Abstract
Background
Exposure to Δ9-tetrahydrocannabinol (THC) is an established risk factor for later-life neuropsychiatric vulnerability, including mood- and anxiety-related symptoms. The psychotropic effects of THC on affect and anxiogenic behavioral phenomena are known to target the striatal network, particularly the nucleus accumbens, a neural region linked to mood and anxiety disorder pathophysiology. THC may increase neuroinflammatory responses via the redox system and dysregulate inhibitory and excitatory neural balance in various brain circuits, including the striatum. Thus, interventions that can induce antioxidant effects may counteract the neurodevelopmental impacts of THC exposure.
Methods
In the current study, we used an established preclinical adolescent rat model to examine the impacts of adolescent THC exposure on various behavioral, molecular, and neuronal biomarkers associated with increased mood and anxiety disorder vulnerability. Moreover, we investigated the protective properties of the antioxidant N-acetylcysteine against THC-related pathology.
Results
We demonstrated that adolescent THC exposure induced long-lasting anxiety- and depressive-like phenotypes concomitant with differential neuronal and molecular abnormalities in the two subregions of the nucleus accumbens, the shell and the core. In addition, we report for the first time that N-acetylcysteine can prevent THC-induced accumbal pathophysiology and associated behavioral abnormalities.
Conclusions
The preventive effects of this antioxidant intervention highlight the critical role of redox mechanisms underlying cannabinoid-induced neurodevelopmental pathology and identify a potential intervention strategy for the prevention and/or reversal of these pathophysiological sequelae.
Keywords: Accumbens, Antioxidant, Anxiety, Cannabinoids, Depression, Neurodevelopment
Plain Language Summary
Sustained cannabis use during adolescence increases vulnerability to neuropsychiatric disorders, including anxiety and depression. Pharmacotherapeutic interventions to normalize these pathological outcomes are still limited. We performed a comprehensive and integrative translational analysis of the effects of adolescent exposure to Δ9-tetrahydrocannabinol (THC), the main psychoactive component in cannabis. Moreover, we tested the protective properties of the antioxidant N-acetylcysteine (NAC). THC-treated rats exhibited anxiety and depressive-like phenotypes concomitant with neuronal and molecular dysregulations in the nucleus accumbens, a crucial area for mood disorders. NAC prevented the development of THC-related pathology. These findings identified a potential strategy to prevent the neurodevelopmental disorders induced by cannabis exposure.
Plain Language Summary
Sustained cannabis use during adolescence increases vulnerability to neuropsychiatric disorders, including anxiety and depression. Pharmacotherapeutic interventions to normalize these pathological outcomes are still limited. We performed a comprehensive and integrative translational analysis of the effects of adolescent exposure to Δ9-tetrahydrocannabinol (THC), the main psychoactive component in cannabis. Moreover, we tested the protective properties of the antioxidant N-acetylcysteine (NAC). THC-treated rats exhibited anxiety and depressive-like phenotypes concomitant with neuronal and molecular dysregulations in the nucleus accumbens, a crucial area for mood disorders. NAC prevented the development of THC-related pathology. These findings identified a potential strategy to prevent the neurodevelopmental disorders induced by cannabis exposure.
Adolescent cannabis exposure can increase the risk of developing mood and anxiety disorder symptoms later in life (1, 2, 3). Δ9-tetrahydrocannabinol (THC), the psychoactive constituent of cannabis, is a partial agonist of cannabinoid CB1 receptors and can alter normal neurophysiological brain maturation through their sustained stimulation. The CB1 receptor system is widely distributed in the mesocorticolimbic system (4), which highlights the crucial relevance of these pathways in THC-related pathophysiology. The nucleus accumbens (Acb) is particularly vulnerable to adolescent cannabinoid exposure. Imaging studies have revealed morphometric Acb abnormalities in young cannabis users (5). Moreover, chronic THC exposure in rodents alters Acb neuronal spines and dendritic branching (6,7), perturbs synaptic strength (8,9), and dysregulates inhibitory/excitatory synaptic transmission (10). Adolescent THC exposure also alters intra-Acb transcriptomic markers associated with reward and impulsivity behaviors (11).
The Acb is a critical integration point for emotional and reward-related processing and is a common nexus for signaling pathways related to both affective disorders and addiction (12). Nevertheless, the two major Acb subregions, the shell (AcbSh) and core (AcbC), may differentially contribute to the mechanisms that underlie these pathologies due to their functional and anatomical distinctions (13, 14, 15). Divergent roles for these regions have been identified for processing reward-related stimuli (16), as well as during drug relapse–related behaviors (17, 18, 19). However, how neurodevelopmental cannabinoid exposure may differentially affect these Acb subregions and related pathological phenotypes is not well understood.
During neurodevelopment, the brain is highly sensitive to oxidative stress. Cannabis is known to increase oxidative stress and increase brain levels of oxidative free radicals (20,21). Moreover, mood and anxiety disorders are associated with impaired antioxidant responses and increased oxidative damage in proteomic, lipidomic, and nucleic acid substrates (22,23). Nevertheless, the functional intersection between neurodevelopmental vulnerability and THC-induced oxidative stress as a mechanism for psychiatric risk requires further investigation.
N-acetylcysteine (NAC) is an acetylated form of the amino acid L-cysteine that has well-established antioxidant properties and regulates neurotransmitter balance, neuroinflammatory processes, and oxidative stress (24). Accordingly, NAC has been proposed as a potentially effective treatment for various neuropsychiatric conditions (24), including substance use disorders (25, 26, 27). For example, NAC has been shown to ameliorate cannabis cessation in adolescent (28) but not adult (29) participants with cannabis use disorder. Moreover, NAC treatment prevented the reinstatement of cannabinoid drug seeking in rodents, potentially via restoration of intra-Acb glutamate (GLUT) homeostasis (30). Oxidative disturbances are associated with mood- and anxiety-related pathophysiology (31). However, how oxidative stress induced by adolescent THC exposure may be related to Acb-mediated psychiatric risk is not currently known.
Using a translational rodent model of adolescent THC exposure, we investigated the potential neuroprotective properties of NAC against THC-induced behavioral pathophenotypes and associated molecular adaptations in the AcbSh versus the AcbC. We report that adolescent THC induced long-lasting anxiety- and depressive-like phenotypes concomitant with dissociable molecular and neuronal activity alterations in the AcbSh versus the AcbC. Remarkably, NAC administration normalized these THC-induced phenotypes while preventing dysregulations in metabolomic biomarkers associated with mood- and anxiety-related pathophysiology.
Methods and Materials
Male Sprague Dawley rats were obtained on postnatal day 28 from Charles River Laboratories. The adolescent THC treatment protocol was performed as previously described (32,33). NAC was administered between postnatal days 35 and 65. Experimental procedures started in adulthood. The elevated plus maze, novelty-suppressed feeding, Porsolt forced swimming test, and contextual fear memory conditioning were performed to assess anxiety- and depressive-like manifestations. Molecular investigations in the AcbSh/AcbC were performed using matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS) and Western blotting. Neuronal activity in the AcbSh/AcbC was examined using in vivo electrophysiology. See Supplement 1 for details.
Data from different experiments are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism, version 9. Datasets were tested for outliers, and comparisons between groups were assessed using 2-way analysis of variance (ANOVA). Post hoc analyses were calculated using the 2-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli for controlling the false discovery rate. The significance level was established at p < .05.
Results
NAC Administration Prevents the Development of Several Anxiety- and Depressive-Like Behaviors Induced by Adolescent THC
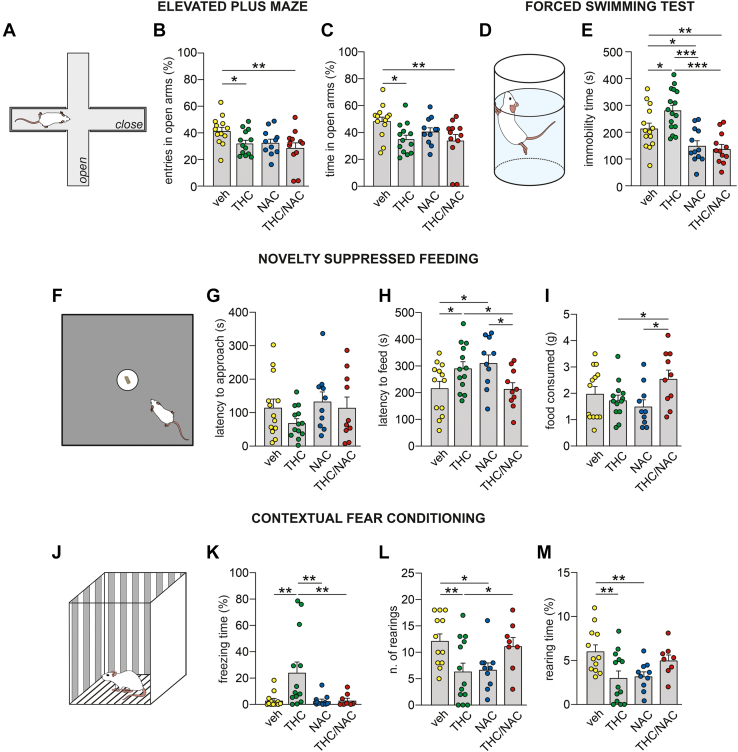
First, we examined the potential neuroprotective properties of NAC on THC-related pathology using 4 tasks validated for anxiety- and depressive-like behaviors (Figure 1; Table S1 in Supplement 2).
