Objective
To compare the impact of two pesticide dose forms on Blattella germanica (B. germanica)resistance enzymes.The micro-drop technique was utilized on subcultured B. germanica, and the metabolic enzyme activity was assessed.Using spectrophotometry, the relative enzyme activities of B.germanica were determined.Profile analysis was used to compare the enzyme activities of beta-cypermethrin in both nanoemulsion and traditional emulsion forms.Findings: The suppression percentages of the acetylcholinesterase enzyme (AchE), GST, and P450–O demethylases across different pesticide formulations were analyzed using regression equations, yielding F = 31.18, P < 0.001; F = 9.18, P < 0.001; and F = 4.58, P < 0.02.Conclusion: The suppression of AchE, GST, and P450–O demethylase by beta-cypermethrin nanoemulsion was more significant compared to the beta-cypermethrin emulsifiable concentrate.The study concluded that beta-cypermethrin nanoemulsion had a significant impact on insect detoxifying enzymes compared to beta-cypermethrin emulsifiable concentrate due to their numerous advantages.
Keywords: Beta-cypermethrin nanoemulsion, Conventional emulsion, Acetylcholinestrase, Glutathione S-Transferase, P450–O demethylase
1. Introduction
Beta-cypermethrin is an insecticide with a broad spectrum that belongs to the cypermethrin pesticide family. Its primary mode of action involves inhibiting nerve conduction and interfering with the insect nervous system to generate its insecticidal effect. Beta-cypermethrin has been widely used in agricultural production.
Presently, the most common forms of beta-cypermethrin preparation are raw powder (containing at least 95 %) and emulsifiable concentrate (content of 27 %) [1]. The powder is utilized after being dissolved in organic solvent acetone and diluted. The conventional emulsion contains more organic solvent, making it difficult to store and transport, as well as harmful to humans, animals, crops, and the environment, and causing the fruit powder to dissolve [2].
Pesticide nanoemulsion is a type of pesticide preparation that uses nanoparticles as a carrier and can enhance the pesticides' stability, dissolving rate, and adsorption performance, as well as their utilization rate and control impact.
By encapsulating pesticide active components in nanoparticles, pesticide nanoemulsion is able to generate a stable emulsion dispersion system.These nanoparticles possess an extensive specific surface area and excellent adsorption capacity, which can improve the interaction between pesticides and crops or pests, thereby boosting pesticide uptake and infiltration.
In addition, nanoparticles as carriers can increase the rate of pesticide dissolution [2,3]. Because nanoparticles are smaller in size and have a bigger surface area, they can dissolve pesticide-active components more quickly and enhance the rate of pesticide release and its effectiveness.
The pesticide nanoemulsion is also stable, can effectively prevent the pesticide's breakdown and degradation, and extends the pesticide's duration of effectiveness. The encapsulation of nanoparticles can protect the active ingredients of pesticides from environmental elements such as light, oxygen, and humidity and prevent pesticide loss during storage and application [4].
After the pesticide has been transformed into a nanoemulsion, it is often dilutable indefinitely with water. At the same concentration, it tends to have a greater insecticidal effect than regular emulsifiable concentrate [[5], [6], [7], [8], [9]]. According to studies, conventional insecticides primarily act on detoxification enzymes (metabolizing enzymes and target enzymes) in the bodies of insects, inhibit the activity of enzymes associated with resistance and reduce the sensitivity of detoxification enzymes to insecticides, thereby achieving their intended effect [10,11].
Therefore, it is crucial to examine the changes in resistance-related enzyme activities of beta-cypermethrin cypermethrin nanoemulsion and conventional emulsion following their application to B. germanica. B.germanica is a common indoor pest, easy to raise, short growth cycle.
2. Materials and methods
2.1. Reagents
TritonX–100: Chemical Reagent Factory of Shanghai.
P-nitroanisole (P-NA) and p-nitrophenol from Yucai Fine Chemical Factory in Beijing.
Acetylthiocholine iodide, 5,5′-dithiobisnitrobenzoic acid (DTNB): Sigma product.
Reduced glutathione (GSH): product of Huamei Bioengineering Co., Ltd.
Sodium dodecyl sulfate (SDS), ethylenediaminetetraacetic acid (EDTA), dithiothreitol (DTT), 2,4-dinitrochlorobenzene (CDNB): Shanghai Reagent No.1 Factory.
Reduced coenzyme II (NADPH): product of Roche company;
Benzyl sulfonyl fluoride (PMSF): product of Nanjing Shengxing Technology Co., Ltd.;
Fluka items include disodium p-nitrophenyl phosphate and Coomassie Brilliant Blue.
