Abstract
Global population is rising, leading to higher demand for meat and concerns on environmental and economic impacts of conventional feedstuffs that corn and soybean meal have. Recently there has been a shift towards more sustainable feedstuffs such as Spirulina (Limnospira platensis) due to its nutritional value and ability to be produced locally. Consumer awareness prompts shifts towards free range poultry production but presents environmental challenges due to climate change. The naked neck (Na) gene, which reduces feather coverage, and enhances growth under adverse conditions offers a possible solution for improved welfare and efficiency. This study aims to investigate the impact of a diet with 15% Spirulina inclusion on growth performance, carcass traits, and meat quality of two slow-growth broiler strains: naked neck (NN) and fully feathered (FF). Forty, 1-day-old male broilers, 20 per strain, were randomly assigned to either a control or a diet containing 15% Spirulina, housed individually in cages and fed ad libitum for 84 d. Growth, carcass, and meat traits were evaluated. Results indicated that animals fed a control diet generally outperformed those fed a Spirulina diet in final body weight (BW), average daily gain (ADG), feed intake (FI), and feed conversion rate (FCR) (P < 0.001). Additionally, Spirulina incorporation led to an increase in the length of the gastrointestinal tract and digesta viscosity in the duodenum plus jejunum (P < 0.05). Although there were no significant differences in breast muscle yield between dietary groups, SP-fed broilers had higher yellowness (*b) values in meat (P < 0.05). Except for the decrease in water holding capacity (WHC) observed in the NN group animals (P < 0.05), there were no significant differences between the strains for the remaining meat quality traits (P > 0.05). The 15% Spirulina inclusion increased the concentrations of n-3 polyunsaturated fatty acids (PUFA) (P < 0.0001) in breast meat and decreased (P < 0.0001) nutritional ratios. Overall, under thermoneutral conditions, animals from the NN strain showed negative effects on growth parameters. Spirulina inclusion improved certain aspects of breast meat quality, particularly fatty acid profiles.
Key words: Slow-growing broiler strain, digesta viscosity, growth performance, meat trait, fatty acid content
INTRODUCTION
In recent decades, poultry meat consumption has gradually increased. In 1990, global per capita consumption was around 9.8 kg, increasing by 60% in 2020, a trend that is expected to continue over the next decades (FAO, 2022). In contrast to other species, poultry production typically requires less land and water resources use. Combined with a faster production cycle resulting from the very efficient feed conversion ratios of broilers, these factors result in a smaller environmental footprint (Siegel, 2014). From a consumer perspective, poultry meat is furthermore readily available in various regions and stands out for its versatility in cooking and appealing taste. Furthermore, by comparison to red meat, poultry is perceived as being healthier and more cost-effective, without being restricted by moral or religious principles (Leinonen et al., 2014). For these reasons, poultry meat is one of the most consumed meats worldwide.
Nowadays, consumers are more concerned and informed about issues related to welfare and sustainability in animal production. Poultry production, particularly in Europe, will tend to move towards outdoor/free range production systems. However, this approach leads to new challenges, particularly environmental issues, since the animals become exposed, for example, to the effects of climate change, particularly high temperatures (Martinelli et al., 2020). The naked neck (Na) gene, which reduces feather coverage, especially in the neck region, becomes relevant in such a context. Several studies (Cahaner et al., 1993; Yalcin et al., 1997; Deeb and Cahaner, 1999; Rajkumar et al., 2011; Adomako et al., 2014) have long suggested that the Na gene improves growth performance in high temperatures by providing resistance to heat stress, resulting in enhanced average daily gain (ADG), feed intake (FI), and feed conversion ratio (FCR) when compared to fully feathered animals. Most broiler strains are generally very sensitive to high environmental temperatures. Therefore, the use of poultry strains that are more adapted and can efficiently dissipate heat offers a straightforward, cost-effective, and welfare-oriented solution (Fernandes et al., 2023). However, studies conducted under thermoneutral conditions generally lack. Studying NN broilers under thermoneutral conditions is nonetheless essential to understand if the benefits of the Na gene, observed under heat stress, also translate to normal temperature conditions. This could provide insights into whether NN broilers have inherent advantages in terms of growth and feed efficiency that are independent of their heat resistance traits. Furthermore, NN animals are often used in alternative outdoor production systems across Europe precisely for such heat tolerance traits.
In the context of animal production, establishing novel sustainable feedstuffs is essential as it has a direct impact on social and environmental effects associated with food production. Poultry diets are generally based on 2 major feedstuffs: soybean meal, as a protein source, and corn as an energy source (Martinelli et al., 2020). Corn and soybean production faces various challenges that span environmental sustainability, as well as social and economic considerations. Monoculture practices, which heavily depend on extensive arable land use, contribute to issues such as soil degradation, deforestation, and biodiversity losses. Moreover, the excessive use of water in such agricultural practices places significant pressure on local water resources. Corn and soybean production extends beyond local environments, with regions like Europe heavily dependent on imports from countries such as Brazil, Argentina, or the United States. This reliance exacerbates the carbon footprint, amplifying the environmental impacts associated with these agricultural practices (Fehlenberg et al., 2017). The increase in both production and consumption of poultry meat leads to pressure on the production of such conventional feedstuffs, it is thus of the utmost importance to find more suitable alternatives (Martinelli et al., 2020). Presently, the scientific community's goal is to find sustainable alternatives that can be of interest in the context of livestock production. Alternatives include microalgae, especially in the context of swine (Martins et al., 2021a,b) and poultry nutrition (Pestana et al., 2020; Alfaia et al., 2021; Cabrol et al., 2022a,b).
Limnospira platensis species, commonly known as Spirulina reaches maximal growth at pH 9.5 to 9.8 (Hu, 2003). Due to its ability to develop in these pH ranges, which inhibits the growth of other microorganisms, it can easily be produced in large-scale outdoor production systems (Chen and Zhang, 1997). Spirulina is classified as a blue-green microalga (Cyanophyceae, also known as cyanobacteria), which is an organism with the properties of bacteria and algae, that contain blue-green and green pigments. In the case of Spirulina, it contains photosynthetic pigments, such as phycocyanin, which are blue. The chemical composition of Spirulina depends on its production process, culture medium, and downstream processes. Generally, it contains about 50-70% crude protein. In addition to its protein content, it is also known for its value in minerals, fatty acids, pigments, and considerable quantities of unique natural antioxidants such as polyphenols, carotenoids, and phycocyanin (El-Shall et al., 2023). Due to its nutritional characteristics, functional properties, and production system, Spirulina has been considered an interesting sustainable alternative to conventional feedstuff for broilers (El-Bahr et al., 2020; Pestana et al., 2020; El-Shall et al., 2023; Khalilnia et al., 2023).
All the studies carried out so far have focused on using fast-growing strains and usually considered Spirulina as a feed supplement in poultry nutrition being incorporated at very low rates typically below 2% (Khan et al., 2020; Hassan et al., 2022; El-Shall et al., 2023). Despite these advancements, a lack of information remains on how Spirulina incorporation affects the growth and meat quality of slow-growth broilers. This study hypothesizes that slow-growing broiler strains will respond better to a diet with Spirulina when compared to other studies conducted on fast-growth strains. Therefore, this study aims to determine the effect of a diet containing Spirulina incorporated as a feed ingredient (15%) on growth, carcass, and meat traits in 2 slow-growth broiler strains, fully feathered (FF) and naked neck (NN). The inclusion level of 15% was chosen to explore the impact of a higher level of Spirulina inclusion, considering its potential benefits and the need to find practical and effective dietary solutions for sustainable poultry production.
MATERIALS AND METHODS
Animal Welfare Declaration
The study was carried out at the research facilities of the School of Agriculture (Instituto Superior de Agronomia - ISA, of the University of Lisbon, Portugal). The procedures were approved by the ISA's Ethics and Research in Animal Welfare Committee (ORBEA) and approved (Authorization Number: 0421/000/000/2021, date of approval 28th September 2021) by the Animal Care Committee of the National Veterinary Authority (DGAV - Direção Geral de Alimentação e Veterinaria, Lisbon, Portugal). This approval was granted following the specific principles and guidelines outlined in Portuguese and European Union legislation (Directive 2010/63/EU).
Animal Housing and Experimental Diets
Twenty FF male 1-day-old chicks of the slow-growth strain C44 × SA31A and twenty NN male 1-day-old chicks of the slow-growth strain XL44N × SA31A (SASSO, Sabres, France) were acquired from a multiplication farm with an initial average body weight (BW) of 42.4 ± 0.60 g were individually identified with wing bands. Animals were randomly allocated and individually housed in wire floor cages measuring 56×50×56 cm. Each cage was equipped with 1 feeder and 2 nipple drinkers. There were 4 groups, with 10 replicates (n = 10) each. This factorial design allowed us to simultaneously evaluate the effects of strain and diet. The study was conducted in an environmentally controlled room, isolated from noise until the age of 84 d (12 wk). Room temperature was kept at 30°C during the initial 15 d and gradually reduced to 20°C by d 49, after which it remained constant. Temperature and ventilation were monitored daily. Throughout the trial, animals had ad libitum access to fresh water and mash feed. Each group received either a Control diet (C) or a diet with 15% Spirulina (SP): Starter (1–28 d), grower (28–70 d), and finisher (70–84 d). To formulate the diets, all feedstuffs were purchased from commercial suppliers. Spirulina, provided in powder form, was supplied by Allmicroalgae (Pataias, Portugal), produced in an open system, and dried using solar energy. All diets were formulated to meet the nutritional needs of the animals. The ingredient composition and nutritional levels and the fatty acid profile of the diets and Spirulina are presented in Table 1, Table 2, respectively.
Table 1.
Ingredient composition and nutrient levels of broiler experimental diets and Spirulina powder (%, as fed basis).
| Dietary treatments1 |
|||||||
|---|---|---|---|---|---|---|---|
| Starter |
Grower |
Finisher |
|||||
| Item | Spirulina | C | SP | C | SP | C | SP |
| Ingredients, % | |||||||
| Corn | - | 52.88 | 59.14 | 59.81 | 66.20 | 60.20 | 69.71 |
| Soybean meal 45% CP | - | 38.83 | 22.20 | 32.40 | 15.40 | 31.90 | 12.37 |
| Spirulina powder 48% CP | - | 0.00 | 15.00 | 0.00 | 15.00 | 0.00 | 15.00 |
| Sunflower oil | - | 4.12 | 0.00 | 4.00 | 0.00 | 4.53 | 0.00 |
| Dicalcium phosphate | - | 1.95 | 1.30 | 1.70 | 1.15 | 1.50 | 0.86 |
| Calcium carbonate | - | 1.30 | 1.60 | 1.20 | 1.50 | 1.00 | 1.30 |
| Vitamin-mineral premix2 | - | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Sodium chloride | - | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| Methionine synthetic | - | 0.12 | 0.01 | 0.10 | 0.00 | 0.12 | 0.00 |
| Synthetic lysine | - | 0.05 | 0.00 | 0.04 | 0.00 | 0.00 | 0.01 |
| Nutrient content | |||||||
| Gross energy, kcal/kg | 4,340 | 4,024 | 3,822 | 3,835 | 3,796 | 4,073 | 3,868 |
| Dry matter, % | 94.5 | 89.6 | 88.3 | 87.4 | 88.7 | 90.5 | 88.1 |
| Crude protein, % | 47.9 | 22.6 | 22.4 | 20.2 | 20.2 | 20.1 | 19.7 |
| Ash, % | 15.8 | 7.0 | 7.2 | 5.9 | 6.8 | 6.6 | 6.5 |
| Sodium, mg/kg | 26,694 | 2,823 | 7,271 | 2,887 | 7,603 | 2,788 | 7,699 |
| Potassium, mg/kg | 25,751 | 12,801 | 11,743 | 10,605 | 10,922 | 11,831 | 9,622 |
| Phosphorus, mg/kg | 9,88 | 8,787 | 6,891 | 6,967 | 6,850 | 8,043 | 5,785 |
| Iron, mg/kg | 953 | 190 | 305 | 160 | 307 | 189 | 286 |
Dietary treatments C: control diet; SP: 15% Spirulina diet. Starter (1–28d), grower (28–70d), and finisher (70–84d).
