Abstract
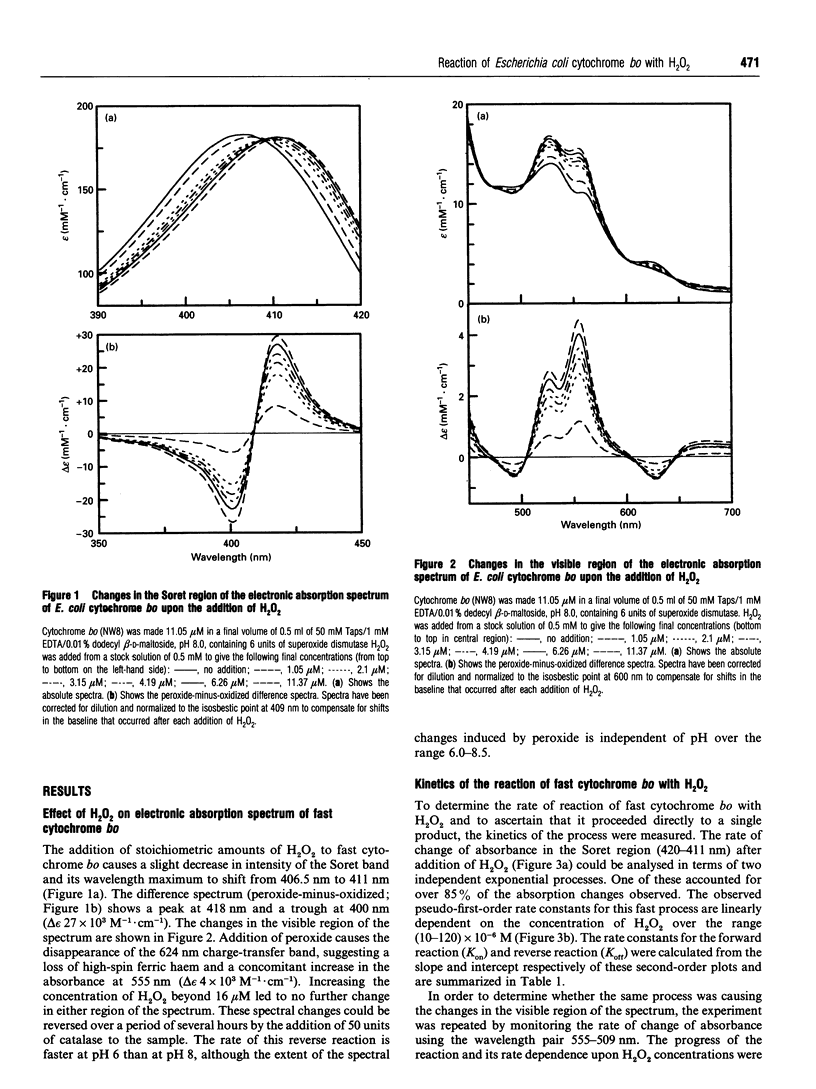
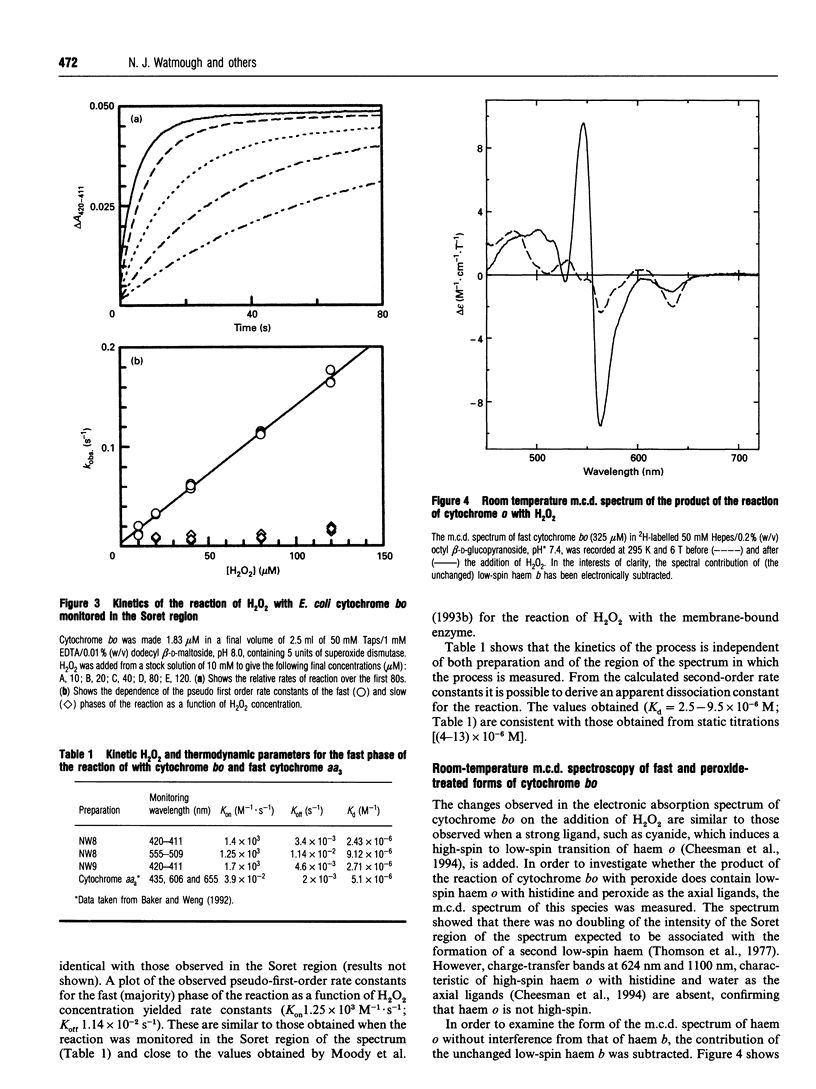
Oxidized cytochrome bo reacts rapidly with micromolar concentrations of H2O2 to form a single derivative. The electronic absorption spectrum of this compound differs from that of the oxidized form of the enzyme reported by this laboratory [Watmough, Cheesman, Gennis, Greenwood and Thomson (1993) FEBS Lett. 319, 151-154]. It is characterized by a Soret maximum at 411 nm, increased absorbance at 555 nm, and reduced intensity at 624 nm. The apparent dissociation constant for this process is of the order of 4 x 10(-6) M, and the bimolecular rate constant for the formation of the new compound is (1.25-1.7) x 10(3) M-1.s-1. Electronic absorption difference spectroscopy shows this product to be identical with the compound formed from the reaction of the mixed-valence form of the enzyme with dioxygen. Investigation of this compound by room-temperature magnetic c.d. spectroscopy shows haem o to be neither high-spin nor low-spin ferric, but to have a spectrum characteristic of an oxyferryl species. There is no evidence for oxidation of the porphyrin ring. Therefore the binuclear centre of this species must consist of an oxyferryl haem (S = 1) coupled to a Cu(II) ion (S = 1/2) to form a new paramagnetic centre. The reaction was also followed by X-band e.p.r. spectroscopy, and this showed the disappearance in parallel with the formation of the oxyferryl species, of the broad g = 3.7, signal which arises from the weakly coupled binuclear centre in the oxidized enzyme. Since no new e.p.r.-detectable paramagnetic species were observed, the Cu(II) ion is presumed to be coupled to another paramagnet, possibly an organic radical. There is no evidence in the electronic absorption spectrum to indicate further reaction of cytochrome bo with H2O2 to form a second species. We argue that the circumstances of formation of this oxyferryl species are the same as those for the P form of cytochrome c oxidase, a species often regarded as containing a bound peroxide ion. The implications of these observations for the reaction mechanism of haem-copper terminal oxidases are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au D. C., Gennis R. B. Cloning of the cyo locus encoding the cytochrome o terminal oxidase complex of Escherichia coli. J Bacteriol. 1987 Jul;169(7):3237–3242. doi: 10.1128/jb.169.7.3237-3242.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock G. T., Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992 Mar 26;356(6367):301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- Baker G. M., Noguchi M., Palmer G. The reaction of cytochrome oxidase with cyanide. Preparation of the rapidly reacting form and its conversion to the slowly reacting form. J Biol Chem. 1987 Jan 15;262(2):595–604. [PubMed] [Google Scholar]
