Abstract
Background
Post-COVID-19 syndrome (PCS) remains a major health issue worldwide, while its pathophysiology is still poorly understood. Systemic oxidative stress (OS) may be involved in PCS, which is reflected by lower circulating free thiols (R–SH, sulfhydryl groups), as they are receptive to rapid oxidation by reactive species. This study aimed to investigate the longitudinal dynamics of serum R–SH after SARS-CoV-2 infection and its association with the development of PCS in individuals with mild COVID-19.
Methods
Baseline serum R–SH concentrations were measured and compared between 135 non-hospitalized COVID-19 subjects and 82 healthy controls (HC). In COVID-19 subjects, serum R–SH concentrations were longitudinally measured during the acute disease phase (up to 3 weeks) and at 3, 6, and 12 months of follow-up, and their associations with relevant clinical parameters were investigated, including the development of PCS.
Results
Baseline albumin-adjusted serum R–SH were significantly reduced in non-hospitalized COVID-19 subjects as compared to HC (p = 0.041), reflecting systemic OS. In mild COVID-19 subjects, trajectories of albumin-adjusted serum R–SH levels over a course of 12 months were longitudinally associated with the future presence of PCS 18 months after initial infection (b = −9.48, p = 0.023).
Conclusion
Non-hospitalized individuals with COVID-19 show evidence of systemic oxidative stress, which is longitudinally associated with the development of PCS. Our results provide a rationale for future studies to further investigate the value of R–SH as a monitoring biomarker and a potential therapeutic target in the development of PCS.
Keywords: Coronavirus disease 2019, COVID-19, Long COVID-19, Post-COVID-19 syndrome, Redox, Oxidative stress
Graphical abstract
Highlights
-
•
Serum free sulfhydryl groups (R–SH) reflect the systemic redox status in health and disease.
-
•
Non-hospitalized individuals with mild coronavirus disease 2019 (COVID-19) show lower serum R–SH levels.
-
•
Serum R–SH levels are longitudinally associated with the development of post-COVID-19-syndrome.
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a significant impact on healthcare since its emergence. Although significant strides have been made in the fight against the virus, the impact of COVID-19 remains significant especially to those experiencing lingering COVID-19 symptoms after initial diagnosis, also known as Post-COVID-19 syndrome (PCS) or long COVID. The World Health Organization (WHO) defines PCS as symptoms that are newly presenting or continuing following initial infection by SARS-CoV-2, and which continue for at least 2 months with no alternative explanation [1]. Although the exact incidence of PCS remains uncertain, it has been estimated that 10–30 % of non-hospitalized COVID-19 cases may develop the condition, for which some studies reported even higher prevalences [2,3], although proportions generally rise to 50–70 % in hospitalized COVID-19 cases [4]. Indeed, although severe COVID-19 has been observed to be a risk factor for the development of PCS, it can also occur in asymptomatic individuals or those with only mild symptoms not requiring hospitalization [5]. To date, PCS remains a significant challenge considering its wide range in symptom patterns and outcomes, while its pathophysiology is still poorly understood [6]. Oxidative stress has been implicated to play a potential role in PCS pathophysiology and may interrelate with other underlying mechanisms including lingering inflammation and coagulopathy [7,8].
Gaining a comprehensive understanding of the pathophysiology and host factors predicting PCS development in specifically non-hospitalized individuals with mild COVID-19 is relevant, since this group comprises the majority of infected individuals in the general population [4]. To date, the complex pathophysiology of PCS is thought to consist of multiple, likely overlapping, mechanisms, among which SARS-CoV-2 persistence in tissue and ongoing inflammation have become major areas of interest [4]. Persistent inflammation with tissue infiltration of immune cells may lead to an overproduction of reactive (oxygen) species and a reduction in antioxidants - thereby altering the redox balance - whereas oxidative stress itself may induce lingering inflammation leading to ongoing tissue damage and neuroaffective toxicity [9]. Furthermore, coagulopathy with microthrombosis and endothelial damage may also be involved in PCS pathogenesis, which may induce oxidative stress through pathological hypoxia-induced redox signaling [4,9]. Another mechanism may be found in impaired cholesterol metabolism, as reflected by lower serum cholesterol and higher levels of the cholesterol oxidation products 7β-hydroxycholesterol (7β-OHC) and 7-ketocholesterol (7 KC) as has been previously described [10]. These oxysterol compounds can give rise to increased ROS production and inflammation and may also be associated with COVID-19-associated organ damage [11]. Altogether, based on theoretical grounds, systemic oxidative stress may be regarded as a potential contributing factor in the pathogenesis of PCS. However, direct evidence for the involvement of oxidative stress is lacking, and biomarkers to assess the redox status in individuals with PCS are highly needed.
Extracellular free thiols, which are organosulfur compounds that contain a free sulfhydryl (R–SH) group, are a reliable reflection of the systemic redox equilibrium as these compounds comprise the main biological targets of reactive species. Reactive species are molecules released during energy production that can lead to cellular and molecular damage – a phenomenon referred to as systemic oxidative stress [12,13]. As a result of their antioxidant capacity, R–SH levels are typically reduced in conditions associated with oxidative stress [[14], [15], [16]]. Next to reactive oxygen species (ROS), other types of reactive species may also react with R–SH, including reactive nitrogen species (RNS) and reactive sulfur species (RSS), collectively referred to as the ‘Reactive Species Interactome’ (RSI) [17,18]. Extracellular free thiols, including both protein-bound free thiols and low-molecular-weight (LMW) free thiols, not only possess potent antioxidant properties, but they also govern various protein functions and are able to transduce multiple biological adaptations on both short-term (e.g., changing protein structure and/or activity) and long-term (affecting gene regulation and/or expression) [17]. Thus, determining R–SH concentrations may be viewed as a robust, minimally invasive, and reproducible read-out of systemic oxidative stress.
In this study, we aimed to determine serum R–SH concentrations - as proxy of systemic oxidative stress - in non-hospitalized individuals with mild COVID-19, while evaluating a potential relationship with the persistence of symptoms at least 6 months after initial SARS-CoV-2 infection. To this end, we hypothesized that decreasing R–SH levels, representing a higher degree of systemic oxidative stress, would be associated with an increased likelihood of developing PCS in individuals with mild COVID-19.
2. Materials and Methods
2.1. Study population
This study used data and serum samples from the COVID-HOME study, an ongoing prospective longitudinal observational study conducted in the Netherlands, aimed at gaining insight into the impact and consequences of COVID-19 in individuals convalescing at home. The study design and procedures of the COVID-HOME study have been described in detail elsewhere [19]. A total of 168 individuals with available serum samples were selected for inclusion in the study, all of whom were considered infected with SARS-CoV-2 based on a positive qRT-PCR test result on a nasopharyngeal/throat swab during the study inclusion period between March 2020 and June 2021. Exclusion criteria consisted of a missing baseline serum sample (n = 17), age <16 years (n = 10) and hospitalization at any time during the study period (n = 6). The final study population consisted of 135 study participants with mild COVID-19 (Fig. 1), comprising non-hospitalized individuals who were either asymptomatic or had only mild symptoms. From these 135 participants, samples were available at baseline/day 0 (n = 135), day 7 (n = 128), day 14 (n = 127), day 21 (n = 126), 3 months (n = 118), 6 months (n = 125), and 12 months (n = 116). Approval of this study was provided by the Institutional Review Board (IRB) of the University Medical Center Groningen (UMCG) (IRB no. 2020/158). Written informed consent was obtained from all study participants for the use of their data and biomaterials. Additionally, blood samples from 82 SARS-CoV-2 uninfected, healthy controls were used for comparison, which were attempted to be made comparable in terms of age, sex, and BMI. These samples were retrieved from a UMCG biobank containing pre-donation samples of living kidney donors (IRB no. 2008/279). These samples were collected in the period from 2018 until 2023, including both pre-pandemic samples and samples retrieved during or after the COVID-19 pandemic. As for the latter samples, the absence of COVID-19 was ascertained through negative PCR testing. Due to the overall younger participants of the COVID-HOME study, the matching result was suboptimal. The study was conducted in accordance with the principles of the Declaration of Helsinki (2013).
