Abstract
Insects belonging to the recent orders of the endopterygote clade (Lepidoptera, Diptera, Hymenoptera and Coleoptera) respond to bacterial challenge by the rapid and transient synthesis of a battery of potent antibacterial peptides which are secreted into their haemolymph. Here we present the first report on inducible antibacterial molecules in the sap-sucking bug Pyrrhocoris apterus, a representative species of the Hemiptera, which predated the Endoptergotes by at least 50 million years in evolution. We have isolated and characterized from immune blood of this species three novel peptides or polypeptides: (i) a 43-residue cysteine-rich anti-(Gram-positive bacteria) peptide which is a new member of the family of insect defensins; (ii) a 20-residue proline-rich peptide carrying an O-glycosylated substitution (N-acetylgalactosamine), active against Gram-negative bacteria; (iii) a 133-residue glycine-rich polypeptide also active against Gram-negative bacteria. The proline-rich peptide shows high sequence similarities with drosocin, an O-glycosylated antibacterial peptide from Drosophila, and also with the N-terminal domain of diptericin, an inducible 9 kDa antibacterial peptide from members of the order Diptera, whereas the glycine-rich peptide has similarities with the glycine-rich domain of diptericin. We discuss the evolutionary aspects of these findings.
Full text
PDF


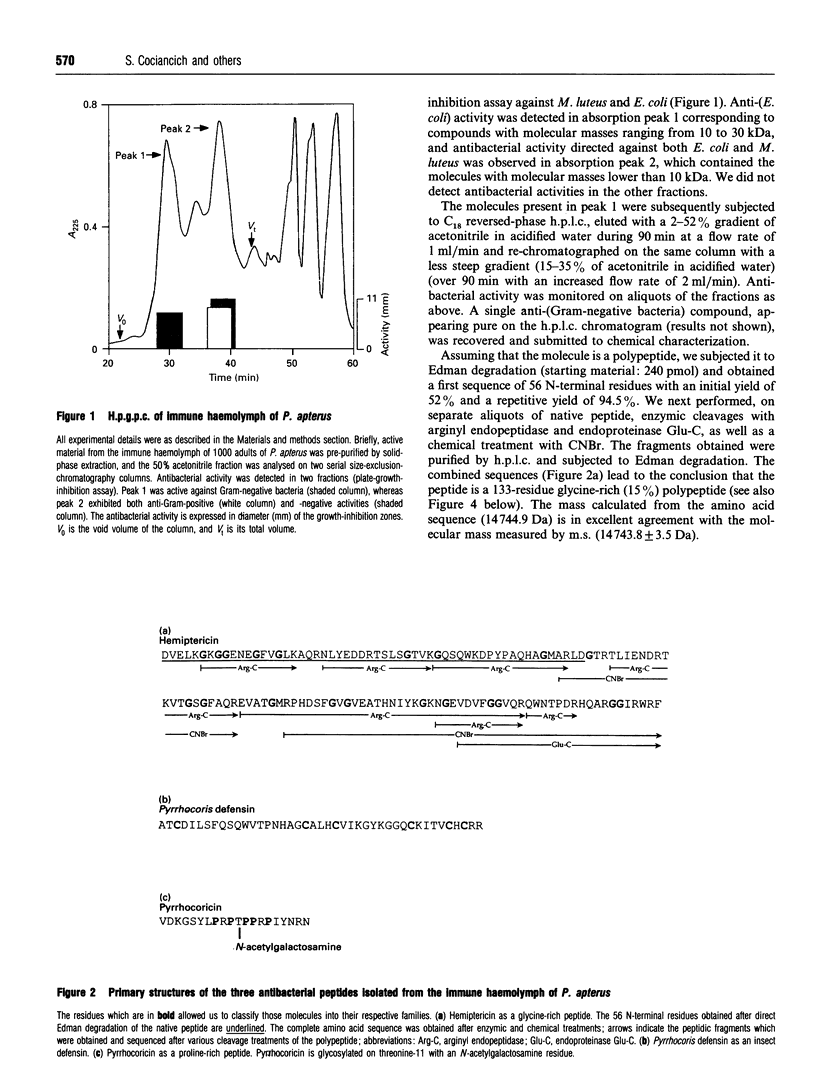
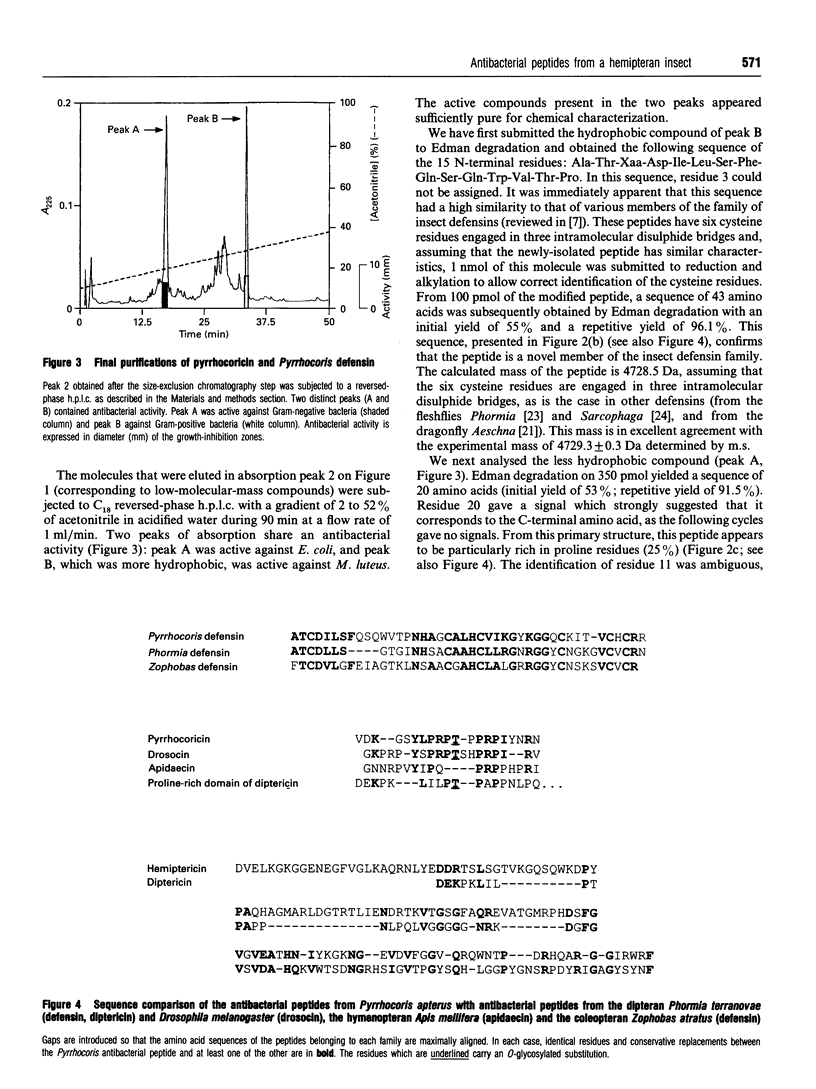



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando K., Natori S. Molecular cloning, sequencing, and characterization of cDNA for sarcotoxin IIA, an inducible antibacterial protein of Sarcophaga peregrina (flesh fly). Biochemistry. 1988 Mar 8;27(5):1715–1721. doi: 10.1021/bi00405a050. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Faye I., Gudmundsson G. H., Lee J. Y., Lidholm D. A. Cell-free immunity in Cecropia. A model system for antibacterial proteins. Eur J Biochem. 1991 Oct 1;201(1):23–31. doi: 10.1111/j.1432-1033.1991.tb16252.x. [DOI] [PubMed] [Google Scholar]
- Bonmatin J. M., Bonnat J. L., Gallet X., Vovelle F., Ptak M., Reichhart J. M., Hoffmann J. A., Keppi E., Legrain M., Achstetter T. Two-dimensional 1H NMR study of recombinant insect defensin A in water: resonance assignments, secondary structure and global folding. J Biomol NMR. 1992 May;2(3):235–256. doi: 10.1007/BF01875319. [DOI] [PubMed] [Google Scholar]
- Bulet P., Cociancich S., Dimarcq J. L., Lambert J., Reichhart J. M., Hoffmann D., Hetru C., Hoffmann J. A. Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J Biol Chem. 1991 Dec 25;266(36):24520–24525. [PubMed] [Google Scholar]
