Abstract
Background
Spinal surgeries are a common procedure, but there is significant risk of adverse events following these operations. While the rate of adverse events ranges from 8% to 18%, surgical site infections (SSIs) alone occur in between 1% and 4% of spinal surgeries.
Methods
We completed a systematic review addressing factors that contribute to surgical site infection after spinal surgery. From the included studies, we separated the articles into groups based on whether they propose a clinical predictive tool or model. We then compared the prediction variables, model development, model validation, and model performance.
Results
About 47 articles were included in this study: 10 proposed a model and 5 validated a model. The models were developed from 7,720 participants in total and 210 participants with SSI. Only one of the proposed models was externally validated by an independent group. The other 4 validation papers examined the performance of the ACS NSQIP surgical risk calculator.
Conclusions
While some preoperative risk models have been validated, and even successfully implemented clinically, the significance of postoperative SSIs and the unique susceptibility of spine surgery patients merits the development of a spine-specific preoperative risk model. Additionally, comprehensive and stratified risk modeling for SSI would be of invaluable clinical utility and greatly improve the field of spine surgery.
Keywords: Surgical site infection, Prediction model, Spine surgery
Introduction
Approximately 313 million surgical procedures were performed worldwide in 2012, and the number continues to increase each year [1]. From 2003 to 2017 the number of spinal surgeries performed increased by 2.4 times, with spinal fusions increasing from 287,600 to 488,300 procedures annually, a nearly 70 percent increase over a 10-year period [2,3]. With increases in the number of spinal procedures there is also an increase in the number of postoperative complications. About 16.4% of patients experienced postoperative complications, 17.8% in thoracolumbar procedures and 8.9% in cervical procedures [4]. Surgical site infections (SSI) continue to be a challenging clinical problem. Ranging from 1% to 4% incidence in spinal surgeries, SSIs can result in increased patient morbidity, mortality, and health care costs [5]. The treatment of SSI can range from administering antibiotics to reoperation. SSIs can also result in readmission and an average increased hospital stay of nearly 10 days. This has led to an approximate $ 345 million spent yearly on preventable SSIs [5]. In an effort to decrease the incidence of SSI, certain maneuvers such as preoperative antibiotics, bacterial screening, betadine irrigation, intrawound vancomycin powder, and surgical drains have been used with varying success.
The use of preoperative risk assessment tools has become increasingly common. Knowledge of risks may alter the pre- and postoperative care and impact whether surgery is recommended. Preoperatively patients may undergo screening through the revised cardiac risk index (RCRI) assessment tool to determine the 30-day risk for adverse cardiac events. Similarly, the ACS NSQIP Surgical Risk Calculator is used to determine the risk of numerous complications based on patient characteristics and comorbidities. Before the introduction of these predictive tools, identification of risk factors was historically determined by the experience of the surgeon [6]. These methods do not account for individual patient risk factors and thus can vary significantly between surgeons. Risk assessment tools also go beyond just recognizing a single factor or patient characteristic that may contribute poorer outcomes. These predictive models are developed to account for confounding variables and to consider several parameters that calculate an overall risk. In addition, risk factors only conclude a correlation between some variable and the outcome whereas a prediction model quantifies the impact that a variable has on a certain outcome [Ref]. This is why predictive risk assessment tools such as the RCRI and NSQIP Surgical Risk Calculator have been instrumental in decreasing adverse events, allowing surgeons to make decisions based on individual patient risks through empirical data.
Although the use of risk calculators has become commonplace, there is a lack of spine surgery-specific risk assessment tools. SpineSage is the only predictive tool used specifically for spine procedures; however, the output of the calculator is nonspecific, looking at major complications, all complications, dural tear, and infection as a whole [41]. Although this is a useful first step, it is not able to differentiate between specific complications such as SSIs, which impacts the specificity of care that the patient may receive. The aim of this study is to address the gaps that are found in the current spine surgery predictive software tools via a systematic review of publications that propose predictive models, specifically for SSI in spine surgery. We found that over the last 14 years, there has been a lack of development for these prediction models. Of the proposed models, few have completed external validation that would be necessary to integrate them into medical practice.
Methods
Study selection
We completed an extensive query of PubMed, Scopus, and Web of Knowledge databases for articles related to surgical site infection after spinal surgery published between January 2008 and December 2022. Detailed search criteria for all databases are outlined in Supplementary Table 1. We included full articles that were available in English. We excluded reviews, meta-analyses, case reports, and abstracts from our review. The most recent PRISMA guidelines were adhered to in this analysis [7]. This project was registered with Prospero ID CRD42023412025.
From the included papers, we completed a manual search to categorize the studies as “doesn't propose model”, “proposes model”, or “validates model”. For this study, a “model” involves a clinical tool that can be utilized to predict outcomes of a patient. We included models for use for any type of SSI, including deep and superficial. We excluded models that didn't separate SSI outcomes from other complications, but we did include models that were not specific to predict SSI alone.
