Abstract
Objective
To conduct a comprehensive analysis of the landscape of gastric cancer (GC)-targeted therapy clinical trials and identify potential therapeutic targets.
Methods
A systematic search and analysis of the Cochrane Central Register of Controlled Trials (CENTRAL) was performed to retrieve all GC clinical trials published up to June 30, 2022. Approved therapeutic targets for 11 common cancers were compiled and analyzed. The role of CSNK2A1 in GC was investigated using bioinformatics tools such as GEPIA, KMPLOT, SangerBox, STRING, ACLBI, and TIMER. Four gastric cancer cell lines (AGS, HGC, MGC, BGC) and one normal gastric mucosa cell line (GES-1) were utilized to assess the sensitivity to the CSNK2A1 inhibitor CX-4945. Quantitative real-time polymerase chain reaction (qPCR) was employed to quantify the cellular expression of CSNK2A1. Cellular apoptosis was evaluated using flow cytometry and Western blot analysis.
Results
The failure rate of GC randomized controlled clinical trials (RCTs) was strikingly high, accounting for 74.29 % (26/35) of the trials. Among the 35 approved targets in 11 different cancers, 13 targets were rigorously evaluated and identified as potential therapeutic targets for GC. Bioinformatics analysis revealed that CSNK2A1 is closely associated with multiple biological characteristics in GC, and its increased expression correlated significantly with enhanced sensitivity to CX-4945 treatment. Flow cytometry and Western blot analysis consistently demonstrated concentration-dependent apoptosis induced by CX-4945 in GC cell lines.
Conclusions
The high failure rate of GC clinical trials highlights the need for a more scientific and precise approach in target identification and clinical trial design. CSNK2A1 emerges as a promising therapeutic target for GC, and its expression level could potentially serve as a biomarker for predicting sensitivity to CX-4945 treatment. Further research is warranted to elucidate the underlying molecular mechanisms and validate the clinical significance of CSNK2A1 in GC therapy.
Keywords: Gastric cancer, Targeted therapy, Biomarker, CSNK2A1, Apoptosis
Highlights
-
•
Through rigorous analysis of extensive clinical trial data, we have observed a remarkably high failure rate in gastric cancer clinical trials.
-
•
CSNK2A1 emerges as a promising therapeutic target for gastric cancer, exhibiting significant correlations with diverse cellular functions.
-
•
The CSNK2A1 inhibitor CX-4945 has demonstrated a marked ability to induce apoptosis in gastric cancer cells, with a significant correlation observed between its effect and the expression level of the CSNK2A1 gene.
1. Introduction
Gastric cancer (GC) is one of the most serious malignancies that threatens human health [1]. Globally, the incidence of GC is higher in East Asian countries, and increasing risks have been observed in younger generations [2]. Among the new incidents, many GC patients were found already in the late stage at the principal diagnosis, which led to the poor overall five-year survival rate [3]. Targeted therapy is considered to be an important and powerful strategy to treat advanced gastric cancer [4]. For example, the ToGA trial showed median overall survival of HER2-positive advanced gastric or gastro-oesophageal junction cancer patients was significantly improved in trastuzumab (a monoclonal antibody against HER2) and a chemotherapy group, compared with those in chemotherapy alone [5]. However, compared with other solid malignancies such as lung cancer, breast cancer, or colon cancer, the therapeutic targets and drugs for GC are scarce [[6], [7], [8]]. Cetuximab, a monoclonal antibody targeting EGFR presented an impressive survival improvement in advanced non-small-cell lung cancer [9], but no improvement in advanced GC [10]. Everolimus, an oral mTOR inhibitor, showed improved progression-free survival in a subgroup of patients with advanced breast cancer [11], but no improvement in advanced GC [12]. According to the National Comprehensive Cancer Network (NCCN) guideline of GC, only drugs targeting HER2, VEGFR, NTRK are suggested [13].
Researching causes of the failure of current treatment and searching for validated biomarkers are as important as discovering new efficient therapeutic targets [[14], [15], [16], [17]]. New research progress in GC-targeted therapy has been reported. A comprehensive view of gastric cancer clinical trials would significantly contribute to clarifying the current status and challenges in treatment, thereby providing crucial direction for fundamental research endeavors. Additionally, a profound understanding of the specific molecular mechanisms underlying therapeutic targets holds the potential to drive breakthroughs in clinical treatment for GC.