Fig. 1.
Flowchart indicating the total number of study participants, number of exclusions and reasons for exclusion.
2.2. Data collection
As part of the COVID-HOME study, demographic and clinical characteristics were collected and included age, gender, body mass index (BMI), smoking history, and medical history. Comorbidities were categorized as cardiovascular disease, pulmonary disease, metabolic disease, inflammatory/autoimmune disease, malignancy, neurological disease, hematological disease, and gastrointestinal disease. Nasopharyngeal/throat swabs were obtained at baseline and then weekly for at least 3 weeks for SARS-CoV-2 testing by qRT-PCR. Cycle threshold (Ct) values < 40 were considered positive. Positive individuals after 3 weeks were invited to continue weekly sampling until they became negative. Blood samples for R–SH measurements were obtained weekly during the acute phase of disease (at baseline up to week 3), as well as at long-term follow-up at 3, 6, and 12 months after enrolment (Fig. 3A). In addition, at 3, 6, 12, and 18 months follow-up, questionnaires on long-term COVID-19-related complaints were applied to gather information on the presence of PCS. The questionnaires were based on questions derived from the ISARIC WHO Clinical Characterisation Protocol [20] used in their questionnaires to assess respiratory diseases and based on questions used in a protocol to assess post-viral fatigue syndrome, details of which can be found in the publication of the original study protocol [19]. The questionnaires used for the present study (as part of the COVID-HOME study) were further modified by adding variables to cover symptoms described by participants in the cohort during the pandemic. The questionnaires assessed general symptoms (e.g., fatigue), but also respiratory, cardiovascular, neurological, gastrointestinal, inflammatory and other symptoms as detailed elsewhere [19]. The presence of PCS was determined according to the 2021 WHO definition [21]. Standard laboratory measurements were performed at baseline, including hemoglobin (Hb), C-reactive protein (CRP), white blood cell counts (WBC), platelet counts, albumin, total bilirubin, liver aminotransferases (ALT, AST), alkaline phosphatase (AP), gamma-glutamyl transferase (GGT), and creatinine. Furthermore, the estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [22].
Fig. 3.
(A) Graphical representation of numbers and proportions of serum samples measured for R–SH (μM) per time point in subjects who classified as having PCS anytime during study follow-up vs. subjects without PCS. (B) Comparison in estimated marginal means (EMM) of serum R–SH (μM) in patients with and without developing PCS at 6 months (B1), 12 months (B2), and 18 months (B3) follow-up. The error bars represent 95 % confidence intervals. (C) Comparison in % changes from baseline in estimated marginal means (EMM) of serum R–SH (μM) derived from linear mixed-effect model analysis in patients with and without developing PCS at 6 months (C1), 12 months (C2), and 18 months (C3) follow-up. Abbreviations: PCS, post-COVID-19 syndrome.
2.3. Measurements of serum R–SH
Previously described methods were used to measure serum R–SH concentrations, albeit with minor modifications [23,24]. At time of sample collection, blood samples were processed under similar and standard conditions according to local laboratory regulations (centrifugation at 1100×g for 10 min) to obtain serum, and the isolated serum was subsequently stored at −80 °C to secure thiol stability until further analysis. Serum samples did not undergo any previous freeze/thaw cycles. Following thawing, the samples were diluted four-fold using a 0.1 M Tris buffer (pH 8.2). Then, the background absorption was measured at 412 nm using the CLARIOstar Plus microplate reading (BMG Labtech, Ortenberg, Germany), simultaneously with a reference measurement at 630 nm. Samples were incubated with 20 μL of 1.9 mM 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB, Ellman's reagent, CAS no. 69-78-3, Sigma-Aldrich Corporation, St. Louis, MO, USA) in 0.1 M phosphate buffer (pH 7.0) for 20 min at room temperature, after which absorption was measured for a second time. Parallel measurement of an l-cysteine (CAS no. 52-90-4, Fluka Biochemika, Buchs, Switzerland) calibration curve (15.6–1000 μM) in 0.1 M Tris/10 mM EDTA (pH 8.2) was used to determine final serum R–SH concentrations. All samples were measured in triplicate. Both intra- and inter-day coefficients of variation (CV) of the sample measurements were less than 10 %. Finally, serum concentrations of R–SH were additionally adjusted for albumin to indirectly account for total thiol content as albumin is the most abundant human plasma protein and may therefore affect the amount of potentially detectable R–SH [12,17]. This adjustment was done both by determining the R–SH/albumin ratio and by incorporating albumin concentrations as covariate in statistical analyses.
2.4. Statistical analysis
Continuous data were presented as means ± standard deviations (SD) in case of normally distributed data, and as medians with interquartile ranges (IQR) in case of skewed data. Categorical data were presented as proportions n with corresponding percentages (%). Normality was assessed visually using histograms and normal probability plots (Q-Q plots). Between-group comparisons for continuous variables were assessed using independent sample t-tests or Mann-Whitney U-tests, depending on data distribution, whereas for categorical variables, chi-square tests or Fisher's exact tests were used, as appropriate. Univariable and multivariable linear regression analyses were performed to identify factors associated with baseline serum R–SH levels. Multivariable analysis included all statistically significantly associated parameters from univariable analysis and was performed by using backward elimination (POUT > 0.2). Linear mixed-effects models were used to assess changes in serum R–SH levels over time. Relevant demographic and clinical variables were entered as fixed effects (with categorical variables as factor and continuous variables as covariate), whereas subject ID was entered as random effect. Serum R–SH levels were corrected for albumin by entering serum albumin as covariate in the linear mixed models. Time was entered as a factor to compare each time point with baseline. Bonferroni correction was applied for multiple testing when appropriate. Estimates of covariates are presented as b with standard errors (SE) and 95 % confidence intervals (CI). Categorical variables (entered as factor) are additionally presented as estimated marginal means (EMM) with corresponding 95 % CI. Data analysis was conducted using IBM SPSS Statistics Version 28.0 (IBM Corp. Armonk, NY, USA). Data visualization was performed using Adobe Illustrator Version 28.2 (Adobe Inc., San Jose, CA, USA) and Python programming language Version 3.9.7 (Python Software Foundation), using the pandas (V.1.3.3), numpy (V.1.21.2), matplotlib (V.3.4.3), and seaborn (V.0.11.2) packages.