Two independent reviewers completed a title and abstract screen on all papers. Disagreements between the 2 reviewers were included in the full text review. Both reviewers then completed a full text review according to the inclusion and exclusion criteria. Any disagreements between reviewers were settled by a third independent reviewer. Forward (cited in the included articles) and backward (cited the included articles) citation screen was completed for any additional articles that fit the scope of the review.
Quality assessment and risk of bias
Publication quality was assessed using a framework based on the Quality in Prognosis Studies (QUIPS) tool and previously used in Velzel et al. to quantify bias for prediction models in a study [8]. This tool analyzes 4 aspects of the study (participants, predictors, outcome, and analysis) to classify the study as low, moderate, or high risk of bias and overall quality. There are 11 criteria within these 4 aspects, and each criterion can be graded as “Yes”, “Partly”, or “No”. For assessing each paper, “yes” was required in at least 10 criteria to be considered low risk of bias and high overall quality. Between 6 and 9 “yes” criteria were deemed moderate risk of bias and moderate overall quality. Fewer than 6 “yes” criteria were considered high risk of bias and low overall quality.
Predictive variables and model assessment
Reviewers extracted information from the included articles relating to article data, variable assessment, participant demographics, and model development, validation, and performance.
Variables were collected from each included study based on if a statistical test was used to assess correlation between the measurement and the incidence of SSI. For each variable, it was indicated if the relationship was found to be statistically significant in univariate and multivariate analysis. For this study “N-'' meant a variable was not found to have a statistically significant relationship in the univariate analysis and therefore was not included in the multivariate. “N+” indicated it was statistically significant in the univariate but not the multivariate analysis. “Y-” indicated a variable was included in the multivariate even though it was not found to be statistically significant in the univariate analysis. “Y+” meant a variable was found to be statistically significant in both the univariate and multivariate analysis. In the variable table, “x” indicates that a variable wasn't studied, and “x(A)” means that every participant was positive for that variable. The predictive variables were separated into 7 domains: patient demographic, comorbidities, pre- and postoperative lab values, imaging studies, assessment scales, pre- and intra-operative characteristics, and postoperative characteristics based on the type and time of the measurement. The lab values domain includes both pre- and postoperative labs, and a study was determined to indicate significance if either pre- or postoperative lab values were significant. In ideal prediction models, variables measured preoperatively will be used to assess patient risk before undertaking the intervention. In the case of SSI, variables that occur intraoperatively or postoperatively can still be utilized to adjust postoperative care as the prediction model indicates.
The development of a model ideally involves completing univariate and multivariate analyses that allows the authors to create a calculator for clinical use. The calculator type can range from score chart to equation to nomogram. To differentiate this from an analysis without clinical applications, we only included studies that included a calculator in our review of model proposing studies.
Validation involves studying the accuracy of the model in a cohort that is distinct from the one used to develop the model. This can be completed internally by cross-validation, bootstrapping, or using a separate training and testing cohort from the original sample. Validation can also occur externally from the same demographic population during a different time period or with different sample demographics.
The overall performance of the models included in this study were assessed based on their reported calibration and discrimination metrics. Calibration of the model is usually reported via a calibration plot or Homer-Lemeshow test, and discrimination is reported as AUC of the ROC curve or c-statistic, which is mathematically equivalent for binary statistics such as the incidence of SSI.
Results
Articles included and descriptions
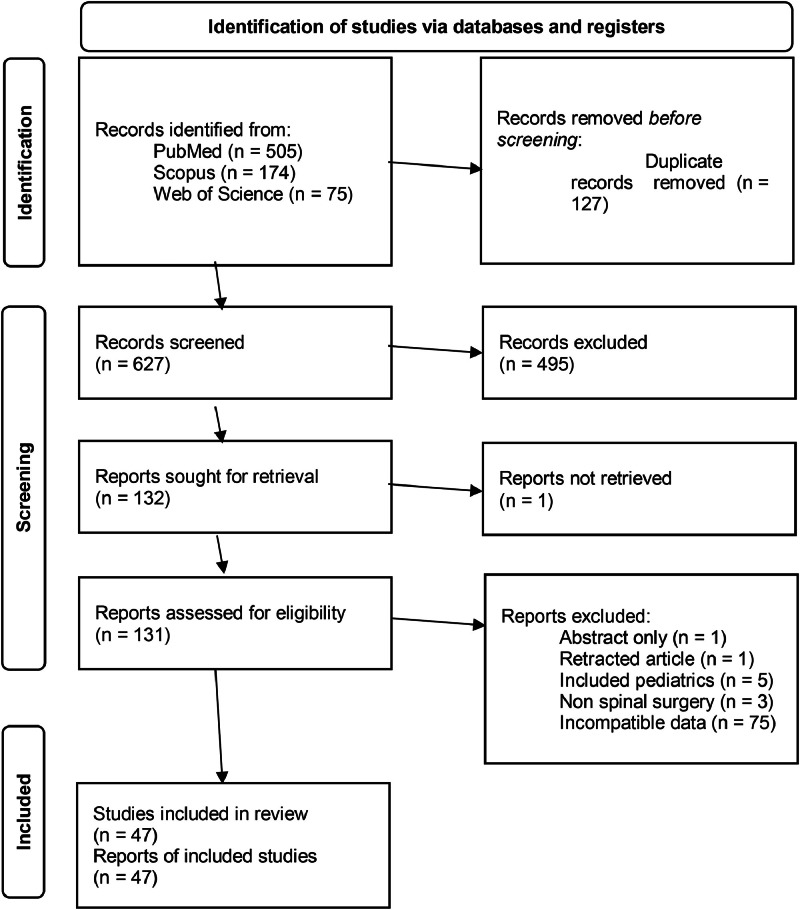
The search for articles between January 2008 and December 2022 returned 754 publications (505 from PubMed, 174 from Scopus, and 75 from Web of Science). About 127 duplicates were removed, 495 publications were removed during the abstract screening, and one was not retrieved because the full text was not available. Of the 131 papers that were assessed, 85 were removed (1 was a retracted publication, 1 was an abstract only, 5 included pediatrics participants, 3 included other types of nonspine surgeries in the analysis, and 75 did not report surgical site infection statistics in a format compatible with this study). Ultimately, 47 publications were included in this systematic review (Fig. 1). The quality of each paper is displayed in Supplementary Table 3. Based on the QUIPS assessment, 35 publications had low bias and high quality, 12 had moderate bias and quality, and no papers had high bias and low quality. All 10 studies that proposed a model had low risk of bias and high overall quality. No papers were excluded from this review based on quality assessment scores.