Figure 1.
Effects of NAC on anxiety- and depressive-like manifestations induced by THC exposure during adolescence. (A) Schematic representation of the elevated plus maze apparatus. (B, C) THC-treated rats made fewer entries and spent less time in the open arms of the apparatus. NAC administration did not prevent these effects (veh, n = 13; THC, n = 13; NAC, n = 11; THC/NAC, n = 12). (D) Schematic representation of the forced swimming test apparatus. (E) NAC administration prevented the increased immobility induced by chronic THC exposure (veh, n = 14; THC, n = 16; NAC, n = 12; THC/NAC, n = 12). (F) Schematic representation of the novelty-suppressed feeding test. (G) No differences between groups were observed in the latency to approach the food during the task. (H) THC-treated rats exhibited a longer latency to start feeding during the test, while NAC administration normalized this effect. (I) The rats exposed to THC and NAC consumed more food than the THC- and NAC-treated groups (veh, n = 13; THC, n = 13; NAC, n = 10; THC/NAC, n = 10). (J) Schematic representation of the contextual fear conditioning box. (K–M) Chronic THC exposure induced an increase in freezing time while reducing the number of rearings and the time spent on rearing behavior. NAC administration prevented the THC-related effects on freezing and number of rearings but was ineffective in restoring the rearing behavior time (veh, n = 12; THC, n = 13; NAC, n = 10; THC/NAC, n = 8). ∗p < .05, ∗∗p < .01, ∗∗∗p < .001. NAC, N-acetylcysteine; THC, Δ9-tetrahydrocannabinol; veh, vehicle.
Elevated Plus Maze
Two-way ANOVA revealed a main effect of adolescent THC exposure on the number of entries and time spent in the open arms (Figure 1B, C). Post hoc comparisons showed that THC- and THC/NAC-treated rats made fewer entries and spent less time in open arms compared with vehicle (veh)–treated rats.
Forced Swim Test
Two-way ANOVA revealed a significant effect of NAC and an interaction between factors on immobility time (Figure 1E). Post hoc comparisons revealed that adolescent THC exposure increased immobility time compared with veh, while THC/NAC co-administration prevented this effect. NAC-treated rats spent less time immobile than veh- and THC-treated rats, while rats in the THC/NAC group showed lower immobility than veh-treated rats.
Novelty-Suppressed Feeding
No differences between groups were observed in the first latency to approach food in the arena (Figure 1G). However, a significant interaction between factors was found in the latency to feed (Figure 1H). Post hoc comparisons revealed that THC-treated rats bit the food later than veh-treated rats, and this effect was prevented by THC/NAC co-administration. NAC-treated rats started feeding later than veh- and THC/NAC-treated rats. Two-way ANOVA also revealed an interaction between factors in food consumption during the test (Figure 1I). Post hoc analysis revealed that the THC/NAC group ate more food than the THC and NAC groups.
Contextual Fear Conditioning
Two-way ANOVA revealed a significant effect of THC and an interaction between factors in freezing time (Figure 1K). Post hoc comparisons showed that THC-treated rats spent longer time in freezing behavior than veh-treated rats, and this effect was prevented by THC/NAC coadministration. THC-treated rats also showed increased freezing time compared with NAC-treated rats. A significant interaction between factors was observed in the number of rearings and in the time spent in rearing behavior (Figure 1L, M). Post hoc analysis revealed that adolescent THC exposure decreased the number of rearings and the time spent rearing compared with veh. THC/NAC prevented THC-induced reduction in rearing events but not in the time spent rearing. NAC-treated rats showed lower rearing numbers and rearing time than veh-treated rats.
NAC Administration Normalizes Neurotransmitter Alterations in the AcbSh Versus the AcbC Induced by Adolescent THC Exposure
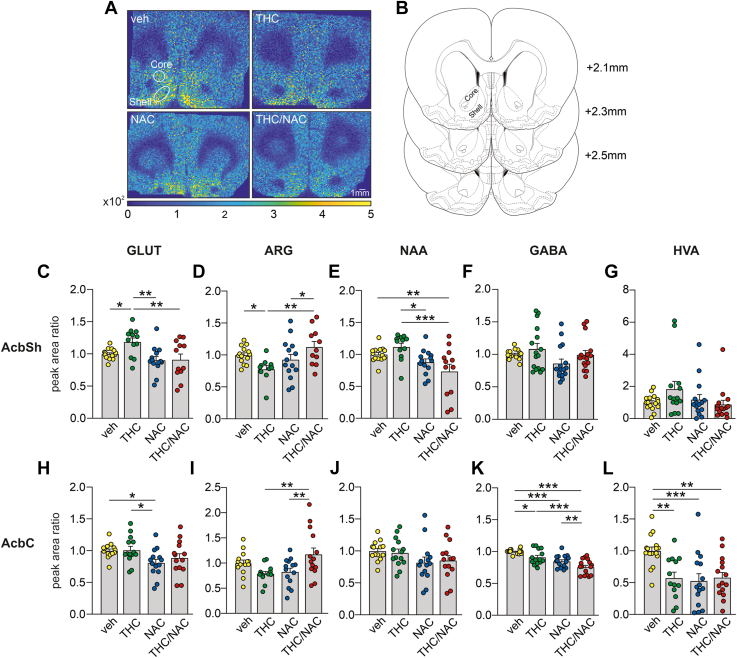
We performed MALDI-IMS in Acb tissue samples to further investigate the neuroprotective properties of NAC at the metabolomic level (Figure 2; Table S2 in Supplement 2). Two-way ANOVA of GLUT levels in the AcbSh revealed a main effect of NAC (Figure 2C). Post hoc comparisons showed that adolescent THC exposure increased GLUT levels compared with veh, while this effect was prevented by THC/NAC coadministration. THC-treated rats had higher GLUT levels than NAC-treated rats. Analysis of arginine (Arg) levels in the AcbSh showed a significant interaction between factors (Figure 2D). Post hoc comparisons revealed that Arg levels were reduced in the AcbSh of THC-treated rats, while this was prevented by THC/NAC coadministration. The THC/NAC group exhibited higher levels of Arg than the NAC group. Two-way ANOVA of N-acetylaspartate (NAA) levels in the AcbSh revealed a main effect of NAC treatment (Figure 2E). Post hoc comparisons showed that NAA levels in THC/NAC group were lower than in the veh and THC groups. NAC-exposed rats exhibited reduced levels of NAA compared with THC-exposed rats. No differences between groups were detected in AcbSh levels of GABA (gamma-aminobutyric acid) (Figure 2F) and HVA (homovanillic acid) (Figure 2G).
Figure 2.
Effects of NAC on THC-induced dysregulations of neurotransmitter levels. (A) MALDI-IMS images of a representative rat brain section including AcbSh and AcbC from each group. (B) Representative localizations of the AcbSh and AcbC on rat brain coronal sections adapted from (76). The numbers indicate the distance from bregma. (C, D) Adolescent THC exposure increased GLUT levels (m/z = 146.95; veh, n = 14; THC, n = 12; NAC, n = 14; THC/NAC, n = 12) and decreased ARG levels (m/z = 213.30; veh, n = 14; THC, n = 10; NAC, n = 14; THC/NAC, n = 11) in AcbSh, while the concomitant administration of NAC prevented these THC-related dysregulations. (E) NAA concentrations (m/z = 198.13; veh, n = 14; THC, n = 12; NAC, n = 14; THC/NAC, n = 12) in the AcbSh were reduced by administration of NAC. (F, G) No differences between groups were observed in GABA (m/z = 353.16; veh, n = 16; THC, n = 16; NAC, n = 16; THC/NAC, n = 16) and HVA (m/z = 450.17; veh, n = 16; THC, n = 14; NAC, n = 15; THC/NAC, n = 15) relative quantifications in the AcbSh. (H) Administration of NAC alone reduced GLUT levels (m/z = 146.95; veh, n = 14; THC, n = 14; NAC, n = 14; THC/NAC, n = 14) in the AcbC compared with the veh and THC groups. (I) ARG concentration (m/z = 213.30; veh, n = 14; THC, n = 13; NAC, n = 14; THC/NAC, n = 14) in the AcbC was higher in the THC/NAC group than the NAC- and THC-treated rats. (J) No differences between groups were detected in NAA levels (m/z = 198.13; veh, n = 14; THC, n = 14; NAC, n = 14; THC/NAC, n = 14) in the AcbC. (K, L) Both adolescent THC exposure and NAC administration decreased GABA levels (m/z = 353.16; veh, n = 16; THC, n = 14; NAC, n = 16; THC/NAC, n = 16) and HVA levels (m/z = 450.17; veh, n = 16; THC, n = 13; NAC, n = 15; THC/NAC, n = 15) in AcbC. ∗p < .05, ∗∗p < .01, ∗∗∗p < .001. AcbC, nucleus accumbens core; AcbSh, nucleus accumbens shell; ARG, arginine; GABA, gamma-aminobutyric acid; GLUT, glutamate; HVA, homovanillic acid; MALDI-IMS, matrix-assisted laser desorption/ionization imaging mass spectrometry; NAA, N-acetylaspartate; NAC, N-acetylcysteine; THC, Δ9-tetrahydrocannabinol; veh, vehicle.
