Abstract
Objective
This study aimed to evaluate the effects of microRNA-650 (miR-650) on melanoma metastasis and reveal the regulatory relationship between miR-650 and the inhibitor of growth family member 4 (ING4).
Methods
miR-650 expression was determined in human melanoma WM115 and A-375 cells. WM115 cells were transfected with miR-650 mimic or mimic control. The invasion and migration abilities of transfected WM115 cells were analyzed using Transwell and wound healing assays, respectively. Then, miR-650-overexpression lentivirus vector was constructed and transfected into WM115 cells. After injection into the mice, the number of micro-metastatic foci in the lung tissues was counted. A regulatory relationship between miR-650 and ING4 was identified in WM115 and A-375 cells.
Results
The miR-650 expression was upregulated in WM115 and A-375 cells. WM115 cells transfected with the miR-650 mimic exhibited higher invasive and migratory abilities than mock cells or cells transfected with negative control (NC). The number of micro-metastatic foci was significantly higher in mice injected with Lenti-miR-650 than that in those injected with mock or NC controls. Transfection with miR-650 mimic observably inhibited the expression of ING4 in WM115 and A-375 cells, whereas transfection with miR-650 inhibitor had the opposite effect. Dual-luciferase reporter gene assay showed that the miR-650 mimic inhibited the luciferase activity of ING4.
Conclusion
miR-650 promotes melanoma metastasis by downregulating ING4 expression.
Keywords: miR-650, ING4, Melanoma, Metastasis
1. Introduction
Melanoma, which develops in melanocytes, is a serious type of skin cancer [1]. The incidence of melanoma is increasing worldwide [2]. Melanoma exhibits high metastatic potential even in the early stages of tumorigenesis [3]. In patients with stage I and II localized melanoma, the recurrence rates are 7 % and 30 %, respectively, and most patients progress to metastasis [4]. Metastasis of melanoma to other organs is a major cause of death [5,6]. The 10-year survival rates of melanoma patients with regional and distant metastases are 64 % and 16 %, respectively [7]. The poor prognosis of melanoma may be partly attributed to the limited knowledge of metastasis.
MicroRNAs (miRNAs) are small endogenous non-coding RNAs involved in complex cellular processes. As miRNAs can post-transcriptionally regulate the expression of their target genes, they play important regulatory roles in tumor development [[8], [9], [10]]. Until now, a variety of miRNAs have been revealed and identified to be associated with tumor metastasis, such as miR-9, miR-10b, miR-21, miR-31, miR-126, miR-200, miR-335, and miR-373 [11,12]. Numerous studies have shown that the abnormal expression of specific miRNAs can functionally influence melanoma metastasis through the regulation of diverse signaling genes [[13], [14], [15]]. For example, upregulation of miR-182 promotes the migration of melanoma cells by repressing forkhead box O3 (FOXO3) and microphthalmia associated transcription factor-M [13]; depletion of miR-638 inhibits the metastasis of melanoma cells and promotes p53-mediated apoptosis [15]; and overexpression of miR-365 inhibits the invasion and metastasis of malignant melanoma cells by regulating the expression of neuropilin1 (NRP1) [14]. Although various miRNAs have been identified to be related to melanoma metastasis, some specific miRNAs have not yet been identified.
The miR-650 is regarded as an important regulator involved in the metastasis of diverse tumors. For example, miR-650 overexpression is associated with a high incidence of lymph node metastasis (LNM) in patients with lung adenocarcinoma [16]. The downregulation of miR-650 inhibits the migration and invasion of non-small cell lung cancer (NSCLC) cells [17]. Reduced miR-650 expression facilitates the growth and metastasis of human ovarian cancer cells [18]. Previous studies have shown that miR-650 is overexpressed in melanomas [19,20]. ZFPM2-AS1 promotes cell proliferation and migration in cutaneous malignant melanoma by regulating miR-650/NOTCH1 signaling [21]. However, studies regarding the specific role of miR-650 in melanoma metastasis are limited.
Recent studies have shown that inhibitor of growth family member 4 (ING4), a tumor suppressor protein, is a target gene of miR-650. Reportedly, upregulated miR-650 promotes the proliferation and invasion of NSCLC cells by targeting ING4 [22], and increased miR-650 expression promotes the proliferation and migration of colorectal cancer cells by targeting ING4 [23]. Previous studies have revealed that overexpression of ING4 inhibits cellular proliferation, migration, and invasion, and promotes apoptosis of human melanoma cells [24,25]. We hypothesized that the regulatory effects of miR-650 on melanoma metastasis were associated with ING4 expression. In the present study, miR-650 was overexpressed in melanoma cells, and its effects of miR-650 on melanoma metastasis were analyzed both in vivo and in vitro. Additionally, we identified a regulatory relationship between miR-650 and ING4. Our findings revealed the regulatory effects of miR-650 on melanoma metastasis and clarified the potential mechanism of action of ING4.