Fig. 1.
PRISMA diagram for study inclusion.
Of the 47 included publications, 43 of the studies were retrospective cohort studies and 4 were prospective cohort studies. Ten studies proposed a clinical model to calculate risk of surgical site infection postspinal surgery, 5 assessed the validation of a previously proposed model, and 32 publications assessed risk factors associated with SSI, but did not develop a clinical model to predict the risk.
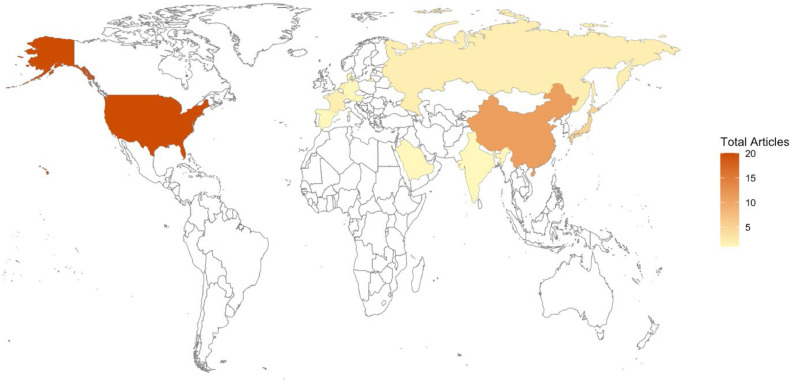
Supplementary Table 2 outlines the key aspects of each study [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], including inclusion and exclusion criteria, location, and demographics. Out of the included articles, 19 were completed in the United States, 11 were completed in China, 4 in Japan, 2 each in Russia, France, and the Netherlands, 1 each in German, Austria, Spain, Singapore, Saudi Arabia, and India, and 1 was a multicountry study including the USA, Denmark, and Japan (Table 1 and Fig. 2). All studies included a total of 1391,793 participants. The 10 models proposed were based on 7,720 participants in total and 210 participants with SSI (Supplementary Table 1).
Table 1.
Breakdown of included studies by the location of each study.
| Region | Primary Research Articles | Models developed | Models validated |
|---|---|---|---|
| Europe | |||
| Netherlands | 2 | 1 | 1 |
| France | 2 | 0 | 0 |
| Denmark | 1 | 0 | 0 |
| Germany | 1 | 0 | 0 |
| Austria | 1 | 0 | 0 |
| Spain | 1 | 0 | 0 |
| Asia | |||
| China | 11 | 2 | 1 |
| Japan | 5 | 1 | 0 |
| Singapore | 1 | 0 | 0 |
| Other | |||
| USA | 20 | 5 | 3 |
| Russia | 2 | 1 | 0 |
| Saudi Arabia | 1 | 0 | 0 |
| India | 1 | 0 | 0 |
Fig. 2.
Map indicating the location of the publications for this paper.
Predictor variables
From all 47 studies, 113 different variables were evaluated as a potential factor in SSI. The possible predictor variables are broken into 7 domains: patient demographic, comorbidities, pre- and postoperative lab values, imaging studies, assessment scales, pre- and intraoperative characteristics, and postoperative characteristics (Supplementary Table 3). Supplementary Table 4 outlines the factors that are included in the assessment scales used in the included articles. The most commonly assessed variable was age with 42 out of 47 of the studies, followed by BMI (35/47) and sex (33/47). However, age and sex were found to be insignificant in the univariate analysis in 26 and 24 studies, respectively. BMI was significantly different between patients with and without SSI in 20 of the studies. In these studies, increased BMI was associated with increased risk of SSI.