The Cochrane Library is a collection of databases that contain high-quality, independent evidence to inform healthcare decision-making. While Cochrane Central Register of Controlled Trials (CENTRAL) is a rich database of bibliographic reports of randomized controlled trials. This study presents an outlook of GC clinical trials based on CENTRAL, focus on the existing conditions and issues, and screen potential targets from other common cancers for GC. Our study might offer a comprehensive view of GC clinical trials and find new targets for further trials.
2. Materials and methods
2.1. Search strategy and inclusion criteria
A rigorous systematic search was conducted in the Cochrane Central Register of Controlled Trials (CENTRAL) database, encompassing PubMed, Embase, ICTRP, and CT.gov, for clinical trials on gastric cancer (GC) published up to June 30, 2022. The search strategy utilized comprehensive medical terminology, including "gastric," "stomach," "cancer," "malignancy," "carcinoma," "tumor," "neoplasm," and "adenocarcinoma," to capture all relevant texts. Inclusion criteria were strictly defined as: (a) phase I, II, or III clinical trials; (b) trials focused on targeted therapy; and (c) English-language publications. The retrieved studies were compiled in the reference management software EndNote for detailed analysis. Exclusion criteria were precisely applied to filter out: (a) duplicate research; (b) studies unrelated to GC; (c) studies involving endoscopy or surgical interventions; (d) reviews or meta-analyses; (e) non-randomized controlled trials; (f) studies involving chemotherapy, radiotherapy, or immunotherapy; and (g) studies with limited details beyond the title. Furthermore, phase II clinical trials that were discontinued or failed to progress to the next phase of clinical testing, yet remained of interest, were also included in the analysis, and their pertinent information was extracted.
2.2. Data collection
Eligible studies were independently scrutinized by two authors, and the following data were systematically extracted: therapy targets, drugs, trial name (or registration number), clinical trial phase, study design, screened biomarkers, study initiation year, outcomes, and study DOI. The references were organized and maintained using Endnote version X9. Any inconsistencies in data extraction between the reviewers were resolved through mutual consensus. The quantities of reported drugs and targets were enumerated. Comprehensive information on all phase III and relevant phase II clinical trials was gathered. Notably, the phase II trials that did not proceed to phase III specifically represented the evaluation of certain therapeutic targets. Additionally, for therapeutic targets and drugs targeting other prevalent cancer types, we gathered data from reliable sources such as the Food and Drug Administration (FDA) websites and the National Comprehensive Cancer Network (NCCN) guidelines. The expression patterns and prognostic associations of these targets in GC were thoroughly analyzed. Genes that met the following criteria were identified as potential therapeutic targets for GC: 1. Genes that exhibited significant differential expression in GC tissues compared to adjacent non-cancerous tissues. 2. Genes that displayed a significant correlation with the prognosis of GC patients.
2.3. Bioinformatics analysis
The analysis of differential gene expression and prognostic correlations in GC was conducted utilizing the web-based tools GEPIA (http://gepia.cancer-pku.cn/index.html) and KMPLOT (http://kmplot.com/analysis). Data pertaining to 412 GC patients were retrieved from the Cancer Genome Atlas (TCGA) database and processed using the SangerBox platform (http://www.sangerbox.com). For the assessment of protein-protein interaction networks, the STRING database was employed. Genetic mutations, tumor mutational burden (TMB), microsatellite instability (MSI), and stemness scores were comprehensively analyzed using the SangerBox web application. Additionally, gene co-expression analyses and pathway exploration were conducted utilizing the ACLBI web tool (www.aclbi.com). Finally, the infiltration of tumor immune cells was analyzed with the TIMER platform (https://cistrome.shinyapps.io/timer/).
2.4. Cell proliferation assay
To evaluate the effects of the CSNK2A1 inhibitor CX-4945 (HY-50855, MedChemExpress, USA) on cellular viability, five distinct cell lines (AGS, BGC, MGC, HGC, and GES-1) were seeded in 96-well plates at a precise density of 5000 cells per well. These cells were cultured overnight under standard conditions of 37 °C and 5 % CO2 to allow for cell attachment and recovery. Subsequently, the culture medium was replaced with RPMI-1640 medium (Gibco, USA) supplemented with 10 % fetal bovine serum (FBS, Gibco, USA) to provide essential nutrients for cell growth. To assess the impact of CX-4945 on cellular viability, the inhibitor was administered at varying concentrations in a gradient fashion. Following a 24-h incubation period, the Cell Counting Kit-8 (CCK-8, Dojinkagaku, Japan) was utilized in accordance with the manufacturer's standardized protocol to quantify cellular viability.