Premix provided the following per kilogram of diet: vitamin A 8000 IU, vitamin D3 2200 IU, vitamin E 30 IU, vitamin B1 1 mg, vitamin B2 3 mg, vitamin B6 2 mg, vitamin B12 0.01 mg, vitamin H2 0.05 mg, vitamin K3 4 mg, Calcium-D-pantothenate 8 mg, Niacinamide 20 mg, folic acid 0.5 mg, Choline chloride 350 mg, Cu 7 mg, Fe 30 mg, I 0.5 mg, Mn 60 mg, Se 0.15 mg, Zn 50 mg.
Table 2.
Fatty acid profile, (% total fatty acids) of experimental diets and Spirulina powder.
| Dietary treatments1 |
|||||||
|---|---|---|---|---|---|---|---|
| Starter |
Grower |
Finisher |
|||||
| Item | Spirulina | C | SP | C | SP | C | SP |
| Fatty acid profile, % total fatty acids | |||||||
| C14:0 | 0.33 | 0.14 | 0.15 | 0.08 | 0.11 | 0.10 | 0.11 |
| C16:0 | 56.32 | 16.77 | 25.87 | 9.84 | 20.07 | 11.03 | 19.34 |
| C16:1c9 | 8.02 | 0.33 | 1.62 | 0.13 | 1.38 | 0.14 | 1.18 |
| C17:0 | 0.29 | 0.10 | 0.18 | 0.06 | 0.12 | 0.08 | 0.13 |
| C18:0 | 1.88 | 5.94 | 3.08 | 3.29 | 2.22 | 3.84 | 2.18 |
| C18:1c9 | 4.13 | 34.28 | 25.59 | 32.95 | 22.39 | 26.23 | 22.82 |
| C18:1c11 | 2.12 | 1.20 | 1.33 | 0.88 | 1.09 | 0.88 | 1.05 |
| C18:2n-6 | 22.84 | 35.31 | 36.47 | 48.99 | 48.63 | 53.73 | 48.99 |
| C18:3n-3 | 2.38 | 1.01 | 1.94 | 1.29 | 2.48 | 1.35 | 2.35 |
| C20:0 | 0.21 | 0.58 | 0.49 | 0.35 | 0.36 | 0.39 | 0.37 |
| C20:1c11 | 0.04 | 0.56 | 0.54 | 0.29 | 0.26 | 0.22 | 0.25 |
| C22:0 | 0.18 | 1.20 | 0.47 | 0.64 | 0.28 | 0.71 | 0.29 |
| C22:1n-9 | 0.54 | 1.06 | 0.91 | 0.28 | 0.16 | 0.29 | 0.32 |
| C23:0 | 0.18 | 0.78 | 0.47 | 0.64 | 0.28 | 0.71 | 0.29 |
| C24:0 | 0.54 | 0.75 | 0.91 | 0.28 | 0.16 | 0.29 | 0.32 |
Dietary treatments C: control, corn-soybean basal diet; SP: basal diet plus 15% Spirulina. Starter (1–28d), grower (28–70d), and finisher (70–84d).
Spirulina (powder) and diet samples, after grinding using a 1 mm sieve (SK100 comfort miller, Retsch, Haan, Germany), were analyzed for dry matter (103°C oven drying) and ash (incineration at 550°C) according to AOAC methods (AOAC, 2000). To determine protein content, 100 mg of diet sample and Spirulina were weighed in tin foil and analyzed using the Dumas method and a Thermo Quest NA 2100 Nitrogen and Protein Analyser (Interscience, Breda, Netherlands). Crude protein was calculated as 6.25 × N. Gross energy was determined through adiabatic bomb calorimetry using a Parr 6,400 instrument (Parr Instrument Company, Moline, IL). The mineral profile was determined according to the procedures described by Ribeiro et al. (2020) with minerals being extracted from 0.3 g sample. Samples underwent digestion with concentrated nitric and hydrochloric acids, followed by the addition of hydrogen peroxide. After dilution, the samples were filtered using 90 mm diameter paper filters and analyzed using inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Thermo Scientific, Waltham, MA). Each analysis was performed in triplicate and the average value was calculated.
Collection and Analysis of Excreta Samples
Excreta was collected during 2 different periods: from d 21 to 28 to coincide with the initial diet, and from d 56 to 63 to coincide with the grower diet. Excreta was collected using trays covered with aluminum foil positioned under each cage. Any spilled feed or feathers were meticulously removed. Before being ground to achieve a 1 mm uniform particle size, each sample was subjected to oven-drying at 60°C for 72 h, with ventilation. Dry matter (DM) and ash contents were assessed using 2 g of the sample, following the previously mentioned procedures. The final DM content was calculated by multiplying the percentage of DM after drying at 60°C by the percentage of DM after drying at 103°C.
Animal Slaughtering and Sampling
Every day, a known amount of feed was administered, and weekly BW and FI were monitored and ADG and FCR were subsequently calculated. On d 84, before slaughter, all animals were weighed.
Animals were electrically stunned and manually exsanguinated following commercial practices and as described by Cabrol et al. (2022a). After scalding in water at 65°C for 10 s, the animals were subjected to automatic plucking for approximately 30 s (CHZ-N50, Incomax, Leiria, Portugal). Breast (Pectoralis major) muscles were removed and weighed to determine breast muscle yield and divided into sub-samples. After removing feathers and internal organs, carcass weight was measured to calculate carcass yield. This calculation included the weight of the legs and head. The crop, heart, proventriculus, gizzard, pancreas, spleen, liver, duodenum, jejunum, ileum, cecum, and testicles were removed, emptied (when required), and weighed. The length of intestinal compartments was additionally measured. To evaluate the viscosity of the small intestine contents, samples of the digesta were collected from both the duodenum plus jejunum and ileum. After centrifugation for 5 min at 5,000 rpm (J2-HS, Beckman-Coulter, Brea, CA), the apparent viscosity of the supernatant from the samples was measured, using a single point mode, at 6 rpm using a viscometer (LVDVCP-II, Brookfield Engineering Laboratories, Middleboro, MA).
Meat Traits
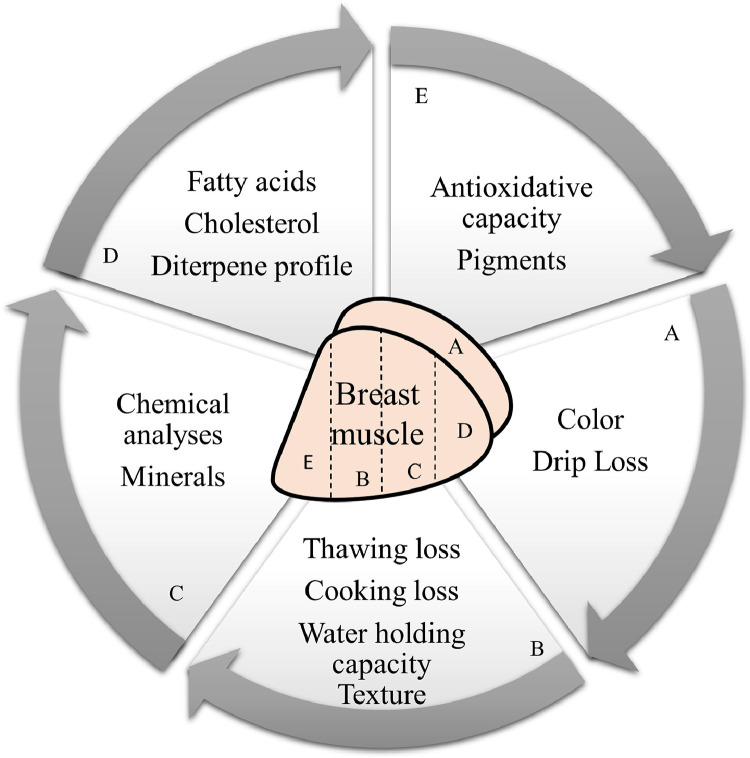
To determine drip loss and color, fresh Pectoralis major muscles were used. The remaining samples were frozen at −20°C and used for subsequent analysis: evaluation of thawing and cooking losses, water holding capacity (WHC), chemical analyses, mineral profiles, fatty acids profiles, cholesterol content, diterpene profiles, pigment concentrations, and antioxidant capacity as explained in Figure 1.
Figure 1.
Sampling plan from broiler breast muscle.
Breast meat color was assessed using a colorimeter (CR−400, Konica Minolta Sensing, Inc., Marunouchi, Japan) standardized with a white calibration plate (Y = 93.5; x = 0.3114; y = 0.3190) according to the CIELAB system (L* for lightness, a* for redness, and b* for yellowness). The evaluation took place 30 min after air exposure to allow blooming. Measurements were conducted at 3 different points, and the average value was calculated. The total color difference in the breast meat samples between the control diet and the diet with 15% Spirulina, for both strains, was calculated according to the following Equation (1).
| (1) |
For the calculation of drip loss (Honikel, 1987), approximately 100 g of fresh breast muscle was used. Each piece was suspended inside a plastic bag, inflated, and stored for 24 h, in a chamber at 4°C. After 24 h, samples were weighed. The percentage of the initial weight was used to measure drip loss.
Following the procedure outlined by El-Bahr et al. (2020), with slight modifications, WHC was calculated. Approximately 10 ±1 g of breast meat was put into 50 mL centrifuge tubes. The samples were centrifuged at 10,000 rpm (J2-HS, Beckman-Coulter, Brea, CA) for 20 min at 5°C. After centrifugation, samples were dried gently with paper and then weighed. After thawing the frozen breast meat for 24 h at 4°C, the final weight was recorded. The value of the thawing loss was the proportion of the difference between the initial and thawed weights.
Afterwards, samples were placed in PA/PE bags (90 ECO 200 × 300 mm, Tecnipeso, V.N. Famalicão, Portugal), sealed, and cooked sous vide at 80°C for 55 min in a hot water bath to reach a core temperature of 77°C to measure cooking losses. Subsequently, samples were cooled down to room temperature, kept at 4°C for 24 h, and weighed, as described in a previous study (Cabrol et al., 2022b). Cooking loss was calculated as a percentage of the initial weight. After calculating cooking losses, samples were used for texture analysis.
Texture Analyses
For texture evaluation, samples were cut into 3 cubes of approximately 1 cm3 each. Measurements were collected for each cube, and the average value was recorded. Texture profile analysis (TPA) was conducted using a TA-XT plus texture analyzer (Stable Micro Systems, Godalming, UK), with a P/50 probe (50 mm diameter cylinder aluminum), to compress the samples and with a load cell of 5 kg, in compression mode. Based on the force/distance information gathered during the 2-cycle compression test, with a 5 s hold time between compressions, and with the samples being compressed to 45% of the original height, hardness, chewiness, springiness, and cohesiveness were determined (Bourne, 1978).