In the AcbC, analysis of GLUT levels revealed a main effect of NAC (Figure 2H). Post hoc comparisons showed that the NAC group had lower GLUT levels than the veh and THC groups. Two-way ANOVA of Arg relative quantification in the AcbC showed a significant interaction between factors (Figure 2I). Post hoc analysis revealed that THC/NAC coexposure increased Arg levels compared with THC and NAC exposure. Statistical analysis of NAA levels in the AcbC revealed a main effect of NAC treatment (Figure 2J). However, no differences between groups were found in the post hoc analysis. Analysis of AcbC GABA levels revealed main effects of NAC and THC (Figure 2K). Post hoc comparisons showed that GABA levels were reduced in THC-treated rats compared with veh-treated rats. The NAC and THC/NAC groups also had lower GABA levels than the veh group. Lastly, the THC/NAC groups showed decreased GABA levels compared with the THC and NAC groups. Analysis of HVA levels in the AcbC revealed main effects of NAC, THC, and interaction between factors (Figure 2L). Post hoc comparisons showed that AcbC HVA levels were decreased following THC, NAC, and THC/NAC exposure compared with veh. Additional biomarkers are presented in Table S2 in Supplement 2.
NAC Administration Restores Several THC-Related Molecular Abnormalities in the AcbSh and AcbC
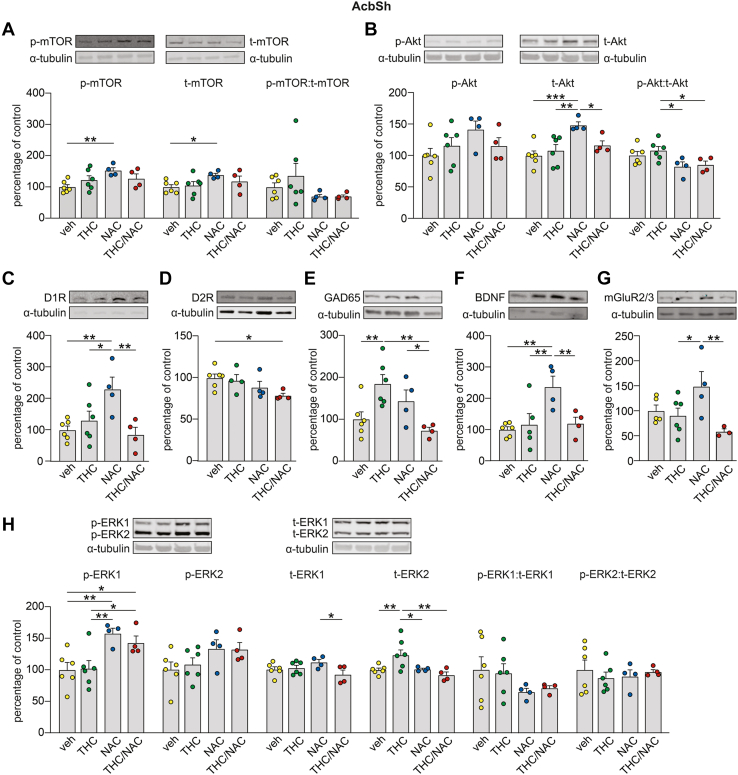
We examined proteomic profiles in the AcbSh and AcbC, focusing on an array of protein markers associated with THC exposure and affective disturbances (Figures 3 and 4; Table S3 in Supplement 2). Western blotting analysis of mTOR (mechanistic target of rapamycin) levels in the AcbSh revealed a main effect of NAC on phosphorylated mTOR (p-mTOR) and total mTOR (t-mTOR), but no changes were found in the p-mTOR:t-mTOR ratio (Figure 3A). Post hoc comparisons showed that NAC administration increased the expression levels of p-mTOR and t-mTOR compared with veh. Protein analysis of AcbSh Akt-Thr308 did not reveal any changes in p-Akt, while a significant effect of NAC and an interaction between factors was found for t-Akt (Figure 3B). Post hoc comparisons showed that NAC administration increased t-Akt levels compared with veh, THC, and THC/NAC. A main effect of NAC was observed on the p-Akt:t-Akt ratio (Figure 3B). Post hoc comparisons showed that THC exposure increased the p-Akt:t-Akt ratio in the AcbSh compared with the NAC and THC/NAC groups. Two-way ANOVA of D1 receptor (D1R) expression levels in the AcbSh revealed a significant interaction between factors (Figure 3C). Post hoc comparisons showed that D1R levels were increased following NAC administration compared with veh, THC, and THC/NAC. Analysis of D2R expression levels in the AcbSh showed a main effect of NAC (Figure 3D). Post hoc comparisons revealed that THC/NAC coexposure decreased D2R expression levels compared with veh. Protein analysis of GAD65 in the AcbSh revealed a significant interaction between factors (Figure 3E). Post hoc comparisons revealed that GAD65 expression levels were significantly increased following THC exposure, and this effect was prevented by THC/NAC coadministration. THC/NAC rats exhibited lower GAD65 levels than NAC rats. Western blotting analysis of BDNF (brain-derived neurotrophic factor) expression levels in the AcbSh revealed a main effect of NAC and an interaction between factors (Figure 3F). Post hoc comparisons showed that the NAC group exhibited higher BDNF levels in the AcbSh than the veh, THC, and THC/NAC groups. Protein analysis of AcbSh mGluR2/3 (metabotropic glutamate receptor 2/3) revealed a significant effect of THC and an interaction between factors (Figure 3G). Post hoc comparisons showed that NAC administration increased mGluR2/3 expression levels compared with THC and THC/NAC. Two-way ANOVA of p-ERK1 (extracellular signal-regulated kinase 1) in the AcbSh revealed a main effect of NAC (Figure 3H). Post hoc comparisons showed that NAC and THC/NAC administration increased p-ERK1 compared with veh and THC. A significant interaction between factors was observed for t-ERK1 (Figure 3H). Post hoc comparisons showed that THC/NAC rats exhibited lower t-ERK1 levels than NAC group rats. No effects were found on the p-ERK1:t-ERK1 ratio (Figure 3H). Lastly, protein analysis of p-ERK2 in the AcbSh revealed a main effect of NAC (Figure 3H); however, no changes between groups were found in the post hoc test. Two-way ANOVA of t-ERK2 showed a significant effect of NAC and an interaction between factors (Figure 3H). Post hoc comparisons showed that THC exposure increased t-ERK2 levels compared with veh, and this effect was prevented by THC/NAC administration. t-ERK2 levels in THC rats were also higher than in NAC group rats. No changes were observed in the p-ERK2:t-ERK2 ratio (Figure 3H).
Figure 3.