2.5. Colony formation assay
To further investigate the effects of the CSNK2A1 inhibitor CX-4945 on cellular proliferation, cells were harvested during the logarithmic growth phase. They were then digested, accurately counted, and plated into 12-well plates at a density of 500 cells per well. After a 10-day culturing period, CX-4945 was introduced into the culture medium at predefined concentrations of 0, 5, 10, and 20 μM. Twenty-four hours later, the culture medium was discarded, and the cells were washed twice with phosphate buffered saline (PBS) to remove any residual CX-4945 or culture medium components. Following this, the cells were fixed in 4 % paraformaldehyde for 30 min to preserve their structure and morphology. Subsequently, the cells were stained with 0.5 % crystal violet for 30 min to allow for visualization of the cellular colonies. Photographs were taken to document the colony formation and distribution.
2.6. Fluorescence quantitative real-time polymerase chain reaction (qPCR)
Total RNA was extracted using TRIZOL reagent (Life Technologies, USA), and measured via a spectrophotometer. The extraction of total RNA was performed using TRIZOL reagent (Life Technologies, USA), followed by a precise determination of its concentration and purity via a spectrophotometric assay. RNA reverse transcription was carried out utilizing the HiScript III 1st Strand cDNA Synthesis Kit (Vazyme Biotechnology, China). Subsequently, the synthesized cDNA samples were analyzed through qPCR using Hieff® qPCR SYBR Green Master Mix (Yeasen Biotechnology, China) on the Applied Biosystems QuantStudio6 Flex platform (Life Technologies, USA). The primers were synthesized by Shanghai Sangon Biotech, and their sequences are detailed in Table S1. The qPCR reaction protocol entailed an initial denaturation step at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 10 s and annealing/extension at 60 °C for 30 s. For each sample, triplicate wells were included, and the average cycle threshold (CT) value was recorded as the definitive CT value. Relative mRNA expression was calculated as 2−ΔΔCT.
2.7. Flow cytometry
A total of 200,000 AGS or BGC cells were seeded into six-well plates and cultured overnight to allow for cell attachment and recovery. Following this initial culturing period, CX-4945 was added to the medium at specific concentrations tailored for each cell line: 5 μM for AGS cells and 10 μM for BGC cells. After an additional 24-h incubation with CX-4945, all cells, along with the supernatant, were carefully collected to ensure the capture of both adherent and non-adherent cells.The collected cells were washed twice with binding buffer to remove any residual culture medium or CX-4945. Following the washes, 250 μL of fresh binding buffer was added to resuspend the cells. Subsequently, 5 μL of Annexin V-FITC and 10 μL of propidium iodide (PI) solution at a concentration of 20 μg/mL were added to the cell suspension. The cells were then incubated at room temperature in the dark for 10 min to allow for the Annexin V-FITC and PI to bind to their respective targets, indicating early and late apoptosis. Finally, the apoptosis rate was quantitatively determined using the BD-FACSVerse flow cytometry system (BD Biosciences, USA).
2.8. Western blot
Cells were collected, lysed in RIPA buffer with protease inhibitors and PMSF (MedChemExpress, China). Proteins were separated by SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, USA). Membranes were blocked in TBS-T with milk, then incubated with primary antibodies against PAR, caspase 3, cleaved caspase 3 (#9542, #9662 and #9661 Cell Signaling Technology, USA), and GAPDH (60004-1-Ig, Proteintech, China) overnight at 4 °C. After washing, membranes were incubated with secondary antibody for 2 h at room temperature. Protein bands were detected using ECL and the ChemiDoc Touch system (Bio-Rad, USA), and their gray values were analyzed using ImageJ software (NIH, USA).
2.9. Statistical analysis
IBM SPSS Statistics (version 19.0) software was used for the data analysis. Data are expressed as mean ± standard deviation. Student's t-test was used for comparisons between two groups, whereas one-way analysis of variance was used for comparisons between over two groups. Statistical significance was set at P < 0.05. Graphs were generated using GraphPad Prism (version 9.3.1).