Proximate Chemical Composition and Mineral Profiling of Meat
To determine the proximate chemical composition, breast muscles were previously thawed. By drying samples for 24 h at 105°C, until constant weight, moisture content was calculated. Afterward, ash content was calculated by incinerating the dried samples at 550°C in a muffle oven until constant weight. Protein content was determined according to the method previously described.
Mineral analysis was determined in milled raw meat after drying the samples for 48 h in an oven at 50°C. Total mineral contents were determined as described in a previous study (Cabrol et al., 2022a). Briefly, by inductively coupled plasma atomic emission spectrometer (ICP-AES-Inductively Coupled Plasma-Atomic Emission Spectrometry: Thermo System, ICAP-7000 series) after, a digest of 0.3 g of sample in a MARS 240/50 microwave digester (CEM Corporation, Mathews, NC). The concentration of sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), phosphorous (P), sulfur (S), iron (Fe), copper (Cu), zinc (Zn), and manganese (Mn) was provided as mg/100 g of wet weight after being adjusted for the dilution variables brought on by the digestive process.
Determination of Total Cholesterol and Diterpenes in Meat
Total cholesterol and vitamin E homologs (tocopherols and tocotrienols) were measured at the same time in 0.75 g of fresh meat (previously thawed at room temperature) according to a method adapted from Prates et al. (2006). Samples were incubated for 15 min at 80°C in a shaking water bath with agitation, using a saponification solution composed of potassium hydroxide, ethanol, and deionized distilled water. Following centrifugation at 2,500 rpm (Biofuge 28 RS, Heraeus Sepatech, Germany) for 10 min, the n-hexane layer was filtered and subsequently injected into an HPLC (High-Performance Liquid Chromatography) system (Agilent 1100 Series; Agilent Technologies Inc., Palo Alto, CA) using a normal phase silica column (Zorbax RX-Sil, 250 mm × 4.6 mm i.d., 5-µm particle size; Agilent Technologies Inc., Palo Alto, CA). Using 2 detectors set on series, a UV-visible photodiode array detector for cholesterol determination (λ = 202 nm) and a fluorescence detector for tocopherols and tocotrienols (excitation λ = 295 nm and emission λ = 325 nm) quantification. Based on the external standard technique, the contents of total cholesterol, tocopherols, and tocotrienols were determined in duplicate using a standard curve of peak area vs. concentration.
Determination of Antioxidant Capacity and Pigment Contents
The antioxidant tests were estimated with the extract obtained from the meat muscle. Briefly, meat extract was prepared by dissolving 2 g of fresh sample in 10 mL of ethanol. Then by measuring its ability to neutralize stable radicals of 2,2-diphenyl-1-picrylhydrazyl (DPPH), following a protocol adapted from Brand-Williams et al. (1995). Inhibition of free radicals scavenging activity (RSA) by DPPH in percentage was calculated as follows (2):
| (2) |
The total phenolic compounds (TPC) were assessed using a method adapted from Mohankumar et al. (2018), which involves a reaction with the Folin−Ciocalteu reagent as described in Cabrol et al. (2022b). To determine DPPH and TPC, absorbances were recorded at 517 nm and 725 nm, respectively, with a 100 UV-visible spectrophotometer (Agilent, Santa Clara, CA). TPC values were calculated based on the calibration curve for gallic acid (0−350 mg/ mL) and expressed as g of gallic acid equivalents per gram of meat extract (g GAE/g).
Pigment contents were determined in breast muscle samples previously freeze-dried using a CoolSafe Superior Touch 95 freeze dryer (Labogene, Alleroed, Denmark) at -92°C and 0.2 hPa. For the analysis of total carotenoids, 5mL of acetone was added to 0.10 mg of freeze-dried sample following the technique designed by Teimouri et al. (2013), with slide modification detailed in Pestana et al. (2020). Total carotenoids were calculated using the following Equation (3) (Hynstova et al., 2018):
| (3) |
Due to the sensitivity of pigments and antioxidants to light, all extraction and analysis procedures were conducted in low-light conditions to minimize potential degradation.
Determination of Total Lipids in Meat and Fatty Acid Composition in Meat and Experimental Diets
For lipids and fatty acid composition, breast muscle samples were used freeze-dried, as described earlier.
Total lipids were extracted according to the method by Folch et al. (1957) with slight modifications. In duplicate, 0.15 g of freeze-dried meat samples were gravimetrically quantified by weighing the fatty residue obtained upon solvent evaporation using dichloromethane: methanol (2:1, v/v) solution.
By converting the fatty acid residue into fatty acid methyl esters (FAME), the fatty acid contents of breast meat (0.15 g), feed (0.10 g), and Spirulina (0.10 g) samples were evaluated, using a 2-stage transesterification procedure (basic and acid transesterification). Briefly, the analysis used a Gas Chromatography system (GC System, 7890A, Agilent Technologies, CA) equipped with a Supelcowax 10 capillary column (30 m × 0.20 mm internal diameter, 0.20 µm film thickness; Supelco, Bellefonte, PA) and a flame ionization detector, under specified parameters (Pestana et al. 2020). The percentage of total fatty acids was calculated using heneicosanoic acid (21:0) methyl ester as an internal reference for both meat and feed samples.
Statistical Analysis
All statistical analyses were performed based on a 2 × 2 factorial treatment arrangement considering the main effects of the strain (FF vs. NN) and diet (C vs. SP), as well as their respective interaction. The individual animal was considered the experimental unit, for all variables. Data were analyzed using the generalized linear mixed (GLM) model of SAS software version 9.4 (SAS Institute Inc., 2013). Strain, diet, and their interaction were included as fixed effects in the model, with nonsignificant interactions subsequently removed. Statistical significance was declared when P < 0.05.
RESULTS
Production Performances and Carcass Yields
The performance metrics of the animals are presented in Table 3. The results for BW, ADG, FI, and FCR are outlined for the starter, grower, and finisher phases and consider the different diets used. On d 84, birds fed the SP diet showed a lower BW, with a reduction of 516 g, compared to birds fed the control diet (P < 0.0001). Such a trend was seen across the different production phases (P < 0.0001) in the SP diet, regardless of strain, when compared to the control diet. The impact of strain was evident over the final BW, with FF birds showing a 222 g increase compared to NN animals (P < 0.05). These significant differences between strains, where NN birds exhibited lower BW, were noted from d 70 (finisher phase) onwards (P < 0.05). In the first 2 phases, starter and grower, the 15% dietary incorporation of Spirulina negatively affected the ADG, FI, and FCR (P < 0.05) compared to controls. In the grower phase, NN animals showed lower ADG (-3.12 g/ d) and worse FCR (1.49 vs. 1.34) when compared to FF animals. During the finisher phase, neither diet nor strain influenced growth performance parameters (P > 0.05). Overall, all performance traits were affected by both strain and diet, except for FI, which was not influenced by strain (P > 0.05).
Table 3.
Effect of strain and diet on growth performance from Fully Feathered (FF) and Naked Neck (NN) broilers fed Control (C) or Spirulina (SP) diets in the different phases.
| Strain |
Diet |
P-value |
|||||
|---|---|---|---|---|---|---|---|
| Parameter | FF | NN | C | SP | SEM | Strain | Diet |
| Body weight, g | |||||||
| D 1 | 42 | 42 | 42 | 43 | 0.8 | 0.9081 | 0.6950 |
| D 28 | 661 | 607 | 753 | 515 | 22.3 | 0.0941 | <0.0001 |
| D 70 | 2,502 | 2,316 | 2,650 | 2,168 | 55.0 | 0.0221 | <0.0001 |
| D 84 | 3,126 | 2,904 | 3,273 | 2,757 | 62.8 | 0.0176 | <0.0001 |
| Average daily gain, g/d | |||||||
| Starter (1 – 28 d) | 22.10 | 20.17 | 25.40 | 16.87 | 0.793 | 0.0928 | <0.0001 |
| Grower (28–70 d) | 43.83 | 40.71 | 45.18 | 39.36 | 1.050 | 0.0422 | 0.0004 |
| Finisher (70–84 d) | 44.94 | 42.42 | 44.91 | 42.45 | 1.972 | 0.3741 | 0.3846 |
| Overall (1–84 d) | 37.21 | 34.58 | 38.96 | 32.83 | 0.747 | 0.0178 | <0.0001 |
| Feed intake, g/d | |||||||
| Starter (1–28 d) | 37.51 | 35.92 | 42.07 | 31.35 | 1.266 | 0.3804 | <0.0001 |
| Grower (28–70d) | 58.31 | 60.17 | 61.79 | 56.69 | 1.166 | 0.2650 | 0.0037 |
| Finisher (70–84 d) | 147.51 | 150.22 | 150.69 | 147.04 | 3.776 | 0.6147 | 0.4995 |
| Overall (1–84 d) | 84.57 | 82.52 | 87.77 | 79.32 | 1.660 | 0.3882 | 0.0010 |
| Feed Conversion Ratio | |||||||
| Starter (1–28 d) | 1.73 | 1.83 | 1.66 | 1.90 | 0.037 | 0.0494 | <0.0001 |
| Grower (28 – 70 d) | 1.34 | 1.49 | 1.37 | 1.46 | 0.027 | 0.0002 | 0.0358 |
| Finisher (70–84 d) | 3.40 | 3.59 | 3.42 | 3.57 | 0.121 | 0.2684 | 0.3962 |
| Overall (1–84 d) | 2.31 | 2.43 | 2.29 | 2.46 | 0.024 | 0.0009 | <0.0001 |
The impact of strain and diet on DM and ash (in DM) contents in broiler excreta are presented in Table 4. The analysis revealed significant effects of diet on both DM and ash contents (P < 0.05) across both starter and grower periods, while strain did not show a significant effect (P > 0.05). During the starter period, broilers fed the SP diet showed a 17% decrease in DM content and a 25% increase in ash content compared to those fed the C diet. Similarly, in the grower period, birds on the SP-diet showed a 37% reduction in DM content and a 33% increase in ash content relative to the C-diet group.
Table 4.
Effect of strain and diet (Control (C) or Spirulina (SP)) on dry matter (DM) and ash content in Fully Feathered (FF) and Naked Neck (NN) broilers excreta.
| Strain |
Diet |
P-value |
|||||
|---|---|---|---|---|---|---|---|
| Parameter | FF | NN | C | SP | SEM | Strain | Diet |
| D 21 - 28 | |||||||
| DM, % | 64.1 | 65.8 | 70.9 | 59.0 | 2.415 | 0.6372 | 0.0012 |
| Ash in DM, % | 17.7 | 17.6 | 15.7 | 19.6 | 0.119 | 0.7332 | <0.0001 |
| D 56 - 63 | |||||||
| DM, % | 47.8 | 49.3 | 59.4 | 37.6 | 2.422 | 0.6543 | <0.0001 |
| Ash in DM, % | 21.1 | 20.8 | 18.0 | 23.9 | 0.253 | 0.4395 | <0.0001 |
Carcass traits and relative organ measurements are shown in Table 5. Carcass yield differed significantly among the experimental groups (FFC: 79.0%, NNSP: 78.3%, FFSP: 78.1%, NNC: 76.7%). Statistical analysis revealed significant differences in carcass yield between the FFC group and the NNC group (P < 0.05). However, no significant differences were observed between the FFSP and NNSP groups. Although the broilers in the control group showed higher breast muscle weights (P < 0.05), breast muscle yield did not differ (P > 0.05) either for NN or for FF broilers. In terms of relative organ measurements, crop, and gizzard weights were higher in the groups with SP dietary inclusion by comparison to control groups (P < 0.05). The incorporation of SP increased the duodenum, jejunum, ileum, and cecum, sections of the intestine (P < 0.05). Birds fed with the SP diet contributed to a greater increase in the viscosity of the duodenum plus jejunum contents (P < 0.0001) by comparison to animals fed the control diet.