2. Materials and methods
2.1. Cell culture
The human immortalized keratinocyte cell line HaCaT and the human melanoma cell lines WM115 and A-375 were cultured in Dulbecco's modified Eagle's medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) containing 10 % fetal bovine serum (FBS). The cells were placed in an incubator containing 5 % CO2 at 37 °C, and passed to 80–90 % cells for fusion. Cells in logarithmic growth phase were used for subsequent assays. Notably, WM115 and A-375 cell lines were short tandem repeats (STR) authenticated by Shanghai Biowing Applied Biotechnology Co., Ltd. (Shanghai, China). STR authentication results confirmed that neither cell line was contaminated with other cell lines.
2.2. Cell transfection
Cells were cultured in a 6-well plate containing Opti-MEM at a density of 2.4 × 105 cells/well. The miR-650 mimic, miR-650 inhibitor, and mimic negative control (NC) were synthesized by Biomic Biotechnology (USA), and the sequence of primers were as follows: miRNA-650 sense chain of mimic: 5′- AGAGGAGGCAGCGCTCT-3’, antisense chain: 5’ -CAGTGCGTGTCGTGGAGT-3’; miRNA-650 sense chain of inhibitor: 5′- GTCGTATCCAGTGCAGGGTCCGAGTCGCACTGGATACGACCG CCAA-3′, antisense chain: 5’ -GTATCCAGTGCAGGGTCCGAGGT-3’. Next, the miR-650 mimic, miR-650 inhibitor, mimic NC, and inhibitor NC (Biotend, Shanghai, China) were transfected into WM115 and A-375 cells using Lipofectamine 2000. Cells treated with Lipofectamine 2000 alone were considered the mock group. Cells were cultivated in fresh medium for another 48 h prior to subsequent assays.
2.3. Cell invasion assay
Cell invasion experiments were performed using transwell chambers (Corning, NY, USA). Transfected WM115 cells from each group were digested at a density of 7 × 105 cells/well. A volume of 100 μL cell suspension was added to the upper compartment of the chamber, and DMEM containing 10 % FBS was placed into the lower compartment of the chamber. After culturing for 24 h, the cells on the upper surface of the membrane were removed, and the cells on the lower surface were fixed with 4 % paraformaldehyde. The cells were stained with 0.1 % crystal violet (Invitrogen) and observed under a microscope (IX73; Olympus, Tokyo, Japan). The number of invasive cells was counted in ten random fields of view at 200 × magnification.
2.4. Cell migration assay
A wound-healing assay was used to analyze cell migration. Transfected WM115 cells from each group were cultured until they reached 90 % confluence. After washing with phosphate-buffered saline to remove debris, the cells were cultured at 37 °C with 5 % CO2 for 24 h. The migration distance was photographed under microscope (IX73, Olympus).
2.5. Western blot
The expression of ING4 in transfected WM115 and A-375 cells in each group was detected by western blotting. Total cellular protein was extracted using RIPA lysis buffer containing 1 mM phenylmethanesulfonyl fluoride. Total protein was separated by 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5 % skim milk in TBST for 2 h, and incubated with specific diluted primary antibody (anti-GAPDH, 1:1000; anti-ING4, 1:200) (Proteintech, Rosemont, IL, USA) overnight at 4 °C. After three rinses with TBST, the membrane was incubated with HRP-labeled Goat Anti-rabbit IgG (1:1000) (Beyotime, China) for 2 h at 37 °C. Protein bands were visualized using electrochemiluminescence (ECL) (Millipore, USA) and quantified using a UV gel imager (ChemiDoc; BIO-RAD, Hercules, CA, USA).
2.6. Dual luciferase reporter gene assay
The interaction between miR-650 and ING4 was detected using a dual-luciferase reporter gene assay kit (Beyotime) according to the manufacturer's instructions. The 3′UTR of ING4 containing putative binding sites for miR-650 was amplified with specific primers (ING4-F, 5′-CAACACAGTTTCTTCCACATCC-3’; ING4-R, 5′-CTCTACAATAAACACAG CAGGC-3′), and cloned into pmirGLO (Lus-ING4). WM115 cells were co-transfected with miR-650 mimic and Lus-ING4 for 24 h. After 15 min of lysis, the supernatant was collected by centrifugation at 10, 000 g for 5 min. A fluorescein detection solution was then added to the supernatant. Two seconds later, fluorescence intensity was detected using a microplate reader (TECAN, Shanghai, China).
2.7. Lentivirus vector construction
The sequence of miR-650 (ACGCGTCGCAGTGCTGGGGTCTCAGGAGGCAGCGCTCTCAGGACGTCACCACCATGGCCTGGGCTCTGCTCCTCCTCACCCTCCTCACTCAGGGCACAGGTGATTTTTTTCCATCGAT) was directly synthesized. MiR-650 overexpression vector was constructed by inserting miR-650 into the lentiviral vector PLVTHM (Tronolab, Switzerland) between MluI and ClaI. Positive clones (Lenti-miR-650) were identified in competent cells Stbl3 (Biotend) by sequencing. The lentivirus was packaged and used to infect the cells according to a previously described method [26]. The lentiviral vectors (Lenti-miR-650 and Lenti-mimic NC) and packing systems (psPAX2 and Pmd2G) were co-transfected into WM115 cells using Lipofectamine 2000. Cells treated with Lipofectamine 2000 were used as mock controls.