- Baker G. M., Weng L. Apparent first-order behavior under second-order kinetic conditions: a general concept illustrated by the reversible binding of hydrogen peroxide to cytochrome c oxidase. J Theor Biol. 1992 Sep 21;158(2):221–229. doi: 10.1016/s0022-5193(05)80720-9. [DOI] [PubMed] [Google Scholar]
- Brown S., Moody A. J., Mitchell R., Rich P. R. Binuclear centre structure of terminal protonmotive oxidases. FEBS Lett. 1993 Feb 1;316(3):216–223. doi: 10.1016/0014-5793(93)81296-c. [DOI] [PubMed] [Google Scholar]
- Calhoun M. W., Gennis R. B., Salerno J. C. The formate complex of the cytochrome bo quinol oxidase of Escherichia coli exhibits a 'g = 12' EPR feature analogous to that of 'slow' cytochrome oxidase. FEBS Lett. 1992 Sep 7;309(2):127–129. doi: 10.1016/0014-5793(92)81079-2. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Compound C2, a product of the reaction of oxygen and the mixed-valence state of cytochrome oxidase. Optical evidence for a type-I copper. Biochem J. 1979 Mar 1;177(3):931–941. doi: 10.1042/bj1770931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman M. R., Watmough N. J., Gennis R. B., Greenwood C., Thomson A. J. Magnetic-circular-dichroism studies of Escherichia coli cytochrome bo. Identification of high-spin ferric, low-spin ferric and ferryl [Fe(IV)] forms of heme o. Eur J Biochem. 1994 Jan 15;219(1-2):595–602. doi: 10.1111/j.1432-1033.1994.tb19975.x. [DOI] [PubMed] [Google Scholar]
- Cheesman M. R., Watmough N. J., Pires C. A., Turner R., Brittain T., Gennis R. B., Greenwood C., Thomson A. J. Cytochrome bo from Escherichia coli: identification of haem ligands and reaction of the reduced enzyme with carbon monoxide. Biochem J. 1993 Feb 1;289(Pt 3):709–718. doi: 10.1042/bj2890709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Andréasson L. E., Karlsson B., Aasa R., Malmström B. G. Characterization of the intermediates in the reaction of mixed-valence state soluble cytochrome oxidase with oxygen at low temperatures by optical and electron-paramagnetic-resonance spectroscopy. Biochem J. 1980 Jan 1;185(1):155–167. doi: 10.1042/bj1850155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennis R. B. Site-directed mutagenesis studies on subunit I of the aa3-type cytochrome c oxidase of Rhodobacter sphaeroides: a brief review of progress to date. Biochim Biophys Acta. 1992 Jul 17;1101(2):184–187. [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Ching Y. C., Rousseau D. L. Ferryl and hydroxy intermediates in the reaction of oxygen with reduced cytochrome c oxidase. Nature. 1990 Nov 1;348(6296):89–90. doi: 10.1038/348089a0. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Ksenzenko MYu, Vygodina T. V., Berka V., Ruuge E. K., Konstantinov A. A. Cytochrome oxidase-catalyzed superoxide generation from hydrogen peroxide. FEBS Lett. 1992 Feb 3;297(1-2):63–66. doi: 10.1016/0014-5793(92)80328-e. [DOI] [PubMed] [Google Scholar]
