Graphical Abstract
Aortic valve replacement (AVR) is only indicated in patients with moderate aortic stenosis (AS) when another indication coexists for open heart surgery (ie coronary artery bypass, other valve disease, aortopathy). Recent data consistently showed that moderate AS is associated with a high risk of mortality, especially in patients with heart failure and reduced ejection fraction.1,2 Such observations led to the design of the TAVR UNLOAD (Transcatheter Aortic Valve Replacement to Unload the Left Ventricle in Patients With Advanced Heart Failure; NCT02661451) investigating the benefit of AVR in moderate AS with reduced left ventricular ejection fraction (LVEF). Hence, we sought to perform a reconstructed Kaplan-Meier meta-analysis to compare AVR to clinical surveillance in moderate AS with LVEF ≤50%.
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and was prospectively registered in the PROSPERO database (CRD42023483420). Electronic data sets were systematically searched from inception to November 10, 2023, by 2 independent investigators (O.M.A. and X.J.) using the key terms “aortic valve stenosis,” and “moderate,” with no language restrictions. Disagreements were resolved by consensus with a third investigator (P.P.). Inclusion criteria included: 1) observational studies of patients with moderate AS; 2) reduced ejection fraction at baseline (LVEF ≤50%); 3) populations were divided into 2 treatment groups (AVR versus clinical surveillance); and 4) time to event follow-up data were present for at least >2 years. The primary outcome of interest was all-cause mortality. The secondary outcome was cardiovascular mortality. Individual patient data based on published Kaplan-Meier graphs from included studies were reconstructed using the “curve approach.” Two investigators assessed the reconstructed patient data accuracy at each read-in point. In the Kaplan-Meier–based meta-analysis, the mean ± SD survival times, median (IQR) survival times, and survival percentage at different time points with 95% CIs were calculated. The differences in survival between the groups were assessed using the log-rank test for differences and a Cox proportional hazards regression model. Truncated survival analysis at longest follow-up was performed as a prespecified outcome and respective HRs and 95% CI were calculated. Sensitivity analysis was performed for adjusted cohorts. P values were 2-sided, and statistical significance was set at P < 0.05. All analyses were completed with R, version 4.2.1 (Foundation for Statistical Computing).