2.8. Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the transfected cells using TRIzol reagent (TAKARA, Dalian, China). Then 500 ng of total RNA of each sample was reversely transcribed by PrimeScript™ RT Master Mix (TAKARA). qRT-PCR was performed using a PCR instrument ViiA7 (Thermo Fisher Scientific) with specific primers (miR-650-F, 5′-AGGAGGCAGCGCTCTCAGGAC-3′, miR-650-R, 5′-GCTGTCAACGATACGCTACCTA-3’; U6-F, 5′-CTCGCTTCGGCAGCACA-3′, U6-R, 5′-AACGCTTCACGAATTTGCGT-3’; ING4-F, 5′-TGCAAGGAATTTGGTGACGAC-3′, ING4-R, 5′-GCCGCCGAATGTGTTTGTC-3’; and GAPDH-F: 5′-TGACAACTTTGGTATCGTGGAAGG-3′, GAPDH-R, 5′- AGGCAGGGATGATGTTCTGGAGAG-3′). The PCR program included 95 °C for 3 min, 40 cycles at 95 °C for 10 s, 60 °C for 30 s. U6 and GAPDH was used as the internal controls for miR-650 and ING4, respectively. Relative gene expression was calculated by 2−ΔΔCt method.
2.9. Construction of metastatic melanoma model in mouse
The 4-week-old male nude mice (weighing 15 ± 1 g), purchased from Slaccas (Shanghai, China) were feeding at 26 ± 1 °C in a standalone environment with free access to water and food. All mice were anesthetized by an intraperitoneal injection of 3 % chloral hydrate (0.1 mL/10 g), and fixed in the prone position. In the Lenti-miR-65 group, mice were injected with 100 μL the suspension of WM115 cells transfected with Lenti-miR-650 (1 × 107 cells) through tail vein injection. In the control group, the mice were injected with either NC or mock. Each group contained 10 mice. Twenty days later, five mice in each group were euthanized by CO2 asphyxiation and lung tissues were collected. The number of micrometastatic foci in the lungs of the mice was counted in five random fields of view at 10 × magnification. The other five mice were fed until they died from natural causes. All the manipulations relevant to animal experiments were approved by the Ethics Committee of The First Affiliated Hospital of Harbin Medical University (No. IRB-AF/SC-04/01.0), and conducted according to the recommendations of the University of Pennsylvania’s Institutional Animal Care and Use Committee.
2.10. Hematoxylin and eosin (HE) staining
The lung tissues of mice from each group were dehydrated, paraffin-embedded, and sliced at 4 μm. After dewaxing in xylene and rehydration in graded ethanol, the tissue sections were stained with hematoxylin (Baso, Zhuhai, China) for 4 min and eosin (Baso) for 90 s. Following dehydration with graded ethanol and vitrification with dimethylbenzene, pathological changes in the lung tissues were observed under a microscope (IX73, Olympus).
2.11. Statistical analyses
All experiments were repeated three times, and the obtained data were expressed as mean ± standard deviation (SD). Statistical analysis was performed using SPSS (version 17.0; SPSS Inc., Chicago, IL, USA). Comparisons between different groups were performed using one-way ANOVA. Differences were considered statistically significant at P < 0.05.
3. Results
3.1. miR-650 promoted the invasion and migration of melanoma cells
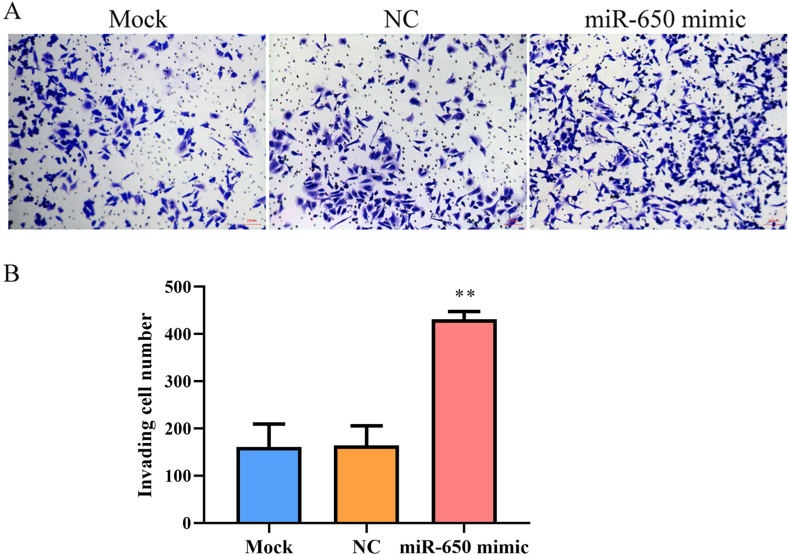
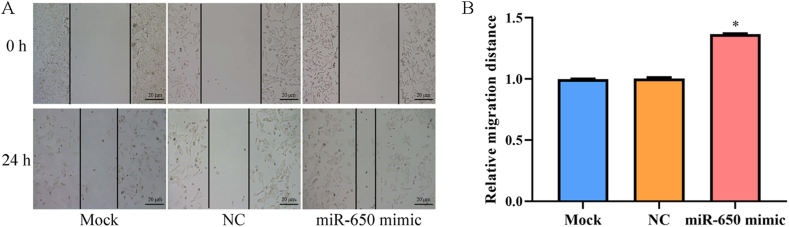
