Abstract
This research evaluates the use of cassava bagasse starch and oregano essential oil (OEO) in an active film. For comparison, films of cassava starch (CS) and cassava bagasse starch (BS) were prepared with OEO at 1, 2, and 3 %. Physical, thermal, mechanical, antioxidant, and antimicrobial properties were determined. BS films presented higher thickness, WVP, ΔE, modulus of elasticity, and maximum stress, but lower strain at break compared to CS films. Adding OEO into the films increased their thickness, moisture, solubility, WVP and strain at break. However, maximum stress, modulus of elasticity, and Tdmax decreased. The CS films added with 3 % of OEO showed higher WVP (6.32 × 10−14 kg m/m2.s.Pa), intermediate solubility of 39 % and low maximum stress (0.19 MPa) while the BS film with 3 % of OEO presented 5.73 × 10−14 kg m/m2.s.Pa, 30 % and 0.39 MPa, respectively. The increase from 1 % to 3 % of OEO increased the total phenolic compound content and antioxidant activity of the films by 1.3-fold and 3.7-fold, respectively. The incorporation of 3 % OEO in the films inhibited the growth of S. aureus and E. coli. Therefore, BS and OEO films offer a promising solution as biodegradable active food packaging, providing a more sustainable alternative to traditional non-biodegradable plastic packaging.
Keywords: Starch-riche residues, Biodegradable films, Casting, Films properties, Food preservation
Highlights
-
•
Active films successfully prepared from Cassava bagasse starch and Oregano essential oil.
-
•
The starch purity influences the film color and the maximum strain property.
-
•
Oregano essential oil increases thickness, permeability, and strain at break of films.
-
•
Cassava bagasse starch films with oregano essential oil show antioxidant activity.
-
•
High antibacterial activity of bagasse starch films with 3 % oregano essential oil.
1. Introduction
An alternative to reduce pollution created due to fossil-based plastic is to develop bio-based and biodegradable packaging based on sustainable raw materials that can be recovered through organic recycling [1]. Starch is a renewable, abundant, readily biodegradable, and low-cost natural polymer that can be used in packaging [2,3]. However, the non-food use of starch is controversial, as its priority should be reducing global hunger problems [4]. For this reason, considerable attention has turned to using bio-based feedstocks not used for human consumption, alternative raw materials, or residues generated during starch processing to develop biodegradable packaging.
Cassava (Manihot esculenta) is an attractive source of starch and starch-rich residues [5] grown in tropical and subtropical regions. Ecuador harvests approximately 96.2 thousand tons annually of cassava, being the INIAP 651 variety, one of the most promising starchy crops due to its high total starch content (around 88.82 %) [6]. INIAP 651 is a variety derived from the clone CM-1335-4, which has CM-462-1 as the mother and MCol-1292 as the father. Its dry matter content is 35.5 % [7]. Obtaining starch from cassava involves peeling, soaking, crushing, washing, settling, and drying. Cassava bagasse, a waste product from cassava processing, contains 50–70 % starch and 20–30 % lignocellulosic content [8]. From cassava starch and glycerol as a plasticizer, thermoplastic starch (TPS) films can be prepared [[9], [10], [11]]. The content of amylose and amylopectin influences the characteristics of the film produced. Amylose plays a crucial role in forming the starch film and its mechanical properties, such as tensile strength and flexibility. On the other hand, the molecular profile of amylopectin and plasticizer content also impact the properties of the films [12]. Few papers in the literature reported the use of cassava bagasse to obtain TPS films. The presence of bagasse fiber on the TPS films increased the maximum stress and the modulus of elasticity [13].
Biodegradable active packaging is a trending and innovative solution to extend the shelf life of food products [14]. Some active packaging contains antimicrobial agents such as essential oils because they interact with food components and can inactivate food-borne pathogens [[14], [15], [16]]. Oregano (Origanum vulgare) essential oil (OEO) has antimicrobial and antioxidant properties related to its main active compounds, including carvacrol and thymol [17,18], that are generally recognized as safe (GRAS) substances [19].
Studies have been conducted on films of starch added with essential oils. Shen et al. (2022) reported a moderate inhibition against Gram-positive (Staphylococcus aureus and Bacillus subtilis) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacteria due to the addition of 3 % OEO into starch films. Essential oils and their components are characterized by their hydrophobicity. This property allows them to interact with the lipids in the cell membrane of bacteria and mitochondria, making them more permeable by disturbing the cell structure. Besides, the incorporation of essential oils into the film led to a decrease in mechanical strength. Moreover, strong intermolecular polysaccharide interactions can be partially replaced by weak polysaccharide-essential oil interactions, resulting in more flexible domains within the film [20].
Kang & Song (2019) prepared starch films from Job's tears and clove essential oil at 0.25 %, 0.5 %, and 0.75 %, showing that the higher the amount of essential oil incorporated into the films, the moisture content and solubility decreased. In contrast, the water vapor permeability, strain at break, and antioxidant activity increased. One of the characteristics of starch films is their high hydrophilicity, which limits their potential application in food packaging. Several studies have been conducted to solve this problem by incorporating hydrophobic compounds like essential oils. Moisture content and water solubility showed a decreasing trend since essential oil affects the water retention of starch films [21].
Likewise, in films based on corn starch and Zanthoxylum bungeaniun essential oil (0.5 %, 1 %, and 2 % v/v), water solubility and water vapor permeability decreased with the addition of essential oil; however, strain at break increased [22]. The primary function of food packaging is to contain and to extend shelf life, which often involves mitigating water transfer between the packaged food and its surrounding environment, as well as between different components within the food itself. Therefore, minimizing the WVP of food packaging materials is necessary. The incorporation of EO considerably decreased the WVP of the films, a reduction that has risen with higher concentrations. This might be attributed to the hydrogen bonding interaction between the starch network and EO. This interaction potentially mitigates the hydrophilic connection between hydrogen groups and water molecules, reducing the film's affinity for water. Different results were obtained in corn starch and OEO films (0.3, 0.5, and 0.7 mL/kg), where at higher the amount of essential oil, the moisture content, water solubility, and water vapor permeability increased, but tensile strength and strain at break decreased [23]. Essential oils exhibit a plasticizing effect on films due to their increased elongation properties. Furthermore, the increase in essential oil leads to a decrease in the strength of the films while enhancing their flexibility.
So, previous studies have used pure cassava starch to prepare thermoplastic materials and active films; however, to the best of our knowledge, few literature have assessed the feasibility of cassava bagasse starch as an alternative matrix to prepare active films with potential applications in food packaging, and no studies using cassava INIAP 651 variety from Ecuador to prepare active films have been reported.
In this study, films of pure cassava and cassava bagasse starch of the INIAP 651 variety were prepared with the addition of different concentrations of OEO (1 %–3 %) and glycerol (0.5 %) as a plasticizer. This research aimed to evaluate the effect of cassava starch purity (pure starch and cassava bagasse starch) and the percentage of essential oil incorporated on the physical, antioxidant, and antimicrobial properties of active films for their potential use as active packaging.
2. Materials and methods
2.1. Materials
Pure cassava starch (CS) and cassava bagasse starch (BS) variety INIAP 651 was provided by producers from Manabí, Ecuador. The purity of cassava starch (CS) was 88.82 % (24.46 % amylose and 75.54 % amylopectin) (Cornejo et al., 2022). Glycerol (J.T. Baker avantor, USA) was used as a plasticizer. Oregano (O. vulgare) essential oil (OEO) was supplied by Now (Illinois, USA). Tryptic soy broth (TSB), peptone water (BPW), and Plate Count Agar (PCA) were purchased from Merck (Darmstadt, Germany). Reagent-grade sodium chloride (NaCl) was purchased from Scharlau (Barcelona, Spain). Bacterial strains S. aureus (ATCC No. 12600) and E. coli (ATCC No. 25922) were purchased from Microbiologics (St. Cloud, MN, USA).