- Bulet P., Cociancich S., Reuland M., Sauber F., Bischoff R., Hegy G., Van Dorsselaer A., Hetru C., Hoffmann J. A. A novel insect defensin mediates the inducible antibacterial activity in larvae of the dragonfly Aeschna cyanea (Paleoptera, Odonata). Eur J Biochem. 1992 Nov 1;209(3):977–984. doi: 10.1111/j.1432-1033.1992.tb17371.x. [DOI] [PubMed] [Google Scholar]
- Bulet P., Dimarcq J. L., Hetru C., Lagueux M., Charlet M., Hegy G., Van Dorsselaer A., Hoffmann J. A. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J Biol Chem. 1993 Jul 15;268(20):14893–14897. [PubMed] [Google Scholar]
- Casteels P., Ampe C., Jacobs F., Tempst P. Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J Biol Chem. 1993 Apr 5;268(10):7044–7054. [PubMed] [Google Scholar]
- Casteels P., Ampe C., Jacobs F., Vaeck M., Tempst P. Apidaecins: antibacterial peptides from honeybees. EMBO J. 1989 Aug;8(8):2387–2391. doi: 10.1002/j.1460-2075.1989.tb08368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels P., Ampe C., Riviere L., Van Damme J., Elicone C., Fleming M., Jacobs F., Tempst P. Isolation and characterization of abaecin, a major antibacterial response peptide in the honeybee (Apis mellifera). Eur J Biochem. 1990 Jan 26;187(2):381–386. doi: 10.1111/j.1432-1033.1990.tb15315.x. [DOI] [PubMed] [Google Scholar]
- Cociancich S., Bulet P., Hetru C., Hoffmann J. A. The inducible antibacterial peptides of insects. Parasitol Today. 1994 Apr;10(4):132–139. doi: 10.1016/0169-4758(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Dimarcq J. L., Keppi E., Dunbar B., Lambert J., Reichhart J. M., Hoffmann D., Rankine S. M., Fothergill J. E., Hoffmann J. A. Insect immunity. Purification and characterization of a family of novel inducible antibacterial proteins from immunized larvae of the dipteran Phormia terranovae and complete amino-acid sequence of the predominant member, diptericin A. Eur J Biochem. 1988 Jan 15;171(1-2):17–22. doi: 10.1111/j.1432-1033.1988.tb13752.x. [DOI] [PubMed] [Google Scholar]
- Engström P., Carlsson A., Engström A., Tao Z. J., Bennich H. The antibacterial effect of attacins from the silk moth Hyalophora cecropia is directed against the outer membrane of Escherichia coli. EMBO J. 1984 Dec 20;3(13):3347–3351. doi: 10.1002/j.1460-2075.1984.tb02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley A. A., Classon B. J., Marschalek R., Williams K. L. Glycosylation sites identified by detection of glycosylated amino acids released from Edman degradation: the identification of Xaa-Pro-Xaa-Xaa as a motif for Thr-O-glycosylation. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1194–1201. doi: 10.1016/0006-291x(91)91019-9. [DOI] [PubMed] [Google Scholar]
