:S212–S219. doi: 10.1111/j.1750-3841.2010.01579.x. [DOI] [PubMed] [Google Scholar]
- 7.Carter B.G., Cheng N., Kapoor R., Meletharayil G.H., Drake M.A. Microfiltration-derived casein and whey proteins from milk. J. Dairy Sci. 2021;104:2465–2479. doi: 10.3168/jds.2020-18811. [DOI] [PubMed] [Google Scholar]
- 8.Vaucher A.C.D.S., Dias P.C., Coimbra P.T., Costa I.D.S.M., Marreto R.N., Dellamora-Ortiz G.M., Ramos M.F. Microencapsulation of fish oil by casein-pectin complexes and gum Arabic microparticles: oxidative stabilisation. J. Microencapsul. 2019;36:459–473. doi: 10.1080/02652048.2019.1646335. [DOI] [PubMed] [Google Scholar]
- 9.Sardar H., Nisar A., Anjum M., Naz S., Ejaz S. Foliar spray of moringa leaf extract improves growth and concentration of pigment, minerals and stevioside in stevia (Stevia rebaudiana Bertoni) Ind. Crops Prod. 2021;16:113–485. [Google Scholar]
- 10.Abedini A., Pourahmad R., Hashemi Ravan M. The effect of replacing rhabdiosides A and maltodextrin on the physicochemical and sensory properties of cocoa milk. Journal of Innovation in Food Science and Technology. 2020;11 142-131. [In Persian] [Google Scholar]
- 11.Phuong Ta L., Bujna E., Antal O., Ladányi M., Juhász R., Szécsi A., Kun S., Sudheer S., Gupta V., Nguyen Q. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021;183:1136–1144. doi: 10.1016/j.ijbiomac.2021.04.170. [DOI] [PubMed] [Google Scholar]
- 12.Chavan V.R., Gadhe K.S., Hingade S.T. Studies on extraction utilization of chia seed Gel in ice cream as a stabiliazer. Journal of Pharmacogncy and Phytochemestry. 2017:1367–1370. [Google Scholar]
- 13.Zhu Y., Bhandari B., Prakash S. Relating the tribo-rheological properties of chocolate flavoured milk to temporal aspects of texture. Int. Dairy J. 2020;110 [Google Scholar]
- 14.Prakash S., Huppertz T., Karvchuk O., Deeth H. Ultra-high temperature processing of chocolate flavoured milk. J. Food Eng. 2010;96:179–184. [Google Scholar]
- 15.Mahato D.K., Keast R., Liem D., Russell C.G., Cicerale S., Gamlath S. Optimisation of natural sweeteners for sugar reduction in chocolate flavoured milk and their impact on sensory attributes. Int. Dairy J. 2021;115 [Google Scholar]
- 16.Razavizadeh A., Tabrizi P. Characterization of fortified compound milk chocolate with microcapsulated chia seed oil. LWT- Food Science and Technology. 2021;150 [Google Scholar]
- 17.Nakkarach A., Withayagiat U. Comparison of synbiotic beverages produced from riceberry malt extract using selected free and encapsulated probiotic lactic acid bacteria. Agriculture and Natural Resources. 2018;52:467–476. [Google Scholar]
- 18.Moumita S., Goderska K., Johnson E.M., Das B., Indira D., Yadav R., Kumari S., Jayabalan R. Evaluation of the viability of free and encapsulated lactic acid bacteria using in vitro gastro intestinal model and survivability studies of symbiotic microcapsules in dry food matrix during storage. LWT--Food Sci. Technol. 2017;77:460–467. [Google Scholar]
- 19.Li Q., Duan M., Hou D., Chen X., Shi J., Zhou W. Fabrication and characterization of Ca (II)-alginate-based beads combined with different polysaccharides as vehicles for delivery, release and storage of tea polyphenols. Food Hydrocolloids. 2021;112 [Google Scholar]
- 20.Sharifi S., Rezazad Bari M., Alizadeh M., Almasi H., Amiri S. Use of whey protein isolate and gum Arabic for the co-encapsulation of probiotic Lactobacillus plantarum and phytosterols by complex coacervation: enhanced viability of probiotic in Iranian white cheese. Food Hydrocolloids. 2020;10:64–96. [Google Scholar]
- 21.Maleki O., Khaledabad M.A., Amiri S., Asl A.K., Makouie S. Microencapsulation of Lactobacillus rhamnosus ATCC 7469 in whey protein isolate- crystalline nanocellulose-inulin composite enhanced gastrointestinal survivability. LWT. 2020;126 [Google Scholar]
- 22.Altamirano-Fortoul R., Moreno-Terrazas R., Quezada-Gallo A., Rosell C.M. Viability of some probiotic coatings in bread and its effect on the crust mechanical properties. Food Hydrocolloids. 2012;29:166–174. [Google Scholar]
- 23.Rao A.V., Shiwnarain N., Maharaj I. Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Can. Inst. Food Sci. Technol. J. 1989;22:345–349. [Google Scholar]
- 24.Schultheiss N., Newman A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009;9:2950–2967. doi: 10.1021/cg900129f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakirul Islam M., Masum A.K.M., Harun-ur-Rashid M. Milk chocolate matrix as a carrier of novel Lactobacillus acidophilus LDMB-01: physicochemical analysis, probiotic storage stability and in vitro gastrointestinal digestion. Journal of Agriculture and Food Research. 2022;7:100–263. [Google Scholar]
- 26.ISO 16297 Bacterial count in milk. 2020. www.iso.org Available at:
- 27.ISO 11866-1 Enumeration of presumptive E. coli. 2005. Available at: www.iso.org.