From the studies that proposed a model, 53 different variables were assessed for potential inclusion. Investigating the 10 models proposed by the included studies revealed that age and BMI were assessed in all the studies. Diabetes mellitus and surgery type were assessed in 9 and 8 studies, respectively. However, diabetes mellitus (DM) and BMI was the most included factor in the final model (7 studies each), followed by cardiovascular pathology, serum albumin, type of surgery, and operation time (3 studies each). A diagnosis of diabetes mellitus or a cardiovascular pathology was associated with increased risk of SSI. Increased BMI, decreased serum albumin, and increased operation time increased SSI risk. For surgery type, traumatic or emergency spine surgeries were more likely to result in SSI. The odds ratios ranged between 1.5 and 3.833 for DM and between 1.1 and 1.98 for BMI in these models. For the rest of the common variables the odd ratio ranges were as follows: cardiovascular pathology (2.19–3.067), serum albumin (0.546–0.872), type of surgery (1.723–4.63), and operation time (1.013–1.29). The assessment of each variable is displayed in Supplementary Table 4. The proposed models are included in Table 2.
Table 2.
Summary of the models proposed for SSI prediction.
| Dataset Reference | Proposed Model |
|---|---|
| Equations | |
| Kmlemencsics 2016 | 0.5 (if age > 54 years) + 1.13 (if arrhythmia) + 0.68 (if BMI > 28 kg/m2) + 2.66 (if chronic liver disease) + 1.1 (if insulin-dependent diabetes mellitus) + 0.78 (if ischemic heart disease) + 2.07 (if systemic immune disease) |
| Janssen 2019 | – 4.159 + 0.014 * Age + – 0.631 (if BMI 25 – 30) + 0.510 (if obese) + 0.524 * ASA + – 0.578 (if Degenerative or revision) + 0.226 (if NSAIDs used) |
| Lubelski 2021 | –9.708 + 1.112 (if female) + 0.064 * BMI + 1.021 (if actively smoking) + 0.400 (if diabetes mellitus) + 0.754 * ASA status class + 0.087 * surgical invasiveness score |
| Stratified Risk | |
| Namba 2020 | + 3 (if skin disease) + 3 (if low albumin) + 2 (if emergency operation) + 2 (if blood loss) + 1 (if diabetes mellitus) Groups: 0–1 = Normal risk, 2 = Moderate, ≥3 = High) |
| Stepanov 2021 | +1 (BMI) +1 (diabetes mellitus) +1 (preoperative serum calcium) +1 (preoperative serum glucose) +1 (preoperative albumin) +1 (number of operative segments) +1 (operation time) +1 (estimate blood loss) +1 (postoperative hemoglobin) +1 (postoperative albumin) +1 (drain time) Groups: 0 = Low, 1–4 = Intermediate, 5–8 = High, >8 = Extremely high) |
| Web Applications | |
| Lee 2014 | https://depts.washington.edu/spinersk/ |
| Hersh 2021 | https://jhuspine4.shinyapps.io/MetsWoundComplications/ |
| Liu 2022 | https://liuwencai3-ssi-ssi-3pmjcp.streamlit.app/ |
| Nomograms | |
| Lubelski 2018 | Nomogram using race, BMI, disk herniation, myelopathy, surgical approach, number of levels, terminal level, and history of cancer |
| Chen 2022 | Nomogram using preoperative albumin, operative time, number of lesion segments, and incision length |
Model development, validation, and performance assessment
Supplementary Table 5 summarizes the stage of model development, validation, and performance as well as conclusions of each study. Of the 10 models included, 8 studies had completed internal validation, but did not complete external validation. One model completed external validation without internal validations. Only 1 model had internal and external validation completed. Five studies underwent internal validation via bootstrapping and 4 were done via cross-validation. Two studies had temporal validation completed at 2 different timeframes, but only one had external validation from a separate publication using a different cohort [40,42]. One of the 5 included validation papers completed external validation on the Lee model [41], and the other 4 papers validated the ACS NSQIP Surgical Risk Calculator.
The 10 models were presented as either equations (3), stratified score charts (2), web applications (3), or nomograms (2). Table 2 shows the calculator of each model in a standardized format or the link at which the web application can be found.
Only 1 model demonstrated calibration via a calibration plot [52], which is the gold standard. Five models performed a Hosmer-Lemeshow statistical test. Three studies calibrated their models by comparing AUC between different cohorts, and 1 completed a LASSO calibration. Two studies did not complete calibration [54,55].
A metric for model discrimination was reported by a value for the AUC of the ROC curve (or equivalent c-statistic) was reported in all 10 studies that proposed a model. The AUC was greatest in Liu 2022 at 0.923 and lowest in Janssen 2019 (0.72). All but 1 study included the ROC curve as a figure.
Discussion
Recommendations for clinical practice
This systematic review found that nearly all predictive modeling studies of SSI isolated contributory variables through correlational studies of large databases. However, most did not include a true model, but rather an evaluation of statistically significant variables correlated with SSI in a given analysis. While many such variables are already well-established indicators of surgical risk (i.e. diabetes, smoking, anemia, etc.), the statistical significance of these biopsychosocial factors reaffirms their clinical use as rapid risk assessment factors.