2.2. Characterization of cassava bagasse starch
The determination of total starch, ash, fat, protein, and fiber was carried out according to AOAC 996.11, AOAC 923.03, AOAC18th 922.06, AOAC 21st 920.87 and AOAC 21st 978.10, respectively. For moisture analysis, the thermogravimetric method used a thermobalance (Sartorius MA37, Göttingen, Germany). Water activity (Aw) was measured with an Aqualab Series 3 TE (Decagon Devices Inc., Pullman, USA). The amylose content was measured according to Hoover & Ratnayake (Hoover & Ratnayake, 2002); while amylopectin content was obtained by amylose difference.
2.3. Film preparation
The films were prepared by the casting method following the methodology of Shen et al. with some modifications [20]. A starch-water solution (2 % cassava starch) was prepared and heated in a magnetic stirrer at 75 °C for 20 min, the temperature was lowered, and essential oil was added in different percentages 1, 2, and 3 % regarding the total solution, and 0.5 % glycerol. These components were homogenized at 7000 rpm for 12 min. Then, the filmogenic solution was poured into Petri dishes (approximately 20 mL) which were left to stand at room temperature for three days until the films were formed. The film was then unmolded from the Petri dish and used for the respective analyses. The films obtained were coded as detailed in Table 1.
Table 1.
Coding of films made with cassava starch and cassava bagasse starch.
| Code | Cassava starch (%) | Cassava Bagasse starch (%) | OEO (%) | Water (%) | Glycerol (%) |
|---|---|---|---|---|---|
| CS | 2 | – | 0 | 97.5 | 0.5 |
| C1 | 2 | – | 1 | 96.5 | 0.5 |
| C2 | 2 | – | 2 | 95.5 | 0.5 |
| C3 | 2 | – | 3 | 94.5 | 0.5 |
| BS | – | 2 | 0 | 97.5 | 0.5 |
| B1 | – | 2 | 1 | 96.5 | 0.5 |
| B2 | – | 2 | 2 | 95.5 | 0.5 |
| B3 | – | 2 | 3 | 94.5 | 0.5 |
OEO: Oregano Essential Oil; CS: cassava starch film; BS: cassava bagasse starch film; C: Films of cassava starch with 1, 2 or 3 % of OEO; B: Films of cassava bagasse starch with 1, 2 or 3 % of OEO.
The content of OEO or glycerol in the films obtained were calculated using the following equation:
where Cs is the concentration of compound in the filmogenic solution expressed in %, Msm is the deposited solution per mold in grams, and Mf is the weight of the film obtained in grams.
2.4. Physical properties of films
2.4.1. Thickness measurement
The average thickness of each film was obtained by measuring at five different points with a digital micrometer (Rexbeti, China) with a precision of ±0.001 mm.
2.4.2. Moisture content
For moisture determination, 0.1 g of the film sample was placed in a thermobalance (Sartorius MA37, Germany) at a temperature of 130 °C. Analyzes were performed in triplicate.
2.4.3. Solubility in water
The films' water solubility (WS) was determined in triplicate, according to the method described by Shen et al., with some modifications [20]. Films were cut (10 × 30 mm) and accurately weighed. Subsequently, they were immersed in 50 mL of distilled water under constant stirring at 180 rpm for 24 h at room temperature. The films were removed after immersion, dried at 105 °C for 24 h, and weighed (final dry weight M2). The initial dry weight (M1) was determined by drying the films at 105 °C until constant weight. The percentage of water solubility in the films was calculated using the following equation:
2.4.4. Water vapor permeability (WVP)
The water vapor permeability of the films was determined according to the ASTM E−96 method with some modifications [24]. Bags of 5 × 5 cm were made with the films of the different treatments studied. Then, 1 g of silica gel was placed inside each bag, heat-sealed, and the mass of each one (initial mass) was recorded. Each formulation was tested in triplicate. The sealed bags were placed in a Memmert brand climatic chamber, model ICH750 (Schwabach, Germany), at a relative humidity of 50 ± 2 % and a temperature of 23 ± 2 °C. The samples were weighed periodically for three days.
The water vapor transmission rate (WVTR) was calculated according to the following equation:
where WVTR is expressed in kg/s.m2, G is the weight change in grams, t is the time in seconds, and A is the surface area of the bags in m2. The G/t term was calculated by linear regression of the curve.
The Water Vapor Permeability (WVP) was calculated with the following equation:
| WVP = WVTR × l ⁄ Δp |
where WVP is expressed in kg.m/s.m2.Pa, Δp is the vapor pressure difference in Pa, and l is the film thickness in m.
2.5. Thermogravimetric analysis (TGA)
An SDT model Q600 thermogravimetric analyzer (TA Instruments, New Castle, DE, USA) was used for TGA analyses. Samples were analyzed under a nitrogen atmosphere (100 mL/min) in a temperature range from room temperature to 600 °C with a heating rate of 10 °C/min.
2.6. Mechanical properties
The mechanical properties of the films were carried out according to the ASTM D-882 standard. A Shimadzu® UTM-600KN universal testing equipment (Shimadzu Co., Kyoto, Japan) was used to perform tensile tests on cassava starch and essential oil films at a speed of 5 mm/min. The films were cut into 20 × 120 mm strips and placed between clamps at 50 mm. For each formulation, five samples were analyzed to measure the maximum stress, strain at break, and modulus of elasticity.
2.7. Color
The color values of the films were determined with a NH300 Portable Digital Colorimeter Color Analyzer (Shenzhen ThreeNH Technology Co., Ltd., Shenzhen, China). The films were placed on a standard white plate (L* = 98.72, a* = −10.53, and b* = −2.37), and three measurements were made. Each formulation was tested four times.
The total color difference (ΔE) was calculated using the following equation:
where L, a, and b are the color parameter values of the film sample and L*, a*, and b* are the color parameter values of the standard white plate.
2.8. Antioxidant properties of films
2.8.1. Oxygen radical absorbance capacity (ORAC) assay
ORAC method was used to evaluate the antioxidant activity with slight modifications [25], using a microplate reader (Biotek Instruments, Winooski, VT, USA) controlled by the Gene 5™ version 1.1 software (BioTek Instruments). For this, the films were cut into sizes of 2 × 2 cm, and then they were placed in a flask containing 10 mL of distilled water, shaken at 150 rpm for 1 h at 25 °C and centrifuged at 3000 g for 10 min [20]. Next, a 100 μL aliquot of the supernatant was taken to measure antioxidant activity. The absorbance was measured in triplicate at wavelengths of 485 and 528 nm with a kinetic incubation time of 2.5 h and readings every 2 min at 37 °C. A Trolox standard curve was made with a linear range (0–160 μM Trolox). The results were expressed as mg of Trolox equivalents (TE) per g of film (g TE/g of film).
2.8.2. Total phenolic content (TPC)
The total phenolic content determination was based on the procedure reported by Zhao et al. with some modifications [26]. Aliquots of 100 μL corresponding to each treatment were taken from the supernatant obtained in the extraction previously mentioned. Absorbance was measured in triplicate at 739 nm using the microplate reader and Gene 5™ software version 1.1. (BioTek Instruments). A gallic acid (GA) standard curve with a linear range (0–90 mg gallic acid/mL) was prepared from a 1 mg/mL GA stock solution. Results were expressed as mg GA equivalents (GAE) per g of film (mg GAE/g of film).