95 % beta-cypermethrin powder: Jiangsu Pesticide Research Institute Nanjing Pesticide Factory;
Our laboratory created a 2.85 % beta-cypermethrin nanoemulsion.The emulsion was light yellow in color, transparent, uniform, and fluid.The specimen was enclosed in a container. The item can be kept at ambient temperature for an extended duration, maintaining its clear appearance without any sedimentation or layering, and exhibiting fluidity and emulsifying characteristics. The mean particle size of the nanoemulsion measured 11.2 nm.The diluent is produced by adding additional reagents of domestic analytical purity.
2.2. Examine the origin of the insect and the treatment approach
For a duration of three months, our lab received B. germanica from the Insect Breeding Facility at the Jiangsu Provincial Disease Control Center, ensuring they were not exposed to any pesticides.The microdrop method is used to select and treat healthy adult males.To avoid unnecessary damage to breeding individuals, use male adults for experimental operations rather than females, since they are more convenient in terms of reproduction (including egg production and hatching).A microinjector was used to inject 1 μL of the medication over the chest and abdomen back plates of the test insects.They were then stored in a clean jam jar without treatment.B. germanica without any medication was used as a control, and B. germanica treated with various amounts of medication was used as an experimental group.
2.3. Determination of enzyme activity
Visible light absorption variations resulting from enzyme-catalyzed reactions are used to quantify enzyme activity. In determining enzyme activity, measurements are often taken in an area where the enzyme's reaction rate is constant with increasing reaction time and can be linearly correlated in the reaction system [12,13]. This is due to the fact that the enzyme activity has ideal working conditions within a defined range, and increasing or not exceeding this range will alter its activity stability and the precision of the test. Therefore, a suitable reaction time is chosen to assess the product yield in order to reflect the enzyme's activity level.
2.3.1. Preparation of enzyme solution
-
(1)
Preparation of AchE enzyme solution: Following a 24-h fasting period, B. germanica was washed with distilled water for 2 min, dried using filter paper, and a phosphate buffer solution [pH 8.0, 1/15 (mol/L), with 0.5 % TritonX-100] was applied to its head.Once finely chopped and blended, the enzyme mixture was spun at 4000 rpm for 15 min, and the resulting supernatant was utilized.
-
(2)
To prepare the GST enzyme solution, B. germanica was starved for a day post-treatment, washed under flowing distilled water for 2 min, and then dried on filter paper.We dissected it at 4 °C, removed food from the digestive tract, prepared an enzyme solution and analyzed it.The mixture, after being finely chopped and blended in a phosphate buffer (pH 6.5, 0.1 mol/L), was centrifuged at 10,000 rpm for 15 min, and the resulting supernatant was utilized as the enzyme solution.
-
(3)
Preparing the P450–O demethylase enzyme solution: Following treatment, B. germanica was deprived of food for a day, washed with distilled water for 2 min, dried on filter paper, dissected at 4 °C, and had its digestive tract contents removed.A solution of enzymes was created by mincing and homogenizing midgut tissue in a phosphate buffer (pH 7.0, 0.1 mol/L, with 1 mmol/L EDTA, 1 mmol/L DTT, and 1 mmol/L PMSF) on ice, followed by centrifugation at 10,000 rpm for 15 min, and using the supernatant.
2.3.2. Determination of enzyme activity
-
(1)
Following Groun's adaptation of the Ellman method [14], the reaction mixture includes 0.2 mL, 100 μL of 1/15 M pH 8.0 phosphate buffer, and 50 μL of 0.75 mM substrate.Following a 15-min incubation of 50 μL of enzyme solution at 30 °C, 1.8 mL of DTNB reagent was introduced, and the absorbance was measured at 412 nm.
-
(2)
To measure GST activity [15], a reaction mixture was prepared by adding 100 μL of enzyme solution, 2.5 mL of phosphate buffer (0.1 mol/L, pH 6.5), 0.1 mL of reduced GSH (100 mmol/L), and 20 μL of 2,4-Dinitrobenzene (CDNB) acetone solution (50 mmol/L).0.5 mL SDS (2.5 %) was added and mixed for 10 min.Following a 10-min period of steady installation, the absorbance at 340 nm was recorded using a UV spectrophotometer at 1-min intervals for a duration of 3 min.In order to calculate enzyme activity, measure the change in absorbance in 3 min.The reaction rate is expressed as [mOD/(mgmin)].
-
(3)
Assessment of P450–O demethylase function [16] involved a 96-well enzyme plate containing 100 μL of 2.0 mmol/L p-nitroanisole (P-NA), 10 μL of 9.6 mmol/L NADPH (reduced coenzyme II), and 90 μL of the enzyme mixture.The optical density values were measured every 25 s at a wavelength of 412 nm for a total of 10 min.During the enzymatic reaction, the temperature was 30 °C.The enzyme activity, measured in nOD/(mg min), was calculated from the reaction rate observed within the 0 to 0.2 range.