Several studies did establish and internally validate preoperative risk assessment tools, though few included external validation. Of the studies with predictive models, most assessed SSI as an outcome. Of note, not all studies with predictive models analyzed SSIs homogeneously; some included all SSI as 1 outcome, while others stratified SSI by depth of infection. Variation in SSI categorization and analysis may be due to inconsistent data availability between different studies. As the majority of the risk assessment tools evaluated in this study predicted total complication risk—rather than exclusively SSI risk—the clinical significance variable SSI definition in predictive modeling remains unclear.
As surgical risk assessment is of critical importance for the provision of optimal care, only the most well-established and externally validated models warrant widespread clinical use. To date, the ACS NSQIP Surgical Risk Calculator remains the most validated risk assessment tool for surgical candidates and merits continued use until another model proves more accurate or specific. Although, there are still limitations of this calculator, including that it utilizes a single CPT code for its calculations, which limits the accuracy of the prediction and constraints the surgeon to select certain criteria over another. It also does not account for a difference in approach to the procedure, which can severely affect risk and outcomes. Overall, this indicates a need for improved risk assessment models in the context of spinal surgeries.
Areas for improvement and future research
Despite the large number of SSI papers proposing predictive models, there is still much that can be improved upon. Nine out of the 10 true predictive model publications performed internal validation, while only 2 performed external validation. More consistent external validation can help to prove the effectiveness of the proposed model compared to similar pre-existing models, such as ACS NSQIP Surgical Risk Calculator.
In addition, the predictive model studies were performed in the USA, China, Netherlands, Japan, and Russia with over half being performed in the USA. Only 1 study had a diverse cohort during external validation [41]. Different geographical locations could have significant differences in physician practices, patient demographics, and culture that could make a predictive model inaccurate. Moving forward, there should not only be an increase in external validation in general, but validation on patients from different demographic groups. This would help validate that the predictive model could be used on the greater population rather than the population used in their cohort.
Although there are several risk assessment tools and calculators for surgical risk in general, such as ACS NSQIP Surgical Risk Calculator and Revised Cardiac Risk Index, there are no spine specific risk assessment tools. The publications reviewed in this study are making efforts toward creating a true predictive tool for the risk factors associated with spinal surgery, however, further prospective studies should continue to be performed to increase the number of predictive models published. This will allow for comparison between these various models and further the efficacy of spinal surgery risk assessment tools proposed in the future. Future attempts of model development should also consider useability of their tool both by ease of access and data input. This may be possible by integrating the system with current electronic medical records that will allow it pull patient data from the chart and does not require the physician to separately navigate to an online tool. Improved integration with patient records will also allow the calculator to account for multiple injuries, procedures, or approaches that may be used.
Limitations
While each of the reviewed publications included predictions of SSIs based on various factors, the definitions of SSI between each paper were variable. Each publication used some combination of deep, superficial, or organ space SSIs to define their variable while some studies analyzed each type individually. It is difficult to meaningfully perform a meta-analysis comparing the effects of patient characteristics and comorbidities on the various definitions of SSI. In that same vein, this study looks into spinal surgeries as a whole and does not take into consideration the variation in the data based on the approach or type of surgery performed. To improve accuracy of the risk assessment, models should have clearly defined populations, procedures, and approaches that they were developed on. This would also assist physicians in understanding which patients that model may appropriately be used for. Since these publications came from a variety of centers, there parameters for patients may differ, such as considering HA1C instead of diabetes mellitus overall. From the limited data in the publications, it is not possible to account for these variations in data use. Due to the heterogeneity of the data acquired based on the definition of SSI, variable predictive factors, and differences in type of surgery we were unable to perform a meta-analysis.
Conclusion
Research has established that SSIs have deleterious effects on postoperative restoration of function, reduction of pain, and reoperation risk in patients who undergo spine surgery. Furthermore, spine surgeries continue to increase worldwide, and those performed by posterolateral approaches uniquely predispose patients to decubitus wound pressure that can increase the risks of SSI and other wound complications. While some preoperative risk models (i.e. ACS NSQIP) have been validated, and even successfully implemented clinically, the significance of postoperative SSIs and the unique susceptibility of spine surgery patients thereto merits the development of a spine-specific preoperative risk model. Additionally, comprehensive and stratified risk modeling (i.e. SSI risk, sepsis risk, reoperation risk, mortality risk, overall complication risk) would be of invaluable clinical utility and greatly improve outcomes in patients undergoing spine surgery.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: ARL: Nothing to disclose. SB: Nothing to disclose. AF: Nothing to disclose. GP: Nothing to disclose. JNG: Nothing to disclose. PMA: Nothing to disclose.
Given his role as Editor in Chief, Jonathan Grauer, MD had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Tobias Mattei, MD.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2024.100518.