2.9. Antimicrobial properties by the disk diffusion method
The film samples were cut into circles with a diameter of approximately 25 mm, disinfected with an ultraviolet light lamp, and placed on the lid of Petri dishes. Previously, the bacterial strains were inoculated in TSB and incubated at 37 °C for 24 h. Subsequently, peptone inoculation was carried out until reaching an approximate concentration of 1.5 × 108 CFU/mL, using the McFarland standard as a reference. Then, the bacterial concentration was adjusted to 105 CFU/mL in a sodium chloride solution for both S. aureus and E. coli. A solution of 1 mL was placed in Petri dishes with PCA and incubated at 37 °C for 24 h. Once this time had elapsed, the diameters of the inhibition zones were measured. The tests were performed in triplicate.
2.10. Statistical analysis
Statistical analysis was performed by simple analysis of variance (ANOVA) using Statgraphics Centurion software (The Plains, VA, USA). Duncan's test was used to evaluate and identify significant differences between the data groups, considering a confidence level of p < 0.05. The Pearson correlation coefficients (r) were used to analyze the correlation among variables, considering values higher than 0.67.
3. Results and discussion
3.1. Characterization of cassava bagasse starch
Table 2 shows the physicochemical composition of cassava bagasse starch, which contains 83.23 % total starch, 13.74 % amylose, and 86.26 % amylopectin. Similar results were reported by Kayode et al., with a starch content of 87.44 % and 26.41 % amylose present in starch samples from cassava roots [27]. In contrast, Teixeira et al. mentioned that cassava bagasse has 50 % starch and 10 % fiber [13]. In another study, cassava bagasse starch was analyzed, containing 26.50 % amylose, 6 % fiber, 1.50 % lipids, 2.10 % protein, and 14.5 % moisture [28]. In the present study, the protein, fat, ash, and moisture content in the cassava bagasse starch were 1.05, 0.008, 0.024, and 10.10 %, respectively. No fiber values were detected in the sample. Similarly, Kayode et al. obtained 0.40 % protein and 0.13 % fat; no ash or fiber values were detected [27]. Generally, a significant difference is observed in the starch purity (total starch content) between cassava and bagasse starch.
Table 2.
Physicochemical composition of cassava bagasse starch (BS) and cassava starch (CS) variety INIAP 651.
| Analysis | Cassava bagasse starch (BS) | Cassava starch (CS) |
|---|---|---|
| Moisture % | 10.10 ± 0.11 | – |
| Aw | 0.47 ± 0.002 | – |
| Ash % | 0.025 ± 0.0003 | – |
| Fat % | 0.0079 ± 0.0003 | – |
| Protein % | 1.05 | – |
| Total starch % | 83.23 ± 0.55 | 88.82 ± 1.57 |
| Amylose % | 13.74 ± 2.44 | 24.46 ± 0.19 |
| Amylopectin % | 86.26 ± 2.44 | 75.54 ± 0.19 |
Data from the literature [6].
3.2. OEO and glycerol content in the films
The concentrations of OEO and glycerol in the films were estimated using the theoretical equation previously described. For the C1, C2, and C3 film samples, the calculated OEO contents were 26.4 ± 2.8 %, 39.6 ± 3.6 %, and 46.8 ± 3.4 %, respectively. Concurrently, the corresponding glycerol contents were determined to be 13.2 ± 1.4 %, 9.9 ± 0.9 %, and 8.1 ± 1.0 %. In the case of B1, B2, and B3 film samples, the estimated OEO contents were 25.7 ± 0.8 %, 38.0 ± 1.0 %, and 46.6 ± 4.8 %, respectively. Additionally, the glycerol content in the C samples was found to be 15.1 ± 0.6 %, while in the B samples, it was 15.1 ± 0.2 %. It is important to note that these values represent theoretical maximums and are nominal content, as they do not account for potential OEO losses due to evaporation during drying phase. Therefore, these calculations primarily serve to indicate the maximum possible content of OEO and glycerol present in each film type, rather than their actual concentrations.
3.3. Films thickness
Table 3 shows the thickness, moisture percentage, and solubility of the cassava starch and OEO films. Additionally, the images of films obtained can be seen in Fig. S1 (in the Supplementary Materials file). A strong positive correlation was found between the starch films thickness and the percentage of essential oil (r = 0.97; p < 0.01), obtaining thickness values ranging from 66.73 μm to 164.73 μm. This could be attributed to the interaction of the essential oil with the matrix of the films, which causes the entrapment of OEO microdroplets and gives rise to thicker films, as has been reported for other biopolymers [29]. Similar results have been presented by dos Santo Caetano et al. in cassava starch films, pumpkin residue extract, and oregano essential oil with percentages of 1.0, 1.6 and 2.0 %, who showed an increase in film thickness with the amount of OEO added [10].
Table 3.
Characterization of films: thickness, moisture content, solubility, water vapor permeability (WVP), mean maximum stress, maximum strain, modulus of elasticity and diameter of inhibition zone against bacteria.
| Films | Thickness (μm) | Moisture (%) | Solubility (%) | WVP ( × 10−11 g m/m2.s.Pa) | Mean maximum stress (Mpa) | Maximum strain (%) | Modulus of elasticity (Mpa) | Inhibition zone (cm) against S. aureus | Inhibition zone (cm) against E. coli |
|---|---|---|---|---|---|---|---|---|---|
| CS | 66.7 ± 0.7h | 8.74 ± 0.28f | 21.99 ± 0.76e | 1.22 ± 0.11a | 0.93 ± 0.57a | 26.8 ± 9.2bc | 5.1 ± 2.0a | Not present | Not present |
| C1 | 98.8 ± 0.8f | 9.49 ± 0.25e | 31.33 ± 1.25b | 3.35 ± 0.63b | 0.50 ± 0.03a | 42.0 ± 13.5cd | 10.3 ± 1.8a | Not present | Not present |
| C2 | 138.0 ± 0.5d | 11.03 ± 0.64c | 38.38 ± 0.95a | 6.05 ± 0.20cd | 0.21 ± 0.01a | 48.4 ± 11.1de | 1.3 ± 0.1a | Total Inhibition | 7.0 ± 0.4a |
| C3 | 161.2 ± 0.8b | 12.89 ± 0.17a | 39.02 ± 1.36a | 6.32 ± 0.98d | 0.19 ± 0.04a | 61.6 ± 9.3e | 1.2 ± 0.1a | Total Inhibition | Total Inhibition |
| BS | 92.8 ± 0.6g | 8.10 ± 0.14g | 20.75 ± 0.51e | 3.50 ± 0.43b | 6.48 ± 1.02b | 1.2 ± 0.3a | 690.9 ± 110.1b | Not present | Not present |
| B1 | 104.7 ± 0.5e | 9.87 ± 0.23de | 23.52 ± 0.77d | 3.67 ± 0.56b | 0.96 ± 0.11a | 14.8 ± 8.8ab | 46.7 ± 17.9a | Not present | Not present |
| B2 | 142.9 ± 0.4c | 10.39 ± 0.47d | 29.45 ± 0.47c | 4.95 ± 0.86c | 0.83 ± 0.115a | 25.9 ± 11.36bc | 38.5 ± 5.14a | Total Inhibition | 6.6 ± 0.2a |
| B3 | 164.7 ± 0.6a | 11.81 ± 0.21b | 30.27 ± 0.59bc | 5.73 ± 0.99cd | 0.39 ± 0.05a | 37.9 ± 9.6cd | 7.6 ± 0.68a | Total Inhibition | Total Inhibition |
* Data are presented as mean ± standard deviation (n = 3). Different lowercase letters within the same column indicate significant differences p ≤ 0.05 (Duncan).
* CS: cassava starch film; BS: cassava bagasse starch film; C: Films of cassava starch with 1, 2 or 3 % of OEO; B: Films of cassava bagasse starch with 1, 2 or 3 % of OEO.