2.3.3. Determination of protein concentration
According to the Bradford method [17], 0.1 mL of the enzyme solution was mixed with 5 mL of Coomassie Brilliant Blue, and the optical density was recorded at 595 nm.The protein levels were measured using the calibration curve.
2.4. Data processing
Utilizing SPSS 22.0, we performed correlation and regression analyses, variance analysis, and Dunnett's t-test, and plotted the regression curves.
2.5. Characterization of beta-cypermethrin nanoemulsion
2.5.1. Morphology and structure of nanoemulsion observed by transmission electron microscope
Fig. 1 shows an image of beta-cypermethrin nanoemulsion captured by a transmission electron microscope (TEM).Under an electron microscope, the majority of nanoemulsion particles had a spherical shape and a homogeneous size distribution.
Fig. 1.

TEM image of beta-cypermethrin nanoemulsion at 10,000× magnification.
2.5.2. Determination of particle size and distribution of nanoemulsion
Using an ESL-8000 particle size analyzer, the beta-cypermethrin nanoemulsion was tested and analyzed.Fig. 2 illustrates that the nanoemulsion's mean particle size was determined to be 11.2 nm through particle size analysis.
Fig. 2.
Particle size distribution of beta-cypermethrin nanoemulsion.
3. Results and analysis
3.1. A comparison of the impact of the dosages of nanoemulsion and conventional
Emulsion's impact on AchE activity in B. germanica (see Table 1)
Table 1.
Effect of different concentrations of nanoemulsion and conventional emulsion on the activity of AchE in B. germanica.
| Nanoemulsion |
Conventional emulsion |
||||||
|---|---|---|---|---|---|---|---|
| Medication concentration (%) | Number of insects | AchE activity (nmol/mg·min) | Inhibition ratio (%) | Medication concentration (%) | number of insects | AchE activity (nmol/mg·min) | Inhibition ratio (%) |
| Control (0) | 10 | 81.15 ± 1.40 | 0 | Control (0) | 10 | 81.15 ± 1.40 | 0 |
| 0.00001 % (1) | 10 | 68.11 ± 2.08 | 16.07 | 0.0001 % (1) | 10 | 73.00 ± 1.40 | 10.04 |
| 0.00002 % (2) | 10 | 60.36 ± 1.98 | 25.62 | 0.0002 % (2) | 10 | 65.50 ± 1.79 | 19.25 |
| 0.00003 % (3) | 10 | 53.94 ± 2.00 | 33.53 | 0.0003 % (3) | 10 | 58.66 ± 1.87 | 27.71 |
| 0.00004 % (4) | 10 | 46.03 ± 2.69 | 43.28 | 0.0004 % (4) | 10 | 52.57 ± 2.12 | 35.22 |
The nanoemulsion cohort was evaluated against the control set, yielding F = 846.774 and P < 0.001.
The conventional emulsion group was evaluated against the control group, yielding F = 844.657, P < 0.001.
The percentage of enzyme activity inhibition is calculated as: ((control group value - treatment group value)/control group value) × 100 %.Table 1 displays the impact of nanoemulsion and conventional emulsion on the acetylcholinesterase activity of B. germanica.When nanoemulsion is applied to B. germanica, it is evident that AchE activity diminishes with increasing drug concentration, and Dunnett's t-test confirms that the experimental group significantly differs from the control group (Table 2).The conventional emulsion group had a similar inhibitory impact on B. germanica as the nanoemulsion group.When its levels exceeded those of the nanoemulsion group, the AchE activity inhibition rate was lower compared to the nanoemulsion group.This suggested that nanoemulsion is more effective against insects than conventional emulsion.
Table 2.
Comparison of AchE activity between the medication group and the control group (Dunnett's t-Test).
| Nanoemulsion |
Conventional emulsion |
|||||
|---|---|---|---|---|---|---|
| comparison among groups |
95%BMDL |
deviation |
95%upper confidence |
95%BMDL |
deviation |
95%upper confidence |
| limit | limit | |||||
| (1)–(0) −14.6599 | −13.0330a | −11.4061 | −9.5193 | −13.0330a | −6.7827 | |
| (2)–(0) −22.4189 | −20.7920a | −19.1651 | −17.0128 | −20.7920a | −14.2762 | |
| (3)–(0) −28.8359 | −27.2090a | −25.5821 | −23.8533 | −27.2090a | −21.1167 | |
| (4)–(0) −36.7464 | −35. 1195a | −33.4926 | −29.9498 | −35. 1195a | −27.2132 | |
BMDL:Benchmark Dose Lower Limit.
denotes a notable variation from the control group, with P < 0.05.