Appendix. Supplementary materials
References
- 1.Weiser T.G., Haynes A.B., Molina G., et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. The Lancet. 2015;385(Supplement 2):S11. doi: 10.1016/S0140-6736(15)60806-6. ISSN 140-6736. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi K., Sato K., Kato F., et al. Trends in the numbers of spine surgeries and spine surgeons over the past 15 years. Nagoya J Med Sci. 2022;84(1):155–162. doi: 10.18999/nagjms.84.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss A.J., Elixhauser A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Agency for Healthcare Research and Quality (US); RockvilleMD: 2006. Trends in operating room procedures in U.S. Hospitals, 2001–2011.https://www.ncbi.nlm.nih.gov/books/NBK201926/ Statistical Brief #171. Available from: [PubMed] [Google Scholar]
- 4.Nasser R., Yadla S., Maltenfort M.G., et al. Complications in spine surgery. J Neurosurg Spine. 2010;13(2):144–157. doi: 10.3171/2010.3.SPINE09369. [DOI] [PubMed] [Google Scholar]
- 5.Aleem I.S., Tan L.A., Nassr A., et al. Surgical site infection prevention following spine surgery. Global Spine J. 2020;10(1 Suppl):92S–98S. doi: 10.1177/2192568219844228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilimoria K.Y., Liu Y., Paruch J.L., et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velzel J., de Hundt M., Mulder F.M., et al. Prediction models for successful external cephalic version: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2015;195:160–167. doi: 10.1016/j.ejogrb.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Chen K.W., Yang H.L., Lu J., et al. Risk factors for postoperative wound infections of sacral chordoma after surgical excision. J Spinal Disord Tech. 2011;24(4):230–234. doi: 10.1097/BSD.0b013e3181ea478a. [DOI] [PubMed] [Google Scholar]
- 10.Bekelis K., Desai A., Bakhoum S.F., et al. A predictive model of complications after spine surgery: the National Surgical Quality Improvement Program (NSQIP) 2005-2010. Spine J. 2014;14(7):1247–1255. doi: 10.1016/j.spinee.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Ee W.W., Lau W.L., Yeo W., et al. Does minimally invasive surgery have a lower risk of surgical site infections compared with open spinal surgery? Clin Orthop Relat Res. 2014;472(6):1718–1724. doi: 10.1007/s11999-013-3158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad S., Millhouse P.W., Maltenfort M., et al. Diagnosis and neurologic status as predictors of surgical site infection in primary cervical spinal surgery. Spine J. 2016;16(5):632–642. doi: 10.1016/j.spinee.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Jalai C.M., Worley N., Poorman G.W., et al. Surgical site infections following operative management of cervical spondylotic myelopathy: prevalence, predictors of occurrence, and influence on peri-operative outcomes. Eur Spine J. 2016;25(6):1891–1896. doi: 10.1007/s00586-016-4501-9. [DOI] [PubMed] [Google Scholar]
- 14.Macki M., Uzosike A., Kerezoudis P., et al. Duration of indwelling drain following instrumented posterolateral fusion of the lumbar spine does not predict surgical site infection requiring reoperation. J Clin Neurosci. 2017;40:44–48. doi: 10.1016/j.jocn.2016.12.008. https://www.sciencedirect.com/science/article/pii/S0967586816310396 ISSN 0967-5868. [DOI] [PubMed] [Google Scholar]
- 15.Glassman S., Carreon L.Y., Andersen M., et al. Predictors of hospital readmission and surgical site infection in the United States, Denmark, and Japan: is risk stratification a universal language? Spine (Phila Pa 1976) 2017;42(17):1311–1315. doi: 10.1097/BRS.0000000000002082. [DOI] [PubMed] [Google Scholar]
- 16.Hijas-Gómez A.I., Egea-Gámez R.M., Martínez-Martín J., et al. Surgical wound infection rates and risk factors in spinal fusion in a University Teaching Hospital in Madrid, Spain. Spine (Phila Pa 1976) 2017;42(10):748–754. doi: 10.1097/BRS.0000000000001916. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K., Ando K., Ito K., et al. Prediction of surgical site infection in spine surgery from tests of nasal MRSA colonization and drain tip culture. Eur J Orthop Surg Traumatol. 2018;28:1053–1057. doi: 10.1007/s00590-018-2163-5. [DOI] [PubMed] [Google Scholar]
- 18.Haydarov V.M., Tkachenko A.N., Kirilova I.A., et al. Prediction of surgical site infection in spine surgery. Russ J Spine Surg (Khirurgiya Pozvonochnika) 2018;15(2):84–90. doi: 10.14531/ss2018.2.84-90. [DOI] [Google Scholar]
- 19.Peng W., Liang Y., Lu T., et al. Multivariate analysis of incision infection after posterior lumbar surgery in diabetic patients: a single-center retrospective analysis. Medicine (Baltimore) 2019;98(23):e15935. doi: 10.1097/MD.0000000000015935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennington Z., Lubelski D., Molina C., et al. Prolonged post-surgical drain retention increases risk for deep wound infection after spine surgery. World Neurosurg. 2019;130:e846–e853. doi: 10.1016/j.wneu.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 21.El-Kadi M., Donovan E., Kerr L., et al. Risk factors for postoperative spinal infection: a retrospective analysis of 5065 cases. Surg Neurol Int. 2019;10:121. doi: 10.25259/SNI-284-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopkins B.S., Mazmudar A., Driscoll C., et al. Using artificial intelligence (AI) to predict postoperative surgical site infection: a retrospective cohort of 4046 posterior spinal fusions. Clin Neurol Neurosurg. 2020;192 doi: 10.1016/j.clineuro.2020.105718. [DOI] [PubMed] [Google Scholar]