3. Results
3.1. Baseline cohort characteristics
Baseline characteristics of non-hospitalized subjects with mild COVID-19 (n = 135) and healthy controls (HC) (n = 82) are presented in Table 1. COVID-19 cases were significantly younger (mean (SD) 42.2 ± 15.5) as compared to HC (49.4 ± 14.1, p < 0.001), while no significant differences were observed in the female/male-ratio (p = 0.221). COVID-19 subjects were more likely to have lower mean body mass index (BMI) values (25.0 ± 4.0) than HC (26.4 ± 2.9, p = 0.016). There was no difference in the prevalence of comorbidities between the two groups. However, multiple biochemical parameters demonstrated significant differences between mild COVID-19 subjects and HC, including increased concentrations of CRP (median [IQR] 1.8 [0.7–4.4] vs. 1.0 [0.6–1.8] mg/L, p = 0.002), AST (25.1 [20.8–28.9] vs. (22.0 [18.5–26.5] U/L, p = 0.002) and eGFR (101.3 ± 18.9 vs. 90.0 ± 14.9 mL/min x 1.73 m2, p < 0.001), and decreased concentrations of total bilirubin (7.2 [5.0–8.7] vs. 8.0 [6.0–12.0] μmol/L, p = 0.003) and creatinine (68.4 [59.0–81.1] vs 77.0 [69.0–87.0] μmol/L, p < 0.001) in subjects with mild COVID-19. Among COVID-19 subjects with available data and serum samples, almost half reported having PCS at 3-months (n = 49, 48.5 %) and 6-months (n = 52, 49.5 %) follow-up, with decreasing proportions at 12-months (n = 34, 33.7 %) and 18-months (n = 18, 26.9 %) follow-up. Fig. 3A demonstrates a graphical representation of serum R–SH measurements per time point in individuals developing PCS at any time during study participation (n = 69) and individuals without PCS (n = 46).
Table 1.
Baseline demographic and clinical characteristics of non-hospitalized COVID-19 subjects and healthy controls.
| COVID-19 (n = 135) | HC (n = 82) | p-value | |
|---|---|---|---|
| Age (years) | 42.2 ± 15.5 | 49.4 ± 14.1 | <0.001 |
| Female sex, n (%) | 79 (58.5) | 41 (50.0) | 0.221 |
| BMI (kg/m2) | 25.0 ± 4.0 | 26.4 ± 2.9 | 0.016 |
| Current smoking, n (%) | 7/131 (5.3) | 12/79 (15.2) | 0.016 |
| Level of education, n (%) | – | ||
| Up to and including high school education | 47,127 (37.0) | ||
| College level education or higher | 80/127 (63.0) | ||
| Employment status, n (%) | – | ||
| Student/pupil | 52/127 (40.9) | ||
| Employed part-time | 43/127 (33.9) | ||
| Employed full-time | 21/127 (16.5) | ||
| Other | 11/127 (8.7) | ||
| Biometrics | – | ||
| Respiratory rate (br/min) (n = 111) | 16.0 [14.0–18.0] | ||
| Pulse (BPM) (n = 110) | 78.8 ± 11.1 | ||
| Number of comorbidities | 0.360 | ||
| 0 | 83/132 (62.9) | 58/82 (70.7) | |
| 1 | 32/132 (24.2) | 18/82 (22.0) | |
| 2–5 | 17/132 12.9) | 6/82 (7.3) | |
| Type of comorbidities | |||
| Cardiovascular, n (%) | 14/131 (10.7) | 11/82 (13.4) | 0.547 |
| Pulmonary, n (%) | 13/130 (10.0) | 6/82 (7.3) | 0.505 |
| Metabolic, n (%) | 10/130 (7.7) | 6/82 (7.3) | 0.920 |
| Inflammatory/autoimmune, n (%) | 4/130 (3.1) | 3/82 (3.7) | 0.817 |
| Malignancy, n (%) | 4/120 (3.3) | 4/82 (4.9) | 0.718 |
| Neurological, n (%) | 4/121 (3.3) | 0/82 (0.0) | 0.149 |
| Hematological, n (%) | 3/129 (2.3) | 0/82 (0.0) | 0.284 |
| Gastrointestinal, n (%) | 5/121 (4.1) | 3/82 (3.7) | 0.865 |
| Laboratory measurements | |||
| Hemoglobin (g/dL) | 8.7 ± 0.8 | 8.9 ± 0.7 | 0.072 |
| CRP (mg/L) | 1.8 [0.7–4.4] | 1.0 [0.6–1.8] | 0.002 |
| WBC (x109/L) | 5.4 [4.4–6.8] | 5.9 [4.8–7.1] | 0.085 |
| Platelets (x109/L) | 271.0 ± 77.3 | 253.3 ± 60.3 | 0.080 |
| Albumin (g/L) | 45.0 ± 2.7 | 45.4 ± 2.5 | 0.359 |
| Total bilirubin (μmol/L) | 7.2 [5.0–8.7] | 8.0 [6.0–12.0] | 0.003 |
| ALT (U/L) | 22.5 [16.9–33.6] | 22.0 [18.5–31.0] | 0.974 |
| AST (U/L) | 25.1 [20.8–28.9] | 22.0 [18.5–26.5] | 0.002 |
| AP (U/L) | 69.5 [59.0–86.0] | 67.0 [57.5–78.5] | 0.161 |
| GGT (U/L) | 23.5 [17.0–42.8] | 21.0 [15.0–35.0] | 0.309 |
| eGFR (mL/min/1.73 m2) | 101.3 ± 18.9 | 90.0 ± 14.9 | <0.001 |
| Creatinine (μmol/L) | 68.4 [59.0–81.1] | 77.0 [69.0–87.0] | <0.001 |
| PCS | – | ||
| at 3 months, n (%) | 49/101 (48.5) | ||
| at 6 months, n (%) | 52/105 (49.5) | ||
| at 12 months, n (%) | 34/101 (33.7) | ||
| at 18 months, n (%) | 18/67 (26.9) | ||
Data are presented as means ± SD, medians [interquartile ranges], or proportions n with corresponding percentages (%). P-values <0.05 were considered statistically significant (indicated in bold). Abbreviations: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; br/min, breaths per minute; BPM, beats per minute; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyl transferase; HC, healthy controls; PCS, post-COVID-19 syndrome; WBC, white blood cell count.
3.2. Baseline serum R–SH concentrations in COVID-19 and HC, and their associations with clinical disease parameters
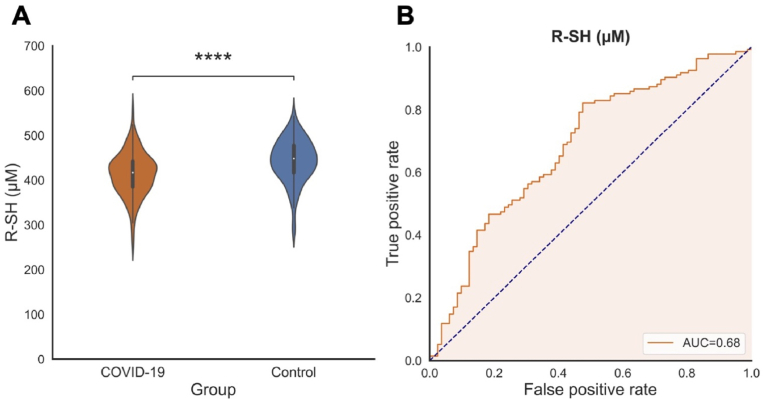
Significantly reduced baseline serum R–SH concentrations were observed in subjects with mild COVID-19 (413.3 ± 48.3 μM) as compared to healthy controls (443.0 ± 48.0 μM, p < 0.001) (Fig. 2A). Similar results were observed when correction for albumin was applied (by calculating the R–SH/albumin ratio), with lower serum R–SH concentrations in mild COVID-19 subjects (9.6 [9.0–10.1] μM/g) as compared to HC (9.8 [9.1–10.5] μM/g, p = 0.041). Furthermore, baseline serum R–SH significantly discriminated between subjects with mild COVID-19 and healthy controls (area under the curve (AUC) = 0.68, 95 % confidence interval (CI) = 0.61–0.76, p < 0.001) (Fig. 2B), corroborating our previous observations in a smaller subset of this cohort [25]. The discriminative capacity of albumin-adjusted R–SH was lower than that of serum R–SH concentrations alone (AUC = 0.58, 95 % CI 0.50–0.66, p = 0.041).