We determined miR-650 expression in melanoma cells and found that miR-650 expression was significantly increased in melanoma WM115 and A-375 cells compared to that in HaCaT cells (p < 0.001, Fig. 1A). Moreover, miR-650 expression in WM115 cells was markedly higher than that in A-375 cells; therefore, WM115 cells were selected to investigate the role of miR-650 in subsequent experiments. To determine the specific role of miR-650 in melanoma cells, miR-650 was overexpressed in WM115 cells by transfection with an miR-650 mimic. As shown in Fig. 1B, miR-650 expression was significantly higher in the miR-650 mimic group than in the mock and NC groups (p < 0.001), indicating that miR-650 was successfully overexpressed in the WM115 cells. Subsequently, the invasive ability of WM115 cells was analyzed using a transwell assay. The results showed that the number of invasive cells was significantly higher in the miR-650 mimic group than in the mock or NC groups (p < 0.01, Fig. 2), indicating that the invasive ability of WM115 cells was enhanced after overexpression of miR-650. The migratory ability of WM115 cells was further analyzed using a wound-healing assay. WM115 cells transfected with the miR-650 mimic exhibited longer cell migration distance when compared with cells in the mock or NC group (p < 0.05, Fig. 3), indicating that the migration ability of WM115 cells was also enhanced after overexpression of miR-650.
Fig. 1.
qRT-PCR showed the relative expression of miR-650. A, The relative expression of miR-650 in normal HaCaT cells and human melanoma WM115 and A-375 cells. ***, p < 0.001 compared to HaCaT cells; ###, p < 0.001 compared to A-375 cells. B, The relative expression of miR-650 in WM115 cells after transfection. Mock, cells treated with lipofectamine 2000; NC, mimic negative control. The experiments were repeated three times. ***, p < 0.001 compared to mock or NC.
Fig. 2.
The invasion of WM115 cells analyzed by transwell assay. A, Invasive cells under microscope; B, Quantization of invasive cells. Mock, cells treated with lipofectamine 2000; NC, mimic negative control. The experiments were repeated three times. The number of invasive cells was counted in 10 random fields of views at 200 × magnifications. **, p < 0.01 compared to mock or NC.
Fig. 3.
The migration of WM115 cells analyzed by wound healing assay. A, Migrated cells under microscope; B, Quantization of migrated cells. Mock, cells treated with lipofectamine 2000; NC, mimic negative control. The experiments were repeated three times. *, p < 0.05 compared to mock or NC.
3.2. Overexpression of miR-650 promoted the metastasis of melanoma in mice
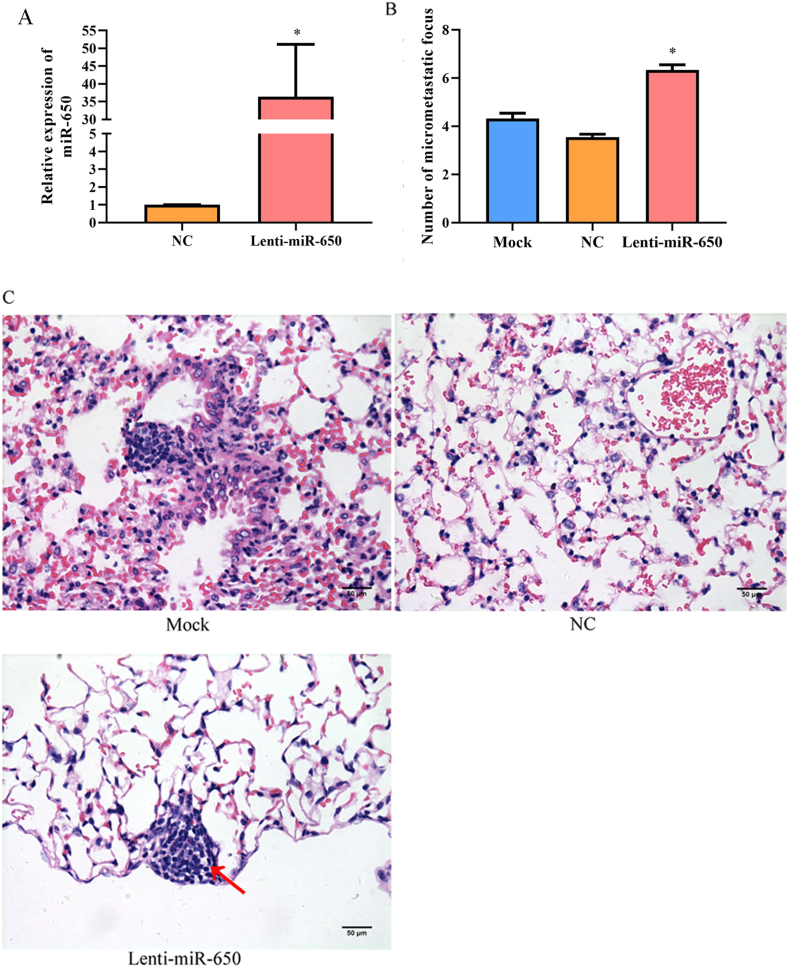
Lenti-miR-650 was constructed to evaluate the effect of miR-650 on melanoma metastasis in vivo. qRT-PCR showed that the expression of miR-650 was significantly increased in WM115 cells after transfection with Lenti-miR-650 (p < 0.05) (Fig. 4A). Lenti-miR-650-transfected WM115 cells were injected into mice, and the number of micrometastatic foci was counted. As shown in Fig. 4B, the number of micrometastatic foci in mice in the Lenti-miR-65 group was significantly higher than that in the mock or NC groups (P < 0.05). In addition, HE staining showed that melanoma cells were scattered in the lung tissues of mice and aggregated in the vicinity of the bronchus (Fig. 4C).