3.4. Moisture content and solubility in water
Moisture content and water solubility percentage of films are fundamental parameters in the development of food packaging and constitute one of the main challenges when making films [19]. The moisture percentage is related to the total free volume occupied by the water molecules in the film network. As shown in Table 3, the moisture content of the prepared films varied significantly between 8.10 % and 12.89 %. The humidity of the control films (CS and BS) presented the lowest values, while an increase in humidity was observed in the films with the addition of essential oil (r = 0.96; p < 0.01). This could be attributed to the formation of porous structure in the starch films with OEO, facilitating the insertion of water molecules between the polymeric chains, as reported by do Evangelho et al. In their work on corn starch films and orange essential oil, the authors reported that adding essential oil to the films increases the percentage of moisture, which is consistent with the results obtained in our study [23]. No correlation was found between humidity and starch purity.
Some studies on starch films incorporating essential oils have observed the presence of micropores in the cross-section of the film through scanning electron microscopy (SEM) or optical microscopy [18,[30], [31], [32], [33], [34]]. It was reported that although the essential oil was dispersed efficiently during the film preparation process, essential oil droplets could form agglomerates or migrate towards the surface of the films during the drying phase. Then, some essential oil could evaporate, contributing to the pore's formation in the structure of the films [[31], [32], [33]]. Furthermore, the increasing concentrations of essential oil increase the size and number of the micropores in the films [33].
Table 3 shows the water solubility of the films prepared from pure and bagasse starch with OEO incorporation. The statistical analysis revealed no significant correlation between solubility and starch purity in pure cassava and bagasse starch samples. However, a significant correlation (r = 0.78; p < 0.01) between OEO concentration and solubility was observed. Indeed, the films presented an increase in the solubility percentage (from 21.99 % to 39.02 % in native starch and from 20.75 % to 30.27 % in bagasse starch) as the incorporation of the concentration of OEO in the film matrix increased, which agrees with the results reported by other authors [20,23]. This result could reveal that interaction between OEO and the starch could influence the film water solubility, and it will depend on the starch's purity level; when the starch is purer, the solubility increases. do Evangelho et al. presented results in films of corn starch and orange essential oil, noting that adding EO in the films increased the solubility percentage from 15.25 % to 18.67 % [23]. Likewise, Shen et al. (2022) reported that by increasing the amount of OEO (1, 2, and 3 %) in the starch films of Dioscorea zingiberensis, the solubility percentage increased from 17.04 % to 22.71 %. The inclusion of EO can cause the formation of starch films with heterogeneous structures and microcavities or pores, affecting the integrity of the films and facilitating the entry of water into the polymeric matrix [20,23].
3.5. Water vapor permeability
As shown in Table 3, the WVP values of the produced films ranged from 1.22 to 6.32 × 10−14 kg m/m2.s.Pa. The CS films presented a lower WVP value than the BS films. In addition, the WVP of the pure starch/OEO films was increased relative to that of the CS control; a similar trend was observed in the WVP of the films made with cassava bagasse starch and OEO compared to the BS control. This could be attributed to the effect of OEO on the integrity of the films prepared with pure starch, which was previously mentioned, presenting a higher solubility in water and, consequently, a higher WVP. According to the literature, adding essential oils can form agglomerates and micropores in starch films, favoring WVP [21]. In fact, a strong correlation was found between essential oil concentration and WVP (r = 0.88; p < 0.01).
Our results for CS film are lower than those reported by Luchese et al., who showed a WVP of 14.12 × 10−14 kg m/m2.s.Pa in cassava starch films [4]. Besides, Caetano et al. reported an increase of WVP values measured at 25 °C and 75 %RH in cassava starch films (11.11 × 10−14 kg m/m2.s.Pa) when OEO (2 %) and pumpkin pomace extract (3 %) were incorporated into the films (26.11 × 10−14 kg m/m2.s.Pa) [35]. Another study also showed the same trend by adding carvacrol (around 0.12 %) in cassava starch films, where the WVP values increased from 25.97 to 36.67 × 10−14 kg m/m2.s.Pa at 27 °C and 60 %RH [36]. The variation in the order of magnitude between these results and the values of the current study could be attributed to differences in temperature and relative humidity utilized during sample testing, as well as to the higher glycerol content in those films (30–42.5 % w/w of total starch) compared to our films (25 % w/w of total starch). This higher glycerol content reduces the matrix density and consequently increases the WVP, as reported in the literature [37]. Finally, in our study, no correlation was found between the starch purity and the WVP.
3.6. Thermogravimetric analysis (TGA)
The thermal stability of films and OEO was studied through thermogravimetric analysis. The TGA and TGA curves derivatives (DTG) are shown in Fig. 1. In the OEO sample (Fig. 1a and c), a single thermal event was observed between 28 °C and 179 °C, with a Tdmax (temperature at which the maximum mass loss occurs) of 162 °C, which is in agreement with previous studies [38]. In their thermal results of pure OEO, those authors reported a single thermal degradation event in ranges between 36 °C and 180 °C, with a maximum temperature of 168 °C [38]. In addition, complete volatilization was observed since it had a mass loss of 99 % and presented a residue of 0.314 % at a temperature of 216 °C, which can be attributed to the decomposition of components present in the OEO such as carvacrol, thymol, linalool, and phenolic terpenes [38,39].
Fig. 1.
TGA and DTGA curves of films: a) and c) cassava starch-based films and OEO pure; b) and d) cassava bagasse-based films. OEO: Oregano Essential Oil; CS: cassava starch film; BS: cassava bagasse starch film; C: Films of cassava starch with 1, 2 or 3 % of OEO; B: Films of cassava bagasse starch with 1, 2 or 3 % of OEO.
The CS and BS control films (Fig. 1c and d) presented two thermal events. The first event was observed between 40 °C and 120 °C with a mass loss of 10 % attributed to water evaporation [39]. The second mass loss was between 174 °C and 430 °C, with a Tdmax of 308 °C for CS and 314 °C for BS, which can be attributed to glycerol degradation (125 °C–290 °C) and starch oxidation [30,39,40].
On the other hand, in Fig. 1a, the CS films with OEO compared to the pure starch film sample showed a shift of the curves towards lower values, indicating that the higher the amount of OEO in the films, the greater its displacement, which is consistent with the literature [39]. In addition, a third thermal event was observed in CS films incorporating OEO, which was clearer at higher OEO concentrations. This mass loss was observed between 196 and 255 °C (see Fig. 1c), which can be attributed to the decomposition of glycerol and all the substances contained in the OEO, such as carvacrol, whose boiling point is estimated at 233 °C [41].
The addition of OEO in the CS films decreased the Tdmax compared with CS, which suggests an easier degradation of the starch chains due to the presence of OEO. This phenomenon has been reported in starch films plasticized with glycerol [42].
Fig. 1b shows that the BS films with OEO presented a similar thermal behavior to the CS films with OEO since there is evidence of a shift to lower temperatures with increasing OEO content. Likewise, a third thermal event was observed in B2 and B3 films in the ranges between 202 and 261 °C (see Fig. 1d), which can be attributed to glycerol and OEO decomposition; however, the peak temperature of this thermal event was higher in B2 and B3 films (10 °C difference) than in C2 and C3 films.
On the other hand, in BS films with OEO, no significant variation of Tdmax was observed, so their thermal stability was not affected by OEO. This could be related to the lower amount of amylose in the BS interacting with the added OEO.
According to the literature, glycerol is completely degraded up to 290 °C [30,43]. Therefore, the mass losses in the CS (35 %) and BS (28 %) samples would be associated with water, glycerol, and starch oxidation losses at such temperatures. In the case of CS and BS films with OEO, mass losses at 290 °C would be associated with water, OEO, glycerol, and starch oxidation losses. Consequently, the OEO content present in the starch films could be estimated by the difference in the mass losses between the starch film with OEO and the mass losses in the starch control film, as reported in the case of other polymers [41]. So, the C1, C2, and C3 films presented an OEO estimated content of 3.4 %, 13.0 %, and 17.8 %, respectively, while the B1, B2, and B3 films had an OEO estimated content of 3.8 %, 15.9 %, and 29.3 %. These results were lower than the values obtained with the theoretical equation previously described, which could be attributed to high OEO losses by evaporation during drying phase of film preparation. Unfortunately, estimating the glycerol content in the film proved challenging. It is an issue that many authors have struggled with. To enhance our understanding, future investigations will involve a comparative TGA analysis between the OEO-starch-water-glycerol film, starch-water-glycerol films, and starch-water films as references and Soxhlet extractions [44].