Fig. 2.
(A) Baseline serum R–SH (μM) are significantly reduced in mild COVID-19 subjects as compared to healthy controls (****p < 0.0001). (B) Baseline serum R–SH (μM) significantly discriminated between subjects with mild COVID-19 and healthy controls (area under the curve = 0.68). Abbreviations: PCS, post-COVID-19 syndrome; R–SH, free sulfhydryl groups.
Univariable and multivariable linear regression analyses were performed to identify associates of serum R–SH concentrations at baseline (Table 2). Univariable linear regression demonstrated that age (standardized beta (St.β) = −0.55, p < 0.001), BMI (St.β = −0.24, p = 0.008), and respiratory rate (St.β = −0.21, p = 0.025) were inversely associated with baseline serum R–SH, whereas a college level education or higher demonstrated a positive association (St.β = 0.20, p = 0.023). Interestingly, additional logistic regression was performed and demonstrated that a college level education or higher was significantly inversely associated with the development of PCS at 12 months (OR = 0.33, 95 % CI 0.14–0.80), p = 0.013). Moreover, several biochemical parameters were significantly associated with baseline serum R–SH, including inverse associations with CRP (St.β = −0.36, p < 0.001), ALT (St.β = −0.34, p < 0.001), AST (St.β = −0.31, p < 0.001), AP (St.β = −0.28, p = 0.002), GGT (St.β = −0.49, p < 0.001), and creatinine (St.β = −0.19, p = 0.033), as well as positive associations with albumin (St.β = 0.59, p < 0.001), and eGFR (St.β = 0.53, p < 0.001). No significant associations were observed between baseline serum R–SH concentrations and the presence of PCS at 3 months (p = 0.920), 6 months (p = 0.996), 12 months (p = 0.278) and 18 months (p = 0.511) follow-up. In multivariable linear regression analyses, BMI (St.β = −0.29, p = 0.049) and AST concentrations (St.β = −0.75, p < 0.001) were independently associated with baseline serum R–SH. For most parameters, similar results were observed when baseline serum R–SH were adjusted for albumin (by calculating the R–SH/albumin ratio) (Supplementary Table S1). However, in univariable linear regression, the associations with BMI (p = 0.224), level of education (p = 0.140), respiratory rate (p = 0.359), and creatinine (p = 0.079) were no longer statistically significant, whereas in multivariable linear regression, only AST concentrations (St.β = −0.73, p < 0.001) showed an independent association with albumin-adjusted serum R–SH.
Table 2.
Univariable and multivariable linear regression analyses of serum R–SH at baseline in mild COVID-19 with clinical and biochemical parameters.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| St. βa | p-value | St. βa | p-value | |
| Age | −0.55 | <0.001 | ||
| Female sex | −0.10 | 0.273 | ||
| BMI | −0.24 | 0.008 | −0.29 | 0.049 |
| Current smoking | −0.06 | 0.476 | ||
| Level of education – college level or higher | 0.20 | 0.023 | ||
| Biometrics | ||||
| Respiratory rateb | −0.21 | 0.025 | ||
| Pulse | −0.07 | 0.489 | ||
| Comorbidities | ||||
| Cardiovascular | −0.15 | 0.085 | ||
| Pulmonary | −0.07 | 0.443 | ||
| Metabolic | −0.08 | 0.353 | ||
| Inflammatory/autoimmune | −0.10 | 0.274 | ||
| Malignancy | −0.07 | 0.481 | ||
| Neurological | −0.06 | 0.506 | ||
| Hematological | −0.01 | 0.873 | ||
| Gastrointestinal | 0.16 | 0.073 | ||
| Laboratory measurements | ||||
| Hemoglobin (g/dL) | 0.05 | 0.580 | ||
| CRP (mg/L)b | −0.36 | <0.001 | ||
| WBC (x109/L)b | −0.12 | 0.199 | ||
| Platelets (x109/L) | −0.01 | 0.933 | ||
| Albumin (g/L) | 0.59 | <0.001 | ||
| Total bilirubin (μmol/L)b | 0.13 | 0.159 | ||
| ALT (U/L)b | −0.34 | <0.001 | ||
| AST (U/L)b | −0.31 | <0.001 | −0.75 | <0.001 |
| AP (U/L)b | −0.28 | 0.002 | ||
| GGT (U/L)b | −0.49 | <0.001 | ||
| eGFR (mL/min x 1.73 m2) | 0.53 | <0.001 | ||
| Creatinine (μmol/L)b | −0.19 | 0.033 | ||
| PCS | ||||
| at 3 months | 0.01 | 0.920 | ||
| at 6 months | 0.00 | 0.996 | ||
| at 12 months | −0.11 | 0.278 | ||
| at 18 months | −0.08 | 0.511 | ||
P-values <0.05 were considered statistically significant (indicated in bold).
Abbreviations: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyl transferase; HC, healthy controls; PCS, post-COVID-19 syndrome; WBC, white blood cell count.
Standardized beta (St. β) coefficient represents the difference in serum R–SH concentrations per 1-SD increment/decrement for continuous variables and the difference in serum R–SH concentrations compared to the reference group for categorical variables.
Skewed variables were log-transformed before entering the linear regression analyses.
3.3. Longitudinal serum R–SH concentrations in COVID-19 and their associations with clinical disease parameters
Fig. 3 shows the changes in serum R–SH concentrations over a 12-month time period (measured weekly during acute disease and then at 3-, 6-, and 12-months follow-up, see Fig. 3A), stratified by reporting the presence of PCS at 6 months (Fig. 3B1-C1), 12 months (Fig. 3B2-C2), and 18 months (Fig. 3B3-C3). In the entire mild COVID-19 cohort, serum R–SH significantly changed over time (p = 0.011) (Table 3, Supplementary Table S2). Interestingly, when comparing estimated marginal means (EMM) over a period of 12 months post-infection, significantly lower longitudinal serum R–SH were observed in individuals experiencing PCS at 12 months (EMM [95 % CI] 403.2 [396.9–409.5]) as compared to individuals without PCS at 12 months (416.4 [411.9–420.8], p < 0.001). Similar results were observed when stratifying by the future presence of PCS at 18 months (p < 0.001), with lower longitudinal serum R–SH in individuals with PCS (395.7 [387.3–404.1]) as compared to those without (413.5 [408.4–418.5]). However, when adjustment for albumin was applied by adding this variable as covariate to the model, only the future presence of PCS at 18 months remained significantly associated with longitudinal serum R–SH concentrations (p = 0.023) (Supplementary Table S3). No significant differences in longitudinal serum R–SH were observed when comparing individuals with and without PCS at 3 months (p = 0.643) and 6 months (p = 0.981) based on the data (day 0, 7, 14, and 21) preceding these timepoints.