- Larsen R. W., Li W., Copeland R. A., Witt S. N., Lou B. S., Chan S. I., Ondrias M. R. Room temperature characterization of the dioxygen intermediates of cytochrome c oxidase by resonance Raman spectroscopy. Biochemistry. 1990 Oct 30;29(43):10135–10140. doi: 10.1021/bi00495a018. [DOI] [PubMed] [Google Scholar]
- Lauraeus M., Morgan J. E., Wikström M. Peroxy and ferryl intermediates of the quinol-oxidizing cytochrome aa3 from Bacillus subtilis. Biochemistry. 1993 Mar 16;32(10):2664–2670. doi: 10.1021/bi00061a026. [DOI] [PubMed] [Google Scholar]
- Mitchell P. A new redox loop formality involving metal-catalysed hydroxide-ion translocation. A hypothetical Cu loop mechanism for cytochrome oxidase. FEBS Lett. 1987 Oct 5;222(2):235–245. doi: 10.1016/0014-5793(87)80378-2. [DOI] [PubMed] [Google Scholar]
- Moody A. J., Cooper C. E., Rich P. R. Characterisation of 'fast' and 'slow' forms of bovine heart cytochrome-c oxidase. Biochim Biophys Acta. 1991 Aug 23;1059(2):189–207. doi: 10.1016/s0005-2728(05)80204-x. [DOI] [PubMed] [Google Scholar]
- Moody A. J., Rumbley J. N., Gennis R. B., Ingledew W. J., Rich P. R. Ligand-binding properties and heterogeneity of cytochrome bo from Escherichia coli. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):321–329. doi: 10.1016/0005-2728(93)90060-s. [DOI] [PubMed] [Google Scholar]
- Moody A. J., Rumbley J. N., Ingledew W. J., Gennis R. B., Rich P. R. The reaction of hydrogen peroxide with cytochrome bo from E. coli. Biochem Soc Trans. 1993 Aug;21(3):255S–255S. doi: 10.1042/bst021255s. [DOI] [PubMed] [Google Scholar]
- Nicholls P. A new carbon monoxide-induced complex of cytochrome c oxidase. Biochem J. 1978 Dec 1;175(3):1147–1150. doi: 10.1042/bj1751147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P., Chanady G. A. Interactions of cytochrome aa3 with oxygen and carbon monoxide. The role of the 607 nm complex. Biochim Biophys Acta. 1981 Feb 12;634(2):256–265. doi: 10.1016/0005-2728(81)90144-4. [DOI] [PubMed] [Google Scholar]
- Nozawa T., Kobayashi N., Hatano M., Ueda M., Sogami M. Magnetic circular dichroism on oxygen complexes of hemoproteins: correlation between magnetic circular dichroism magnitude and electronic structures of oxygen complexes. Biochim Biophys Acta. 1980 Dec 16;626(2):282–290. doi: 10.1016/0005-2795(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Puustinen A., Morgan J. E., Verkhovsky M., Thomas J. W., Gennis R. B., Wikström M. The low-spin heme site of cytochrome o from Escherichia coli is promiscuous with respect to heme type. Biochemistry. 1992 Oct 27;31(42):10363–10369. doi: 10.1021/bi00157a026. [DOI] [PubMed] [Google Scholar]