- 23.Bratschitsch G., Puchwein P., Zollner-Schwetz I., et al. Spinal surgery site infection leading to implant loosening is influenced by the number of prior operations. Global Spine J. 2022;12(3):458–463. doi: 10.1177/2192568220957268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi Y., Inose H., Ushio S., et al. Body mass index and modified glasgow prognostic score are useful predictors of surgical site infection after spinal instrumentation surgery. Spine. 2020;45(3):E148–E154. doi: 10.1097/BRS.0000000000003226. [DOI] [PubMed] [Google Scholar]
- 25.Sang C., Chen X., Ren H., et al. Correlation between lumbar multifidus fat infiltration and lumbar postoperative infection: a retrospective case-control study. BMC Surg. 2020;20(1):35. doi: 10.1186/s12893-019-0655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pradip I., Dilip Chand Raja S., Rajasekaran S., et al. Presence of preoperative Modic changes and severity of endplate damage score are independent risk factors for developing postoperative surgical site infection: a retrospective case-control study of 1124 patients. Eur Spine J. 2021;30:1732–1743. doi: 10.1007/s00586-020-06581-7. [DOI] [PubMed] [Google Scholar]
- 27.Ushirozako H., Hasegawa T., Yamato Y., et al. Does preoperative prognostic nutrition index predict surgical site infection after spine surgery? Eur Spine J. 2021;30:1765–1773. doi: 10.1007/s00586-020-06622-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhong W., Liang X., Luo X., et al. Complications rate of and risk factors for the unplanned reoperation of degenerative lumbar spondylolisthesis in elderly patients: a retrospective single-Centre cohort study of 33 patients. BMC Geriatr. 2020;20:301. doi: 10.1186/s12877-020-01717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amelot A., Riche M., Latreille S., et al. Antimicrobial prophylaxis in noninstrumented spine surgery: a prospective study to determine efficacy and drawbacks. J Neurosurg Spine. 2021;35(3):366–375. doi: 10.3171/2020.11.SPINE201891. [DOI] [PubMed] [Google Scholar]
- 30.Hoeller S., Roch P.J., Weiser L., et al. C-reactive protein in spinal surgery: more predictive than prehistoric. Eur Spine J. 2021;30(5):1261–1269. doi: 10.1007/s00586-021-06782-8. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W., Shi H., Deng X., et al. The incidence of incision infections after lumbar fusion and the significance of dynamically monitoring serum albumin and C-reactive protein levels. Ann Palliat Med. 2021;10(10):10870–10877. doi: 10.21037/apm-21-2512. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Fan T., Yang B., et al. Development and internal validation of supervised machine learning algorithms for predicting the risk of surgical site infection following minimally invasive transforaminal lumbar interbody fusion. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.771608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AlSaleh K., Aldowesh A., Alqhtani M., et al. Subcutaneous fat thickness on erect radiographs is a predictor of infection following elective posterior lumbar fusion. Int J Spine Surg. 2022;16(4):660–665. doi: 10.14444/8295. [published online ahead of print, July 14, 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aynaszyan S., Udoeyo I.F., DelSole E.M., et al. The effect of low preoperative platelet count on adverse outcomes following lumbar microdiscectomy. North Am Spine Soc J. 2022;10 doi: 10.1016/j.xnsj.2022.100116. ISSN 2666-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnally C.J., Henstenburg J.M., Pezzulo J.D., et al. Increased surgical site subcutaneous fat thickness is associated with infection after posterior cervical fusion. Surg Infect (Larchmt) 2022:364–371. doi: 10.1089/sur.2021.271. [DOI] [PubMed] [Google Scholar]
- 36.Pulido L.C., Meyer M., Reinhard J., et al. Hospital frailty risk score predicts adverse events in spine surgery. Eur Spine J. 2022;31:1621–1629. doi: 10.1007/s00586-022-07211-0. [DOI] [PubMed] [Google Scholar]
- 37.Lainé G., Le Huec J.C., Blondel B., et al. Factors influencing complications after 3-columns spinal osteotomies for fixed sagittal imbalance from multiple etiologies: a multicentric cohort study about 286 cases in 273 patients. Eur Spine J. 2022;31:3673–3686. doi: 10.1007/s00586-022-07410-9. [DOI] [PubMed] [Google Scholar]