- 28.ISO 21527-1 (2008) Microbiology of food and animal feeding stuffs, Horizontal method for enumeration of yeasts and moulds.Available at: www.iso.org.
- 29.ISO 21528-2 (2004) Microbiology of food and animal feeding stuffs, Horizontal method for enumeration of Entrobacteriaceae.Available at: www.iso.org.
- 30.Yanes M., Duran L., Costell E. Rheological and optical properties of commercial chocolate milk beverages. J. Food Eng. 2002;51:229–234. [Google Scholar]
- 31.Syrbe A., Bauer W.J., Klostermeyer H. Polymer science concepts in dairy systems- an overview of milk protein and food hydrocolloid interaction. Int. Dairy J. 1998;8:179–193. [Google Scholar]
- 32.Ozen A.E., Kilic M. Improvement of physical properties of nonfat fermented milk drink by using whey protein concentrate. J. Texture Stud. 2009;40:288–299. [Google Scholar]
- 33.Barros Verde A., Dutra Alvim I., Luccas V., Vercelino Alves R. Stability of milk chocolate with hygroscopic fibers during storage. LWT- Food Science and Technology. 2021;137 [Google Scholar]
- 34.Hadidi M., Motamedzadegan A., Jeylani A.Z., Khashadeh S. Nanoencapsulation of hyssop essential oil in chitosan-pea protein isolate nano-complex. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2020;144:111–254. [Google Scholar]
- 35.Paixão J., Rodrigues J., Esmerino E., Cruz A., Bolini H. Influence of temperature and fat content on ideal sucrose concentration, sweetening power, and sweetness equivalence of different sweeteners in chocolate milk beverage. J. Dairy Sci. 2014;97:7344–7353. doi: 10.3168/jds.2014-7995. [DOI] [PubMed] [Google Scholar]
- 36.Li k, Jelicic I., Tratnik L., Bozanic R. Influence of sweetener stevia on the quality of strawberry flavoured fresh yoghurt. Quality of flavoured fresh yoghurt. Mljekarstvo. 2011;61:220–225. [Google Scholar]
- 37.Ferreira J.M., Azevedo B.M., Luccas V., Bolini H.M.A. Sensory profile and consumer acceptability of prebiotic white chocolate with sucrose substitutes and the addition of Goji Berry (Lycium barbarum) J. Food Sci. 2017;82:818–824. doi: 10.1111/1750-3841.13632. [DOI] [PubMed] [Google Scholar]
- 38.Qi W., Liang X., Yun T., Guo W. Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J. Food Sci. Technol. 2019;56:1398–1404. doi: 10.1007/s13197-019-03616-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosnea L.A., Moschakis T. Complex coacervation as a novel microencapsulation technique to improve viability of probiotics under different stresses. Food Bioprocess Technol. 2014:2767–2781. [Google Scholar]
- 40.Lumay G., Boschini F., Traina K., Bontempi S., Remy J.C., Cloots R., Vandewalle N. Measuring the flowing properties of powders and grains. Powder Technol. 2012;224:19–27. [Google Scholar]
- 41.Muzaffar K., Wani S.A., Dinkarrao B.V., Kumar P. Determination of production efficiency, color, glass transition, and sticky point temperature of spray-dried pomegranate juice powder. Cogent Food Agric. 2016;2:114–120. [Google Scholar]
- 42.Nilfrooshzadeh H., Jahadi M. Production of functional mocha milk containing encapsulated Lactobacillus rhamnosus GG. J. Food Sci. Technol. 2024;149:1–12. [Google Scholar]
- 43.De Araújo Etchepare M., Nunes G.L., Nicoloso B.R., Barin J.S., Flores E.M.M., de Oliveira Mello R., de Menezes C.R. Improvement of the viability of encapsulated probiotics using whey proteins. LWT--Food Sci. Technol. 2020;117 [Google Scholar]
- 44.Gandomi H., Abbaszadeh S., Misaghi A., Bokaie S., Noori N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT--Food Sci. Technol. 2016;69:365–371. [Google Scholar]
- 45.Pradeep Prasanna P.H., Charalampopoulos D. Encapsulation in an alginate–goats’ milk–inulin matrix improves survival of probiotic Bifidobacterium in simulated gastrointestinal conditions and goats’milk yoghurt. Int. J. Dairy Technol. 2019;72:132–141. [Google Scholar]
- 46.Tijssen R.L.M., Canabady-Rochelle L.S., Mellema M. Gelation upon long storage of milk drinks with carrageenan. J. Dairy Sci. 2007;90:2604–2611. doi: 10.3168/jds.2006-854. [DOI] [PubMed] [Google Scholar]
- 47.Guggisberg D., Piccinali P., Schreier K. Effects of sugar substitution with Stevia, Actilight and Stevia combinations or Palatinose on rheological and sensory characteristics of low-fat and whole milk set yoghurt. Int. Dairy J. 2011;21:636–644. [Google Scholar]
- 48.Lisak k, Jelicic I., Tratnik L., Bozanic R. Influence of sweetener stevia on the quality of strawberry flavoured fresh yoghurt. Quality of flavoured fresh yoghurt. Mljekarstvo. 2011;61(3):220–225. [Google Scholar]
- 49.Schmidt K.A., Smith D.E. Milk reactivity of gum and milk protein solutions. J. Dairy Sci. 1992;75:3290–3295. [Google Scholar]
- 50.Zarabadipour F., Piravi-vanak Z., Aminifar M. Optimization of chocolate milk formulation containing gum tragacanthin using response surface method. J. Food Res. 2019;29:131–144. (In Persian) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.