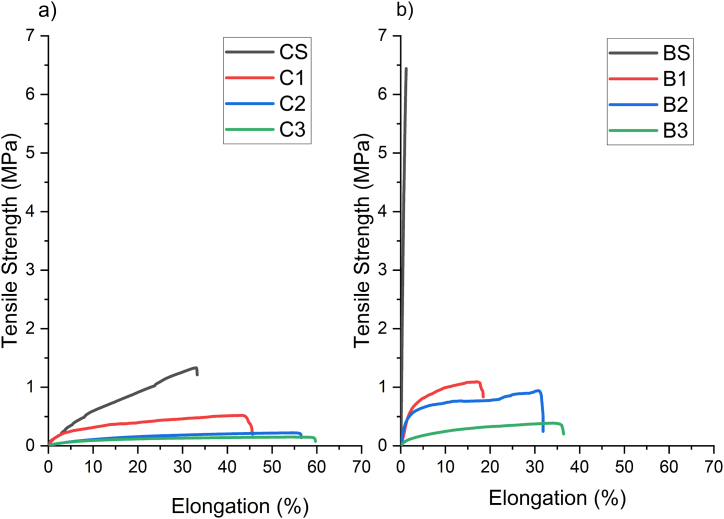
3.7. Mechanical properties
Table 3 and Fig. 2 detail the data obtained on the tensile tests on the films made, describing the maximum stress, maximum strain, and modulus of elasticity. The results show that while the BS had a higher modulus of elasticity and maximum stress, the CS had a higher strain at break. The maximum tensile stress and the modulus of elasticity in BS films were around 7 -fold and 136-fold higher, respectively, than in the CS samples. The CS film presented a relatively low maximum stress (0.93 ± 0.57 MPa), but it was a ductile material (strain at break: 26.8 ± 9.2 %). On the contrary, the films made with BS presented a brittle or rigid behavior, with a relatively high maximum stress (6.48 ± 1.02 MPa) and a low strain at break (1.2 ± 0.3 %). A strong correlation was found between maximum strain and the starch purity (r = 0.87; p < 0.001), as well as with the amylose content (r = 0.88; p < 0.01).
Fig. 2.
Stress – strain curves of films: a) cassava starch based films and b) cassava bagasse based films (right). CS: cassava starch film; BS: cassava bagasse starch film; C: Films of cassava starch with 1, 2 or 3 % of OEO; B: Films of cassava bagasse starch with 1, 2 or 3 % of OEO.
In the CS and OEO films (C1 to C3), the maximum stress values slightly decreased compared to CS films, whereas the strain at break values presented a significant increase related to the CS films. This can be attributed to a plasticizing effect of the OEO, as reported in the literature [45]. The correlation analysis between the OEO concentration and the maximum stress bears out this result (r = −0.70; p < 0.001). However, the addition of OEO could also generate micropores in the films [46], limiting the increase in strain at break.
The films prepared with cassava bagasse starch with OEO (B1 to B3) showed significant differences compared to BS films. It was observed that the maximum stress decreased, and the strain at break increased with the OEO addition. In this case, incorporating OEO into the BS allowed obtaining ductile films. The decrease in the mechanical resistance of the films could be explained by the generation of discontinuities in the polymer matrix when the essential oil is added, which results in the replacement of stronger polymer-polymer interactions with weaker polymer-oil interactions [24].
The results regarding the modulus of elasticity are consistent with those reported in the literature. This trend was similar to that of Wigati et al. in jicama starch and agarwood bouya essential oil films, the modulus of elasticity decreased with the addition of EO from 6.77 to 3.16 MPa [47]. Ardjoum et al. presented a similar behavior in corn starch and essential oil films, where the modulus of elasticity decreased from 452 to 376 MPa with the addition of EO. The authors attributed this behavior to the formation of discontinuous areas caused by weaker interactions between polysaccharides and polyphenols [48]. So, adding EO to the film-forming solution leads to a lower modulus of elasticity value [49].
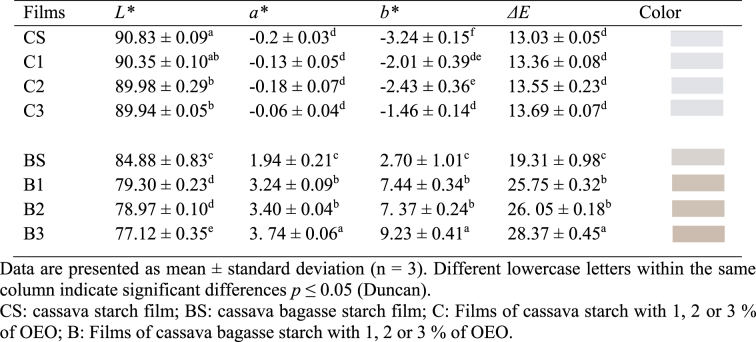
3.8. Color
The values of the optical properties, such as brightness (L), red – green (a), yellow – blue (b), and the total color difference (ΔE) of the cassava starch and oregano essential oil films, are summarized in Table 4 and the images of films obtained can be seen in Fig. S1. The brightness of the films varied from 90.83 to 89.94 for the treatments of pure cassava starch and OEO; and from 84.44 to 77.12 for the samples of cassava bagasse starch and OEO; the L value decreased with the OEO concentration. The films made with cassava bagasse starch presented the lowest gloss values compared with the pure cassava starch films. The bagasse starch films with 3 % OEO presented the highest values of a and b, corresponding to the red and yellow colors; these treatments presented a light brown-yellow color, whereas the pure cassava starch films were more transparent. This can be better appreciated in the values of the total color difference of CS and BS, which presented a significant difference (p < 0.05). The color difference is a numerical comparison between the color of a sample with a reference color, which shows the divergences in the absolute color. Generally, ΔE formulas calculate the difference between two colors to recognize color versatility [50].
Table 4.
Optical properties of cassava starch and OEO films.
The results did not show any difference (p > 0.05) in ΔE between the pure starch films with different concentrations of OEO (C1, C2, C3) and the control (CS), indicating that the addition of OEO between 1.0 % and 3.0 % did not affect the ΔE value of films, these results are consistent with those reported by Kang & Song [21]. On the contrary, in the bagasse starch films with different concentrations of OEO (B1, B2, B3), significant differences (p < 0.05) were observed in the value of ΔE between them and the control without OEO (BS), which suggests an effect of the starch purity on the color of cassava bagasse starch films, indeed a strong correlation negative was found between the color and the starch purity (r = −0.87; p < 0.01).
3.9. Antioxidant properties
TPC and ORAC values (see Fig. 3) increased significantly with the addition of OEO. TPC values increased 1.3 times from 576.73 to 737.46 mg GAE/g in the case of CS/OEO films, while they increased 1.2 times, from 572.28 to 671.57 mg GAE/g for BS/OEO films. Similarly, ORAC values increased 3.5 times from 809.35 to 2825.82 mg TE/g, and 3.7 times from 644.87 to 2357.90 mg TE/g in films made with CS/OEO and BS/OEO, respectively.
Fig. 3.
Total phenolic content (left) and oxygen radical absorbance capacity (right) of cassava starch and OEO films and cassava bagasse-based films. Different lowercase letters within the same column indicate significant differences p ≤ 0.05 (Duncan). CS: cassava starch film; BS: cassava bagasse starch film; C: Films of cassava starch with 1, 2 or 3 % of OEO; B: Films of cassava bagasse starch with 1, 2 or 3 % of OEO.