- Hanzawa H., Shimada I., Kuzuhara T., Komano H., Kohda D., Inagaki F., Natori S., Arata Y. 1H nuclear magnetic resonance study of the solution conformation of an antibacterial protein, sapecin. FEBS Lett. 1990 Sep 3;269(2):413–420. doi: 10.1016/0014-5793(90)81206-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann J. A., Hetru C. Insect defensins: inducible antibacterial peptides. Immunol Today. 1992 Oct;13(10):411–415. doi: 10.1016/0167-5699(92)90092-L. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Andersson K., Steiner H., Bennich H., Boman H. G. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 1983;2(4):571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuhara T., Nakajima Y., Matsuyama K., Natori S. Determination of the disulfide array in sapecin, an antibacterial peptide of Sarcophaga peregrina (flesh fly). J Biochem. 1990 Apr;107(4):514–518. doi: 10.1093/oxfordjournals.jbchem.a123077. [DOI] [PubMed] [Google Scholar]
- Labandeira C. C., Sepkoski J. J., Jr Insect diversity in the fossil record. Science. 1993 Jul 16;261(5119):310–315. doi: 10.1126/science.11536548. [DOI] [PubMed] [Google Scholar]
- Lambert J., Keppi E., Dimarcq J. L., Wicker C., Reichhart J. M., Dunbar B., Lepage P., Van Dorsselaer A., Hoffmann J., Fothergill J. Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(1):262–266. doi: 10.1073/pnas.86.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage P., Bitsch F., Roecklin D., Keppi E., Dimarcq J. L., Reichhart J. M., Hoffmann J. A., Roitsch C., Van Dorseelaer A. Determination of disulfide bridges in natural and recombinant insect defensin A. Eur J Biochem. 1991 Mar 28;196(3):735–742. doi: 10.1111/j.1432-1033.1991.tb15872.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Okada M., Takahashi H., Ming Q. X., Nakajima Y., Nakanishi Y., Komano H., Natori S. Molecular cloning of a cDNA and assignment of the C-terminal of sarcotoxin IA, a potent antibacterial protein of Sarcophaga peregrina. Biochem J. 1986 Nov 1;239(3):717–722. doi: 10.1042/bj2390717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K., Natori S. Purification of three antibacterial proteins from the culture medium of NIH-Sape-4, an embryonic cell line of Sarcophaga peregrina. J Biol Chem. 1988 Nov 15;263(32):17112–17116. [PubMed] [Google Scholar]
- O'Connell B. C., Hagen F. K., Tabak L. A. The influence of flanking sequence on the O-glycosylation of threonine in vitro. J Biol Chem. 1992 Dec 15;267(35):25010–25018. [PubMed] [Google Scholar]
- Steiner H., Hultmark D., Engström A., Bennich H., Boman H. G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981 Jul 16;292(5820):246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- Wicker C., Reichhart J. M., Hoffmann D., Hultmark D., Samakovlis C., Hoffmann J. A. Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J Biol Chem. 1990 Dec 25;265(36):22493–22498. [PubMed] [Google Scholar]
- Wilson I. B., Gavel Y., von Heijne G. Amino acid distributions around O-linked glycosylation sites. Biochem J. 1991 Apr 15;275(Pt 2):529–534. doi: 10.1042/bj2750529. [DOI] [PMC free article] [PubMed] [Google Scholar]