Table 5.
Effect of strain (S) and diet (D) on carcass traits, relative organ weight and length of the gastrointestinal tract, and intestinal content apparent viscosity (6 rpm) from Fully Feathered (FF) and Naked Neck (NN) broilers fed Control (C) or Spirulina (SP) diets.
| FF |
NN |
P value |
||||||
|---|---|---|---|---|---|---|---|---|
| C | SP | C | SP | |||||
| Parameter | n = 10 | n = 9 | n = 9 | n = 10 | RSD | Strain | Diet | S×D |
| Carcass traits | ||||||||
| Carcass weight, g | 2,653 | 2,264 | 2,446 | 2,055 | 241.0 | 0.0120 | <0.0001 | 0.9876 |
| Carcass yield, % | 79.0a | 78.1ab | 76.7b | 78.3ab | 1.71 | 0.0704 | 0.5117 | 0.0370 |
| Breast muscle, g | 442.8 | 354.8 | 385.4 | 320.6 | 57.10 | 0.0188 | 0.0002 | 0.5363 |
| Breast muscle yield, % | 16.7 | 15.7 | 15.7 | 15.6 | 1.41 | 0.2662 | 0.2281 | 0.3695 |
| Relative organ weight, g/kg BW | ||||||||
| Crop | 2.83 | 4.02 | 2.33 | 3.34 | 1.171 | 0.1305 | 0.0067 | 0.8088 |
| Heart | 4.43 | 4.27 | 4.39 | 4.78 | 0.671 | 0.2864 | 0.6047 | 0.2121 |
| Proventriculus | 2.79 | 3.16 | 3.04 | 3.12 | 0.535 | 0.5335 | 0.2135 | 0.4104 |
| Gizzard | 11.00 | 12.72 | 11.98 | 13.85 | 2.020 | 0.1180 | 0.0098 | 0.9097 |
| Pancreas | 1.30b | 1.63a | 1.56a | 1.56a | 0.202 | 0.1739 | 0.0146 | 0.0171 |
| Spleen | 1.38 | 1.64 | 1.21 | 1.26 | 0.302 | 0.0079 | 0.1190 | 0.2790 |
| Liver | 12.85b | 15.61a | 14.10ab | 14.79a | 1.322 | 0.6209 | 0.0003 | 0.0215 |
| Duodenum | 3.11 | 3.41 | 4.44 | 3.41 | 1.146 | 0.0822 | 0.3316 | 0.0817 |
| Jejunum | 6.97 | 7.36 | 7.33 | 7.11 | 1.187 | 0.8796 | 0.8347 | 0.4355 |
| Ileum | 5.47 | 6.12 | 6.49 | 6.10 | 0.943 | 0.1132 | 0.6702 | 0.0986 |
| Cecum1 | 3.21 | 3.87 | 3.53 | 3.67 | 0.628 | 0.7580 | 0.0600 | 0.2109 |
| Testicles | 3.73 | 1.94 | 2.23 | 1.92 | 1.782 | 0.1977 | 0.0787 | 0.2121 |
| Relative length of GI tract, cm/kg BW | ||||||||
| Duodenum | 9.68 | 10.89 | 9.04 | 11.57 | 1.675 | 0.9669 | 0.0016 | 0.2346 |
| Jejunum | 23.02 | 24.97 | 21.12 | 25.95 | 2.951 | 0.6365 | 0.0012 | 0.1422 |
| Ileum | 21.10 | 24.66 | 20.73 | 25.82 | 2.827 | 0.6669 | <0.0001 | 0.4104 |
| Cecum | 5.90 | 6.56 | 5.57 | 7.01 | 1.025 | 0.8567 | 0.0034 | 0.2449 |
| Content viscosity, cP | ||||||||
| Duodenum + jejunum | 4.83 | 9.60 | 4.90 | 11.20 | 1.609 | 0.1459 | <0.0001 | 0.1806 |
| Ileum | 15.74 | 15.42 | 7.16 | 20.13 | 10.266 | 0.6392 | 0.1334 | 0.1155 |
Different superscripts within a row indicate a significant difference (P < 0.05).
RDS, relative standard deviation.
Cecum: weight of 2 cecum.
Meat Traits and Texture Parameters
The results regarding the effects of strain and diet on meat traits and texture parameters are shown in Table 6. Drip loss increased with SP dietary inclusion (P = 0.0103). In terms of color, the SP-fed group exhibited a significant increase in yellowness (b*) of the muscle (P = 0.0038), when compared to the C-fed group. The incorporation of SP did not impact thawing loss but led to an increase in cooking losses (P < 0.05). The NN animals showed a significant (P = 0.0347) decrease in WHC. Texture parameters were not affected by either strain or diet (P > 0.05), except for cohesiveness. Indeed, a significant interaction between diet and strain (P < 0.05) was noted for this parameter. Among NN broilers, SP inclusion resulted in a trend of a 10% improvement in the cohesiveness of cooked meat. Conversely, in FF broilers, the inclusion of SP led to a 5% reduction trend in cohesiveness when compared to the control groups.
Table 6.
Effect of strain (S) and diet (D) on the meat quality traits from Fully Feathered (FF) and Naked Neck (NN) broilers fed Control (C) or Spirulina (SP) diets.
| FF |
NN |
P value |
||||||
|---|---|---|---|---|---|---|---|---|
| C | SP | C | SP | |||||
| Parameter | n = 10 | n = 9 | n = 9 | n = 10 | RSD | Strain | Diet | S×D |
| Drip loss, % | 3.29 | 4.65 | 3.45 | 3.88 | 1.218 | 0.3530 | 0.0103 | 0.1646 |
| Color | ||||||||
| L*-lightness | 44.83 | 47.49 | 48.55 | 48.21 | 3.496 | 0.0685 | 0.3308 | 0.2119 |
| a*-redness | 4.19 | 4.76 | 3.54 | 4.87 | 2.067 | 0.7034 | 0.1827 | 0.5914 |
| b*-yellowness | 6.82 | 12.64 | 11.14 | 14.24 | 4.244 | 0.0468 | 0.0038 | 0.3476 |
| Thawing loss, % | 6.50 | 7.16 | 5.24 | 6.88 | 1.882 | 0.2190 | 0.0680 | 0.4307 |
| Cooking loss, % | 23.70 | 25.57 | 22.17 | 24.19 | 2.939 | 0.1381 | 0.0499 | 0.9416 |
| WHC, % | 90.69 | 90.95 | 84.47 | 90.02 | 2.903 | 0.0347 | 0.1454 | 0.2340 |
| Texture parameters | ||||||||
| Hardness, N | 13.36 | 15.27 | 19.92 | 14.92 | 6.157 | 0.1298 | 0.4445 | 0.0934 |
| Chewiness | 5.71 | 6.20 | 7.80 | 6.28 | 2.565 | 0.2018 | 0.5424 | 0.2376 |
| Springiness | 0.73 | 0.72 | 0.69 | 0.70 | 0.058 | 0.0626 | 0.9881 | 0.6530 |
| Cohesiveness | 0.59ab | 0.56ab | 0.55b | 0.61a | 0.036 | 0.8173 | 0.0898 | 0.0013 |
Different superscripts within a row indicate a significant difference (P < 0.05).
RDS, relative standard deviation.
WHC, Watter holding capacity.
Meat Chemical Composition
Data on the effect of strain and diet on the chemical composition of breast muscle is detailed in Table 7. No significant variations among experimental groups were identified for moisture, ash, and protein contents (P > 0.05). Regarding mineral contents, an interaction between strain and diet was observed, specifically for K and P. For the former, NN animals exhibited a significant increase (P < 0.05) of 38 mg/100g in breast meat when SP-fed, while for FF animals it was decreased by 23 mg/100g when animals were SP-fed. Regarding P, NN animals had a significant increase (P < 0.05) of 14 mg/100g in breast meat when SP-fed, while FF animals had a decrease of 15 mg/100g when SP-fed. No significant differences were found for the other minerals (P > 0.05).
Table 7.
Effect of strain (S) and diet (D) on the mineral composition of breast meats from Fully Feathered (FF) and Naked Neck (NN) broilers fed Control (C) or Spirulina (SP) diets.
| FF |
NN |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|
| C | SP | C | SP | |||||
| Parameter | n = 10 | n = 9 | n = 9 | n = 10 | RSD | Strain | Diet | S×D |
| Moisture, % | 72.3 | 72.6 | 72.2 | 71.9 | 0.790 | 0.1313 | 0.9486 | 0.2779 |
| Ash, % | 1.26 | 1.22 | 1.17 | 1.25 | 0.125 | 0.4873 | 0.5997 | 0.1322 |
| Proteins, % | 26.6 | 26.1 | 26.4 | 26.2 | 1.220 | 0.9579 | 0.3970 | 0.7685 |
| Macrominerals, mg/100g fresh weight | ||||||||
| Sodium (Na) | 29.82 | 28.72 | 29.41 | 28.70 | 3.723 | 0.8595 | 0.4605 | 0.8731 |
| Potassium (K) | 369.96b | 346.67c | 342.98c | 381.25a | 40.287 | 0.7730 | 0.5710 | 0.0246 |
| Calcium (Ca) | 3.36 | 3.39 | 3.12 | 3.11 | 0.390 | 0.0507 | 0.9621 | 0.8907 |
| Magnesium (Mg) | 35.37 | 32.62 | 31.83 | 32.56 | 3.210 | 0.0936 | 0.3405 | 0.1041 |
| Phosphorous (P) | 256.02a | 240.69ab | 233.98b | 247.73ab | 13.920 | 0.1065 | 0.8625 | 0.0029 |
| Sulfur (S) | 215.98 | 216.25 | 221.15 | 220.24 | 7.442 | 0.0667 | 0.8964 | 0.8087 |
| Microminerals, mg/100g fresh weight | ||||||||
| Iron (Fe) | 0.89 | 0.94 | 1.05 | 0.88 | 0.495 | 0.7914 | 0.7051 | 0.4989 |
| Copper (Cu) | 0.08 | 0.10 | 0.11 | 0.09 | 0.043 | 0.5886 | 0.9304 | 0.1118 |
| Zinc (Zn) | 0.50 | 0.51 | 0.53 | 0.46 | 0.064 | 0.6575 | 0.1799 | 0.0601 |
| Manganese (Mn) | 0.01 | 0.01 | 0.01 | 0.01 | 0.004 | 0.6579 | 0.9931 | 0.6530 |
Different superscripts within a row indicate a significant difference (P < 0.05).
RDS, relative standard deviation.
Diterpenes, Pigments, and Antioxidant Capacity of Meat
Table 8 illustrates the impact of strain and diet on the content of diterpenes, pigments, and antioxidant capacity of broilers' breast meat. Spirulina diets significantly decreased (P < 0.0001) the levels of α-tocopherol, as well as the minor diterpenes, γ-tocopherol+β-tocotrienol. Interestingly, α-tocotrienol and β-tocopherol were undetectable in all meat groups. Total carotenoid content increased (P < 0.05) in the breast muscle of the animals fed the Spirulina diet when compared to those fed the control diet, regardless of the strain. No significant variations among experimental groups were identified for the antioxidant capacity of the meat (P > 0.05).