Table 3.
Estimated marginal means of serum R–SH derived from linear mixed model analyses.
| EMM (95 % CI) | F-statistic | p-value | |
|---|---|---|---|
| Time point | 2.770 | 0.011 | |
| Day 0 | 413.3 (404.9–421.6) | ||
| Day 7 | 402.9 (394.3–411.5) | 0.089a | |
| Day 14 | 408.5 (399.9–417.1) | 0.437a | |
| Day 21 | 412.2 (403.5–420.8) | 0.855a | |
| 3 months | 419.4 (410.5–428.3) | 0.326a | |
| 6 months | 420.7 (412.0–429.4) | 0.226a | |
| 12 months | 423.9 (414.9–433.0) | 0.089a | |
| PCS | |||
| 3 months | 0.215 | 0.643 | |
| No | 411.2 (406.2–416.1) | ||
| Yes | 412.8 (407.8–417.9) | ||
| 6 months | 0.001 | 0.981 | |
| No | 410.9 (405.9–415.9) | ||
| Yes | 410.8 (405.8–415.9) | ||
| 12 months | 11.233 | <0.001 | |
| No | 416.4 (411.9–420.8) | ||
| Yes | 403.2 (396.9–409.5) | ||
| 18 months | 12.743 | <0.001 | |
| No | 413.5 (408.4–418.5) | ||
| Yes | 395.7 (387.3–404.1) | ||
Time point and presence of PCS at 3-, 6-, 12-, and 18-months follow-up were entered as factors to the model. Estimated marginal means (EMM) with corresponding 95 % confidence intervals (CI) are presented. P-values <0.05 were considered statistically significant (indicated in bold).
Abbreviations: PCS, post-COVID-19 syndrome; R–SH, free sulfhydryl groups.
P-values comparison with baseline.
Age (estimate of fixed effect (b) = −1.74, p < 0.001) and BMI (b = −3.36, p < 0.001) were both inversely associated with longitudinal serum R–SH concentrations, whereas no significant association was observed with gender (p = 0.456) (Supplementary Table S2). Furthermore, individuals with college level education or higher demonstrated higher serum R–SH over time (EEM [95 % CI] 423.0 [418.8–427.1]) than individuals with an education level up to and including high school education (397.3 [391.8–402.7], p < 0.001). Interestingly, viral RNA shedding in the nasopharynx/throat was negatively associated with longitudinal serum R–SH concentrations, as indicated by an inverse association with the duration of viral RNA shedding in days (b = −0.46, p = 0.024), as well as with the binary variable long shedding (≥21 days) (b = −7.58, p = 0.028) (Fig. 4). Additional associates of longitudinal serum R–SH included biometrical parameters, including respiratory rate (b = −0.37, p = 0.028) and pulse (b = −0.038, p = 0.006). Moreover, the total number of comorbidities was associated with R–SH over time (p < 0.001), with significant inverse associations with cardiovascular (b = −31.58, p < 0.001), metabolic (b = −16.75, p = 0.008), inflammatory/autoimmune (b = −23.07, p = 0.022), and neurological disease (b = −23.33, p = 0.013). Finally, most of the investigated biochemical parameters were significantly associated with longitudinal serum R–SH concentrations, including inverse associations with CRP (b = −0.78, p < 0.001), WBC (b = −2.94, p = 0.002), ALT (b = −0.43, p < 0.001), AST (b = −0.91, p < 0.001), AP (b = −0.56, p < 0.001), GGT (b = −0.46, p < 0.001), and creatinine (b = −0.61, p < 0.001), whereas positive associations were observed with albumin (b = 9.93, p < 0.001), total bilirubin (b = 1.77, p < 0.001), and eGFR (b = 1.33, p < 0.001). In general, similar results were observed when adjustment for albumin was applied (Supplementary Table S3), with additional significant associations with female gender (b = 9.45, p = 0.002) and hemoglobin (b = −9.70, p < 0.001) as well as hematological (b = −22.89, p = 0.019) and gastrointestinal disease (b = −20.01, p = 0.032). In contrast, the associations with respiratory rate (p = 0.194), metabolic disease (p = 0.657), and total bilirubin (p = 0.770) were no longer statistically significant.
Fig. 4.
Comparison in estimated marginal means (EMM) of serum R–SH (μM) in patients with and without long viral RNA shedding (≥21 days) in the nasopharynx/throat. Abbreviations: NPT, nasopharynx/throat.
4. Discussion
This study investigated the temporal dynamics of serum R–SH concentrations – as proxy of systemic oxidative stress – during acute disease and long-term follow-up. Associations with relevant clinical disease parameters were investigated, including the presence of lingering COVID-19-related symptoms months after initial SARS-CoV-2 infection (also known as PCS). We demonstrated that non-hospitalized individuals with mild COVID-19 show significantly reduced baseline serum R-SH as compared to healthy controls, thereby supporting previous results from our pilot study [25]. We also show that the course of serum R-SH concentrations over a period of 12 months post-infection, measured weekly during acute disease as well as at 3, 6, and 12 months of follow-up, was associated with experiencing PCS at 12 months and 18 months follow-up. To the best of our knowledge, this is the first study to show the temporal dynamics of R–SH in mild COVID-19, providing direct and novel evidence of an association of systemic oxidative stress with the development of PCS.
Systemic oxidative stress has been hypothesized to be one of the involved mechanisms underlying PCS for several reasons. First of all, PCS has been associated with a protracted mild inflammatory state which may drive nitro-oxidative processes, resulting in an excess of reactive species and a decrease in antioxidant defenses [26]. This may cause neuroaffective toxicity, leading to the onset of commonly reported PCS symptoms such as chronic fatigue, concentration and memory problems, insomnia, anxiety, and depression [[27], [28], [29]]. Furthermore, COVID-19-related coagulopathy - including microvascular blood clotting and endothelial dysfunction with resulting alveolar fluid buildup - may lead to lingering systemic hypoxia, which in turn may also affect the systemic redox balance and induce systemic oxidative stress [9]. For example, hypoxia leads to the oxidation of glutathione (GSH), depleting the body glutathione stores, which results in a lower GSH//glutathione disulfide (GSSG) ratio. In this respect, both direct and indirect influences of COVID-19 on GSH metabolism could exist. For example, directly through oxidative stress, GSSG itself may lead to increased ROS production via uncoupling of the endothelial nitric oxide synthase (eNOS) enzyme through S-glutathionylation [30]. Indirect impacts may consist of concurrent inflammation, modulation of the immune response, shifts of in cholesterol metabolism (e.g., through oxysterols) and the various treatments provided to SARS-CoV-2-infected individuals. Finally, a common theory suggests that PCS is caused by persisting viral reservoirs, as the presence of SARS-CoV-2 has been documented in various tissues long after initial infection [4]. SARS-CoV-2 may by itself lead to a disturbance of the redox equilibrium through increased production of reactive species via NADPH oxidase or mitochondria in the infected host cell, as is the case for other viruses too [31]. Our results indeed indicate a negative relationship between the duration of viral RNA shedding and longitudinal serum R–SH concentrations, suggesting that viral presence during the acute phase (up to day 42) may affect the redox equilibrium over time, which may be relevant to PCS pathophysiology. It should be stressed, however, that mechanistic processes other than oxidative stress (such as autoimmune responses, SARS-CoV-2-induced disruption of microbiota, and dysfunctional neurological signaling, among others) are likely to also contribute to PCS pathophysiology.