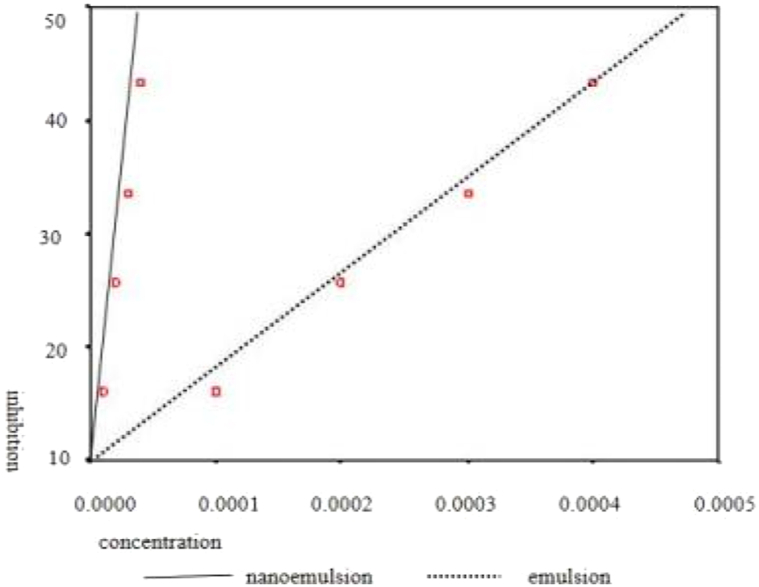
Using SPSS 22.0 software to assess the drug concentration and AchE inhibition ratio of nanoemulsion and conventional emulsion, we first create a scatter plot to determine if there is a linear trend (Fig. 3).After validating the linear trend, we compute the correlation coefficient between the two variables using the CORR procedure and test its correlation coefficient to determine if there is a statistically linear link.The correlation analysis revealed that r1 = 0.999 (P = 0.001 < 0.05) and r2 = 0.998 (P = 0.001 < 0.05), indicating a positive relationship between the concentrations of nanoemulsion and conventional emulsion and the AchE inhibition ratio.
Fig. 3.
Regression curve between pesticide concentration and AchE inhibition ratio.
The regression equation of AchE inhibition ratio and nanoemulsion concentration was produced using the REG procedure:y1 = 895400.00x1+7.240 (F = 1302.543, P < 0.05); regression equation of AchE inhibition ratio and concentration of conventional emulsion: y2 = 84000.00x2 +2.055 (F = 973.913, P < 0.05).
A comparison of the two regression equations showed a statistically significant difference in their coefficients, F = 31.18, P < 0.001.The inhibition ratios of the two samples on AchE may be distinct.It was determined that nanoemulsion suppressed AchE more efficiently than conventional emulsion.
3.2. Comparison of the effects of the dosage of nanoemulsion and conventional emulsion on GST activity of B. germanica (Table 3)
Table 3.
Effect of varying concentrations of nanoemulsion and conventional emulsion on B. germanica GST activity.
| Nanoemulsion |
Conventional emulsion |
||||||
|---|---|---|---|---|---|---|---|
| Medication concentration (%) | Number of insects | GST activity mOD/(mg·min) | Inhibition ratio (%) | Medication concentration (%) | Number of insects | GST activity mOD/(mg·min) | Inhibition ratio (%) |
| Control (0) | 10 | 0.74 ± 0.01 | 0 | Control (0) | 10 | 0.74 ± 0.01 | 0 |
| 0.00001 % (1) | 10 | 0.67 ± 0.02 | 9.46 | 0.0001 % (1) | 10 | 0.62 ± 0.02 | 16.22 |
| 0.00002 % (2) | 10 | 0.54 ± 0.02 | 27.03 | 0.0002 % (2) | 10 | 0.58 ± 0.01 | 21.62 |
| 0.00003 % (3) | 10 | 0.48 ± 0.02 | 35.14 | 0.0003 % (3) | 10 | 0.50 ± 0.01 | 32.43 |
| 0.00004 % (4) | 10 | 0.40 ± 0.02 | 45.95 | 0.0004 % (4) | 10 | 0.42 ± 0.01 | 43.24 |
A comparison was made between the nanoemulsion group and the control group, yielding F = 1558.214 and P < 0.001.
The standard emulsion group was evaluated against the control group, yielding F = 1692.375 and P < 0.001.