Table 8.
Effect of strain (S) and diet (D) on diterpenes, pigment, and antioxidant capacity of breast meats from Fully Feathered (FF) and Naked Neck (NN) broilers fed Control (C) or Spirulina (SP) diets.
| FF |
NN |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|
| C | SP | C | SP | |||||
| Parameter | n = 10 | n = 9 | n = 9 | n = 10 | RSD | Strain | Diet | S×D |
| Diterpene profile, µg/g | ||||||||
| α-Tocopherol | 5.36 | 0.95 | 5.16 | 1.06 | 0.936 | 0.8779 | <0.0001 | 0.6164 |
| ϒ-Tocopherol + β-tocotrienol | 0.11 | 0.03 | 0.12 | 0.03 | 0.013 | 0.2400 | <0.0001 | 0.2166 |
| α-Tocotrienol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| β-Tocopherol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Pigments, µg/100 g | ||||||||
| Total carotenoids | 50.96 | 119.09 | 53.54 | 73.39 | 48.9 | 0.2245 | <0.0171 | 0.1752 |
| Antioxidant Capacity | ||||||||
| DPPH, RSA % | 7.13 | 6.89 | 7.05 | 7.1 | 0.834 | 0.8530 | 0.6922 | 0.4960 |
| TPC, g GAE/g | 37.56 | 35.96 | 38.00 | 35.65 | 3.487 | 0.0899 | 0.9546 | 0.7398 |
DPPH, 2,2-diphenyl-1-picrylhydrazyl.
TPC, total phenolic compounds.
Total Lipid and Cholesterol Contents and Fatty Acid Composition of Meat
The impact of strain and diet on the total lipid and cholesterol contents are outlined in Table 9, as well fatty acid composition of breast meat. The total lipid content remained unaffected by either strain or diet (P > 0.05). However, the inclusion of 15% SP in the diet led to a significant average increase of 0.070 mg/g in cholesterol content (P = 0.0032). Regarding fatty acid composition, total saturated fatty acids (SFA) such as 14:0, 16:0, 17:0, and 23:0 had higher concentrations in SP-fed animals when compared to the C-fed animals (P < 0.0001). A similar trend was observed for monounsaturated fatty acids (MUFA) contents, although not statistically significant (P = 0.0522). The incorporation of Spirulina in broiler diets caused a significant (P < 0.0001) decrease in the polyunsaturated fatty acids (PUFA) contents and the PUFA/SFA ratio of breast meat when compared to the controls. The 15% Spirulina inclusion in the diet resulted in a significant (P < 0.05) increase in total n-3 PUFA and a decrease in total n-6 PUFA contents in the breast muscle. The n-6/n-3 ratio of fatty acids decreased by half in SP-fed compared to C-fed animals.
Table 9.
Effect strain (S) and diet (D) on total lipid and cholesterol contents, and fatty acid profile of breast meats from Fully Feathered (FF) and Naked Neck (NN) broilers fed Control (C) or Spirulina (SP) diets.
| FF |
NN |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|
| C | SP | C | SP | |||||
| Parameter | n = 10 | n = 9 | n = 9 | n = 10 | RSD | Strain | Diet | S×D |
| Total lipids, g/100 g | 0.921 | 0.961 | 0.921 | 0.865 | 0.208 | 0.4842 | 0.9140 | 0.4831 |
| Cholesterol, mg/g | 0.324 | 0.372 | 0.265 | 0.359 | 0.068 | 0.1135 | 0.0032 | 0.2996 |
| Fatty acid composition, g/100 g FA | ||||||||
| C12:0 | 0.056 | 0.055 | 0.056 | 0.056 | 0.016 | 0.9335 | 0.8975 | 0.9801 |
| C14:0 | 0.308 | 0.420 | 0.353 | 0.421 | 0.058 | 0.2289 | <0.0001 | 0.2488 |
| C14:1c9 | 0.059 | 0.109 | 0.079 | 0.112 | 0.025 | 0.1605 | <0.0001 | 0.2947 |
| C15:0 | 0.088 | 0.102 | 0.081 | 0.107 | 0.030 | 0.8860 | 0.0499 | 0.5187 |
| C16:0 | 18.659c | 24.019a | 20.311b | 24.116a | 0.925 | 0.0063 | <0.0001 | 0.0141 |
| C16:1c7 | 0.289 | 0.329 | 0.261 | 0.332 | 0.038 | 0.3078 | <0.0001 | 0.2242 |
| C16:1c9 | 1.378 | 3.259 | 2.086 | 3.318 | 0.695 | 0.0987 | <0.0001 | 0.1598 |
| C17:0 | 0.183 | 0.220 | 0.176 | 0.224 | 0.056 | 0.9082 | 0.0267 | 0.7694 |
| C17:0c9 | 0.052 | 0.097 | 0.058 | 0.106 | 0.031 | 0.4580 | <0.0001 | 0.8610 |
| C18:0 | 9.291 | 9.608 | 8.887 | 9.506 | 0.925 | 0.4070 | 0.1285 | 0.6188 |
| C18:1c9 | 26.944 | 26.296 | 27.078 | 26.525 | 2.469 | 0.8228 | 0.4594 | 0.9534 |
| C18:1c11 | 1.754 | 2.742 | 1.728 | 2.784 | 0.231 | 0.9156 | <0.0001 | 0.6522 |
| C18:2n-6 | 24.215 | 13.372 | 23.579 | 13.315 | 1.580 | 0.5037 | <0.0001 | 0.5767 |
| C18:3n-6 | 0.103 | 0.059 | 0.105 | 0.059 | 0.018 | 0.8220 | <0.0001 | 0.9216 |
| C18:3n-3 | 0.331 | 0.303 | 0.310 | 0.302 | 0.061 | 0.5837 | 0.3792 | 0.6264 |
| C20:0 | 0.126 | 0.118 | 0.115 | 0.130 | 0.032 | 0.9476 | 0.7188 | 0.2800 |
| C20:1c11 | 0.198 | 0.213 | 0.201 | 0.212 | 0.044 | 0.9588 | 0.3830 | 0.9060 |
| C20:2n-6 | 0.416 | 0.222 | 0.357 | 0.219 | 0.073 | 0.1995 | <0.0001 | 0.2484 |
| C20:3n-6 | 0.577 | 1.222 | 0.630 | 1.202 | 0.196 | 0.8025 | <0.0001 | 0.5653 |
| C20:4n-6 | 7.156 | 7.892 | 6.766 | 7.636 | 1.886 | 0.6012 | 0.1987 | 0.9138 |
| C20:3n-3 | 0.079 | 0.067 | 0.064 | 0.072 | 0.025 | 0.5264 | 0.8085 | 0.2263 |
| C20:5n-3 | 0.087 | 0.124 | 0.071 | 0.125 | 0.036 | 0.5262 | 0.0004 | 0.4441 |
| C22:0 | 0.105 | 0.123 | 0.117 | 0.127 | 0.043 | 0.5697 | 0.3247 | 0.7857 |
| C22:1n-9 | 0.023 | 0.035 | 0.030 | 0.031 | 0.061 | 0.9347 | 0.7366 | 0.7982 |
| C22:2n-6 | 0.145 | 0.239 | 0.111 | 0.234 | 0.044 | 0.1779 | <0.0001 | 0.3095 |
| C22:5n-3 | 0.432 | 0.714 | 0.320 | 0.685 | 0.189 | 0.2573 | <0.0001 | 0.5074 |
| C22:6n-3 | 0.371 | 0.522 | 0.275 | 0.482 | 0.175 | 0.2408 | 0.0033 | 0.6330 |
| C23:0 | 0.208 | 0.306 | 0.245 | 0.318 | 0.094 | 0.4236 | 0.0084 | 0.6887 |
| Others | 6.37ab | 6.40ab | 5.55b | 7.25a | 0.993 | 0.9674 | 0.0115 | 0.0146 |
| Partial sums of fatty acids | ||||||||
| SFA | 29.04 | 34.97 | 30.34 | 35.00 | 1.242 | 0.1067 | <0.0001 | 0.1246 |
| MUFA | 30.70 | 33.08 | 31.53 | 33.42 | 3.262 | 0.5828 | 0.0522 | 0.8182 |
| PUFA | 33.91 | 24.72 | 32.59 | 24.32 | 2.198 | 0.2358 | <0.0001 | 0.5242 |
| n-6 PUFA | 32.61 | 23.01 | 31.56 | 22.67 | 1.996 | 0.2895 | <0.0001 | 0.5859 |
| n-3 PUFA | 1.30 | 1.73 | 1.04 | 1.67 | 0.307 | 0.1126 | <0.0001 | 0.3353 |
| Nutritional ratios | ||||||||
| n-6/n-3 ratio | 26.18 | 13.60 | 31.30 | 14.05 | 4.495 | 0.0652 | <0.0001 | 0.1198 |
| PUFA/SFA | 1.17 | 0.71 | 1.07 | 0.70 | 0.075 | 0.0331 | <0.0001 | 0.0945 |
Different superscripts within a row indicate a significant difference (P < 0.05).
RDS, relative standard deviation.
SFA= 12:0+14:0+15:0+16:0+17:0+18:0+20:0+22:0+23:0.
MUFA= 14:1c9+ 16:1c7+16:1c9+17:1c9+18:1c9+18:1c11+20:1c11+22:1n-9.
PUFA= 18:2n-6+18:3n-6+18:3n-3+20:2n-6+20:3n-6+20:4n-6+20:3n-3+20:5n-3+22:5n-3+22:6n-3.
n-6 PUFA= 18:2n-6+18:3n-6+20:2n-6+20:3n-6+20:4n-6.
n-3 PUFA = 18:3n-3+20:3n-3+20:5n-3+22:5n-3+22:6n-3.
DISCUSSION
This study assessed the effect of a diet incorporating Spirulina as a feed ingredient (15% inclusion rate) on production performances and meat characteristics and examined specifically its impact on two strains of slow-growing boilers: FF and NN. During this research, we observed significant differences in animal weights between the 2 strains, particularly noticeable from the grower diet onwards (>28 d). Indeed, on d 84 the final BW of the NN animals was 7% lower than that of the FF animals. According to the literature (Fernandes et al., 2023), NN animals, under thermoneutral temperature conditions, tend to show lower body weights when compared to FF animals.
The challenges associated with producing and incorporating Spirulina in animal feed include its relatively high price compared to other protein feedstuffs like soybean meal (Altmann and Rosenau, 2022), and the variability in its nutritional composition influenced by factors such as drying process and culturing conditions (Gutiérrez-Salmeán et al., 2015). However, local production under controlled conditions and drying with solar-powered dryers can reduce costs, promote sustainability, and ensure product uniformity of the biomass composition. Despite the potential color alteration of the feed due to Spirulina addition, research suggests it is unlikely to significantly impact animal feeding behavior (Gulizia and Downs, 2021).
Previous research on the assessment of productive performances of poultry with the inclusion of Spirulina in their diets used levels of incorporation, ranging from 0.1% to 15%, showing both consistent and inconsistent results. Such contradictory results are possibly due to different incorporation levels of Spirulina in the diet, treatment (e.g. enzymes), age, and strain of the birds used (Pestana et al., 2020; Khan et al., 2020; Alwaleed et al., 2021; Hassan et al., 2022; Abdelfatah et al., 2024). In the present study, it was observed that birds fed with Spirulina exhibited a decrease of BW, ADG, and FI, along with an increased FCR until the end of the growth diet period (70th d). However, in the last 2 wk of the trial, during the finisher diet, no significant differences were observed between the 2 diets (C or SP).