Next to PCS, we also investigated the relationship between systemic oxidative stress and other clinical disease parameters. Following up on our pilot study (including 29 mild COVID-19 subjects and 30 HC), the current study with a larger sample size (including 135 mild COVID-19 subjects and 82 HC) allowed us to validate previous results and provided sufficient power to demonstrate additional cross-sectional associations with other clinical and biochemical parameters. Specifically, next to our previous observations of CRP and albumin as significant associates of R–SH in acute COVID-19, we now demonstrate that other laboratory measurements (including ALT, AST, AP, GGT, eGFR, and creatinine), as well as respiratory rate, age, BMI, and level of education also affect R–SH concentrations in mild acute COVID-19. Age and BMI have both been shown to inversely correlate with R–SH concentrations in other conditions than COVID-19 [32,33]. Of note, despite our attempt to make the groups comparable for age, gender, and BMI, significant differences were observed in our cohort with HC being older with a higher BMI than individuals with mild COVID-19. This is relevant, as previous studies have shown that both BMI and age are negatively associated with serum free thiol levels, with age repeatedly showing an independent relationship (St.β between −0.13 and −0.50) [32,34,35]. Nevertheless, albeit these differences in age and BMI may have influenced our results to some extent, the differences in serum R–SH concentrations between mild COVID-19 subjects and HC were large enough to be statistically significant.
In the present study, we show a positive correlation between serum R–SH and albumin concentrations, as has also been observed in literature before [25,36]. This association can be attributed to the fact that the largest source of free thiols is embedded with the redox-active Cys [34] thiol residue of albumin, which is the most abundant circulating plasma protein [12,37]. Furthermore, albumin also confers transporting capacity for other low molecular weight (LMW) free thiols [12,38]. Indeed, since albumin is the most dominant source of free thiols it may quantitatively affect the amount of free thiols that can be detected. Therefore, instead of analyzing the ratio of free to oxidized thiols, the total thiol content can be indirectly accounted for by solely determining free thiol groups while adjusting for albumin [39]. Nevertheless, experiences from our lab have indicated that this albumin adjustment does not always have a notable impact on the results, and thus does not necessarily result in other outcomes. In the current study, most results were indeed similar after correction for albumin was applied. However, in contrast to the longitudinal associations we observed between serum R–SH and experiencing PCS at both 12 months and 18 months follow-up, albumin-adjusted R–SH concentrations were only significantly correlated to PCS after 18 months of initial infection. This suggests that the association between systemic oxidative stress and the development of PCS may - at least partially - be explained by the level of circulating albumin.
Interestingly, our results demonstrated a significant association between the level of education and the presence of PCS, with a college level education or higher being inversely associated with PCS at 12 months follow-up. This is socially relevant, as it suggests that population groups with lower education levels may be more likely to develop PCS. The relationship between a lower level of education and PCS has also been observed by others [40,41], and is widely known to exist in other disease conditions [[42], [43], [44]]. Indeed, this association is likely to result from a complex interplay of multiple involved factors, with less favorable living standards, lower socioeconomic status, and differences in comorbidities, age, gender, and diet as potential explanations, among others. Although exploring these underlying mechanisms was beyond the scope of the current study, our results suggests that an impaired redox balance may be another factor involved in this association, as the level of education was both cross-sectionally and longitudinally associated with serum R–SH concentrations, with significantly reduced serum R–SH in individuals with a lower level of education.
Following the observed association between systemic oxidative stress and the development of PCS in COVID-19-infected individuals, it is tempting to speculate whether the development of this syndrome could be therapeutically modulated, e.g. through dietary or medical (antioxidant) interventions. The role of diet and specific nutrients in SARS-CoV-2-infected individuals has been extensively described before and generally relates to their impact on regulation of immune responses, the composition and functionality of the gut microbiota, potential antiviral activities, lipid metabolism, and the activation of the endogenous antioxidant machinery [45]. In terms of treatment, it could also be interesting to evaluate the effects of specific antioxidant compounds that may have the potential to attenuate oxidative stress in these individuals. A classical example in this regard constitutes N-acetylcysteine (NAC), a pharmacological antioxidant substance that promotes endogenous GSH biosynthesis as well as the generation of sulfane sulfur species when it is desulfurated to hydrogen sulfide (H2S) [46]. Several potential mechanisms have been described through which H2S could impact SARS-CoV-2-infected individuals, including interference with functional host receptors (i.e., angiotensin-converting enzyme 2 [ACE2]) [47], inhibition of viral replication, and attenuating systemic inflammation. Similar to NAC, also taurine has been extensively discussed as a potential treatment in COVID-19, because of its anti-inflammatory, antioxidant, and H2S-promoting effects [48]. Furthermore, the potential application of oxysterol compounds, such as the cholesterol metabolite 27-hydroxycholesterol (27-OHC), would be worth further investigating [10]. For example, it has been shown that oxysterol-induced oxidative stress (e.g. through 7-KC) can be attenuated by NAC and GSH [49]. In this regard, the quantification of oxysterol compounds (including 27-OHC, 7β-OHC, and 7-KC) in a cohort of individuals developing PCS would be an essential first step in order to determine the rationale for testing such therapies in subsequent interventional studies.