Effect of NAC on molecular adaptations induced by THC in the AcbSh. (A–H) Insets on the top of the bar graphs are representative Western blots for p-mTOR, t-mTOR, p-Akt (Thr308), t-Akt, D1R, D2R, GAD65, BDNF, mGluR2/3, p-ERK1/2, and t-ERK1/2 in AcbSh. (A) p-mTOR and t-mTOR expression levels were increased by NAC administration (veh, n = 6; THC, n = 6; NAC, n = 4; THC/NAC, n = 4). (B) The NAC group showed higher t-Akt levels than the veh, THC, and THC/NAC groups, while the p-Akt:t-Akt ratio was decreased following NAC and THC/NAC compared with THC (veh, n = 6; THC, n = 6; NAC, n = 4; THC/NAC, n = 4). (C) D1R expression was increased by NAC administration (veh, n = 6; THC, n = 6; NAC, n = 4; THC/NAC, n = 4). (D) THC/NAC induced a reduction in D2R level (veh, n = 6; THC, n = 6; NAC, n = 4; THC/NAC; n = 4). (E) Adolescent THC exposure increased GAD65 levels, while the concomitant administration of THC and NAC prevented this effect (veh, n = 6; THC, n = 6; NAC, n = 4; THC/NAC, n = 4). (F) BDNF expression was increased by NAC administration (veh, n = 6; THC, n = 5; NAC, n = 4; THC/NAC, n = 4). (G) The NAC group showed higher mGluR2/3 levels than the THC and THC/NAC groups (veh, n = 5; THC, n = 6; NAC, n = 4; THC/NAC, n = 3). (H) p-ERK1 expression levels were increased by NAC administration, while t-ERK1 was reduced by THC/NAC compared with the NAC group. Moreover, adolescent THC exposure increased t-ERK2 levels, while the concomitant administration of THC and NAC prevented this effect (veh, n = 6; THC, n = 6; NAC, n = 4; THC/NAC, n = 4). ∗p < .05, ∗∗p < .01, ∗∗∗p < .001. AcbSh, nucleus accumbens shell; BDNF, brain-derived neurotrophic factor; D1R, D1 receptor; D2R, D2 receptor; ERK, extracellular signal-regulated kinase; mGluR, metabotropic glutamate receptor; mTOR, mechanistic target of rapamycin; NAA, N-acetylaspartate; NAC, N-acetylcysteine; p, phosphorylated; THC, Δ9-tetrahydrocannabinol; t, total; veh, vehicle.
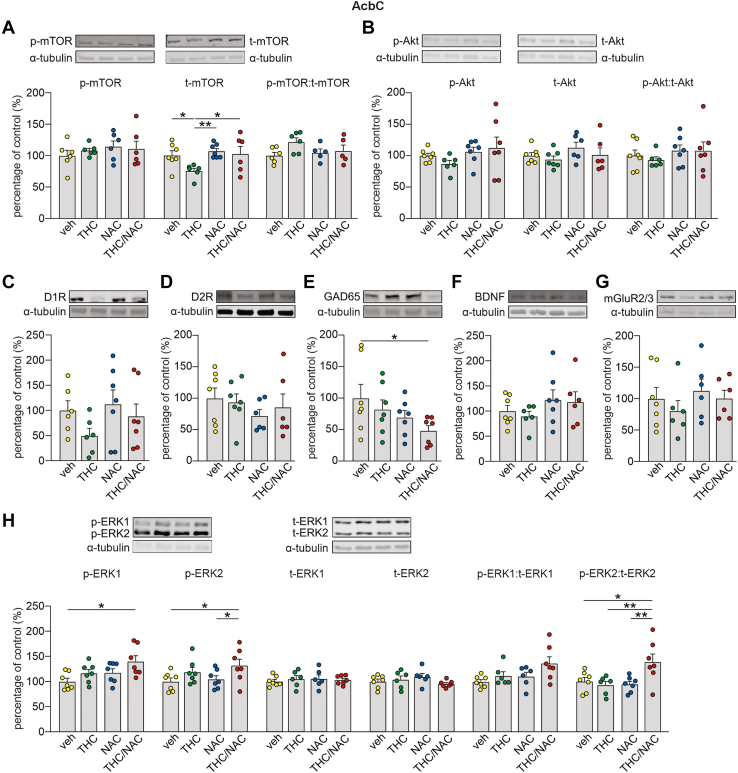
Figure 4.
Effect of NAC on molecular adaptations induced by THC in the AcbC. (A–H) Insets on the top of the bar graphs are representative Western blots for p-mTOR, t-mTOR, p-Akt (Thr308), t-Akt, D1R, D2R, GAD65, BDNF, mGluR2/3, p-ERK1/2 and t-ERK1/2 in AcbC. (A) Adolescent THC exposure decreased t-mTOR expression levels, while the concomitant administration of THC and NAC prevented this dysregulation (veh, n = 7; THC, n = 6; NAC, n = 7; THC/NAC, n = 6). No differences between groups were observed in p-mTOR (veh, n = 6; THC, n = 6; NAC, n = 6; THC/NAC, n = 6) and p-mTOR:t-mTOR ratio (veh, n = 6; THC, n = 6; NAC, n = 5; THC/NAC, n = 5). (B–D) No effects were found in the expression levels of p-Akt (veh, n = 7; THC, n = 6; NAC, n = 7; THC/NAC, n = 7), t-Akt (veh, n = 7; THC, n = 7; NAC, n = 6; THC/NAC, n = 6), p-Akt:t-Akt ratio (veh, n = 7; THC, n = 6; NAC, n = 7; THC/NAC, n = 7), D1R (veh, n = 6; THC, n = 6; NAC, n = 7; THC/NAC, n = 7), and D2R (veh, n = 7; THC, n = 7; NAC, n = 6; THC/NAC, n = 6). (E) GAD65 levels were reduced by NAC administration (veh, n = 7; THC, n = 7; NAC, n = 7; THC/NAC, n = 7). (F, G) No differences between groups were observed in the expression of BDNF (veh, n = 7; THC, n = 6; NAC, n = 7; THC/NAC, n = 6) and mGluR2/3 (veh, n = 7; THC, n = 6; NAC, n = 7; THC/NAC, n = 7). (H) Concomitant administration of THC and NAC increased the levels of pERK1 (veh, n = 7; THC, n = 7; NAC, n = 7; THC/NAC, n = 7), p-ERK2 (veh, n = 7; THC, n = 7; NAC, n = 7; THC/NAC, n = 7), and p-ERK2:t-ERK2 ratio (veh, n = 7; THC, n = 6; NAC, n = 7; THC/NAC, n = 7). No changes were found in t-ERK1 (veh, n = 7; THC, n = 6; NAC, n = 6; THC/NAC, n = 7), t-ERK2 (veh, n = 7; THC, n = 6; NAC, n = 6; THC/NAC, n = 7), and p-ERK1:t-ERK1 (veh, n = 7; THC, n = 6; NAC, n = 6; THC/NAC, n = 7). ∗p < .05. AcbC, nucleus accumbens core; BDNF, brain-derived neurotrophic factor; D1R, D1 receptor; D2R, D2 receptor; ERK, extracellular signal-regulated kinase; mGluR, metabotropic glutamate receptor; mTOR, mechanistic target of rapamycin; NAA, N-acetylaspartate; NAC, N-acetylcysteine; p, phosphorylated; THC, Δ9-tetrahydrocannabinol; t, total; veh, vehicle.
In the AcbC, a 2-way ANOVA comparing mTOR expression levels revealed a main effect of NAC on t-mTOR (Figure 4A). Post hoc comparisons showed that THC exposure reduced t-mTOR levels in the AcbC compared with veh, which was prevented by THC/NAC coadministration. Moreover, THC-treated rats exhibited lower t-mTOR levels than NAC-treated rats. No effects were observed on p-mTOR or on the p-mTOR:t-mTOR ratio (Figure 4A). Protein analysis of GAD65 showed a main effect of NAC (Figure 4E). Post hoc comparisons revealed that THC/NAC administration reduced GAD65 levels compared with veh. A 2-way ANOVA of p-ERK1 in the AcbC revealed main effects of NAC and THC (Figure 4H). Post hoc comparisons revealed that THC/NAC administration increased p-ERK1 levels compared with veh. No changes between groups were observed in t-ERK1 and in the p-ERK1:t-ERK1 ratio (Figure 4H). Protein analysis of AcbC p-ERK2 showed a main effect of THC (Figure 4H). Post hoc comparisons revealed that THC/NAC administration increased p-ERK2 expression compared with veh and NAC. While t-ERK2 expression in the AcbC was not affected by adolescent treatments, analysis of the p-ERK2:t-ERK2 ratio revealed a significant interaction between factors (Figure 4H). Post hoc comparisons showed that THC/NAC administration increased the p-ERK2:t-ERK2 ratio in the AcbC compared with veh, THC, and NAC. No significant differences were found in the levels of Akt-Thr308 (Figure 4B), D1R (Figure 4C), D2R (Figure 4D), BDNF (Figure 4F), and mGluR2/3 (Figure 4G).