Given that previous studies predominantly focused on fast-growing strains over short experimental durations, normally 35 d (5 wk), it could be hypothesized that any disparities evident in the final period could be attributed to the animals' capacity to adapt to the diet over time. This hypothesis gains traction from the observed increased relative size of the crop (by 43%) and gizzard (by 16%), indicating potential adaptation to changes in feed intake, including meal size and/or frequency. Moreover, such a relative increase in crop and gizzard could be linked to the cellular composition and structure of Spirulina. Indeed, and as time progresses, in animals fed with such high levels of inclusion, the additional effort required to digest Spirulina may stimulate hypertrophy of the crop and gizzard muscles (Rodrigues and Choct, 2018). Several researchers have attributed 1 major challenge to the use of microalgae as a feedstuff in poultry feed, particularly when levels exceed 10%: the gelation of its indigestible proteins. This gelation is associated with poorer performance, as it increases digesta viscosity, thereby limiting the access of endogenous enzymes to their target substrates (Pestana et al., 2020; Alfaia et al., 2021; Cabrol et al., 2022b). In this study, the inclusion of 15% Spirulina in the diet led to higher content viscosities when compared to birds fed the control diet, regardless of strain. The observed increase in the length of the gastrointestinal tract (duodenum, jejunum, ileum, and caecum) resulting from the inclusion of Spirulina is likely linked to a slower passage rate of the digesta caused by increased viscosity (Wu et al., 2013). These findings may explain the overall lower bird performance observed in the SP group of the 2 strains.
For the broiler industry, carcass, and breast muscle yields are considered two of the most important parameters (Scheuermann et al., 2003). In this study, when calculating carcass yield, we accounted for the weight of the head and legs, as the slow-growing strains used in our trial are typically marketed with these parts included. This accounts for the higher value obtained in our study compared to those reported in another research (Almeida and Zuber, 2010). In this study, when fed a control diet, the NN broilers had lower carcass yield than the FF broilers. Among other factors, carcass yields are influenced by strain (Baéza et al., 2022) as genetics influence parameters such as growth rate, feed efficiency, and muscle development. We can therefore hypothesize that NN animals will have lower muscle development rates, in line with lower growth rates. However, breast muscle yields are the most valuable part of the carcass (Scheuermann et al., 2003) and in this study, there is no difference between the groups. Unlike overall carcass yield, breast muscle yield may be more standardized and consistent due to selective breeding programs, which focus on traits like breast meat yield (Tickle et al., 2014).
One of the meat quality parameters that most influence consumer acceptance is color (Fletcher, 2002). Like other studies (Altmann et al., 2018, 2020; Pestana et al., 2020; Altmann and Rosenau, 2022), the raw breast meat of the SP-fed animals showed a significant increase in yellowness compared to C-fed animals. This increase in color results in a more intense orange hue that is not only detected by instrumental analytical measurement but also noticeable to the naked eye. The ΔE value, a widely used parameter for assessing overall color variations and quantifying the difference between 2 samples, exceeded 5 in both strains. A ΔE value surpassing 5 indicates that the colors are visibly different to the human eye (Altmann et al., 2022). The intense color of poultry meat, in some markets, may be perceived as unfamiliar by consumers, potentially resulting in reduced meat acceptance (Altmann and Rosenau, 2022). The increased color found in the meat from the SP group in this study suggests a very efficient transfer of Spirulina pigments from the diet to the meat, as animals are not able to synthesize them. Carotenoids, the main Spirulina pigments responsible for the more intense color in meat, offer additional antioxidant protection and support immune function (Maoka, 2020). In this study, the inclusion of 15% Spirulina in the diet, increased in average by 85% the content of carotenoids found in the breast muscle. The inclusion of Spirulina into the diet can thus be advantageous, as it reduces the need to add synthetic pigments to the diet.
The WHC of meat is a complex characteristic influenced by both structural and biochemical alterations that take place during the conversion of muscle to meat. Protein denaturation induces changes in the structure and solubility of proteins, which in turn affect their capacity to bind and retain water (Huff-Lonergan and Lonergan, 2005). In our study, the WHC of the breast meat from the NN animals was observed to be 2% lower compared to the FF animals, suggesting that these animals would have less juicy and tender meat. However, the specific mechanisms by which protein denaturation affects WHC in chicken meat are not fully understood and require further research (Bowker and Zhuang, 2015). Meat with higher WHC tends to have lower drip loss, cooking, and thawing losses because it retains more moisture within the meat structure (Huff-Lonergan and Lonergan, 2005). However, in our study, we found that although strain influenced WHC, it was the diet that had a higher impact on cooking and drip losses. The incorporation of Spirulina in the diet increased drip and cooking loss of the breast meat in comparison to the control diet. Drip loss, which refers to the loss of moisture or liquid from meat during storage or processing, is a crucial measure of meat quality. Increased drip loss often suggests poorer meat quality, as it can result in a drier, less tender texture and hasten spoilage, thereby shortening the shelf life of the meat product (Pang et al., 2020). Despite these results, the present study showed that cooked meat cohesiveness of NN broilers was increased with SP incorporation when compared to the control diet, suggesting that the increase in drip loss will not be high enough to have a major impact. In contrast, no significant differences were observed in cooked meat cohesiveness for FF broilers with SP incorporation compared to the control diet. Cohesiveness, the ability to retain its integrity after a second deformation, relative to its resistance during the initial deformation, is determined by comparing the working area during the second compression to that of the first compression (Kohyama, 2020). When meat exhibits greater cohesiveness, it indicates that its proteins are more tightly bound together, leading to a more cohesive and unified structure. Such cohesive structure contributes to the tenderness of the meat (Paredes et al., 2022). Cooking loss, defined as the proportion of weight lost during the cooking process, is an indicator of meat moisture retention, with a lower cooking loss typically reflecting better moisture retention. Contrary to the results found by Pestana et al. (2020), where the same incorporation of Spirulina at a rate of 15% in the diet showed no significant differences, this study revealed an increase in cooking loss with the SP diet compared to the control diet. However, it is important to acknowledge that comparing cooking losses across studies can be challenging due to variations in experimental parameters and methodologies. The discrepancy in results between studies may also be attributed to differences in the methods of analysis employed. For a thorough comprehension of how Spirulina affects cooking loss, future studies must investigate specific mechanisms, including Spirulina dosage, duration of supplementation, and interaction with other dietary components. More comprehensive research is thus necessary to elucidate the precise impact of Spirulina supplementation on cooking loss in slow-growth poultry meat.
In poultry meat, the amino acid profile may be affected by diet, whereas the crude protein content depends upon factors such as strain and slaughter age (Baéza et al., 2022). In this study, no differences were found in the protein content of breast meat among the different groups, suggesting that there is no impact of strain. However, our figures in protein content were higher than those reported in the literature, typically ranging from 23% to 25% (Baéza et al., 2022). This difference may be attributed to the age of the animals. Our study used slow-growth strains slaughtered at 84 d, whereas previous studies often used fast-growing strains slaughtered at 35 d.
Spirulina contains rich concentrations of minerals such as potassium, calcium, magnesium, selenium, iron, zinc, and manganese. These minerals are typically found in varying concentrations depending on geographical location, harvesting year, and drying technique (Grosshagauer et al., 2020). Supplementation of animal diets with Spirulina could decrease the need for adding trace minerals in the form of feed supplements, thereby reducing premix incorporation, and balancing the final price of feed. In this study, the incorporation of Spirulina increased the final content of the diets in sodium (+62%) and iron (+40%) compared to control diets. Excessive sodium intake in poultry diets can lead to increased water consumption, dehydration, reduced feed intake, and electrolyte imbalances (Alagawany et al., 2021). In both diets, starter, and grower, the excreta of the animals fed an SP-diet had a 17% and 37% (respectively) lower percentage of DM than animals fed the C-diet. Although water intake was not measured in this study, the observed results suggest a potential increase in water intake, possibly influenced by higher sodium levels in the diet. The increase in water consumption may also be related to the decrease in feed intake, which was in line with the results obtained and may have had an impact on production performances. These results suggest that in the future it may be interesting to take sodium content into account in the formulation of the diets to mitigate this impact. Iron overload can result in oxidative stress and damage to tissues and organs, including the liver and kidneys (Alagawany et al., 2021). Within our research, the livers of animals fed a Spirulina-supplemented diet showed increases of 2.78 and 0.69 g/kg BW, respectively for FF and NN animals. Such increase can putatively be explained by the excessive iron intake over a prolonged period can result in the accumulation of iron in the liver, potentially leading to an increase in its weight, as the liver is intrinsically involved in iron metabolism (Alagawany et al., 2021).
Despite variations in the mineral composition of the diets, our study found no significant differences in most of the minerals present in broiler meat across both diets. This finding can be explained by the average 22% increase in the percentage of ash in the excreta of animals fed a Spirulina-based diet compared to those fed the control diet. As has been long described, the higher ash content may indicate a potentially greater excretion of minerals (Mcdonald et al., 2011) suggesting that these substances may not have been fully utilized or absorbed by the animals. However, an intriguing interaction between strain and diet emerged, particularly concerning the potassium and phosphorus content in broiler meat. These findings suggest that genetic disparities among poultry breeds or strains may impact their ability to metabolize and deposit minerals within their tissues. This emphasizes the need for further comprehensive research to elucidate the specific genetic factors and mechanisms influencing mineral bioaccessibility and bioavailability for poultry.
The present study highlights a decrease in α-tocopherol levels in the breast meat compared to the control, attributed to the lower content of this vitamin in Spirulina as documented in the literature (Madeira et al., 2017). These findings are consistent with previous studies (Pestana et al., 2020; Spínola et al., 2024).