The antioxidant activity of OEO is due to the presence of phenolic compounds such as carvacrol, thymol, terpinene, and p-cymene, which are the most active constituents with a broad spectrum of antioxidant capacity [24,51]. These compounds are effective free radical scavengers, neutralizing free radicals that cause oxidation in food products. They donate hydrogen atoms to free radicals, converting them into more stable forms and stopping the chain reaction of lipid peroxidation. This process helps preserve the quality and extend the shelf life of food products, especially those prone to oxidative degradation [52]. Our results are consistent with the literature that reports an increase in antioxidant capacity due to the incorporation of OEO in biodegradable films, which is proportional to the amount of essential oil added, certainly a strong correlation was found between these parameters (r = 0.94; p < 0.01) [18,53,54].
3.10. Antimicrobial properties
Table 3 and Fig. S2 (in the Supplementary Materials file) show the results of antimicrobial properties of films against Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria. The diameters of the inhibition zones increased with the increase in the concentration of OEO, thus showing a total inhibition of the two microorganisms with 3 % OEO, while with 2 % OEO, it presented total inhibition only for S. aureus. The inhibition diameter of treatments C2 and B2 against E. coli was 7.0 mm and 6.6 mm, respectively (see Table 3).
The main antibacterial components of oregano essential oil are carvacrol and other phenols, such as thymol, whose content can reach 93.58 % [55]. They can integrate into the lipid bilayer of microbial cell membranes, increasing permeability and leading to cell contents leakage. So, these compounds can cause the loss of ions, ATP, and other cytoplasm contents, inhibiting their growth [24]. The compounds of OEO can also interfere with microbial enzymatic systems and impede energy production, which is crucial for microbial survival and reproduction [52].
According to the literature, OEO presents a minimum inhibitory concentration (MIC) that varies depending on the type of bacteria, showing a MIC of 0.5 μg/mL and 1.25 μg/mL for S. aureus and E. coli, respectively [56]. The results of the present study showed that S. aureus is more susceptible to OEO than E. coli, which could be attributed to the bacterial membrane components. Gram-negative bacteria have a lipopolysaccharide component in their outer membrane, which constitutes a protective barrier against the entry of antimicrobial substances. However, Gram-positive bacteria have a peptidoglycan layer in their structure, which allows the entry of antibacterial compounds [11,55,57].
Recent studies mentioned the antimicrobial activity of oregano essential oil in films made with different polysaccharides. Shen et al. presented a similar trend, obtaining the best results with 3 % OEO against four types of microorganisms such as B. subtillis, E. coli, S. aureus, and P. aeruginosa, whose inhibition diameters were 33.50, 21.98, 57.81 and 6.41 mm, respectively [20]. In addition, dos Santos Paglione et al. presented similar results in soy protein films and 3 % OEO, showing a more significant action on Gram-positive bacteria (S. aureus) than on Gram-negative bacteria (E. coli) [53]. Furthermore, incorporating OEO into the cassava bagasse starch matrix can lead to a uniform distribution of the oil throughout the film, which can help in the controlled release of OEO [20] This can maintain its effectiveness over an extended period, enhancing food products' shelf life and safety. Here, it should be noted that films can be tailored to target specific types of microorganisms or food products by adjusting the concentration, composition of OEO and its release to a specific food in contact.
4. Conclusions
Pure cassava starch and cassava bagasse starch films with different OEO concentrations were prepared by solvent casting. The cassava bagasse starch of the INIAP 651 variety presented a purity of 83.23 %, with 13.74 % of amylose and 86.26 % of amylopectin. The films prepared with BS presented a higher thickness, WVP, ΔE, modulus of elasticity, and maximum stress, but a lower strain at break than the films prepared with CS, both potentially applicable for developing active packaging. An increase in OEO content resulted in higher thickness, humidity, solubility of the films, WVP and strain at break; however, the maximum stress and the modulus of elasticity decreased. Furthermore, as the amount of OEO increased, the films showed higher content of total phenolic compounds and antioxidant activity, improving until 1.3-fold and 3.7-fold, respectively. In particular, the films with 3 % OEO showed total inhibition against S. aureus and E. coli, which clearly indicated that those films might be used as an active packaging material. Additionally, the results show that the starch purity only influences the film color and the maximum strain property. Therefore, cassava bagasse starch films with OEO produced in this study have a potential application as a biodegradable active packaging for food, being a new alternative for the use of residues from the process of obtaining cassava starch. The present study focused on establishing the percentage of OEO that can inhibit microbial growth in the initial formulation of the film to be transferable to the industry; however, this percentage does not define the actual content of OEO in the film. Although TGA estimated the OEO contents, future works should be done to define this percentage and glycerol content in the films.
Funding
This research received no external funding.
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Juliana Criollo-Feijoo: Writing – original draft, Investigation, Formal analysis. Verónica Salas-Gomez: Writing – original draft, Visualization, Investigation, Formal analysis. Fabiola Cornejo: Writing – review & editing, Formal analysis. Rafael Auras: Writing – review & editing, Supervision, Conceptualization. Rómulo Salazar: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the support from ESPOL through "Maestría en Ciencia de los Alimentos", FIMCP, and "Decanato de Investigación".
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36150.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Souza A.G., Ferreira R.R., Paula L.C., Mitra S.K., Rosa D.S. Starch-based films enriched with nanocellulose-stabilized Pickering emulsions containing different essential oils for possible applications in food packaging. Food Packag. Shelf Life. 2021;27 doi: 10.1016/j.fpsl.2020.100615. [DOI] [Google Scholar]
- 2.Trinh B.M., Chang C.C., Mekonnen T.H. Facile fabrication of thermoplastic starch/poly (lactic acid) multilayer films with superior gas and moisture barrier properties. Polymer (Guildf) 2021;223 doi: 10.1016/j.polymer.2021.123679. [DOI] [Google Scholar]
- 3.Wang L., Yang C., Deng X., Peng J., Zhou J., Xia G., Zhou C., Shen Y., Yang H. A pH-sensitive intelligent packaging film harnessing Dioscorea zingiberensis starch and anthocyanin for meat freshness monitoring. Int. J. Biol. Macromol. 2023;245 doi: 10.1016/j.ijbiomac.2023.125485. [DOI] [PubMed] [Google Scholar]