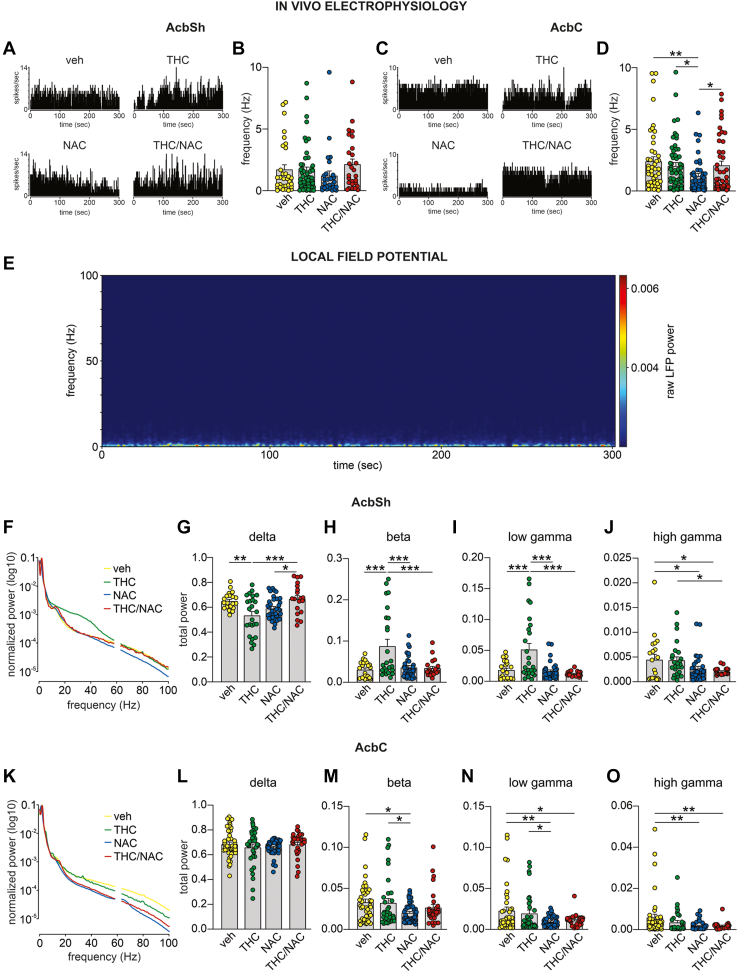
NAC Prevents THC-Induced Neuronal Dysregulations in the AcbSh and AcbC
We investigated the impact of adolescent THC and NAC administration on neural activity of putative medium spiny neurons and oscillation patterns in the AcbSh versus the AcbC (Figure 5; Table S4 in Supplement 2). No group differences were observed in the firing frequency of AcbSh medium spiny neurons (Figure 5B). However, a significant interaction between factors was found for the firing frequency of AcbC medium spiny neuron cells (Figure 5D). Post hoc comparisons revealed that NAC-treated rats exhibited a reduced firing rate compared with veh-, THC-, and THC/NAC-treated rats.
Figure 5.
Effects of NAC on neuronal and oscillatory patterns induced by adolescent THC exposure in the AcbS vs. the AcbC. (A, C) Representative rate histograms of putative GABA cells in the AcbSh and AcbC recorded from each group. (B, D) No differences between groups were found in the firing frequency of AcbSh GABA neurons (veh, n = 32 cells/9 rats; THC, n = 49 cells/15 rats; NAC, n = 27 cells/8 rats; THC/NAC, n = 27 cells/5 rats), while the administration of NAC alone decreased the firing rate of GABA neurons in the AcbC (veh, n = 46 cells/16 rats; THC, n = 55 cells/15 rats; NAC, n = 44 cells/6 rats; THC/NAC, n = 44 cells/8 rats). (E) Representative spectrogram of a 5-minute recording. (F, K) Average normalized LFP power spectra in AcbSh and AcbC. (G) Adolescent THC exposure reduced delta waves in the AcbSh, while this effect was prevented by the concomitant administration of THC and NAC (veh, n = 20 recording sites/8 rats; THC, n = 25 recording sites/9 rats; NAC, n = 33 recording sites/8 rats; THC/NAC, n = 19 recording sites/6 rats). (H, I) THC-treated rats exhibited higher AcbSh beta and low gamma oscillations than the veh group, and these effects were prevented by NAC administration (veh, n = 20 recording sites/8 rats; THC, n = 25 recording sites/9 rats; NAC, n = 33 recording sites/8 rats; THC/NAC, n = 19 recording sites/6 rats). (J) NAC administration reduced high gamma waves in the AchSh (veh, n = 20 recording sites/8 rats; THC, n = 25 recording sites/9 rats; NAC, n = 33 recording sites/8 rats; THC/NAC, n = 19 recording sites/6 rats). (L) No differences between groups were found in delta oscillations in the AcbC (veh, n = 41 recording sites/15 rats; THC, n = 32 recording sites/11 rats; NAC, n = 41 recording sites/7 rats; THC/NAC, n = 30 recording sites/7 rats). (M–O) NAC administration decreased beta, low gamma, and high gamma waves in the AcbC (veh, n = 41 recording sites/15 rats; THC, n = 32 recording sites/11 rats; NAC, n = 41 recording sites/7 rats; THC/NAC, n = 30 recording sites/7 rats). ∗p < .05, ∗∗p < .01, ∗∗∗p < .001. AcbC, nucleus accumbens core; AcbSh, nucleus accumbens shell; GABA, gamma-aminobutyric acid; LFP, local field potential; NAC, N-acetylcysteine; THC, Δ9-tetrahydrocannabinol; veh, vehicle.
Significant differences were observed following analyses of local field potential oscillation patterns in the AcbSh versus the AcbC. An interaction between factors was found for AcbSh delta wave power (Figure 5G). Post hoc comparisons showed that adolescent THC decreased delta oscillations compared with veh, and this effect was prevented by THC/NAC coexposure. Moreover, THC/NAC-treated rats exhibited higher delta oscillations than rats in the NAC group. Analyses of beta waves in the AcbSh revealed main effects of NAC, THC, and an interaction between factors (Figure 5H). Post hoc comparisons showed increased beta oscillations in THC-treated rats compared with veh-treated rats, while THC/NAC coadministration prevented this effect. The THC group exhibited higher beta waves than the NAC group. Similarly, analyses of low gamma waves in the AcbSh revealed main effects of NAC, THC, and an interaction between factors (Figure 5I). Post hoc comparisons showed that adolescent THC exposure increased low gamma oscillations compared with veh, and this effect was prevented by THC/NAC coadministration. Also, THC-treated rats exhibited higher low gamma waves than NAC-treated rats. Two-way ANOVA revealed a main effect of NAC on AcbSh high gamma oscillations (Figure 5J). Post hoc comparisons revealed that NAC- and THC/NAC-treated rats had significantly lower high gamma waves than veh-treated rats. The THC/NAC group showed significantly reduced high gamma oscillations compared with the THC-treated group.
In the AcbC, no differences between groups were observed in delta oscillations (Figure 5L). Analyses of beta waves in the AcbC revealed a main effect of NAC (Figure 5M). Post hoc comparisons showed that beta oscillations were reduced in NAC-treated rats compared with veh- and THC-treated rats. A main effect of NAC was also found on low and high gamma oscillations (Figure 5N, O). Post hoc comparisons showed that NAC administration decreased low gamma waves compared with veh and THC administration. THC/NAC-treated rats exhibited lower low gamma oscillations than veh-treated rats. Post hoc comparisons of high gamma waves revealed a significant reduction following NAC and THC/NAC administration compared with veh administration. No differences were found in alpha and theta oscillations in the AcbSh or AcbC (data not shown).
Discussion
Neurodevelopmental THC exposure induces long-lasting neuropsychiatric disorders and dysregulations in several brain regions. Although still limited, interventions during adolescence have been found to restore THC-related cognitive deficits, anxiety, and depressive-like manifestations (34, 35, 36).
Emerging evidence points to convergent roles of oxidative stress and cannabinoid-induced pathophysiology underlying the etiology of mood and anxiety disorders (20,22). The Acb is a critical neural nexus not only for THC-related neuropsychiatric effects (37,38) but also for mood- and anxiety-related psychopathology (12). This study demonstrates that concurrent administration of the antioxidant NAC can prevent behavioral, neuronal, and molecular pathophenotypes induced by adolescent THC exposure and identifies selective vulnerabilities in the AcbSh versus the AcbC in these phenomena.
Given the critical role of the ventral striatum in affective processing, we focused our molecular and electrophysiological analyses on the AcbSh and AcbC regions. These areas show anatomical and functional dissociations (39,40). For example, the AcbSh and the AcbC play differential roles in Pavlovian conditioning (41), fear processing (42), and responses to drugs of abuse (43,44). THC exposure can induce many neuropsychiatric affective and cognitive disturbances directly in the Acb (37,38,45). Region-specific effects in ΔFosB induction markers have been observed following chronic THC exposure (46). Interestingly, NAC has been shown to prevent accumbal disruptions in various signaling pathways, including GLUT, following chronic cocaine exposure in rats (47) and blocks amphetamine-induced locomotor sensitization in mice (48). This is consistent with our MALDI-IMS findings showing that THC/NAC coexposure prevented elevated intra-AcbSh GLUT levels.