Table 3 shows the effects of different concentrations of nanoemulsion and conventional emulsion.Following a 24-h treatment period, the GST activity in B. germanica is assessed. Both nanoemulsion and traditional emulsion can reduce GST activity in B. germanica as the concentration of the active ingredient rises. Dunnett's t-test results indicated that the enzyme activity in B. germanica, at four different insecticide concentrations for both nanoemulsion and conventional emulsion, was statistically significant compared to the control group (Table 4).
Table 4.
Comparison of GST enzyme activities between the medication group and the control group (Dunnett's t-Test).
| Nanoemulsion |
Conventional emulsion |
|||||
|---|---|---|---|---|---|---|
| comparison among groups | 95%BMD L | deviation | 95%upper confidence |
95%BMDL | deviation | 95%upper confidence |
| limit | limit | |||||
| (1)–(0) | −0.0793 | −0.0670* | −0.0547 | −0.1327 | -. 1225* | −0.1123 |
| (2)–(0) | −0.2188 | −0.2065* | −0.1942 | −0.1732 | -. 1630* | −0.1528 |
| (3)–(0) | −0.2693 | −0.2570* | −0.2447 | −0.2462 | −0.2360* | −0.2258 |
| (4)–(0) | −0.3498 | −0.3375* | −0.3252 | −0.3262 | −0.3160* | −0.3058 |
A significant difference from the control group is indicated by '*', with P < 0.05.
At first, by using SPSS 22.0 software to assess the medicine concentration and GST inhibition ratio of nanoemulsion and conventional insecticide, we first generate a scatter plot to determine if a linear trend exists (Fig. 4).After validating the linear trend, we compute the correlation coefficient between the two variables using the CORR procedure and test its correlation coefficient to determine if there is a statistically linear link.The correlation analysis showed that the coefficient between nanoemulsion concentration and GST inhibition ratio was r1 = 0.987 (P = 0.013 < 0.05), while for conventional emulsion concentration and GST inhibition ratio, the coefficient was r2 = 0.990 (P = 0.010 < 0.05).This reveals a positive association between the concentrations of nanoemulsion and conventional emulsion and the ratio of GST inhibition.
Fig. 4.
Regression curve between pesticide concentration and GST inhibition ratio.
REG method was used to derive the regression equation for the GST inhibition ratio and the concentration of nanoemulsion: y1 = 1175800x1-8.43 × 10–15 (F = 73.468, P < 0.01); regression equation of GST inhibition ratio and conventional emulsion:
y2 = 91870.00 x2 + 5. 10 (F = 96.124, P < 0.01).
Analyzing the two regression models, F = 9.18, P < 0.001 indicates a statistically significant difference between their coefficients.The inhibition ratio of nanoemulsion on GST is deemed to be greater than that of conventional emulsion.
3.3. Comparison of the effects of nanoemulsion and conventional emulsion on P450–O demethylase in B. germanica (Table 5)
Table 5.
Effect of varying nanoemulsion and conventional emulsion concentrations on the P450–O demethylase activity of B. germanica.
| Nanoemulsion |
Conventional emulsion |
||||||
|---|---|---|---|---|---|---|---|
| Medication concentration (%) | Number of insects | P450-OactivitynOD/(mg·min) | Inhibition ratio (%) | Medication concentration (%) | Number of insects | P450–O activity nOD/(mg·min) | Inhibition ratio (%) |
| Control (0) | 10 | 2.33 ± 0.07 | 0 | Control (0) | 10 | 2.33 ± 0.07 | 0 |
| 0.00001 % (1) | 10 | 1.87 ± 0.04 | 19.74 | 0.0001 % (1) | 10 | 2.00 ± 0.07 | 14.16 |
| 0.00002 % (2) | 10 | 1.37 ± 0.04 | 41.20 | 0.0002 % (2) | 10 | 1.43 ± 0.05 | 38.63 |
| 0.00003 % (3) | 10 | 1.11 ± 0.06 | 52.36 | 0.0003 % (3) | 10 | 1.26 ± 0.04 | 45.92 |
| 0.00004 % (4) | 10 | 0.95 ± 0.03 | 59.23 | 0.0004 % (4) | 10 | 1.03 ± 0.05 | 55.79 |
The comparison between the nanoemulsion and control groups yielded F = 1786.062, P < 0.001.
The standard emulsion group was evaluated against the control group, yielding F = 2644.376.P < 0.001.
Table 5 illustrates the impact of different concentrations of nanoemulsion and traditional emulsion on P450–O demethylase activity in 24 h post-treatment.As the drug concentration rises, both nanoemulsions and traditional emulsions can reduce the activity of P450–O demethylase. Dunnett's t-test results show the enzyme activity of B. germanica at four different drug concentrations for both nanoemulsions and conventional emulsions.The variations are statistically significant when compared to the control group, as shown in Table 6.