3. Results
3.1. High failure rate of GC RCTs
Total of 11051 reports were retrieved as of June 30, 2022. After rigorous screening, we excluded duplicates (164), non-gastric cancer studies (2507), endoscopic or surgical studies (2190), review articles or meta-analyses (265), non-randomized controlled trials (1933), and studies investigating non-targeted therapies for gastric cancer, specifically chemotherapy or immunotherapy (3432). As depicted in Fig. 1, 560 trials satisfied the predefined inclusion criteria. The detailed targets and drugs were listed in Supplementary Data 1. Subsequently, we focused on extracting all phase III randomized controlled trials (RCTs; 36 trials) and selected phase II RCTs (17 trials) of interest, resulting in a compilation of 53 studies detailed in Supplementary Data 2. Of the 53 trials, 35 trials had been completed and reported. Finally, twenty-six RCTs reported treatment failure (74.29 %, 26/35; Supplementary Data 2).
Fig. 1.
Flowchart of GC clinical trial systematic review process.
3.2. Thirteen targets applied in other cancers were evaluated as potential targets for GC
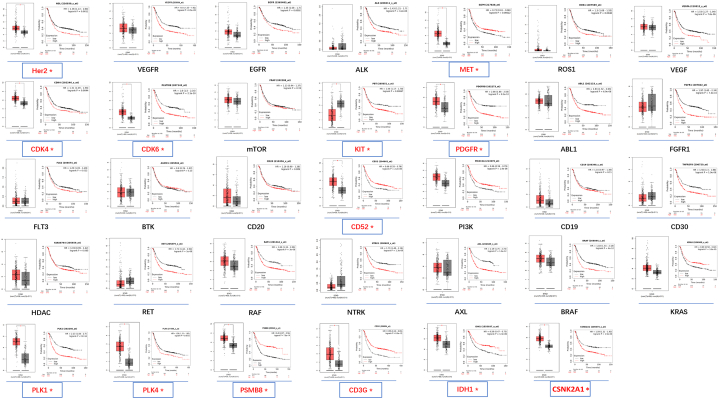
To compare GC-targeted therapy with other cancers, we collected data on therapeutic targets and drugs used in other common cancers. A total of 11 common cancers were enrolled, including 35 targets and 51 approved drugs (Supplementary Data 3). GC had the least number of applied targets, while leukemia had the most (2 vs. 13, Supplementary Data 3). VEGFR is the most popular and effective target for thyroid cancer, renal carcinoma, liver cancer, leukemia, gastrointestinal stromal tumors, stomach cancer, and colorectal cancer. The differential expression of these 35 targets in GC tissues and their association with GC prognosis were analyzed, as shown in Fig. 2. Thirteen targets met the criteria as potential targets for GC (HER2, MET, PLK1/4, CDK4/6, KIT, PDGFR, CD52, CD3G, PSMB8, IDH1, and CSNK2A1). Expression of these genes was higher in cancer tissues than in normal tissues, except for the KIT gene (Fig. 2). The expression of MET, CD52, PSMB8, CD3G, and IDH1 positively correlated with GC prognosis, whereas the remaining genes were negatively correlated (Fig. 2). Clinical trials related to HER2, MET, KIT, PDGFR, and CDK have already been conducted. However, trials on PLK1/4 and CSNK2A1 have not yet been conducted (Supplementary Data 1).
Fig. 2.
Expression and overall survival analysis of 34 collected genes in GC. Gene name in red meet the inclusion criteria: 1. Significant differential expression in GC and para-cancer tissues; 2. Significant correlation with the prognosis of GC. *P < 0.05.
3.3. CSNK2A1 was related to multiple GC biological characteristics
Compared to other target genes, our analysis revealed that CSNK2A1 might play a more stable and independent role in GC progression (Fig. S1). The expression levels of PLK1 and PLK4 in cancer tissues fluctuate more widely than those of CSNK2A1 (Fig. S1A); in terms of protein interactions, CSNK2A1 is more functionally independent (Fig. S1C); in terms of gene mutations, CSNK2A1 is also more stable(Fig. S1D).