- Stillman M. J., Hollebone B. R., Stillman J. S. Characterization of the chromophores in horseradish peroxidase compounds I and II using magnetic circular dichroism. Biochem Biophys Res Commun. 1976 Sep 20;72(2):554–559. doi: 10.1016/s0006-291x(76)80076-9. [DOI] [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. P. Variable-temperature magnetic-circular-dichroism spectra of cytochrome c oxidase and its derivatives. Biochem J. 1977 Aug 1;165(2):327–336. doi: 10.1042/bj1650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubaki M., Mogi T., Anraku Y., Hori H. Structure of the heme-copper binuclear center of the cytochrome bo complex of Escherichia coli: EPR and Fourier transform infrared spectroscopic studies. Biochemistry. 1993 Jun 15;32(23):6065–6072. doi: 10.1021/bi00074a018. [DOI] [PubMed] [Google Scholar]
- Varotsis C., Babcock G. T. Appearance of the v(FeIV = O) vibration from a ferryl-oxo intermediate in the cytochrome oxidase/dioxygen reaction. Biochemistry. 1990 Aug 14;29(32):7357–7362. doi: 10.1021/bi00484a001. [DOI] [PubMed] [Google Scholar]
- Varotsis C., Zhang Y., Appelman E. H., Babcock G. T. Resolution of the reaction sequence during the reduction of O2 by cytochrome oxidase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):237–241. doi: 10.1073/pnas.90.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygodina T., Konstantinov A. A. Evidence for two H2O2-binding sites in ferric cytochrome c oxidase. Indication to the O-cycle? FEBS Lett. 1987 Jul 27;219(2):387–392. doi: 10.1016/0014-5793(87)80258-2. [DOI] [PubMed] [Google Scholar]
- Vygodina T., Konstantinov A. Effect of pH on the spectrum of cytochrome c oxidase hydrogen peroxide complex. Biochim Biophys Acta. 1989 Mar 23;973(3):390–398. doi: 10.1016/s0005-2728(89)80380-9. [DOI] [PubMed] [Google Scholar]
- Watmough N. J., Cheesman M. R., Gennis R. B., Greenwood C., Thomson A. J. Distinct forms of the haem o-Cu binuclear site of oxidised cytochrome bo from Escherichia coli. Evidence from optical and EPR spectroscopy. FEBS Lett. 1993 Mar 15;319(1-2):151–154. doi: 10.1016/0014-5793(93)80056-z. [DOI] [PubMed] [Google Scholar]
- Weng L. C., Baker G. M. Reaction of hydrogen peroxide with the rapid form of resting cytochrome oxidase. Biochemistry. 1991 Jun 11;30(23):5727–5733. doi: 10.1021/bi00237a014. [DOI] [PubMed] [Google Scholar]
- Wikström M. Energy-dependent reversal of the cytochrome oxidase reaction. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4051–4054. doi: 10.1073/pnas.78.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M., Morgan J. E. The dioxygen cycle. Spectral, kinetic, and thermodynamic characteristics of ferryl and peroxy intermediates observed by reversal of the cytochrome oxidase reaction. J Biol Chem. 1992 May 25;267(15):10266–10273. [PubMed] [Google Scholar]
- Wrigglesworth J. M. Formation and reduction of a 'peroxy' intermediate of cytochrome c oxidase by hydrogen peroxide. Biochem J. 1984 Feb 1;217(3):715–719. doi: 10.1042/bj2170715. [DOI] [PMC free article] [PubMed] [Google Scholar]