Table 6.
Comparison of P450–O demethylase activity between the medication group and the control group (Dunnett's t-test).
| nanoemulsion |
conventional emulsion |
|||||
|---|---|---|---|---|---|---|
| comparison among groups | 95%BMDL | deviation | 95%upper confidence limit | 95%BMDL | deviation | 95%upper confidence limit |
| (1)–(0) | −0.4988 | −0.4600* | −0.4212 | −0.3706 | −0.3260* | −0.2814 |
| (2)–(0) | −0.9943 | −0.9555* | −0.9167 | −0.9361 | −0.8915* | −0.8469 |
| (3)–(0) | −1.2523 | −1.2135* | −1.1747 | −1.1136 | −1.0690* | −1.0244 |
| (4)–(0) | −1.4218 | −1.3830* | −1.3442 | −1.3371 | −1.2925* | −1.2479 |
A significant difference from the control group is indicated by '*', with P < 0.05.
By using SPSS 22.0 to assess the insecticide concentrations in nanoemulsion and conventional emulsion and the P450–O demethylase inhibition ratio, we first create a scatter plot to determine if there is a linear trend (Fig. 5). After establishing the linear trend, we compute the correlation coefficient between the two variables using the CORR procedure and do a statistical test on the correlation coefficient to see if there is a linear relationship. According to correlation analysis, the correlation coefficient between the concentration of nanoemulsion and the P450–O demethylase inhibition ratio was r1 = 0.969 (P = 0.031 < 0.05). The correlation coefficient between conventional emulsion concentration and P450–O demethylase inhibition ratio was r2 = 0.961 (P = 0.039 < 0.05). This demonstrates that there is a positive link between the concentrations of nanoemulsion and conventional emulsion and the P450–O demethylase inhibition ratio.
Fig. 5.
Regression curve between pesticide concentration and P450–O inhibition ratio.
REG method was used to construct the regression equation for the inhibitory ratio of P450–O demethylase and the concentration of nanoemulsion:y1 = 1296300x1+10.725 (F = 30.540, P < 0.01).The regression equation for the P450–O demethylase inhibition ratio and conventional emulsion concentration was: y2 = 132180x2 + 5.580 (F = 23.995, P < 0.01).Analyzing the two regression models results in F = 4.58, with 0.01<P < 0.02, suggesting that the disparity between the regression coefficients is statistically meaningful.The inhibition ratio of P450–O demethylase in nanoemulsion appears to be greater than that of conventional emulsion.
4. Discussion
The insect resistance mechanisms to pyrethroid insecticides include a reduction in insecticide penetration by the insect epidermis, an increase in detoxifying metabolism, a decrease in target site sensitivity, and the occurrence of knockdown resistance [[18], [19], [20]].However, the chemical mechanism underlying the decrease in neurosensitivity is rarely understood.The experiment results reveal that AchE is one of the beta-cypermethrin targets of cypermethrin and that the change in enzyme activity represents the dose-effect and time-effect link between beta-cypermethrin cypermethrin and AchE activity.At the same time, this shows that the reduction of AchE sensitivity in resistant B. germanica is also one of the molecular mechanisms of neuro sensitivity reduction, as well as one of 's major resistance mechanisms to pyrethroid pesticides.Examining the principles of various insecticides, the degrees of drug resistance, and the levels and activities of AchE and other detoxifying enzymes, based on the mechanisms of insecticides and the molecular and biochemical mechanisms of insecticide resistance, is crucial for understanding the resistance mechanism of B.germanica to pyrethroid insecticides and for developing biochemical methods to detect this resistance.Implementing suitable and effective management strategies is essential for improving the speed and precision of resistance detection and predicting resistance risks in real-time.
GST is a type of active protein that performs numerous physiological roles in the metabolic process.It not only catalyzes the binding of polar molecules of dangerous chemicals in the body to glutathione (GSH), but it also promotes the non-enzymatic excretion of numerous potentially poisonous compounds in the body.As a result, it is classified as an isozyme with numerous interpretation roles.One of the main causes of mosquito resistance to DDT and organophosphorus is enhanced GST activity, which increases the ability to digest pesticides [21].According to research, the protective enzyme activity in insects is related to the insect's response to the matching intensity of external stimuli and resistance to particular medications [[22], [23], [24]].The cytochrome P450 enzyme system is essential for insects to detoxify pesticides [[25], [26], [27], [28], [29]].resistance in pests is frequently linked to increased cytochrome P450 activity, and cytochrome P450 polymorphism and a wide range of substrates further complicate the cross-resistance interaction across agents.Studies indicate that cytochrome P450 is essential in the mechanism of resistance [30,31].