- 4.Luchese C.L., Rodrigues R.B., Tessaro I.C. Cassava starch-processing residue utilization for packaging development. Int. J. Biol. Macromol. 2021;183:2238–2247. doi: 10.1016/j.ijbiomac.2021.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Matheus J.R.V., de Farias P.M., Satoriva J.M., de Andrade C.J., Fai A.E.C. Cassava starch films for food packaging: trends over the last decade and future research. Int. J. Biol. Macromol. 2022;225:658–672. doi: 10.1016/j.ijbiomac.2022.11.129. [DOI] [PubMed] [Google Scholar]
- 6.Cornejo F., Maldonado-Alvarado P., Palacios-Ponce S., Hugo D., Rosell C.M. Impact of cassava starch varieties on the physiochemical change during enzymatic hydrolysis. Molecules. 2022;27:1–12. doi: 10.3390/molecules27186098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinostroza F., Cárdenas F., Alvarez H., Cobeña G. INIAP-Portoviejo 651: Variedad de yuca para la producción de almidón. 2012. INIAP estación experimental portoviejo.http://repositorio.iniap.gob.ec/handle/41000/1115 [Google Scholar]
- 8.Rattanachomsri U., Tanapongpipat S., Eurwilaichitr L., Champreda V. Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis. J. Biosci. Bioeng. 2009;107:488–493. doi: 10.1016/j.jbiosc.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 9.De Moraes J.O., Scheibe A.S., Sereno A., Laurindo J.B. Scale-up of the production of cassava starch based films using tape-casting. J. Food Eng. 2013;119:800–808. doi: 10.1016/J.JFOODENG.2013.07.009. [DOI] [Google Scholar]
- 10.dos Santos Caetano K., Almeida Lopes N., Haas Costa T.M., Brandelli A., Rodrigues E., Hickmann Flôres S., Cladera-Olivera F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life. 2018;16:138–147. doi: 10.1016/j.fpsl.2018.03.006. [DOI] [Google Scholar]
- 11.Wang Y., Luo J., Hou X., Wu H., Li Q., Li S., Luo Q., Li M., Liu X., Shen G., Cheng A., Zhang Z. Physicochemical, antibacterial, and biodegradability properties of green Sichuan pepper (Zanthoxylum armatum DC.) essential oil incorporated starch films. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2022;161 doi: 10.1016/j.lwt.2022.113392. [DOI] [Google Scholar]
- 12.Auras R., Arroyo B., Selke S. Production and properties of spin-coated cassava-starch-glycerol-beeswax films. Starch/Staerke. 2009;61:463–471. doi: 10.1002/star.200700701. [DOI] [Google Scholar]
- 13.Teixeira E. de M., Curvelo A.A.S., Corrêa A.C., Marconcini J.M., Glenn G.M., Mattoso L.H.C. Properties of thermoplastic starch from cassava bagasse and cassava starch and their blends with poly (lactic acid) Ind. Crops Prod. 2012;37:61–68. doi: 10.1016/j.indcrop.2011.11.036. [DOI] [Google Scholar]
- 14.Han J.W., Ruiz-Garcia L., Qian J.P., Yang X.T. Food packaging: a comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018;17:860–877. doi: 10.1111/1541-4337.12343. [DOI] [PubMed] [Google Scholar]
- 15.Pazmiño A., Campuzano A., Marín K., Coronel J., Salazar R. Evaluation of PLA active biodegradable films incorporated of essential oils to inhibit microbial adhesion. Granja. 2022;36 doi: 10.17163/LGR.N36.2022.02. [DOI] [Google Scholar]
- 16.Sharma S., Barkauskaite S., Jaiswal A.K., Jaiswal S. Essential oils as additives in active food packaging. Food Chem. 2021;343 doi: 10.1016/j.foodchem.2020.128403. [DOI] [PubMed] [Google Scholar]
- 17.Cui H., Zhang C., Li C., Lin L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019;139 doi: 10.1016/j.indcrop.2019.111498. [DOI] [Google Scholar]
- 18.Wang J., Chen C., Xie J. Loading oregano essential oil into microporous starch to develop starch/polyvinyl alcohol slow-release film towards sustainable active packaging for sea bass (Lateolabrax japonicus) Ind. Crops Prod. 2022;188 doi: 10.1016/j.indcrop.2022.115679. [DOI] [Google Scholar]
- 19.Zhou Y., Wu X., Chen J., He J. Effects of cinnamon essential oil on the physical, mechanical, structural and thermal properties of cassava starch-based edible films. Int. J. Biol. Macromol. 2021;184:574–583. doi: 10.1016/j.ijbiomac.2021.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y., Zhou J., Yang C., Chen Y., Yang Y., Zhou C., Wang L., Xia G., Yu X., Yang H. Preparation and characterization of oregano essential oil-loaded Dioscorea zingiberensis starch film with antioxidant and antibacterial activity and its application in chicken preservation. Int. J. Biol. Macromol. 2022;212:20–30. doi: 10.1016/j.ijbiomac.2022.05.114. [DOI] [PubMed] [Google Scholar]
- 21.Kang J.H., Bin Song K. Characterization of Job's tears (Coix lachryma-jobi L.) starch films incorporated with clove bud essential oil and their antioxidant effects on pork belly during storage. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;111:711–718. doi: 10.1016/j.lwt.2019.05.102. [DOI] [Google Scholar]
- 22.Wang B., Sui J., Yu B., Yuan C., Guo L., Abd El-Aty A.M., Cui B. Physicochemical properties and antibacterial activity of corn starch-based films incorporated with Zanthoxylum bungeanum essential oil. Carbohydr. Polym. 2021;254 doi: 10.1016/j.carbpol.2020.117314. [DOI] [PubMed] [Google Scholar]
- 23.do Evangelho J.A., da Silva Dannenberg G., Biduski B., el Halal S.L.M., Kringel D.H., Gularte M.A., Fiorentini A.M., da Rosa Zavareze E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019;222 doi: 10.1016/j.carbpol.2019.114981. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.Y., V Garcia C., Shin G.H., Kim J.T. Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;106:164–171. doi: 10.1016/j.lwt.2019.02.061. [DOI] [Google Scholar]
- 25.Yang W., Fortunati E., Dominici F., Giovanale G., Mazzaglia A., Balestra G.M., Kenny J.M., Puglia D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016;89:360–368. doi: 10.1016/j.ijbiomac.2016.04.068. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y., Teixeira J.S., Gänzle M.M., Saldaña M.D.A. Development of antimicrobial films based on cassava starch, chitosan and gallic acid using subcritical water technology. J. Supercrit. Fluids. 2018;137:101–110. doi: 10.1016/j.supflu.2018.03.010. [DOI] [Google Scholar]
- 27.Kayode B.I., Kayode R.M.O., Salami K.O., Obilana A.O., George T.T., Dudu O.E., Adebo O.A., Njobeh P.B., Diarra S.S., Oyeyinka S.A. Morphology and physicochemical properties of starch isolated from frozen cassava root. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2021;147 doi: 10.1016/j.lwt.2021.111546. [DOI] [Google Scholar]
- 28.Inguillay S., Jadán F., Maldonado-Alvarado P. Fermentation study of Cassava bagasse starch hydrolyzed's using INIAP 650 and INIAP 651 varieties and a strain of Lactobacillus leichmannii for the lactic acid production. Bionatura. 2021;6:1803–1811. doi: 10.21931/RB/2021.06.02.21. [DOI] [Google Scholar]
- 29.Luís Â., Pereira L., Domingues F., Ramos A. Development of a carboxymethyl xylan film containing licorice essential oil with antioxidant properties to inhibit the growth of foodborne pathogens. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;111:218–225. doi: 10.1016/j.lwt.2019.05.040. [DOI] [Google Scholar]
- 30.Hernández M.S., Ludueña L.N., Flores S.K. Citric acid, chitosan and oregano essential oil impact on physical and antimicrobial properties of cassava starch films. Carbohydrate Polymer Technologies and Applications. 2023;5 doi: 10.1016/j.carpta.2023.100307. [DOI] [Google Scholar]
- 31.Kim B.K., Lee H.S., Yang H.S., Bin Song K. Development of ginkgo (Ginkgo biloba) nut starch films containing cinnamon (cinnamomum zeylanicum) leaf essential oil. Molecules. 2021;26 doi: 10.3390/molecules26206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghasemlou M., Aliheidari N., Fahmi R., Shojaee-Aliabadi S., Keshavarz B., Cran M.J., Khaksar R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013;98:1117–1126. doi: 10.1016/j.carbpol.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 33.Song X., Zuo G., Chen F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018;107:1302–1309. doi: 10.1016/j.ijbiomac.2017.09.114. [DOI] [PubMed] [Google Scholar]