To be considered lean meats, fat content should be less than 5% (Food Advisory Committee, 1990). In our case, the total lipid content ranged from 0.865 to 0.961 g/100 g, thus meeting this standard. The fat content found was lower than the one found by Pestana et al. (2020) which evaluated the effect of a diet with 15% Spirulina on fast-growing broilers. Among the various factors influencing fat content, genetics seems to be a determinant. Slow-growing strains generally show lower fat content compared to fast-growing genotypes (Fanatico et al., 2007). The American Heart Association recommends limiting the total amount of cholesterol to less than 300 mg/d (Carson et al., 2020). Despite the breast meat of animals fed Spirulina showing an average increase of 0.070 mg/g, the cholesterol content remains quite low and within the established criteria. Our study suggests that diet had a significantly greater influence on the fatty acid profile of the meat than the strain of the animals. The differences found in the dietary profiles, especially in palmitic acid (16:0), stearic acid (18:0), and oleic acid (C18:1c9), can be attributed to 15% Spirulina incorporation. As the predominant fatty acids found in Spirulina were palmitic acid (>50%) and linoleic acid (23%), the predominant fatty acids found in corn and soybean meal are linoleic acid (>50%) and oleic acid (20-30%). Corn, soybean meal, and Spirulina are poor sources of α-linolenic acid (18:3n-3) (Tokusoglu and Unal, 2003). For breast muscle, Spirulina incorporation increased SFA, mainly palmitic acid (16:0). This fatty acid is not the most predominant in the diets but is not as easy to oxidize and is more readily deposited in the meat (Long et al., 2018). There is no specific recommended dosage for palmitic acid alone. However, it recommends limiting saturated fat intake because of the increased levels of low-density lipoprotein (LDL) cholesterol, which is a risk factor for heart disease (Mensink et al., 2003). Spirulina inclusion resulted in higher SFA and lower total PUFA contents, leading to a reduction in PUFA/SFA ratios compared to breast muscle of the control-feed groups. However, following previous studies, PUFA/SFA ratios remained above the recommended minimum for food of 0.45, which is considered beneficial for cardiovascular health (Burghardt et al., 2010). The higher content of linoleic acid (LA) found in the diets compared to α-linolenic acid (ALA) content might lead to elevated levels of n-6 PUFAs in the meat. However, this increment does not necessarily correspond to a proportional increase due to the complexities of fatty acid metabolism and enzymatic activities. The competition between n-6 and n-3 fatty acid pathways is well-established. The ALA is known to compete with LA for the same enzymes involved in the conversion to long-chain polyunsaturated fatty acids (LC-PUFAs), and it is suggested that ALA may inhibit the formation of n-6 LC-PUFAs more effectively than LA inhibits the formation of n-3 LC-PUFAs (Lee et al., 2019). Both n-6 and n-3 fatty acids are important for human health, thus maintaining a proper balance between them is crucial. The optimal ratio of n-6 to n-3 fatty acids is still a topic of debate among researchers (Sugano, 1996; Burghardt et al., 2010), but general recommendations suggest aiming for around 4:1. Our study observed a favorable decrease in the n-6/n-3 PUFA ratio of the meat from the group fed 15% Spirulina, however, still not meeting the recommended guidelines. In our study, the significant increase in n-3 PUFA content in broiler meat-fed Spirulina relative to the control feed was mainly due to the increase of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA). The health benefits of n-3 PUFAs, particularly EPA and DHA, are linked to their anti-inflammatory properties (Siriwardhana et al., 2012).
Although our study provides valuable insights into the effects of Spirulina on performance and carcass traits, further research with larger sample sizes, particularly involving these specific strains under production conditions, is necessary to fully ascertain the effect of high levels of Spirulina inclusion in these 2 poultry genotypes.
CONCLUSION
The results of this study indicate that incorporating 15% Spirulina into the diets of both strains of birds led to decreased performance and increased weight and size of gastrointestinal compartments, along with increased digesta viscosity. These findings suggest that factors are causing the ineffective results of Spirulina consumption, highlighting the importance of more research, especially on diet formulation considering mineral content. Despite lower production performance, NN broilers showed no significant impact on most meat traits. It is noteworthy to mention that these findings were observed under thermoneutral conditions. The adaptability of NN broilers in maintaining favorable meat traits despite lower performance presents an intriguing opportunity for future research. Investigating their performance under heat stress conditions could yield different outcomes. By expanding our understanding beyond thermoneutrality, we might establish novel strategies for sustainable poultry production in changing environmental conditions. Interestingly, the SP diet improved certain quality parameters and fatty acid composition of breast meat in both strains. Further investigation, particularly focusing on digestibility, is warranted to comprehensively understand the impact of Spirulina supplementation on slow-growth poultry and its influence on meat characteristics.
DISCLOSURES
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Elisabete Fernandes reports financial support was provided by Foundation for Science and Technology. Catia Martins reports financial support was provided by Foundation for Science and Technology. Joana Sales reports financial support was provided by Foundation for Science and Technology. Daniela Carvalho reports financial support was provided by Foundation for Science and Technology. Jose Prates reports was provided by Foundation for Science and Technology. Maria Madalena Lordelo reports financial support was provided by Foundation for Science and Technology. Luisa Louro reports financial support was provided by Foundation for Science and Technology. Anabela Raymundo reports financial support was provided by Foundation for Science and Technology. Andre Almeida reports financial support was provided by Foundation for Science and Technology. Nothing to declare If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGMENTS
This work was funded by national funds through FCT – Fundação para a Ciência e a Tecnologia, I.P., under the project UIDB/04129/2020 of LEAF-Linking Landscape, Environment, Agriculture and Food, Research Unit through the internal project Trop-Plumage. Author E. A. F. acknowledges a PhD fellowship (grant: UI/BD/151166/2021) also from the FCT. Authors acknowledge animal experiment work supported by Miss Andreia Chaves (LEAF/ISA) and Professor Rui Bessa (CIISA/AL4ANIMALS/FMV) in formal analyses.
Author contributions: E. A. Fernandes: Investigation, methodology, validation, formal analyses, data curation, writing original draft, writing – review and editing. C. F. Martins: Investigation, methodology, writing – review and editing. D. F. P. Carvalho: investigation, writing – review and editing. Sales J.R: Methodology, validation, supervision, writing – review and editing. J. A. M. Prates: Methodology, writing – review and editing. M. M. Lordelo: Conceptualization, methodology, writing – review and editing. L. L. Martins: Conceptualization, validation, supervision, methodology, writing - review and editing. A. Raymundo: Conceptualization, validation, supervision, methodology, writing - review and editing. A.M. Almeida: Conceptualization, validation, supervision, methodology, writing - review and editing.
REFERENCES
- Abdelfatah S.H., Yassin A.M., Khattab M.S., Abdel-Razek A.S., Saad A.H. Spirulina platensis as a growth booster for broiler; Insights into their nutritional, molecular, immunohistopathological, and microbiota modulating effects. BMC Vet. Res. 2024;20:11. doi: 10.1186/s12917-023-03858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adomako K., Olympio O.S., Hagan J.K., Hamidu J.A. Growth performance of crossbred naked neck and normal feathered laying hens kept in tropical villages. Br. Poult. Sci. 2014;55:701–708. doi: 10.1080/00071668.2014.960805. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., Tiwari R., Yatoo M.I., Karthik K., Michalak I., Dhama K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—a comprehensive review. Vet. Quart. 2021;41:1–29. doi: 10.1080/01652176.2020.1857887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaia C.M., Pestana J.M., Rodrigues M., Coelho D., Aires M.J., Ribeiro D.M., Major V.T., Martins C.F., Santos H., Lopes P.A., Lemos J.P.C., Fontes C.M.G.A., Lordelo M.M., Prates J.A.M. Influence of dietary Chlorella vulgaris and carbohydrate-active enzymes on growth performance, meat quality and lipid composition of broiler chickens. Poult. Sci. 2021;100:926–937. doi: 10.1016/j.psj.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A.M., Zuber U. The effect of the Naked Neck genotype (Nana), feeding and outdoor rearing on growth and carcass characteristics of free range broilers in a hot climate. Trop. Anim. Health Prod. 2010;42:99–107. doi: 10.1007/s11250-009-9391-y. [DOI] [PubMed] [Google Scholar]
- Altmann B.A., Gertheiss J., Tomasevic I., Engelkes C., Glaesener T., Meyer J., Schäfer A., Wiesen R., Mörlein D. Human perception of color differences using computer vision system measurements of raw pork loin. Meat Sci. 2022;188 doi: 10.1016/j.meatsci.2022.108766. [DOI] [PubMed] [Google Scholar]
- Altmann B.A., Neumann C., Velten S., Liebert F., Mörlein D. Meat quality derived from high inclusion of a micro-alga or insect meal as an alternative protein source in poultry diets: A pilot study. Foods. 2018;7:34. doi: 10.3390/foods7030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann B.A., Rosenau S. Spirulina as animal feed: Opportunities and challenges. Foods. 2022;11:965. doi: 10.3390/foods11070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann B.A., Wigger R., Ciulu M., Mörlein D. The effect of insect or microalga alternative protein feeds on broiler meat quality. J. Sci. Food Agric. 2020;100:4292–4302. doi: 10.1002/jsfa.10473. [DOI] [PubMed] [Google Scholar]
- Alwaleed E.A., El-Sheekh M., Abdel-Daim M.M., Saber H. Effects of Spirulina platensis and Amphora coffeaeformis as dietary supplements on blood biochemical parameters, intestinal microbial population, and productive performance in broiler chickens. Environmental Science and Pollution Research. 2021;28:1801–1811. doi: 10.1007/s11356-020-10597-3. [DOI] [PubMed] [Google Scholar]
- AOAC Official methods of analysis. 2000 [Google Scholar]
- Baéza E., Guillier L., Petracci M. Review: Production factors affecting poultry carcass and meat quality attributes. Animal. 2022;16 doi: 10.1016/j.animal.2021.100331. [DOI] [PubMed] [Google Scholar]
- Bourne M.C. Texture profile analysis. J. Food Sci. 1978;32:62–67. [Google Scholar]
- Bowker B., Zhuang H. Relationship between water-holding capacity and protein denaturation in broiler breast meat1. Poult. Sci. 2015;94:1657–1664. doi: 10.3382/ps/pev120. [DOI] [PubMed] [Google Scholar]
- Burghardt P.R., Kemmerer E.S., Buck B.J., Osetek A.J., Yan C., Koch L.G., Britton S.L., Evans S.J. Dietary n-3:n-6 fatty acid ratios differentially influence hormonal signature in a rodent model of metabolic syndrome relative to healthy controls. Nutr. Metab. (Lond) 2010;7:53. doi: 10.1186/1743-7075-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrol M.B., Martins J.C., Malhão L.P., Alfaia C.M., Prates J.A.M., Almeida A.M., Lordelo M., Raymundo A. Digestibility of meat mineral and proteins from broilers fed with graded levels of chlorella vulgaris. Foods. 2022;11:1345. doi: 10.3390/foods11091345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrol M.B., Martins J.C., Malhão L.P., Alves S.P., Bessa R.J.B., Almeida A.M., Raymundo A., Lordelo M. Partial replacement of soybean meal with Chlorella vulgaris in broiler diets influences performance and improves breast meat quality and fatty acid composition. Poult. Sci. 2022;101:1–16. doi: 10.1016/j.psj.2022.101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahaner A., Deeb N., Gutman M. Effects of the Plumage-Reducing Naked Neck (Na) Gene on the Performance of Fast-Growing Broilers at Normal and High Ambient Temperatures. Poult. Sci. 1993;72:767–775. [Google Scholar]
- Carson J.A.S., Lichtenstein A.H., Anderson C.A.M., Appel L.J., Kris-Etherton P.M., Meyer K.A., Petersen K., Polonsky T., Van Horn L. Dietary cholesterol and cardiovascular risk: a science advisory from the American Heart Association. Circulation. 2020;141:e39–e53. doi: 10.1161/CIR.0000000000000743. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhang Y. High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzyme Microb. Technol. 1997;20:221–224. [Google Scholar]
- Deeb N., Cahaner A. The effects of naked neck genotypes, ambient temperature, and feeding status and their interactions on body temperature and performance of broilers. Poult. Sci. 1999;78:1341–1346. doi: 10.1093/ps/78.10.1341. [DOI] [PubMed] [Google Scholar]
- El-Bahr S., Shousha S., Shehab A., Khattab W., Ahmed-Farid O., Sabike I., El-Garhy O., Albokhadaim I., Albosadah K. Effect of dietary microalgae on growth performance, profiles of amino and fatty acids, antioxidant status, and meat quality of broiler chickens. Animals. 2020;10:761. doi: 10.3390/ani10050761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shall N.A., Jiang S., Farag M.R., Azzam M., Al-Abdullatif A.A., Alhotan R., Dhama K., Hassan F., Alagawany M. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front. Immunol. 2023;14:1–12. doi: 10.3389/fimmu.2023.1072787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Emmert J.L., Owens C.M. Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007;86:2245–2255. doi: 10.1093/ps/86.10.2245. [DOI] [PubMed] [Google Scholar]
- Fehlenberg V., Baumann M., Gasparri N.I., Piquer-Rodriguez M., Gavier-Pizarro G., Kuemmerle T. The role of soybean production as an underlying driver of deforestation in the South American chaco. Glob. Environ. Change. 2017;45:24–34. [Google Scholar]
- Fernandes E., Raymundo A., Martins L.L., Lordelo M., de Almeida A.M. The Naked neck gene in the domestic chicken: a genetic strategy to mitigate the impact of heat stress in poultry production—a review. Animals. 2023;13:1007. doi: 10.3390/ani13061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher D.L. Poultry meat quality. Worlds Poult. Sci. J. 2002;58:131–145. [Google Scholar]
- Folch J., Lees M., Sloane G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Food Advisory Committee . Her Majesty's Stationery Office; London: 1990. Report on review of food labelling and advertising. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). 2022. World food and agriculture – Statistical yearbook 2022. Retrieved from https://www.fao.org/statistics/yearbook.