This post-hoc analysis of the COVID-HOME study is - to the best of our knowledge - the first to show the temporal dynamics of R–SH during acute disease and long-term follow-up in mild COVID-19 subjects. The prospective study design, following individuals from the day of diagnosis of infection, facilitated the analysis of longitudinal associations between R–SH and clinical parameters, including the development of PCS. In addition, this study included a unique cohort of particularly milder COVID-19 cases, which are the majority of SARS-CoV-2 infected individuals in the general population. The sample size, including 135 mild COVID-19 subjects with data and samples at multiple time points and 82 HC, allowed us to reliably validate previous results from our pilot study and additionally provided enough data with sufficient power to demonstrate novel associations between R–SH and clinical parameters in mild COVID-19. Some limitations of the study must also be addressed. First of all, as this was a longitudinal study, our results may have been affected by missing data during follow-up time points, but also by various unseen factors such as vaccination and potential reinfection by SARS-CoV-2, and beyond such COVID-19-specific factors also more generic factors such as dietary habits, environmental exposures, and medication use. Furthermore, we report relatively high proportions of individuals that developed PCS, with percentages reaching almost 50 % at 3 months and 6 months follow-up, which is higher than the average 10–30 % reported in literature [1,50]. However, we have examined 62 symptomatic variables and adhered to the WHO definition of PCS, and therefore believe that the reported prevalence is correct. To illustrate this, there are also studies that have found higher prevalences in non-hospitalized individuals, i.e. prevalences of 55 % [2] or even 69 % [3]. Although we have adhered to the WHO definition of PCS [21], and believe that the reported prevalence is correct, this definition is broad and there may be the likelihood that not all the reported ‘COVID-19’ complaints at the time of the interview were truly the result of PCS as a condition. In addition, the HC samples used for this study comprised samples collected both before, during, and shortly after the COVID-19 pandemic (during the period from 2018 to 2023), whereas a more similar timeline of recruitment and retention as that for the sample collection of the COVID-19 subjects would have been preferred. Similarly, detailed metadata of these HC individuals, e.g. on educational level, employment status, and biometrics, were lacking, precluding detailed comparisons between COVID-19 subjects and controls. Another important limitation of this study is that we only quantified serum R–SH as a single biomarker of systemic oxidative stress. Although serum R–SH have extensively been shown to be a reliable reflection of the whole-body redox status, a combination of redox parameters reflecting multiple redox-regulated metabolic pathways would probably be a more reliable strategy to better understand changes in the redox signaling network [51]. Recent advancements in this field have led to the development of so-called ‘redox metabolomics’ approaches, but their realization comes relatively slowly since well-defined, consensus-based criteria for redox biomarkers to accurately assess the redox status are currently lacking. Moreover, several logistical and technological obstacles may occur hindering their implementation [51,52]. Indeed, the quantification of reactive species is rather difficult because of their short half-lives, low concentrations in biospecimens, and their involvement in a diversity of chemical reactions, requiring sophisticated and well-experienced laboratories to establish these analyses [51]. Nevertheless, the implementation of a structured redox metabolomics approach, focusing on the key components of the Reactive Species Interactome (RSI), including (1) nutritional constituents (e.g., hydrogen sulfide, amino acids, vitamins), (2) transducing constituents, including cysteine-based redox switches such as systemic R–SH, and (3) stable RSI end products, comprising metabolites of oxygen, nitrogen or sulfur, could facilitate gaining a comprehensive understanding of changes in the human redox status while also identifying pivotal players in redox interactions [17]. Beyond the integration of multiple redox-related parameters, the potential utility of other biomarkers, e.g., inflammatory compounds, virus-specific molecules, oxysterols, and others relevant to COVID-19 pathophysiology, should not be overlooked, since this could be useful to more accurately predict the development of PCS after initial infection. Given the multifaceted and heterogeneous nature of the disease, marked by a variety of involved mechanisms and several disease phases, this seems critically important for prediction as well as for determine the most appropriate treatment for each individual patient – following the principles of ‘personalized medicine’.
To conclude, this is the first study that investigates the temporal dynamics of R–SH as a reliable marker of systemic oxidative stress in mild COVID-19, which is associated with the presence of PCS at least one year after initial infection. Our results provide important insights into the complex pathophysiology of PCS, important in the development and implementation of treatment modalities.
Funding
The study received funding from the Netherlands Organisation for Health Research and Development (ZonMw), grant 10430012010023, and from the Horizon 2020 ORCHESTRA project, grant 101016167. The views expressed in this publication are the sole responsibility of the authors and the Commission is not responsible for any use that may be made of the information it contains. ARB is supported by a Rubicon fellowship from NWO (452022317). The funders had no role in study design, data collection and analysis, preparation of the manuscript or decision to publish.
Data availability
All datasets used for the current study are available upon reasonable request to the corresponding author.
CRediT authorship contribution statement
Larissa E. Vlaming-van Eijk: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Marian L.C. Bulthuis: Writing – review & editing, Validation, Methodology, Investigation, Data curation. Bernardina T.F. van der Gun: Writing – review & editing, Validation, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Karin I. Wold: Writing – review & editing, Validation, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Alida C.M. Veloo: Writing – review & editing, Validation, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. María F. Vincenti González: Writing – review & editing, Validation, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Martin H. de Borst: Writing – review & editing, Validation, Resources, Methodology, Investigation. Wilfred F.A. den Dunnen: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Conceptualization. Jan-Luuk Hillebrands: Writing – review & editing, Validation, Supervision, Methodology, Investigation. Harry van Goor: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Adriana Tami: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Arno R. Bourgonje: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
A.R.B. has received a research grant from Janssen Pharmaceuticals and received speaker's fees from AbbVie, outside the submitted work. All other authors have no conflicts of interest to declare.
Acknowledgments
The authors would like to thank all participants of the COVID-HOME study for their contribution to the understanding of COVID-19 short- and long-term consequences.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103310.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.WHO. Post COVID-19 Condition (Long COVID) Available from: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition#:∼:text=It%20is%20defined%20as%20the,months%20with%20no%20other%20explanation. Accessed 24 April 2024.
- 2.Blomberg B., Mohn K.G., Brokstad K.A., et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021 doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchberger I., Meisinger C., Warm T.D., Hyhlik-Dürr A., Linseisen J., Goßlau Y. Post-COVID-19 syndrome in non-hospitalized individuals: healthcare situation 2 Years after SARS-CoV-2 infection. Viruses. 2023;15(6) doi: 10.3390/v15061326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsampasian V., Elghazaly H., Chattopadhyay R., et al. Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis. JAMA Intern. Med. 2023;183(6):566–580. doi: 10.1001/jamainternmed.2023.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deer R.R., Rock M.A., Vasilevsky N., et al. Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majumder N., Deepak V., Hadique S., et al. Redox imbalance in COVID-19 pathophysiology. Redox Biol. 2022;56 doi: 10.1016/j.redox.2022.102465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul B.D., Lemle M.D., Komaroff A.L., Snyder S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. U. S. A. 2021;118(34) doi: 10.1073/pnas.2024358118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGarry T., Biniecka M., Veale D.J., Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018;125:15–24. doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Marcello A., Civra A., Milan Bonotto R., et al. The cholesterol metabolite 27-hydroxycholesterol inhibits SARS-CoV-2 and is markedly decreased in COVID-19 patients. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghzaiel I., Sassi K., Zarrouk A., et al. 7-Ketocholesterol: effects on viral infections and hypothetical contribution in COVID-19. J. Steroid Biochem. Mol. Biol. 2021;212 doi: 10.1016/j.jsbmb.2021.105939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turell L., Radi R., Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013;65:244–253. doi: 10.1016/j.freeradbiomed.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banne A.F., Amiri A., Pero R.W. Reduced level of serum thiols in patients with a diagnosis of active disease. J. Anti Aging Med. 2003;6(4):327–334. doi: 10.1089/109454503323028920. [DOI] [PubMed] [Google Scholar]