- 34.Souza A.C., Goto G.E.O., Mainardi J.A., V Coelho A.C., Tadini C.C. Cassava starch composite films incorporated with cinnamon essential oil: antimicrobial activity, microstructure, mechanical and barrier properties. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2013;54:346–352. doi: 10.1016/j.lwt.2013.06.017. [DOI] [Google Scholar]
- 35.Caetano K. dos S., Hessel C.T., Tondo E.C., Flôres S.H., Cladera-Olivera F. Application of active cassava starch films incorporated with oregano essential oil and pumpkin residue extract on ground beef. J. Food Saf. 2017;37:1–9. doi: 10.1111/jfs.12355. [DOI] [Google Scholar]
- 36.Homayouni H., Kavoosi G., Nassiri S.M. Physicochemical, antioxidant and antibacterial properties of dispersion made from tapioca and gelatinized tapioca starch incorporated with carvacrol. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;77:503–509. doi: 10.1016/j.lwt.2016.12.007. [DOI] [Google Scholar]
- 37.Thakur R., Pristijono P., Scarlett C.J., Bowyer M., Singh S.P., Vuong Q.V. Starch-based films: major factors affecting their properties. Int. J. Biol. Macromol. 2019;132:1079–1089. doi: 10.1016/j.ijbiomac.2019.03.190. [DOI] [PubMed] [Google Scholar]
- 38.Da Silva L.R.C., Da Silva L.O., De Carvalho L.H., De Oliveira A.D., Bardi M.A.G., De Souza Mesquita A.B., Ferreira J.H.L., Alves T.S., Barbosa R. Physical, morphological, structural, thermal and antimicrobial characterization of films based on poly(lactic acid), organophilic montmorillonite and oregano essential oil. Mater. Res. 2022;25 doi: 10.1590/1980-5373-MR-2022-0043. [DOI] [Google Scholar]
- 39.Piñeros-Hernandez D., Medina-Jaramillo C., López-Córdoba A., Goyanes S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017;63:488–495. doi: 10.1016/j.foodhyd.2016.09.034. [DOI] [Google Scholar]
- 40.Mena-Cervantes V.Y., Hernández-Altamirano R., Tiscareño-Ferrer A. Development of a green one-step neutralization process for valorization of crude glycerol obtained from biodiesel. Environ. Sci. Pollut. Control Ser. 2020;27:28500–28509. doi: 10.1007/s11356-019-07287-0. [DOI] [PubMed] [Google Scholar]
- 41.Llana-Ruiz-Cabello M., Pichardo S., Bermúdez J.M., Baños A., Núñez C., Guillamón E., Aucejo S., Cameán A.M. Development of PLA films containing oregano essential oil (Origanum vulgare L. virens) intended for use in food packaging. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2016;33:1374–1386. doi: 10.1080/19440049.2016.1204666. [DOI] [PubMed] [Google Scholar]
- 42.García N.L., Famá L., Dufresne A., Aranguren M., Goyanes S. A comparison between the physico-chemical properties of tuber and cereal starches. Food Res. Int. 2009;42:976–982. doi: 10.1016/J.FOODRES.2009.05.004. [DOI] [Google Scholar]
- 43.Martins P.C., Bagatini D.C., Martins V.G. Oregano essential oil addition in rice starch films and its effects on the chilled fish storage. J. Food Sci. Technol. 2021;58:1562–1573. doi: 10.1007/s13197-020-04668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hablot E., Dewasthale S., Zhao Y., Zhiguan Y., Shi X., Graiver D., Narayan R. Reactive extrusion of glycerylated starch and starch-polyester graft copolymers. Eur. Polym. J. 2013;49:873–881. doi: 10.1016/j.eurpolymj.2012.12.005. [DOI] [Google Scholar]
- 45.Arezoo E., Mohammadreza E., Maryam M., Abdorreza M.N. The synergistic effects of cinnamon essential oil and nano TiO2 on antimicrobial and functional properties of sago starch films. Int. J. Biol. Macromol. 2020;157:743–751. doi: 10.1016/j.ijbiomac.2019.11.244. [DOI] [PubMed] [Google Scholar]
- 46.Marzlan A.A., Muhialdin B.J., Zainal Abedin N.H., Manshoor N., Ranjith F.H., Anzian A., Meor Hussin A.S. Incorporating torch ginger (Etlingera elatior Jack) inflorescence essential oil onto starch-based edible film towards sustainable active packaging for chicken meat. Ind. Crops Prod. 2022;184 doi: 10.1016/j.indcrop.2022.115058. [DOI] [Google Scholar]
- 47.Wigati L.P., Wardana A.A., Tanaka F., Tanaka F. Edible film of native jicama starch, agarwood Aetoxylon Bouya essential oil and calcium propionate: processing, mechanical, thermal properties and structure. Int. J. Biol. Macromol. 2022;209:597–607. doi: 10.1016/j.ijbiomac.2022.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Ardjoum N., Chibani N., Shankar S., Salmieri S., Djidjelli H., Lacroix M. Incorporation of Thymus vulgaris essential oil and ethanolic extract of propolis improved the antibacterial, barrier and mechanical properties of corn starch-based films. Int. J. Biol. Macromol. 2022;224:578–583. doi: 10.1016/j.ijbiomac.2022.10.146. [DOI] [PubMed] [Google Scholar]
- 49.Gómez-Contreras P., Figueroa-Lopez K.J., Hernández-Fernández J., Rodríguez M.C., Ortega-Toro R. Effect of different essential oils on the properties of edible coatings based on yam (Dioscorea rotundata l.) starch and its application in strawberry (fragaria vesca l.) preservation. Appl. Sci. 2021;11:1–15. doi: 10.3390/app112211057. [DOI] [Google Scholar]
- 50.Liu Q.R., Wang W., Qi J., Huang Q., Xiao J. Oregano essential oil loaded soybean polysaccharide films: effect of Pickering type immobilization on physical and antimicrobial properties. Food Hydrocoll. 2019;87:165–172. doi: 10.1016/j.foodhyd.2018.08.011. [DOI] [Google Scholar]
- 51.Dutra T.V., Castro J.C., Menezes J.L., Ramos T.R., do Prado I.N., Machinski M., Mikcha J.M.G., Filho B.A. de A. Bioactivity of oregano (Origanum vulgare) essential oil against Alicyclobacillus spp. Ind. Crops Prod. 2019;129:345–349. doi: 10.1016/j.indcrop.2018.12.025. [DOI] [Google Scholar]
- 52.Rodriguez-Garcia I., Silva-Espinoza B.A., Ortega-Ramirez L.A., Leyva J.M., Siddiqui M.W., Cruz-Valenzuela M.R., Gonzalez-Aguilar G.A., Ayala-Zavala J.F. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 2016;56:1717–1727. doi: 10.1080/10408398.2013.800832. [DOI] [PubMed] [Google Scholar]
- 53.dos Santos Paglione I., Galindo M.V., de Medeiros J.A.S., Yamashita F., Alvim I.D., Ferreira Grosso C.R., Sakanaka L.S., Shirai M.A. Comparative study of the properties of soy protein concentrate films containing free and encapsulated oregano essential oil. Food Packag. Shelf Life. 2019;22 doi: 10.1016/j.fpsl.2019.100419. [DOI] [Google Scholar]
- 54.Jouki M., Mortazavi S.A., Yazdi F.T., Koocheki A. Characterization of antioxidant-antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2014;99:537–546. doi: 10.1016/j.carbpol.2013.08.077. [DOI] [PubMed] [Google Scholar]
- 55.Hao Y., Kang J., Yang R., Li H., Cui H., Bai H., Tsitsilin A., Li J., Shi L. Multidimensional exploration of essential oils generated via eight oregano cultivars: compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131629. [DOI] [PubMed] [Google Scholar]
- 56.Gavaric N., Mozina S.S., Kladar N., Bozin B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. Journal of Essential Oil-Bearing Plants. 2015;18:1013–1021. doi: 10.1080/0972060X.2014.971069. [DOI] [Google Scholar]
- 57.Wang X., Shen Y., Thakur K., Han J., Zhang J.G., Hu F., Wei Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules. 2020;25 doi: 10.3390/molecules25173955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.