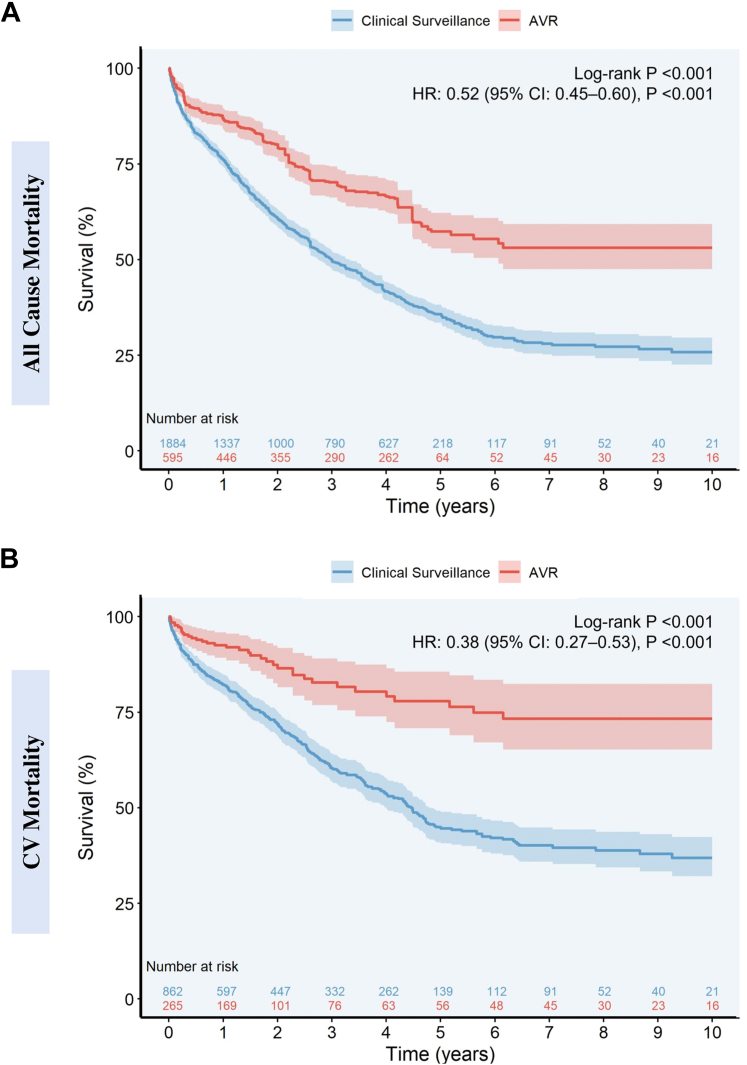
Five observational studies (n = 2,479, AVR; n = 595 [24%] and clinical surveillance; n = 1,884 [76%]) with 2.4 years (IQR: 0.8–5.0 years) median follow-up were included.1, 2, 3, 4, 5 The mean age was 75.7 ± 9.8 years in the AVR cohort and 74.3 ± 11.4 years in the clinical surveillance cohort. Male patients constituted 63.3% and 67.9% in the AVR and clinical surveillance groups, respectively. Ten-year overall survival rates among patients with moderate AS and reduced LVEF undergoing AVR compared to clinical surveillance were 53.1% (95% CI: 47.5%–59.3%) and 25.8% (95% CI: 22.6%–29.6%), respectively. AVR was associated with a decreased risk of all-cause mortality (HR: 0.52; 95% CI: 0.44-0.60; P < 0.001) (proportional hazards, Schoenfeld residual P = 0.55) (Figure 1A). On sensitivity analysis of adjusted studies (n = 3)1,4,5, 825 patients (AVR; n = 234 and clinical surveillance; n = 591) with 1.6 years (IQR: 0.6–2.6 years) follow-up were included. Seven-year overall survival rates among those with moderate AS undergoing AVR compared to clinical surveillance were 54.6% (95% CI: 44.4%–67.1%) and 22.6% (95% CI: 17.4%–29.3%), respectively. AVR was associated with a decreased risk of all-cause mortality (HR: 0.45; 95% CI: 0.33-0.61; P < 0.001) (proportional hazards, Schoenfeld residual P = 0.98). Moreover, to account for immortal time bias, we performed a sensitivity analysis following the exclusion of studies with no clear definition of enrollment time for both groups. AVR remained associated with a decreased risk of all-cause mortality (HR: 0.47; 95% CI: 0.34-0.67; P < 0.001).
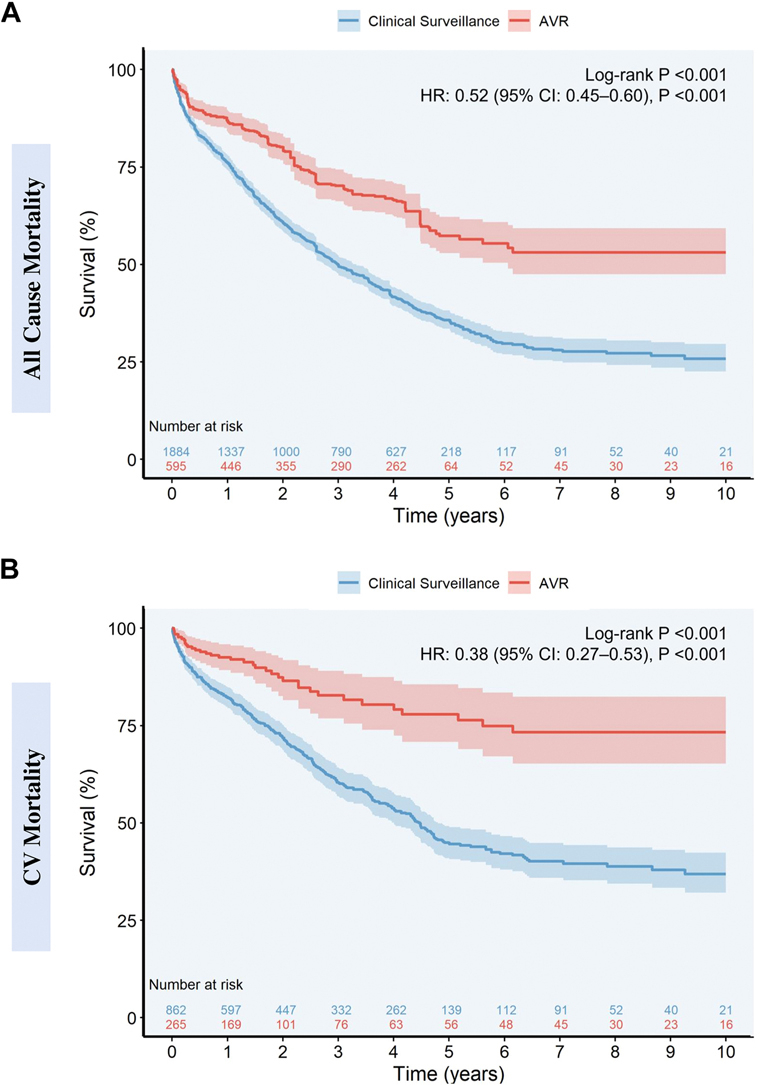
Figure 1.
Mortality According to Aortic Valve Replacement Versus Clinical Surveillance in Patients With Moderate Aortic Stenosis and Reduced Ejection Fraction
Reconstructed Kaplan-Meier analysis in patients with moderate aortic stenosis and reduced left ventricular dysfunction undergoing aortic valve replacement versus clinical surveillance: (A) All-Cause Mortality, (B) Cardiovascular Mortality.
AS = aortic stenosis; AVR = aortic valve replacement; HFrEF = heart failure with reduced ejection fraction.