- 14.Bourgonje A.R., Abdulle A.E., Bourgonje M.F., et al. Serum free sulfhydryl status associates with new-onset chronic kidney disease in the general population. Redox Biol. 2021;48 doi: 10.1016/j.redox.2021.102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boekhoud L., Koeze J., van der Slikke E.C., et al. Acute kidney injury is associated with lowered plasma-free thiol levels. Antioxidants. 2020;9(11):1135. doi: 10.3390/antiox9111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spraakman N.A., Coester A.M., Bourgonje A.R., et al. Systemic and renal dynamics of free sulfhydryl groups during living donor kidney transplantation. Int. J. Mol. Sci. 2022;23(17):9789. doi: 10.3390/ijms23179789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., et al. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxidants Redox Signal. 2017;27(10):684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malard E., Valable S., Bernaudin M., Pérès E., Chatre L. The reactive species interactome in the brain. Antioxidants Redox Signal. 2021;35(14):1176–1206. doi: 10.1089/ars.2020.8238. [DOI] [PubMed] [Google Scholar]
- 19.Tami A., van der Gun B.T.F., Wold K.I., et al. The COVID HOME study research protocol: prospective cohort study of non-hospitalised COVID-19 patients. PLoS One. 2022;17(11) doi: 10.1371/journal.pone.0273599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. Br. Med. J. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO . 6 October 2021. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu M.L., Louie S., Cross C.E., Motchnik P., Halliwell B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993;121(2):257–262. [PubMed] [Google Scholar]
- 24.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.van Eijk L.E., Tami A., Hillebrands J.L., et al. Mild coronavirus disease 2019 (COVID-19) is marked by systemic oxidative stress: a pilot study. Antioxidants. 2021;10(12):2022. doi: 10.3390/antiox10122022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Hakeim H.K., Al-Rubaye H.T., Al-Hadrawi D.S., Almulla A.F., Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol. Psychiatr. 2023;28(2):564–578. doi: 10.1038/s41380-022-01836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatr. 2021;8(5):416–427. doi: 10.1016/s2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris G., Maes M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/Chronic fatigue syndrome (CFS) Curr. Neuropharmacol. 2014;12(2):168–185. doi: 10.2174/1570159x11666131120224653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maes M., Galecki P., Chang Y.S., Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35(3):676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 30.De Pascali F., Hemann C., Samons K., Chen C.A., Zweier J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry. 2014;53(22):3679–3688. doi: 10.1021/bi500076r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieczfinska J., Kleniewska P., Pawliczak R. Oxidative stress-related mechanisms in SARS-CoV-2 infections. Oxid. Med. Cell. Longev. 2022 doi: 10.1155/2022/5589089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourgonje A.R., Abdulle A.E., Al-Rawas A.M., et al. Systemic oxidative stress is increased in postmenopausal women and independently associates with homocysteine levels. Int. J. Mol. Sci. 2020;21(1) doi: 10.3390/ijms21010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frenay A.S., de Borst M.H., Bachtler M., et al. Serum free sulfhydryl status is associated with patient and graft survival in renal transplant recipients. Free Radic. Biol. Med. 2016;99:345–351. doi: 10.1016/j.freeradbiomed.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Bourgonje M.F., Bourgonje A.R., Abdulle A.E., et al. Systemic oxidative stress, aging and the risk of cardiovascular events in the general female population. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.630543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourgonje A.R., Gabriëls R.Y., de Borst M.H., et al. Serum free thiols are superior to fecal calprotectin in reflecting endoscopic disease activity in inflammatory bowel disease. Antioxidants. 2019;8(9) doi: 10.3390/antiox8090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Slikke E.C., Boekhoud L., Bourgonje A.R., et al. Plasma free thiol levels during early sepsis predict future renal function decline. Antioxidants. 2022;11(5) doi: 10.3390/antiox11050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anraku M., Chuang V.T., Maruyama T., Otagiri M. Redox properties of serum albumin. Biochim. Biophys. Acta. 2013;1830(12):5465–5472. doi: 10.1016/j.bbagen.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 38.Hortin G.L., Sviridov D., Anderson N.L. High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin. Chem. 2008;54(10):1608–1616. doi: 10.1373/clinchem.2008.108175. [DOI] [PubMed] [Google Scholar]
- 39.Bourgonje A.R., Geertsema S., Holstein H.J., et al. Evaluating serum free thiols in inflammatory bowel disease: contribution of albumin to extracellular free thiol status. Inflamm. Bowel Dis. 2024 doi: 10.1093/ibd/izae102. [DOI] [PubMed] [Google Scholar]
- 40.Müller S.A., Isaaka L., Mumm R., et al. Prevalence and risk factors for long COVID and post-COVID-19 condition in Africa: a systematic review. Lancet Global Health. 2023;11(11):e1713–e1724. doi: 10.1016/s2214-109x(23)00384-4. [DOI] [PubMed] [Google Scholar]
- 41.Smith P., Charafeddine R., Drieskens S., et al. COVIMPACT Studie: Long COVID en de lichamelijke, psychische en sociale gevolgen – Resultaten van 3 maanden opvolging. Sciensano. 2021 doi: 10.25608/gqcw-yk36. [DOI] [Google Scholar]
- 42.Khan N., Javed Z., Acquah I., et al. Low educational attainment is associated with higher all-cause and cardiovascular mortality in the United States adult population. BMC Publ. Health. 2023;23(1):900. doi: 10.1186/s12889-023-15621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnani J.W., Ning H., Wilkins J.T., Lloyd-Jones D.M., Allen N.B. Educational attainment and lifetime risk of cardiovascular disease. JAMA Cardiol. 2024;9(1):45–54. doi: 10.1001/jamacardio.2023.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.IHME-CHAINCollaborators Effects of education on adult mortality: a global systematic review and meta-analysis. Lancet Public Health. 2024;9(3):e155–e165. doi: 10.1016/s2468-2667(23)00306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brahmi F., Vejux A., Ghzaiel I., et al. Role of diet and nutrients in SARS-CoV-2 infection: incidence on oxidative stress, inflammatory status and viral production. Nutrients. 2022;14(11) doi: 10.3390/nu14112194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourgonje A.R., Offringa A.K., van Eijk L.E., et al. N-acetylcysteine and hydrogen sulfide in coronavirus disease 2019. Antioxidants Redox Signal. 2021 doi: 10.1089/ars.2020.8247. [DOI] [PubMed] [Google Scholar]
- 47.Bourgonje A.R., Abdulle A.E., Timens W., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Eijk L.E., Offringa A.K., Bernal M.E., Bourgonje A.R., van Goor H., Hillebrands J.L. The disease-modifying role of taurine and its therapeutic potential in coronavirus disease 2019 (COVID-19) Adv. Exp. Med. Biol. 2022;1370:3–21. doi: 10.1007/978-3-030-93337-1_1. [DOI] [PubMed] [Google Scholar]
- 49.Lizard G., Gueldry S., Sordet O., et al. Glutathione is implied in the control of 7-ketocholesterol-induced apoptosis, which is associated with radical oxygen species production. Faseb. J. 1998;12(15):1651–1663. doi: 10.1096/fasebj.12.15.1651. [DOI] [PubMed] [Google Scholar]
- 50.Ballering A.V., van Zon S.K.R., Olde Hartman T.C., Rosmalen J.G.M. Persistence of somatic symptoms after COVID-19 in The Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–461. doi: 10.1016/s0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourgonje A.R., Kloska D., Grochot-Przęczek A., Feelisch M., Cuadrado A., van Goor H. Personalized redox medicine in inflammatory bowel diseases: an emerging role for HIF-1α and NRF2 as therapeutic targets. Redox Biol. 2023;60 doi: 10.1016/j.redox.2023.102603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feelisch M., Cortese-Krott M.M., Santolini J., Wootton S.A., Jackson A.A. Systems redox biology in health and disease. EXCLI J. 2022;21:623–646. doi: 10.17179/excli2022-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets used for the current study are available upon reasonable request to the corresponding author.
Data will be made available on request.