Consistent with previous reports (49,50), we found that adolescent THC exposure induced a region-specific increase in AcbSh GLUT. Regulation of AcbSh GLUT is the result of both local release and inputs from surrounding areas, such as the ventral hippocampus (vHipp) and prefrontal cortex. Interestingly, optogenetic stimulation of the vHipp→AcbSh GLUTergic pathway promotes depressive-like behaviors (51), suggesting that overstimulation of GLUT drive into the AcbSh may underlie the depressive-like manifestations observed in this study. Although a previous report did not reveal changes in spontaneous firing frequency of vHipp pyramidal neurons following adolescent THC exposure, we observed THC-induced increases in mGluR2/3 and GluN2B vHipp expression (32), demonstrating long-term GLUTergic dysregulation. We have also previously reported cortical hyperactivity following adolescent THC exposure (33,52) and that pharmacological prefrontal cortex inhibition normalizes THC-induced neurodevelopmental cognitive and affective disturbances (52). The current findings suggest that NAC may normalize AcbSh GLUTergic abnormalities by reducing local GLUT release (53). NAC modulates extracellular GLUT levels via interactions with the cystine/GLUT antiporter system xc−, thereby causing activation of extrasynaptic mGluR2/3 receptors (54, 55, 56) and in turn decreasing neuronal GLUT release (57). Moreover, by increasing cysteine levels, NAC regulates levels of glutathione, leading to a reduction in circulating GLUT. Consistent with this, a previous study reported that higher levels of glutathione limited the hyperglutamatergic state in the dorsal anterior cingulate cortex of participants with a first episode of psychosis (58). NAC also modulates the activity of the GLUT transporter type I, which is primarily responsible for GLUT uptake (59). By restoring glutamatergic homeostasis, NAC might prevent the development of the THC-induced depressive phenotypes. Further investigations are needed to relate these mechanisms more directly to the observed behavioral phenotypes.
We report several novel effects of NAC treatment on THC-induced striatal oxidative stress markers. First, adolescent THC strongly increased GLUT levels in the AcbSh and elevated oscillatory power in beta and gamma waveforms, consistent with a local excitotoxic phenotype and with studies that have shown profound dysregulation of striatal GLUT signaling in depressive disorders (60). Remarkably, NAC treatment was able to completely prevent this elevation in local AcbSh GLUT, consistent with its known antioxidant role in regulating aberrant GLUTergic pathways in psychiatric conditions (57) and in vitro models of GLUT-induced cellular toxicity, where it can reduce reactive oxidative stress responses (61). Arg is synthesized from glutamine, GLUT, and proline via the intestinal-renal axis and is a conditionally essential amino acid that has powerful antioxidant properties (62). In the current study, adolescent THC exposure caused a significant loss of Arg in the AcbSh, and this effect was effectively prevented by NAC treatment. Consistent with our observed behavioral phenotypes, decreased Arg levels are strongly correlated with depressive disorders (63), and Arg supplementation has been shown to reduce anxiety levels in human subjects (64). Given the crucial role of Arg in redox processes and its antioxidant properties (62), the observed reductions in Arg levels in the current study may result from THC-induced oxidative stress, which was prevented by THC/NAC administration. This mechanism may in turn prevent the development of anxiety- and depressive-like behavioral manifestations. Beyond its profound protective effects against THC, we found that the combination of THC/NAC increased basal Arg levels compared with NAC alone in both the AcbSh and AcbC, suggesting a potential synergy between THC and NAC in regulating striatal Arg levels.
NAA is an intermediate metabolite and is found at high concentrations in the brain. It has been proposed that NAA may serve as an available pool of metabolic precursors for GLUT production (65). Given our finding that NAC administration counteracted THC-induced GLUT elevations, one possibility is that the observed decreases in striatal NAA levels with both NAC alone and THC/NAC treatment suggest that NAA forms an integral part of the NAC neuroprotective pathway against hyper-GLUTergic activity. Interestingly, THC alone had no impact on NAA levels compared with vehicle controls, suggesting that NAC specifically was affecting local NAA metabolism. Together, these impacts of NAC on THC-induced pathophysiology identify novel roles for these metabolomic markers in cannabis-induced neurodevelopmental risk pathways.
Striatal oscillatory patterns are well-established markers for mood and anxiety disorders. For example, beta waves have been associated with anxiety processing, with higher levels being linked to states of heightened anxiety (66). Consistent with our behavioral phenotypes, NAC prevented THC-induced anxiogenic effects and blunted increased striatal beta power compared with vehicle controls. Similar effects were observed in the low gamma range. Increased gamma power has been linked to heightened anxiety (67) and increased sensitivity to negative emotional stimuli (68). This is also consistent with our previous behavioral findings showing increased sensitivity to associative fear memory in THC-exposed rats, an effect that, remarkably, was prevented by NAC treatment.
Local proteomic analyses in the AcbSh and AcbC revealed several potential protective molecular mechanisms induced by NAC. Among these, analyses of ERK1/2 revealed that adolescent THC significantly increased t-ERK2 levels in the AcbSh, and this effect was entirely prevented by NAC. ERK signaling abnormalities have been observed in several brain regions following acute or chronic THC exposure (69,70). Thus, NAC may exert its protective properties through modulation of THC-induced ERK signaling dysregulation. NAC was previously reported to inhibit ERK activation by reducing oxidative stress (71). Notably, we observed some differences in ERK expression between the AcbSh and the AcbC, highlighting an overall NAC-induced decrease of ERK levels in the AcbSh and an increase in the AcbC. As previously reported, these two subregions are functionally dissociated, and therefore ERK signaling may differently modulate their roles. For example, it has previously been reported that the shell and core portions of the Acb are differentially involved in fear processing, which is mediated by mGluR1 and ERK/MAPK (mitogen-activated protein kinase) signaling selectively in the AcbSh but not in the AcbC (42).
We also observed that adolescent THC exposure induced a long-lasting increase in GAD65 expression levels in the AcbSh, and the concomitant administration of THC and NAC was protective against these abnormalities. GAD65 plays a crucial role in maintaining the excitatory/inhibitory balance, regulating the conversion from GLUT to GABA. Therefore, the observed increase in AcbSh GLUT levels might have been related to upregulation of GAD65 expression as a local compensatory mechanism for maintaining GLUT/GABA homeostasis.
Lastly, NAC administration prevented THC-induced reductions in mTOR selectively in the AcbC, which is an established biomarker for depressive- and anxiety-related disorders (72,73). Thus, NAC is capable of blocking THC-induced deficits in local mTOR expression, which may partially account for its neuroprotective effects.
In the AcbSh, we also observed that NAC alone increased BDNF and D1R levels. It has been shown that NAC exerts its therapeutic properties through multiple mechanisms, increasing cystine-glutamate antiporter activity, reducing inflammatory cytokines levels, and protecting against oxidative stress (74). These NAC-related effects may converge to promote cell survival and BDNF synthesis (74). Therefore, the increased BDNF levels observed in this study might have resulted from a complex interplay between several NAC-related mechanisms. Increasing cystine-glutamate antiporter activity, NAC has also been reported to modulate the activity of the mGluRs and facilitate dopamine release. This could potentially underlie the observed increase in D1R expression in the current study (74). Moreover, NAC-induced upregulation of D1R and BDNF expression may be interconnected. It has been reported that pharmacological D1R activation can enhance BDNF expression in hippocampal and striatal tissue slices (75). Further studies are required to fully explore the underlying mechanisms associated with these phenomena.
Conclusions
In summary, antioxidant treatment with NAC can powerfully prevent multiple behavioral, neuronal, and molecular pathophysiological effects associated with adolescent THC exposure through modulation of several biomarker pathways associated with oxidative stress mechanisms in the AcbSh or AcbC. The current studies relied exclusively on male rat cohorts, and while previous reports have suggested greater vulnerability of the male adolescent brain to THC, it will be important to extend these analyses to also include female rat cohorts to determine whether there are sex differences in the observed phenomena. Regardless, our study provides several novel discoveries that link the impacts of cannabinoid-induced striatal pathophysiology on markers for affective dysregulation, which is a concerning risk factor for chronic adolescent cannabis use. Importantly, the current findings suggest that antioxidant interventions may serve as a potential pharmacotherapeutic approach to the prevention of neurodevelopmental disorders induced by cannabis exposure.
Acknowledgments and Disclosures
This work was supported by the Canadian Institutes of Health Research (Grant No. MOP-123378 [to SRL]), the Natural Sciences and Engineering Research Council (to SRL), a BrainsCAN Postdoctoral Fellowship funded by Canada First Research Excellence Fund (to MDF), and a Natural Sciences and Engineering Research Council Graduate Fellowship (to MHS).