Fig. 4.
The effects of miR-650 overexpression on the metastasis of melanoma in mice (N = 5 in each group). A, Relative expression of miR-650 in WM115 cells; B, The number of micrometastaic focus; C, HE staining of lung tissues (400 × ). Mock, cells treated with lipofectamine 2000; NC, mimic negative control. The number of micrometastatic focus in the lung of mice was counted in 5 random fields of views at 10 × magnifications. *, p < 0.05 compared to mock or NC.
3.3. ING4 was down-regulated by miR-650
The regulatory effect of miR-650 on ING4 expression was observed in WM115 cells. As shown in Fig. 5A, qRT-PCR showed that there was a slight but not significant downregulation of ING4 mRNA in the miR-650 mimic group compared to that in the mock or NC group. However, western blotting showed that the protein expression level of ING4 in the miR-650 mimic group was markedly downregulated compared to that in the mock or NC groups (p < 0.001) (Fig. 5B, supplementary file 1). The dual-luciferase reporter gene assay showed that the miR-650 mimic significantly inhibited the luciferase activity of ING4 (p < 0.05) (Fig. 5C), indicating that ING4 was a target of miR-650. Additionally, WM115 cells were transfected with an miR-650 inhibitor to confirm the regulatory relationship between miR-650 and ING4. As shown in Fig. 5D, miR-650 expression was dramatically inhibited in the miR-650 inhibitor group compared to that in the mock or NC groups (p < 0.05), indicating high transfection efficiency. Subsequent experiments confirmed that the mRNA and protein expression levels of ING4 were significantly increased in the miR-650 inhibitor group compared to those in the mock or NC groups (p < 0.05) (Fig. 5E and F, supplementary file 1). Furthermore, the negative regulation between miR-650 and ING4 was confirmed in the second melanoma cell line, A-375. The results showed that, in comparison with their respective NC groups, miR-650 was significantly overexpressed in A-375 cells after transfection with the miR-650 mimic and was obviously inhibited in this cell line after transfection with the miR-650 inhibitor (p < 0.05) (Fig. 5G). Further experiments revealed that the expression of ING4 at the mRNA and protein levels was remarkably downregulated in miR-650 mimic-transfected A-375 cells, but was significantly upregulated in miR-650 inhibitor-transfected A-375 cells (p < 0.05) (Fig. 5H and I, supplementary file 1).
Fig. 5.
The regulatory effect of miR-650 on ING4. A, qRT-PCR showed the relative mRNA expression of ING4 in WM115 cells after transfection with miR-650 mimic or mimic NC; B, Western blot showed the protein expression of ING4 in WM115 cells after transfection with miR-650 mimic or mimic NC; C, Dual luciferase reporter gene assay confirmed the target relationship between miR-650 and ING4; D, qRT-PCR showed the miR-650 expression in WM115 cells after transfection with miR-650 inhibitor or inhibitor NC; E, qRT-PCR showed the relative mRNA expression of ING4 in WM115 cells after transfection with miR-650 inhibitor or inhibitor NC; F, Western blot showed the protein expression of ING4 in WM115 cells after transfection with miR-650 inhibitor or inhibitor NC; G, qRT-PCR showed the miR-650 expression in A-375 cells after transfection with miR-650 mimic, miR-650 inhibitor or their NC; H, qRT-PCR showed the relative mRNA expression of ING4 in A-375 cells after transfection with miR-650 mimic, miR-650 inhibitor or their NC; I: Western blot showed the protein expression of ING4 in A-375 cells after transfection with miR-650 mimic, miR-650 inhibitor or their NC. Mock, cells treated with lipofectamine 2000. The experiments were repeated three times. *, p < 0.05, and ***, p < 0.001 compared to mock or NC.
4. Discussion
miRNAs are important regulators of human diseases through post-transcriptional regulation of a variety of target genes [27,28]. The isolation and identification of specific miRNAs are of great value for the characterization, diagnosis, and treatment of tumors [29]. In this study, we found that miR-650 overexpression promoted melanoma metastasis both in vivo and in vitro. The regulatory effects of miR-650 on melanoma metastasis were closely associated with ING4 downregulation.