For cardiovascular mortality, a total of 3 observational studies (n = 1,127, [AVR; n = 265 and clinical surveillance; n = 862]) with 1.9 years (IQR: 0.7–4.5 years) follow-up were included in the analysis.3, 4, 5 Ten-year cardiovascular survival rates among those with moderate AS undergoing AVR compared to clinical surveillance were 73.3% (95% CI: 65.2%–82.4%) and 36.9% (95% CI: 32.1%–42.4%), respectively. AVR was associated with a decreased risk of cardiovascular mortality (HR: 0.38; 95% CI: 0.27-0.53; P < 0.001) (proportional hazards, Schoenfeld residual P = 0.80) (Figure 1B). On sensitivity analysis of adjusted studies (n = 2), 563 patients (AVR; n = 191 and clinical surveillance; n = 372) with 1.4 years (IQR: 0.5–2.0 years) follow-up were included. Seven-year overall survival rates among those with moderate AS undergoing AVR compared to clinical surveillance were 72.3% (95% CI: 60.0%–87.1%) and 44.9% (95% CI: 36.5%–55.3%), respectively. AVR was associated with a decreased risk of cardiovascular mortality (HR: 0.37; 95% CI: 0.24-0.58; P < 0.001) (proportional hazards, Schoenfeld residual P = 0.64).
This Kaplan Meier (KM)-reconstructed meta-analysis of 5 observational studies with 2,479 moderate AS patients with LVEF ≤50% demonstrated that AVR (transcatheter or surgical AVR) is associated with a significantly lower risk of all-cause and cardiovascular mortality, compared to clinical surveillance. However, given the observational nature of the included studies, caution in the interpretation of results is warranted given the possible unaddressed confounders that might introduce bias. Further long-term and randomized data are required to confirm the benefits of early AVR in this unique population and its impact on long-term outcomes. Several limitations are worth mentioning, including the susceptibility to selection bias due to procedural patient selection and referral bias in high-volume centers. Immortal time bias was accounted for in the sensitivity analysis where time zero for AVR was within 90 days from index echocardiography with moderate AS diagnosis.
Footnotes
Dr Pibarot has received funding from Edwards Lifesciences, Medtronic, Pi-Cardia, and Cardiac Phoenix for echocardiography core laboratory analyses and research studies in the field of transcatheter valve therapies, for which he received no personal compensation; and has received lecture fees from Edwards Lifesciences and Medtronic. Dr Clavel holds the Canada Research Chair on Women’s Valvular Heart Health; and has received funding from Edwards Lifesciences, Medtronic, and Pi-Cardia for CT core laboratory analyses and research studies for which she received no personal compensation. Dr Généreux has served as a consultant for Abbott Vascular, Abiomed, BioTrace Medical, Boston Scientific, CARANX Medical, Cardiovascular System Inc (PI Eclipse Trial), Edwards Lifesciences (PI EARLY-TAVR trial, PI PROGRESS trial), GE Healthcare, iRhythm Technologies, Medtronic, Opsens, Pi- Cardia, Puzzle Medical, Saranas, Shockwave, Siemens, Soundbite Medical Inc, Teleflex, and 4C Medical (PI feasibility study); has served as an advisor for Abbott Vascular, Abiomed, BioTrace Medical, Edwards Lifesciences, and Medtronic; has received speaker fees from Abbott Vascular, Abiomed, BioTrace Medical, Edwards Lifesciences, Medtronic, and Shockwave; has served as a proctor for and received an institutional research grant from Edwards Lifesciences; and has equity in Pi-Cardia, Puzzle Medical, Saranas, and Soundbite Medical Inc. Dr Leon serves on the PARTNER Trial Executive Committee for Edwards Lifesciences (nonpaid); and has received institutional research grants from and has served as a nonpaid advisor for Abbott, Boston Scientific, and Medtronic; has served as a nonpaid advisor for Sinomed; and has equity in Medinol. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Jean G., Van Mieghem N.M., Gegenava T., et al. Moderate aortic stenosis in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:2796–2803. doi: 10.1016/S0735-1097(21)04151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samad Z., Vora A.N., Dunning A., et al. Aortic valve surgery and survival in patients with moderate or severe aortic stenosis and left ventricular dysfunction. Eur Heart J. 2016;37:2276–2286. doi: 10.1093/eurheartj/ehv701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariri E.H., Badwan O., Kassab J., et al. Role of aortic valve replacement in moderate aortic stenosis: a 10-year outcomes study. Open Heart. 2024;11 doi: 10.1136/openhrt-2024-002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig S., Schofer N., Abdel-Wahab M., et al. Transcatheter aortic valve replacement in patients with reduced ejection fraction and nonsevere aortic stenosis. Circ Cardiovasc Interv. 2023;16 doi: 10.1161/CIRCINTERVENTIONS.122.012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon I., Kim M., Choi J.-W., et al. Early surgery versus watchful waiting in patients with moderate aortic stenosis and left ventricular systolic dysfunction. Korean Circ J. 2020;50:791. doi: 10.4070/kcj.2020.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]