We thank Western’s MALDI facility for the MALDI-IMS data acquisition.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2024.100361.
Supplementary Material
References
- 1.Hengartner M.P., Angst J., Ajdacic-Gross V., Rössler W. Cannabis use during adolescence and the occurrence of depression, suicidality and anxiety disorder across adulthood: Findings from a longitudinal cohort study over 30 years. J Affect Disord. 2020;272:98–103. doi: 10.1016/j.jad.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 2.Gobbi G., Atkin T., Zytynski T., Wang S., Askari S., Boruff J., et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: A systematic review and meta-analysis. JAMA Psychiatry. 2019;76:426–434. doi: 10.1001/jamapsychiatry.2018.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis J.P., Pedersen E.R., Tucker J.S., Prindle J., Dunbar M.S., Rodriguez A., et al. Directional associations between cannabis use and anxiety symptoms from late adolescence through young adulthood. Drug Alcohol Depend. 2022;241 doi: 10.1016/j.drugalcdep.2022.109704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., De Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilman J.M., Kuster J.K., Lee S., Lee M.J., Kim B.W., Makris N., et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34:5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb B., Gorny G., Limebeer C.L., Parker L.A. Chronic treatment with Δ-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- 7.Kolb B., Li Y., Robinson T., Parker L.A. THC alters morphology of neurons in medial prefrontal cortex, orbital prefrontal cortex, and nucleus accumbens and alters the ability of later experience to promote structural plasticity. Synapse. 2018;72 doi: 10.1002/syn.22020. [DOI] [PubMed] [Google Scholar]
- 8.Hwang E.K., Lupica C.R. Altered corticolimbic control of the nucleus accumbens by long-term Δ9-tetrahydrocannabinol exposure. Biol Psychiatry. 2020;87:619–631. doi: 10.1016/j.biopsych.2019.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafferty C.K., Britt J.P. Cannabis exposure enhances subcortical control of nucleus accumbens activity. Biol Psychiatry. 2020;87:592–594. doi: 10.1016/j.biopsych.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman A.F., Oz M., Caulder T., Lupica C.R. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orihuel J., Capellán R., Roura-Martínez D., Ucha M., Ambrosio E., Higuera-Matas A. Δ9-tetrahydrocannabinol during adolescence reprograms the nucleus accumbens transcriptome, affecting reward processing, impulsivity, and specific aspects of cocaine addiction-like behavior in a sex-dependent manner. Int J Neuropsychopharmacol. 2021;24:920–933. doi: 10.1093/ijnp/pyab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L., Nan J., Lan Y. The nucleus accumbens: A common target in the comorbidity of depression and addiction. Front Neural Circuits. 2020;14:37. doi: 10.3389/fncir.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahm D.S. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- 14.Salgado S., Kaplitt M.G. The nucleus accumbens: A comprehensive review. Stereotact Funct Neurosurg. 2015;93:75–93. doi: 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- 15.Maria-Rios C.E., Murphy G.G., Morrow J.D. Subregional differences in medium spiny neuron intrinsic excitability properties between nucleus accumbens core and shell in male rats. eNeuro. 2023;10:1–13. doi: 10.1523/ENEURO.0432-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Chiara G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 17.Bossert J.M., Poles G.C., Wihbey K.A., Koya E., Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassoler F.M., Schmidt H.D., Gerard M.E., Famous K.R., Ciraulo D.A., Kornetsky C., et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28:8735–8739. doi: 10.1523/JNEUROSCI.5277-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi L.M., Reverte I., Ragozzino D., Badiani A., Venniro M., Caprioli D. Role of nucleus accumbens core but not shell in incubation of methamphetamine craving after voluntary abstinence. Neuropsychopharmacology. 2020;45:256–265. doi: 10.1038/s41386-019-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarafian T.A., Magallanes J.A., Shau H., Tashkin D., Roth M.D. Oxidative stress produced by marijuana smoke. An adverse effect enhanced by cannabinoids. Am J Respir Cell Mol Biol. 1999;20:1286–1293. doi: 10.1165/ajrcmb.20.6.3424. [DOI] [PubMed] [Google Scholar]
- 21.Bayazit H., Dulgeroglu D., Selek S. Brain-derived neurotrophic factor and oxidative stress in cannabis dependence. Neuropsychobiology. 2020;79:186–190. doi: 10.1159/000504626. [DOI] [PubMed] [Google Scholar]
- 22.Hovatta I., Juhila J., Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68:261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Fedoce A.D.G., Ferreira F., Bota R.G., Bonet-Costa V., Sun P.Y., Davies K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic Res. 2018;52:737–750. doi: 10.1080/10715762.2018.1475733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smaga I., Frankowska M., Filip M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br J Pharmacol. 2021;178:2569–2594. doi: 10.1111/bph.15456. [DOI] [PubMed] [Google Scholar]
- 25.Bradlow R.C.J., Berk M., Kalivas P.W., Back S.E., Kanaan R.A. The potential of N-acetyl-L-cysteine (NAC) in the treatment of psychiatric disorders. CNS Drugs. 2022;36:451–482. doi: 10.1007/s40263-022-00907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squeglia L.M., Fadus M.C., McClure E.A., Tomko R.L., Gray K.M. Pharmacological treatment of youth substance use disorders. J Child Adolesc Psychopharmacol. 2019;29:559–572. doi: 10.1089/cap.2019.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma R., Tikka S.K., Bhute A.R., Bastia B.K. N-acetyl cysteine in the treatment of cannabis use disorder: A systematic review of clinical trials. Addict Behav. 2022;129 doi: 10.1016/j.addbeh.2022.107283. [DOI] [PubMed] [Google Scholar]
- 28.Gray K.M., Carpenter M.J., Baker N.L., DeSantis S.M., Kryway E., Hartwell K.J., et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray K.M., Sonne S.C., McClure E.A., Ghitza U.E., Matthews A.G., McRae-Clark A.L., et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249–257. doi: 10.1016/j.drugalcdep.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer S., Neuhofer D., Chioma V.C., Garcia-Keller C., Schwartz D.J., Allen N., et al. A model of Δ9-tetrahydrocannabinol self-administration and reinstatement that alters synaptic plasticity in nucleus accumbens. Biol Psychiatry. 2018;84:601–610. doi: 10.1016/j.biopsych.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yager S., Forlenza M.J., Miller G.E. Depression and oxidative damage to lipids. Psychoneuroendocrinology. 2010;35:1356–1362. doi: 10.1016/j.psyneuen.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 32.De Felice M., Chen C., Rodríguez-Ruiz M., Szkudlarek H.J., Lam M., Sert S., et al. Adolescent Δ-9-tetrahydrocannabinol exposure induces differential acute and long-term neuronal and molecular disturbances in dorsal vs. ventral hippocampal subregions. Neuropsychopharmacology. 2023;48:540–551. doi: 10.1038/s41386-022-01496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Felice M., Renard J., Hudson R., Szkudlarek H.J., Pereira B.J., Schmid S., et al. L-theanine prevents long-term affective and cognitive side effects of adolescent Δ-9-tetrahydrocannabinol exposure and blocks associated molecular and neuronal abnormalities in the mesocorticolimbic circuitry. J Neurosci. 2021;41:739–750. doi: 10.1523/JNEUROSCI.1050-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Felice M., Laviolette S.R. Reversing the psychiatric effects of neurodevelopmental cannabinoid exposure: Exploring pharmacotherapeutic interventions for symptom improvement. Int J Mol Sci. 2021;22:1–16. doi: 10.3390/ijms22157861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castelli V., Lavanco G., D’Amico C., Feo S., Tringali G., Kuchar M., et al. CBD enhances the cognitive score of adolescent rats prenatally exposed to THC and fine-tunes relevant effectors of hippocampal plasticity. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1237485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pintori N., Caria F., De Luca M.A., Miliano C. THC and CBD: Villain versus hero? Insights into adolescent exposure. Int J Mol Sci. 2023;24:5251. doi: 10.3390/ijms24065251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudson R., Renard J., Norris C., Rushlow W.J., Laviolette S.R. Cannabidiol counteracts the psychotropic side-effects of Δ-9-tetrahydrocannabinol in the ventral hippocampus through bidirectional control of ERK1-2 phosphorylation. J Neurosci. 2019;39:8762–8777. doi: 10.1523/JNEUROSCI.0708-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson R., Norris C., Szkudlarek H.J., Khan D., Schmid S., Rushlow W.J., Laviolette S.R. Anxiety and cognitive-related effects of Δ 9-tetrahydrocannabinol (THC) are differentially mediated through distinct GSK-3 vs. Akt-mTOR pathways in the nucleus accumbens of male rats. Psychopharmacol (Berl) 2022;239:509–524. doi: 10.1007/s00213-021-06029-w. [DOI] [PubMed] [Google Scholar]