An increasing number of studies have confirmed that the prognosis of patients with melanoma with local or distant metastases remains poor [30]. Studies of metastasis-associated miRNAs have provided important guidance for the suppression of melanoma metastasis [[31], [32], [33]]. miR-650 is an important miRNA involved in tumor aggressiveness [34]. Increasing evidence has shown that miR-650 upregulation is associated with high metastatic potential [16,17,35]. In miR-650 inhibitor-transfected NSCLC cells, the metastatic ratio is decreased to 40.46 ± 5.72 % or 45.53 ± 4.63 % in H23 cells or A549 cells, and the invasive ratio is decreased to 53.98 ± 4.16 % or 55.37 ± 4.45 % in H23 cells or A549 cells, respectively [17]. Approximately 31 % of mice experience metastasis of prostate cancer, whereas none of the mice treated with doxycycline, an miR-650 inhibitor, experience metastasis [35]. A higher incidence of LNM (70.7 %) was observed in lung adenocarcinoma patients with high miR-650 expression than in those with low miR-60 expression (51.2 %) [16]. In this study, we evaluated the regulatory effects of miR-650 on melanoma metastasis, both in vivo and in vitro. These results revealed that the invasive and migratory abilities of WM115 cells transfected with miR-650 were significantly higher than those of the control group. Meanwhile, miR-650 overexpression promoted melanoma metastasis in mice, exhibiting significantly more micrometastatic foci than the control group. Our data are consistent with those of previous studies and further illustrate that miR-650 promotes melanoma metastasis. The inhibition of miR-650 may be an effective strategy for preventing melanoma metastasis.
As miRNAs can regulate various target genes involved in diverse cellular signals, the regulatory mechanisms of miR-650 in tumor metastasis are complex. Some target genes of miR-650 involved in tumors have been identified, including KLF12 [18], PPP2CA [36], LATS2 [17], and ING4 [16]. ING4 is a direct target of miR-650 in tumors. A previous study showed that miR-650 expression is negatively correlated with ING4 expression in lung adenocarcinoma tissues, and the effects of miR-650 inhibitors or mimics can be reversed by inhibiting or enhancing ING4 expression in lung adenocarcinoma cells [16]. Another study showed that upregulation of miR-650 promotes the proliferation of gastric cancer cells, and enhances the tumorigenicity of gastric cancer cells via targeting ING4 [37]. Consistent with previous studies, we found an inverse expression of miR-650 and ING4 in WM115 and A-375 cells. Our findings indicate that the promotion effects of miR-650 on melanoma metastasis are closely associated with ING4 inhibition.
As a member of the ING family, ING4 is involved in various biological and pathological processes in humans, including oncogenesis [38]. Increasing evidence has shown that ING4 is involved in the regulation of tumorigenesis and melanoma progression through the regulation of diverse genes [24,39,40]. It has been reported that ING4 inhibits the angiogenesis of melanoma angiogenesis through suppressing the expression of NF-κB and IL-6 [39]. ING4 inhibits the migration of melanoma cells by regulating p65 expression [40]. ING4 inhibits the proliferation of melanoma cells and promotes apoptosis by activating FAS via a caspase-8-dependent pathway [24]. We suspect that miR-650 promotes melanoma metastasis through the regulation of diverse tumor-associated factors by targeting ING4.
This study has some limitations. First, the association between miR-650 expression and clinical outcomes of melanoma was not analyzed in this study, which cripples the miR-650 clinical potential. Second, although HE staining has shown melanoma metastasis in mice, the nature of the cellular clusters has not been precisely evaluated. Further immunostaining should be performed to prove that these cells are melanoma cells, and that miR-650 function in vivo needs to be validated by further experiments. Besides, the regulatory effects of overexpression or knockdown of ING4 on melanoma metastasis and the role of ING4 in relation to miR-650's oncogenic mechanisms are not investigated, which reduce the impact of this study. In the future, conducting experiments involving mutations in the miRNA-binding site on ING4 using consistent in vitro and in vivo assays will offer valuable insights into the mechanism of the interaction between ING4 and miR-650.
5. Conclusion
In conclusion, miR-650 promotes both the invasion and migration of melanoma cells and metastasis of melanoma in mice. Regulatory effects of miR-650 on melanoma metastasis may be associated with the negative regulation of ING4.
Ethics approval and consent to participate
Animal experiments were approved by the ethical committee of The First Affiliated Hospital of Harbin Medical University (No. IRB-AF/SC-04/01.0).