- 38.Xiong C., Zhao R., Xu J., et al. Construct and validate a predictive model for surgical site infection after posterior lumbar interbody fusion based on machine learning algorithm. Comput Math Methods Med. 2022;2022 doi: 10.1155/2022/2697841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen X., Gao Y., Zhang P., et al. Subcutaneous Lumbar Spine Index (SLSI) as a risk factor for surgical site infection after lumbar fusion surgery: a retrospective matched case–control study. Global Spine J. 2022;0(0) doi: 10.1177/21925682221146503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghenbot Y., Wathen C., Gutierrez A., et al. Effectiveness of oral antibiotic therapy in prevention of postoperative wound infection requiring surgical washout in spine surgery. World Neurosurg. 2022;163:e275–e282. doi: 10.1016/j.wneu.2022.03.106. ISSN 1878-8750. [DOI] [PubMed] [Google Scholar]
- 41.Lee M.J., Cizik A.M., Hamilton D., et al. Predicting surgical site infection after spine surgery: a validated model using a prospective surgical registry. Spine J. 2014;14(9):2112–2117. doi: 10.1016/j.spinee.2013.12.026. ISSN 1529-9430. [DOI] [PubMed] [Google Scholar]
- 42.Klemencsics I., Lazary A., Szoverfi Z., et al. Risk factors for surgical site infection in elective routine degenerative lumbar surgeries. Spine J. 2016;16(11):1377–1383. doi: 10.1016/j.spinee.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Lubelski D., Alentado V., Nowacki A.S., et al. Preoperative nomograms predict patient-specific cervical spine surgery clinical and quality of life outcomes. Neurosurgery. 2018;83(1):104–113. doi: 10.1093/neuros/nyx343. [DOI] [PubMed] [Google Scholar]
- 44.Janssen D.M.C., van Kuijk S.M.J., d'Aumerie B., et al. A prediction model of surgical site infection after instrumented thoracolumbar spine surgery in adults. Eur Spine J. 2019;28:775–782. doi: 10.1007/s00586-018-05877-z. [DOI] [PubMed] [Google Scholar]
- 45.Namba T., Ueno M., Inoue G., et al. Prediction tool for high risk of surgical site infection in spinal surgery. Infect Control Hospital Epidemiol. 2020;41(7):799–804. doi: 10.1017/ice.2020.107. [DOI] [PubMed] [Google Scholar]
- 46.Lubelski D., Feghali J., Ehresman J., et al. Web-based calculator predicts surgical-site infection after thoracolumbar spine surgery. World Neurosurg. 2021;151:e571–e578. doi: 10.1016/j.wneu.2021.04.086. ISSN 1878-8750. [DOI] [PubMed] [Google Scholar]
- 47.Hersh A.M., Feghali J., Hung B., et al. A web-based calculator for predicting the occurrence of wound complications, wound infection, and unplanned reoperation for wound complications in patients undergoing surgery for spinal metastases. World Neurosurg. 2021;155:e218–e228. doi: 10.1016/j.wneu.2021.08.041. ISSN 1878-8750. [DOI] [PubMed] [Google Scholar]
- 48.Stepanov I.A., Beloborodov V.A., Shameeva M.A., et al. A scoring system to predict the risk of surgical site infections after spinal surgery. SciELO. 2021;20(3) doi: 10.1590/S1808-185120212003251045. [DOI] [Google Scholar]
- 49.Chen L., Liu C., Ye Z., et al. Predicting surgical site infection risk after spinal tuberculosis surgery: development and validation of a nomogram. Surg Infect (Larchmt) 2022;23(6):564–575. doi: 10.1089/sur.2022.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W.C., Ying H., Liao W.J., et al. Using preoperative and intraoperative factors to predict the risk of surgical site infections after lumbar spinal surgery: a machine learning–based study. World Neurosurg. 2022;162:e553–e560. doi: 10.1016/j.wneu.2022.03.060. ISSN 1878-8750. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Hu Y., Zhao B., et al. Predictive validity of the ACS-NSQIP surgical risk calculator in geriatric patients undergoing lumbar surgery. Medicine (Baltimore) 2017;96(43):e8416. doi: 10.1097/MD.0000000000008416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janssen D.M.C., van Kuijk S.M.J., d'Aumerie B.B., et al. External validation of a prediction model for surgical site infection after thoracolumbar spine surgery in a Western European cohort. J Orthop Surg Res. 2018;13:114. doi: 10.1186/s13018-018-0821-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sebastian A., Goyal A., Alvi M.A., et al. Assessing the performance of national surgical quality improvement program surgical risk calculator in elective spine surgery: insights from patients undergoing single-level posterior lumbar fusion. World Neurosurg. 2022;126:e323–e329. doi: 10.1016/j.wneu.2019.02.049. ISSN 1878-8750. [DOI] [PubMed] [Google Scholar]
- 54.Narain A., Kitto A.Z., Braun B., et al. Does the ACS NSQIP surgical risk calculator accurately predict complications rates after anterior lumbar interbody fusion procedures? Spine. 2021;46(12):E655–E662. doi: 10.1097/BRS.0000000000003893. [DOI] [PubMed] [Google Scholar]
- 55.Pierce K.E., Kapadia B.H., Naessig S., et al. Validation of the ACS-NSQIP risk calculator: a machine-learning risk tool for predicting complications and mortality following adult spinal deformity corrective surgery. Int J Spine Surg. 2021;15(6):1210–1216. doi: 10.14444/8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.