- 39.Meredith G.E., Agolia R., Arts M.P.M., Groenewegen H.J., Zahm D.S. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience. 1992;50:149–162. doi: 10.1016/0306-4522(92)90389-j. [DOI] [PubMed] [Google Scholar]
- 40.Castro D.C., Bruchas M.R. A motivational and neuropeptidergic hub: Anatomical and functional diversity within the nucleus accumbens shell. Neuron. 2019;102:529–552. doi: 10.1016/j.neuron.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corbit L.H., Muir J.L., Balleine B.W. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutta S., Beaver J., Halcomb C.J., Jasnow A.M. Dissociable roles of the nucleus accumbens core and shell subregions in the expression and extinction of conditioned fear. Neurobiol Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zocchi A., Girlanda E., Varnier G., Sartori I., Zanetti L., Wildish G.A., et al. Dopamine responsiveness to drugs of abuse: A shell-core investigation in the nucleus accumbens of the mouse. Synapse. 2003;50:293–302. doi: 10.1002/syn.10271. [DOI] [PubMed] [Google Scholar]
- 44.Chaudhri N., Sahuque L.L., Schairer W.W., Janak P.H. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitoussi A., Zunder J., Tan H., Laviolette S.R. Delta-9-tetrahydrocannabinol potentiates fear memory salience through functional modulation of mesolimbic dopaminergic activity states. Eur J Neurosci. 2018;47:1385–1400. doi: 10.1111/ejn.13951. [DOI] [PubMed] [Google Scholar]
- 46.Perrotti L.I., Weaver R.R., Robison B., Renthal W., Maze I., Yazdani S., et al. Distinct patterns of ΔFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madayag A., Lobner D., Kau K.S., Mantsch J.R., Abdulhameed O., Hearing M., et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrmann A.P., Andrejew R., Benvenutti R., Gama C.S., Elisabetsky E. Effects of N-acetylcysteine on amphetamine-induced sensitization in mice. Braz J Psychiatry. 2018;40:169–173. doi: 10.1590/1516-4446-2017-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Secci M.E., Mascia P., Sagheddu C., Beggiato S., Melis M., Borelli A.C., et al. Astrocytic mechanisms involving kynurenic acid control Δ9-tetrahydrocannabinol-induced increases in glutamate release in brain reward-processing areas. Mol Neurobiol. 2019;56:3563–3575. doi: 10.1007/s12035-018-1319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mason N.L., Theunissen E.L., Hutten N.R.P.W., Tse D.H.Y., Toennes S.W., Stiers P., Ramaekers J.G. Cannabis induced increase in striatal glutamate associated with loss of functional corticostriatal connectivity. Eur Neuropsychopharmacol. 2019;29:247–256. doi: 10.1016/j.euroneuro.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Bagot R.C., Parise E.M., Peña C.J., Zhang H.X., Maze I., Chaudhury D., et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renard J., Szkudlarek H.J., Kramar C.P., Jobson C.E.L., Moura K., Rushlow W.J., Laviolette S.R. Adolescent THC exposure causes enduring prefrontal cortical disruption of GABAergic inhibition and dysregulation of sub-cortical dopamine function. Sci Rep. 2017;7 doi: 10.1038/s41598-017-11645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anwyl R. Metabotropic glutamate receptors: Electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 54.Baker D.A., Xi Z.X., Shen H., Swanson C.J., Kalivas P.W. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xi Z.X., Shen H., Baker D.A., Kalivas P.W. Inhibition of non-vesicular glutamate release by group III metabotropic glutamate receptors in the nucleus accumbens. J Neurochem. 2003;87:1204–1212. doi: 10.1046/j.1471-4159.2003.02093.x. [DOI] [PubMed] [Google Scholar]
- 56.Mohan A., Pendyam S., Kalivas P.W., Nair S.S. Molecular diffusion model of neurotransmitter homeostasis around synapses supporting gradients. Neural Comput. 2011;23:984–1014. doi: 10.1162/NECO_a_00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McQueen G., Lally J., Collier T., Zelaya F., Lythgoe D.J., Barker G.J., et al. Effects of N-acetylcysteine on brain glutamate levels and resting perfusion in schizophrenia. Psychopharmacol (Berl) 2018;235:3045–3054. doi: 10.1007/s00213-018-4997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Limongi R., Jeon P., Théberge J., Palaniyappan L. Counteracting effects of glutathione on the glutamate-driven excitation/inhibition imbalance in first-episode schizophrenia: A 7T MRS and dynamic causal modeling study. Antioxidants (Basel) 2021;10:75. doi: 10.3390/antiox10010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raghu G., Berk M., Campochiaro P.A., Jaeschke H., Marenzi G., Richeldi L., et al. The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr Neuropharmacol. 2021;19:1202–1224. doi: 10.2174/1570159X19666201230144109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCullumsmith R.E., Meador-Woodruff J.H. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26:368–375. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- 61.Park E., Yu K.H., Kim D.K., Kim S., Sapkota K., Kim S.J., et al. Protective effects of N-acetylcysteine against monosodium glutamate-induced astrocytic cell death. Food Chem Toxicol. 2014;67:1–9. doi: 10.1016/j.fct.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 62.Liang M., Wang Z., Li H., Cai L., Pan J., He H., et al. L-arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem Toxicol. 2018;115:315–328. doi: 10.1016/j.fct.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 63.Ali-Sisto T., Tolmunen T., Viinamäki H., Mäntyselkä P., Valkonen-Korhonen M., Koivumaa-Honkanen H., et al. Global arginine bioavailability ratio is decreased in patients with major depressive disorder. J Affect Disord. 2018;229:145–151. doi: 10.1016/j.jad.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 64.Smriga M., Ando T., Akutsu M., Furukawa Y., Miwa K., Morinaga Y. Oral treatment with L-lysine and L-arginine reduces anxiety and basal cortisol levels in healthy humans. Biomed Res. 2007;28:85–90. doi: 10.2220/biomedres.28.85. [DOI] [PubMed] [Google Scholar]
- 65.Clark J.F., Doepke A., Filosa J.A., Wardle R.L., Lu A., Meeker T.J., Pyne-Geithman G.J. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. 2006;67:506–512. doi: 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 66.Satapathy S.K., Dehuri S., Jagadev A.K., Mishra S. Academic Press; Cambridge: 2019. EEG Brain Signal Classification for Epileptic Seizure Disorder Detection; pp. 1–25. [Google Scholar]
- 67.Liu J., Li J., Peng W., Feng M., Luo Y. EEG correlates of math anxiety during arithmetic problem solving: Implication for attention deficits. Neurosci Lett. 2019;703:191–197. doi: 10.1016/j.neulet.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 68.Schneider T.R., Hipp J.F., Domnick C., Carl C., Büchel C., Engel A.K. Modulation of neuronal oscillatory activity in the beta- and gamma-band is associated with current individual anxiety levels. Neuroimage. 2018;178:423–434. doi: 10.1016/j.neuroimage.2018.05.059. [DOI] [PubMed] [Google Scholar]
- 69.Rubino T., Forlani G., Viganò D., Zippel R., Parolaro D. Modulation of extracellular signal-regulated kinases cascade by chronic Δ9-tetrahydrocannabinol treatment. Mol Cell Neurosci. 2004;25:355–362. doi: 10.1016/j.mcn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Valjent E., Pagès C., Rogard M., Besson M.J., Maldonado R., Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 71.Wang D., Yu X., Brecher P. Nitric oxide and N-acetylcysteine inhibit the activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J Biol Chem. 1998;273:33027–33034. doi: 10.1074/jbc.273.49.33027. [DOI] [PubMed] [Google Scholar]
- 72.Réus G.Z., Quevedo J., Rodrigues A.L.S. MTOR signaling in the neuropathophysiology of depression: Current evidence. J Recept Ligand Channel Res. 2015;8:65–74. [Google Scholar]
- 73.Wang A., Zou X., Wu J., Ma Q., Yuan N., Ding F., et al. Early-life stress alters synaptic plasticity and mTOR signaling: Correlation with anxiety-like and cognition-related behavior. Front Genet. 2020;11 doi: 10.3389/fgene.2020.590068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dean O., Giorlando F., Berk M. N-acetylcysteine in psychiatry: Current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36:78–86. doi: 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams S.N., Undieh A.S. Dopamine D1-like receptor activation induces brain-derived neurotrophic factor protein expression. NeuroReport. 2009;20:606–610. doi: 10.1097/WNR.0b013e32832a0a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paxinos G., Watson C. Academic Press; San Diego, CA: 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.