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Chen Chen: Writing – original draft, Conceptualization. Jing Liu: Data curation. Yanli Ma: Formal analysis. Yu Wang: Investigation. Limin Cai: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Research Innovation Fund of the First Affiliated Hospital of Harbin Medical University (Program No. 2019M07).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36199.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pawelec G. Immune correlates of clinical outcome in melanoma. Immunology. 2018;153(4):415–422. doi: 10.1111/imm.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes J., Rodrigues C.M., Gaspar M.M., Reis C.P.J.C. Melanoma management: from epidemiology to treatment and latest advances. 2022;14(19):4652. doi: 10.3390/cancers14194652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y., Meng D., Lu A., Yu E., Merlino G. PHLPP1 mediates melanoma metastasis suppression through repressing AKT2 activation. Oncogene. 2018;37(17):2225–2236. doi: 10.1038/s41388-017-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiter U., Buettner P.G., Eigentler T.K., Bröcker E.B., Voit C., Gollnick H., et al. Hazard rates for recurrent and secondary cutaneous melanoma: an analysis of 33,384 patients in the German Central Malignant Melanoma Registry. J. Am. Acad. Dermatol. 2012;66(1):37–45. doi: 10.1016/j.jaad.2010.09.772. [DOI] [PubMed] [Google Scholar]
- 5.Del V.H., Van L.D.W., Nsengimana J., Speak A.O., Sjöberg M.K., Bishop D.T., et al. Comparative genomics reveals that loss of lunatic fringe (LFNG) promotes melanoma metastasis. Mol. Oncol. 2017;12(2) doi: 10.1002/1878-0261.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Raimondo C., Lozzi F., Di Domenico P.P., Campione E., Bianchi L.J. The diagnosis and management of cutaneous metastases from melanoma. 2023;24(19) doi: 10.3390/ijms241914535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., et al. Cancer statistics. Ca - Cancer J. Clin. 2008;58(2):71. doi: 10.3322/CA.2007.0010. 2008. [DOI] [PubMed] [Google Scholar]
- 8.Chen L., Zhou Y., Sun Q., Zhou J., Pan H., Sui X. Regulation of autophagy by miRNAs and their emerging roles in tumorigenesis and cancer treatment. International Review of Cell & Molecular Biology. 2017;334:1. doi: 10.1016/bs.ircmb.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Menon A., Abd-Aziz N., Khalid K., Poh C.L., Naidu R.J. miRNA: a promising therapeutic target in cancer. 2022;23(19) doi: 10.3390/ijms231911502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poniewierska-Baran A., Słuczanowska-Głąbowska S., Małkowska P., Sierawska O., Zadroga Ł., Pawlik A., et al. Role of miRNA in melanoma development and progression. 2022;24(1):201. doi: 10.3390/ijms24010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valastyan S., Weinberg R.A. MicroRNAs: crucial multi-tasking components in the complex circuitry of tumor metastasis. Cell Cycle. 2009;8(21):3506–3512. doi: 10.4161/cc.8.21.9802. [DOI] [PubMed] [Google Scholar]
- 12.Penna E., Orso F., Cimino D., Tenaglia E., Lembo A., Quaglino E., et al. microRNA‐214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 2011;30(10):1990–2007. doi: 10.1038/emboj.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segura M.F., Hanniford D., Menendez S., Reavie L., Zou X., Alvarez-Diaz S., et al. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proc. Natl. Acad. Sci. U.S.A. 2009;106(6):1814–1819. doi: 10.1073/pnas.0808263106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai J., Zhang Z., Li X., Liu H. MicroRNA-365 inhibits growth, invasion and metastasis of malignant melanoma by targeting NRP1 expression. Cancer Biomarkers. 2015;15(5):599–608. doi: 10.3233/CBM-150500. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya A., Schmitz U., Raatz Y., Schönherr M., Kottek T., Schauer M., et al. miR-638 promotes melanoma metastasis and protects melanoma cells from apoptosis and autophagy. Oncotarget. 2015;6(5):2966. doi: 10.18632/oncotarget.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J.Y., Cui S.Y., Chen Y.T., Song H.Z., Huang G.C., Feng B., et al. MicroRNA-650 was a prognostic factor in human lung adenocarcinoma and confers the docetaxel chemoresistance of lung adenocarcinoma cells via regulating Bcl-2/Bax expression. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Y., Zhuang J., Wang G., He S., Ni J., Xia W., et al. microRNA-605 promotes cell proliferation, migration and invasion in non-small cell lung cancer by directly targeting LATS2. Exp. Ther. Med. 2017;14(1):867–873. doi: 10.3892/etm.2017.4538. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Lu X., Han Y., Han Y., Huang M., You J., Liu Y., et al. MicroRNA-650 suppresses KLF12 expression to regulate growth and metastasis of human ovarian cancer cells. Acta Biochim. Pol. 2022;69(4):745–751. doi: 10.18388/abp.2020_5987. [DOI] [PubMed] [Google Scholar]