The findings indicate that nanoemulsion suppresses the three enzymes associated with resistance—AchE, GST, and P450–O demethylase—more effectively at lower concentrations compared to traditional emulsion.This suggests that nanoemulsion can increase the sensitivity of target area.This could be owing to the nanoemulsion's small particle size and penetrating strength.nanoemulsion enters the insect body at a faster and larger dose than conventional emulsion.A greater inhibitory impact requires a higher effective dose of the insecticide targeting the resistance-related enzyme compared to the emulsifiable concentrate.Insect target area sensitivity is directly connected to insect resistance [32,33].The nanoemulsion increases the sensitivity of target area, which may result in a faster decrease in insect resistance, but this needs to be proven by future research.
Funding
This research was funded by [station and vehicle health research fund of Railway Department] grant number [J99Z217] and The APC was funded by [J99Z217].
Data availability statement
Data included in article/supp. material/referenced in article.
Ethics statement
Provide the name of Ethics Committee for approval and original approval.
CRediT authorship contribution statement
Yan Shen: Writing – original draft. Qiong Li: Investigation. Fujin Fang: Data curation. Chuanli Yang: Methodology. Yu Dong: Software. Xiaoqin Li: Visualization. Zhizhi Luo: Validation. Xiaobing Shen: Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Xiaobing Shen reports financial support was provided by station and vehicle health research fund of Railway Department. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
The writer(s) state(s) that there are no conflicts of interest related to the release of this paper.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36372.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Fan Yang, Xia Shuai. Relationship between beta-cypermethrin resistance and acetylcholinesterase in Myzus persicae. Plant Prot. 2018;34:60–62. [Google Scholar]
- 2.Benxin Zhou. Chemical Industry Press; Beijing: 1994. New Formulation of Pesticides; p. 192. [Google Scholar]
- 3.Menglan Liang. Publishing House; 1990. Surface Active Agents and detergents,Beijing:Science and Technology Literature; p. 392. [Google Scholar]
- 4.Chunfeng Li, Luo Xinmin, Yanqing Li. vol. 10. 2016. pp. 8–11. (Progress in Microemulsion Pesticide Technology , Pesticide Research and Application). [Google Scholar]
- 5.Gao Yangfan, Wang Jianhua, Wang Zhenhe, Zhang Suqin. Study on the development status of pesticide microemulsion and the influencing factors of its preparation. Journal of Henan University of Science and Technology(Natural Science Edition) 2017;35:44–46. [Google Scholar]
- 6.Dong Guangxin, Zhou Liangjia, Du Wei, Cui Yong. Preparation of 10% pyroxazole microemulsion. Agriculture. 2016;45:311–315. [Google Scholar]
- 7.Chen Lin, Yin Hong, Deng Defeng, Cheng Wang, Xue Guangcai, Xiao Hongbo. vol. 10. 2016. pp. 24–26. (Study on 30% Chlorpyrifos Microemulsion and Field Efficacy Test,Pesticide Research and Application). [Google Scholar]
- 8.Han Meimei, Xie Guangli, Lu Rongsheng, Meili Du. Experiment of 2.5% beta-permethrin microemulsion against Pieris rapae. Agricultural Sciences of Guangxi. 2015;36:555–556. [Google Scholar]
- 9.Chen Wei, Hu Hongtao, Zhou Ronghua, Zhu Zhigang, Cao Chunxia. Application of 20% Acitretin·Dimethaphane microemulsion on rice. Hubei Agric. Sci. 2005;5:65–66. [Google Scholar]
- 10.Hui Wei, Shen Jinliang, Wu Wei, Zhao Jianwei, Zhan Zhixiong. Purification, biochemical properties and insecticide sensitivity of acetylcholinesterase from Musca domestica. Journal of Agro-Environment Science. 2019;28:156–160. [Google Scholar]
- 11.Kristoff G., Guerrero N.V., de D., Angelo A.M., de D., Angelo A.M.P., Cochón A.C. Inhibition of cholinesterase activity by azinphos-methyl in two freshwater invertebrates: Biomphalaria glabrata and Lumbriculus variegatus. Toxicol. Sci. 2006;222:185–194. doi: 10.1016/j.tox.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Michael E.S., Walid K. Changes in an insecticide-resistant field population of German cockroach (Dictyoptera: blattelidae) after expose to an insecticide mixture. Econ. Entomol.Sci. 1997;90:38–48. [Google Scholar]