- Grosshagauer S., Kraemer K., Somoza V. The true value of Spirulina. J. Agric. Food Chem. 2020;68:4109–4115. doi: 10.1021/acs.jafc.9b08251. [DOI] [PubMed] [Google Scholar]
- Gulizia J.P., Downs K.M. The effects of feed color on broiler performance between day 1 and 21. Animals. 2021;11:1511. doi: 10.3390/ani11061511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Salmeán G., Fabila-Castillo L., Chamorro-Cevallos G. Nutritional and toxicological aspects of Spirulina (Arthrospira) Nutr. Hosp. 2015;32:34–40. doi: 10.3305/nh.2015.32.1.9001. [DOI] [PubMed] [Google Scholar]
- Hassan R., Refaie M., El-Shoukary R., Rehan I., Zigo F., Karaffová V., Amer H. Effect of dietary microalgae (spirulina platensis) on growth performance, ingestive behavior, hemato-biochemical parameters, and economic efficiency of fayoumi broilers. Life. 2022;12:1892. doi: 10.3390/life12111892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honikel K.O. Springer; Netherlands, Dordrecht: 1987. How to measure the water-holding capacity of meat? recommendation of standardized methods. Pages 129–142 in evaluation and control of meat quality in pigs. [Google Scholar]
- Hu Q. John Wiley & Sons; Ltd: 2003. Industrial production of microalgal cell-mass and secondary products - major industrial species: Arthrospira (Spirulina) platensis. Pages 264–272 in handbook of microalgal culture. [Google Scholar]
- Huff-Lonergan E., Lonergan S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Hynstova V., Sterbova D., Klejdus B., Hedbavny J., Huska D., Adam V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using high performance thin layer chromatography. J. Pharm Biomed. Anal. 2018;148:108–118. doi: 10.1016/j.jpba.2017.09.018. [DOI] [PubMed] [Google Scholar]
- Khalilnia F., Mottaghitalab M., Mohiti M., Seighalani R. Effects of dietary supplementation of probiotic and Spirulina platensis microalgae powder on growth performance immune response, carcass characteristics, gastrointestinal microflora and meat quality in broilers chick. Vet. Med. Sci. 2023;9:1666–1674. doi: 10.1002/vms3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Mobashar M., Mahsood F.K., Javaid S., Abdel-Wareth A.A., Ammanullah H., Mahmood A. Spirulina inclusion levels in a broiler ration: evaluation of growth performance, gut integrity, and immunity. Trop. Anim. Health Prod. 2020;52:3233–3240. doi: 10.1007/s11250-020-02349-9. [DOI] [PubMed] [Google Scholar]
- Kohyama K. Wiley; Hoboken, NJ, USA: 2020. Food texture – sensory evaluation and instrumental measurement. Pages 1–13 in textural characteristics of world foods. [Google Scholar]
- Lee S.A., Whenham N., Bedford M.R. Review on docosahexaenoic acid in poultry and swine nutrition: Consequence of enriched animal products on performance and health characteristics. Ani. Nutr. 2019;5:11–21. doi: 10.1016/j.aninu.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen I., Williams A.G., Kyriazakis I. The effects of welfare-enhancing system changes on the environmental impacts of broiler and egg production. Poult. Sci. 2014;93:256–266. doi: 10.3382/ps.2013-03252. [DOI] [PubMed] [Google Scholar]
- Long S.F., Kang S., Wang Q.Q., Xu Y.T., Pan L., Hu J.X., Li M., Piao X.S. Dietary supplementation with DHA-rich microalgae improves performance, serum composition, carcass trait, antioxidant status, and fatty acid profile of broilers. Poult. Sci. 2018;97:1881–1890. doi: 10.3382/ps/pey027. [DOI] [PubMed] [Google Scholar]
- Madeira M.S., Cardoso C., Lopes P.A., Coelho D., Afonso C., Bandarra N.M., Prates J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest Sci. 2017;205:111–121. [Google Scholar]
- Maoka T. Carotenoids as natural functional pigments. J. Nat. Med. 2020;74: 1– 16. doi: 10.1007/s11418-019-01364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli G., Vogel E., Decian M., Farinha M.J.U.S., Bernardo L.V.M., Borges J.A.R., Gimenes R.M.T., Garcia R.G., Ruviaro C.F. Assessing the eco-efficiency of different poultry production systems: an approach using life cycle assessment and economic value added. Sustain. Prod. Consum. 2020;24:181–193. [Google Scholar]
- Martins C.F., Pestana J.M., Alfaia C.M., Costa M., Ribeiro D.M., Coelho D., Lopes P.A., Almeida A.M., Freire J.P.B., Prates J.A.M. Effects of chlorella vulgaris as a feed ingredient on the quality and nutritional value of weaned piglets’. Meat. Foods. 2021;10:1155. doi: 10.3390/foods10061155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins C.F., Pestana J.A., Ribeiro D.M.S., dos S. Madeira M.S.M., Alfaia C.M.R.P.M., Lopes P.A.A.B., Coelho D.F.M., Cardoso Lemos J.P., de Almeida A.M., Prates J.A.M., Freire J.P.B. Effect of dietary inclusion of Spirulina on production performance, nutrient digestibility and meat quality traits in post-weaning piglets. J. Anim. Physiol Anim. Nutr. (Berl) 2021;105:247–259. doi: 10.1111/jpn.13470. [DOI] [PubMed] [Google Scholar]
- Mcdonald P., Edwards R.A., Greenhalgh J.F.D., Morgan C.A., Sinclair L.A., Wilkinson R.G., Greenhalgh M.E., Wilkinson M.S. 7th ed. Pearson; Harlow, UK: 2011. Animal nutrition. [Google Scholar]
- Mensink R.P., Zock P.L., Kester A.D., Katan M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Mohankumar J.B., Uthira L., Maheswari S.U. Total phenolic content of organic and conventional green leafy vegetables. J. Nutr. Hum. Health. 2018;2:1–6. [Google Scholar]
- Pang B., Bowker B., Zhuang H., Yang Y., Zhang J. Research Note: Comparison of 3 methods used for estimating cook loss in broiler breast meat. Poult. Sci. 2020;99:6287–6290. doi: 10.1016/j.psj.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes J., Cortizo-Lacalle D., Imaz A.M., Aldazabal J., Vila M. Application of texture analysis methods for the characterization of cultured meat. Sci. Rep. 2022;12: 1– 10. doi: 10.1038/s41598-022-07785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestana J.M., Puerta B., Santos H., Madeira M.S., Alfaia C.M., Lopes P.A., Pinto R.M.A., Lemos J.P.C., Fontes C.M.G.A., Lordelo M.M., Prates J.A.M. Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult. Sci. 2020;99:2519–2532. doi: 10.1016/j.psj.2019.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prates J.A.M., Quaresma M.A.G., Bessa R.J.B., Fontes C.M.G.A., Alfaia C.M.P.M. Simultaneous HPLC quantification of total cholesterol, tocopherols and β-carotene in Barrosã-PDO veal. Food Chem. 2006;94:469–477. [Google Scholar]
- Rajkumar U., Reddy M.R., Rao S.V.R., Radhika K., Shanmugam M. Evaluation of growth, carcass, immune response and stress parameters in naked neck chicken and their normal siblings under tropical winter and summer temperatures. Asian-Austral. J. Anim. Sci. 2011;24:509–516. [Google Scholar]
- Ribeiro D.M., Scanlon T., Kilminster T., Martins C.F., Greeff J., Milton J., Oldham C., Freire J.P.B., Mourato M.P., de Almeida A.M. Mineral profiling of muscle and hepatic tissues of Australian merino, Damara and dorper Lambs: Effect of weight loss. J Anim. Physiol Anim. Nutr. (Berl) 2020;104:823–830. doi: 10.1111/jpn.13339. [DOI] [PubMed] [Google Scholar]
- Rodrigues I., Choct M. The foregut and its manipulation via feeding practices in the chicken. Poult. Sci. 2018;97:3188–3206. doi: 10.3382/ps/pey191. [DOI] [PubMed] [Google Scholar]
- Scheuermann G., Bilgili S., Hess J., Mulvaney D. Breast muscle development in commercial broiler chickens. Poult. Sci. 2003;82:1648–1658. doi: 10.1093/ps/82.10.1648. [DOI] [PubMed] [Google Scholar]
- Siegel P.B. Evolution of the Modern Broiler and Feed Efficiency. Annu Rev. Anim. Biosci. 2014;2:375–385. doi: 10.1146/annurev-animal-022513-114132. [DOI] [PubMed] [Google Scholar]
- Siriwardhana N., Kalupahana N.S. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012;65:211–222. doi: 10.1016/B978-0-12-416003-3.00013-5. [DOI] [PubMed] [Google Scholar]
- Spínola M.P., Costa M.M., Prates J.A.M. Effect of cumulative spirulina intake on broiler meat quality, nutritional and health-related attributes. Foods. 2024;13:799. doi: 10.3390/foods13050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano M. Characteristics of fats in Japanese diets and current recommendations. Lipids. 1996;31:283. doi: 10.1007/BF02637092. [DOI] [PubMed] [Google Scholar]
- Teimouri M., Amirkolaie A.K., Yeganeh S. The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2013;396–399:14–19. [Google Scholar]
- Tickle P.G., Paxton H., Rankin J.W., Hutchinson J.R., Codd J.R. Anatomical and biomechanical traits of broiler chickens across ontogeny. Part I. Anatomy of the musculoskeletal respiratory apparatus and changes in organ size. PeerJ. 2014;2 doi: 10.7717/peerj.432. e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusoglu O., Unal M.K. Biomass nutrient profiles of three microalgae: spirulina platensis, chlorella vulgaris, and Isochrisis Galbana. J. Food. Sci. 2003;68:1144–1148. [Google Scholar]
- Wu Q.J., Zhou Y.M., Wu Y.N., Wang T. Intestinal development and function of broiler chickens on diets supplemented with clinoptilolite. Asian-Austral. J. Anim. Sci. 2013;26:987–994. doi: 10.5713/ajas.2012.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin S., Testik A., Ozkan S., Settar P., Celen F., Cahaner A. Performance of naked neck and normal broilers in hot, warm, and temperate climates. Poult. Sci. 1997;76:930–937. doi: 10.1093/ps/76.7.930. [DOI] [PubMed] [Google Scholar]