- 19.Yun J.H., Moon S., Lee H., Yeong Hwang M.I., Kim Y., Hoyeong Y.U., et al. MicroRNA-650 in a copy number-variable region regulates the production of interleukin 6 in human osteosarcoma cells. Oncol. Lett. 2015;10(4):2603–2609. doi: 10.3892/ol.2015.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan E., Patel R., Nallur S., Ratner E., Bacchiocchi A., Hoyt K., et al. MicroRNA signatures differentiate melanoma subtypes. Cell Cycle. 2011;10(11):1845–1852. doi: 10.4161/cc.10.11.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W., Hu X., Mu X., Tian Q., Gao T., Ge R., et al. ZFPM2‐AS1 facilitates cell proliferation and migration in cutaneous malignant melanoma through modulating miR‐650/NOTCH1 signaling. 2021;34(2) doi: 10.1111/dth.14751. [DOI] [PubMed] [Google Scholar]
- 22.Tang X., Ding Y., Wang X., Wang X., Zhao L., Bi HJOl. miR-650 promotes non-small cell lung cancer cell proliferation and invasion by targeting ING4 through Wnt-1/β-catenin pathway. 2019;18(5):4621–4628. doi: 10.3892/ol.2019.10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You Q., Li H., Liu Y., Xu Y., Miao S., Yao G., et al. MicroRNA-650 targets inhibitor of growth 4 to promote colorectal cancer progression via mitogen activated protein kinase signaling. 2018;16(2):2326–2334. doi: 10.3892/ol.2018.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y., Cheng X., Wang F., Pan J., Liu J., Chen H., et al. ING4 inhibits proliferation and induces apoptosis in human melanoma A375 cells via the Fas/caspase-8 apoptosis pathway. Dermatology. 2016;232(3):265–272. doi: 10.1159/000444050. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Martinka M.G. Role of ING4 in human melanoma cell migration, invasion and patient survival. Carcinogenesis. 2008;29(7):1373. doi: 10.1093/carcin/bgn086. [DOI] [PubMed] [Google Scholar]
- 26.Yu B.-L., Peng X.-H., Zhao F.-P., Liu X., Lu J., Wang L., et al. MicroRNA-378 functions as an onco-miR in nasopharyngeal carcinoma by repressing TOB2 expression. 2014;44(4):1215–1222. doi: 10.3892/ijo.2014.2283. [DOI] [PubMed] [Google Scholar]
- 27.Ho P.T.B., Clark I.M., Le L.T.T. MicroRNA-based diagnosis and therapy. Int. J. Mol. Sci. 2022;23(13) doi: 10.3390/ijms23137167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bushati N., Cohen S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 29.Di Leva G., Garofalo M., Croce C.M. MicroRNAs in cancer. Annu. Rev. Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloot S., Chen Y.A., Zhao X., Weber J.L., Benedict J.J., Mulé J.J., et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer. 2017;124(2):297–305. doi: 10.1002/cncr.30946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaziel A., Menendez S., Segura M.F., Zakrzewski J., Rose A., Kerbel R.S., et al. Abstract 1946: identification of miRNAs that contribute to melanoma brain metastasis. Cancer Res. 2011;70(8 Supplement):1946. [Google Scholar]
- 32.Mannavola F., Tucci M., Felici C., Stucci S., Silvestris F. miRNAs in melanoma: a defined role in tumor progression and metastasis. Expet Rev. Clin. Immunol. 2016;12(1):79–89. doi: 10.1586/1744666X.2016.1100965. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y., Xu J., Li H., Hu Y., Yu G. Identification of metastasis-associated MicroRNAs in metastatic melanoma by miRNA expression profile and experimental validation. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.663110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su Y., Zheng Q., Zhu L., Gu X., Lu J., Li L. Functions and underlying mechanisms of miR-650 in human cancers. Cancer Cell Int. 2022;22(1):132. doi: 10.1186/s12935-022-02551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuo Z.H., Yu Y.P., Ding Y., Liu S., Martin A., Tseng G., et al. Oncogenic activity of miR-650 in prostate cancer is mediated by suppression of CSR1 expression. Am. J. Pathol. 2015;185(7):1991–1999. doi: 10.1016/j.ajpath.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlandella F.M., Mariniello R.M., Iervolino P.L.C., Imperlini E., Mandola A., Verde A., et al. miR-650 promotes motility of anaplastic thyroid cancer cells by targeting PPP2CA. Endocrine. 2019;65(3):582–594. doi: 10.1007/s12020-019-01910-3. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Zhu W., Zhang J., Huo S., Zhou L., Gu Z., et al. MicroRNA-650 targets ING4 to promote gastric cancer tumorigenicity. Biochem. Biophys. Res. Commun. 2010;395(2):275–280. doi: 10.1016/j.bbrc.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Cui S., Gao Y., Zhang K., Chen J., Wang R., Chen L. The emerging role of inhibitor of growth 4 as a tumor suppressor in multiple human cancers. Cell. Physiol. Biochem. 2015;36(2):409–422. doi: 10.1159/000430108. [DOI] [PubMed] [Google Scholar]
- 39.Li J., Li G. Cell cycle regulator ING4 is a suppressor of melanoma angiogenesis that is regulated by the metastasis suppressor BRMS1. Cancer Res. 2010;70(24):10445–10453. doi: 10.1158/0008-5472.CAN-10-3040. [DOI] [PubMed] [Google Scholar]
- 40.Tang Y., Cheng Y., Martinka M., Ong C.J., Li G. Prognostic significance of KAI1/CD82 in human melanoma and its role in cell migration and invasion through the regulation of ING4. Carcinogenesis. 2014;35(1):86–95. doi: 10.1093/carcin/bgt346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.