- 13.Valles S.M., Koehler P.G., Brenner R.J.C. Omparative insecticide susceptibility and detoxification enzyme activities among pestiferous Blattodea Comparative Biochemistry and Physiology, part C. Sci . 1999;124:227–232. doi: 10.1016/s0742-8413(99)00076-6. [DOI] [PubMed] [Google Scholar]
- 14.Gorun V., Proinov L., Baltescu V., et al. Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Anal. Chem. 1978;86:324–326. doi: 10.1016/0003-2697(78)90350-0. [DOI] [PubMed] [Google Scholar]
- 15.Wu Xingfu, Jianhua Deng, Dejun Wang, Hongdong Li, Kuanfeng Zhao. Effect of pesticide combination on resistance development of Myzus persicae. Chinese Journal of Tobacco. 2014;10:38–42. [Google Scholar]
- 16.Shijun Xia, Wu Zhongliang. Science and Technology Press; Hubei: 2010. Foundation of Molecular Toxicology; pp. 66–67. [Google Scholar]
- 17.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantites of protein utilizing the principle of protein dybinging. Anal. Chem.Sci. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Qu Qihui. Some advances in insect molecular biology: molecular basis of insecticide resistance. Journal of insect. 1995;38:493–501. [Google Scholar]
- 19.Sun Yunqin, Yuan Jia, Jing Li. Resistance mechanism of Musca domestica to pyrethroids. Journal of insect. 1990;33:265–272. [Google Scholar]
- 20.Chang C.P., Plapp F.W. DDT and pyrethroids:receptor binding in relation to knockdown resistance (kdr) in the housefly. Pestic. Biochem. Physiol. 1983;20:86–91. [Google Scholar]
- 21.Zhang Hongying, Guotong Chi, Zhang Jinlin. Journal of Agricultural University of Hebei; 2012. Advances in Insect Interpretation Enzymes and Insecticide Resistance; pp. 193–195. (supplementary issue) [Google Scholar]
- 22.Wu Xiaofeng, Junliang Xu, Weizheng Cui. Catalase activity in blood of silkworm and its relationship with resistance of silkworm body. Journal of insect. 1998;41:124–128. [Google Scholar]
- 23.Gao Xiwu, Dong Xiangli, Bingzong Zheng, S-Transferases Glutamine. GSTs) of cotton boll worm: induction of pesticides and plant secondary substances and metabolism of pesticides by GSTs. Journal of insect. 1997;40:122–127. [Google Scholar]
- 24.Moran Rick J. Ross-effects of infection of cucumber with fungal and bacterial pathogens, and the role of peroxidase. Bull. Ecol. Soc. Am. 1997;78:287. [Google Scholar]
- 25.Junwen Ai, Dong Yuanling, Weiqing Kong. vol. 30. Journal of Southwest University Natural Science Edition; 2018. pp. 51–58. (Molecular Cloning and Sequence Analysis of CYP337A1, the First Gene of a Novel Cytochrome P450 Family). [Google Scholar]
- 26.Zhang Shuang, Yang Yihua, Shuwen Wu. Metabolic effects of yeast expression product of P450 gene CYP9A12 of helicoverpa armigera on pyrethroids. Journal of insect. 2018;51:1255–1259. [Google Scholar]
- 27.Zhang Ning, Yu Wenjuan, Xiangling Wang. Extraction of liver microsomes from grass carp and determination of CYP enzyme activities. Marine Fisheries. 2017;29:148–152. [Google Scholar]
- 28.Yang Haifeng, Jiang Shanxiang. Establishment of a method for detecting cytochrome P450 enzyme system in chicken liver microsomes. Chin. J. Vet. Sci. 2016;36:147–150. [Google Scholar]
- 29.Zheng Liangpu, Lin Wei, Ye Yuzhi. Experimental study on detection method of CYP450 enzymes in mouse liver microsomes. Journal of Fujian College of Traditional Chinese Medicine. 2016;16:50–51. [Google Scholar]
- 30.Wu D., Scharf M.E., Neal J.J., et al. Mechanisms of fenvalerate resistance in the German cockroach, Blattella germanica (L.) Pestic. Biochem. Physiol. 1998;61:53–62. [Google Scholar]
- 31.Peng Cheng, Cao Yinguang, Maoqing Gong. Advances in cytochrome P450-mediated insect insecticide resistance. Chinese Journal of Pathogenic Biology. 2019;4:62–65. [Google Scholar]
- 32.He Shuhai, Han Bingjun, Peng Lixu. Advances in insect resistance research and development strategies of new pesticides. Tropical Agricultural Sciences. 2016;26:75–80. [Google Scholar]
- 33.Zhiwei Ni, Guanqin Pu. vol. 4. Jiangsu Sericulture; 2006. Advances in the Mechanism of Insect Resistance; pp. 6–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.