Thus, CSNK2A1 was selected as a potential target for further analysis. We found that CSNK2A1 expression was associated with tumor differentiation (P < 0.05; Fig. 3D). As shown in Fig. 3A–F, expression of CSNK2A1 was not related to the depth of infiltration (P = 0.34), lymphatic metastasis (P = 0.91), distant metastasis (P = 0.1), cancer stage (P = 0.74), or patient sex (P = 0.91). Regarding immune infiltration, CD8+ T cells, macrophages, neutrophils, and dendritic cells negatively correlated with CSNK2A1 expression (Fig. 3G). No correlation was observed between CSNK2A1 expression and TMB or MSI (Fig. 3H and I). However, CSNK2A1 expression was positively associated with the DNA and RNA stemness scores (Fig. 3J and K).
Fig. 3.
Relation of CSNK2A1 expression with GC characteristic. Relation of CSNK2A1 expression with GC at T (A), lymphatic metastasis (B), distant metastasis (C), tumor differentiation (D), and immune infiltration (E) stages. Relation of CSNK2A1 expression with pan-cancers TMB (F), MSI (G), DNA stemness (H), and RNA stemness (I). P < 0.05 was regarded as statistically significant.
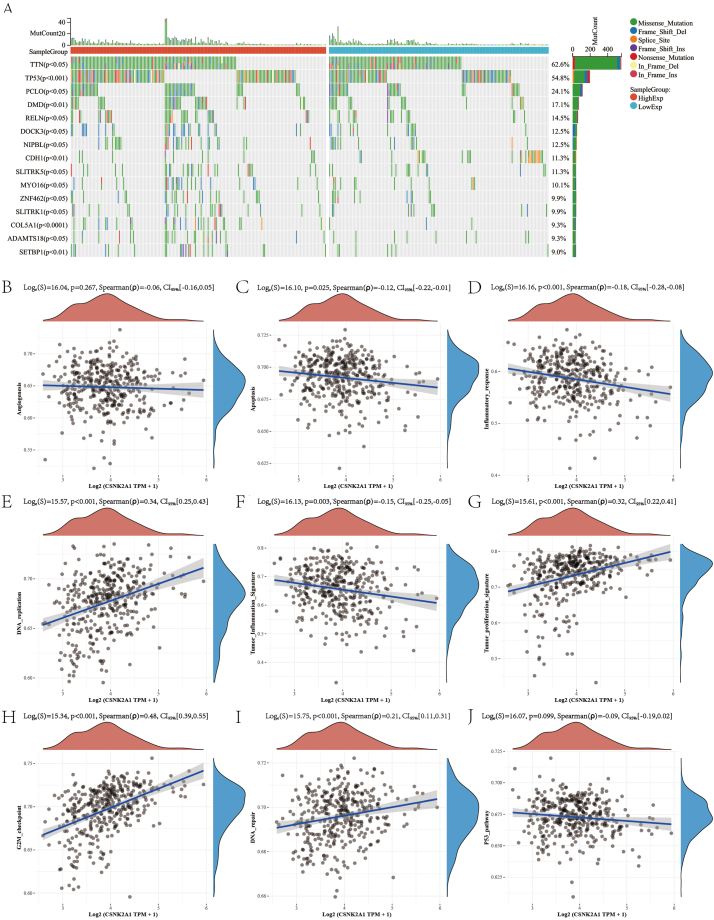
We further analyzed the correlation between gene mutation patterns and CSNK2A1 expression and found the top five discrepancy genes: TTN, TP53, PCLO, DMD, and RELN (Fig. 4A). Pathway analysis showed that CSNK2A1 expression was positively correlated with DNA replication (Fig. 4E), tumor proliferation (Fig. 4G), the G2/M checkpoint (Fig. 4H), and DNA repair (Fig. 4I), whereas CSNK2A1 expression was negatively correlated with apoptosis (Fig. 4C), inflammatory response (Fig. 4D), tumor inflammation signature (Fig. 4F), and the P53 pathway (Fig. 4J). No significant correlation was observed with the angiogenesis pathway (Fig. 4B).
Fig. 4.
Relation of CSNK2A1 expression with gene mutations and pathways. (A) Discrepancy of genome mutation between high and low CSNK2A1 expression. Relation of CSNK2A1 expression with angiogenesis (B), apoptosis (C), inflammatory response (D), DNA replication (E), tumor inflammation signature (F), tumor proliferation signature (G), G2M checkpoint (H), DNA repair (I), and p53 (J) pathways. P < 0.05 was regarded as statistically significant.
3.4. CX-4945 inhibits GC cell proliferation and induces apoptosis and its sensitivity is related to CSNK2A1 expression
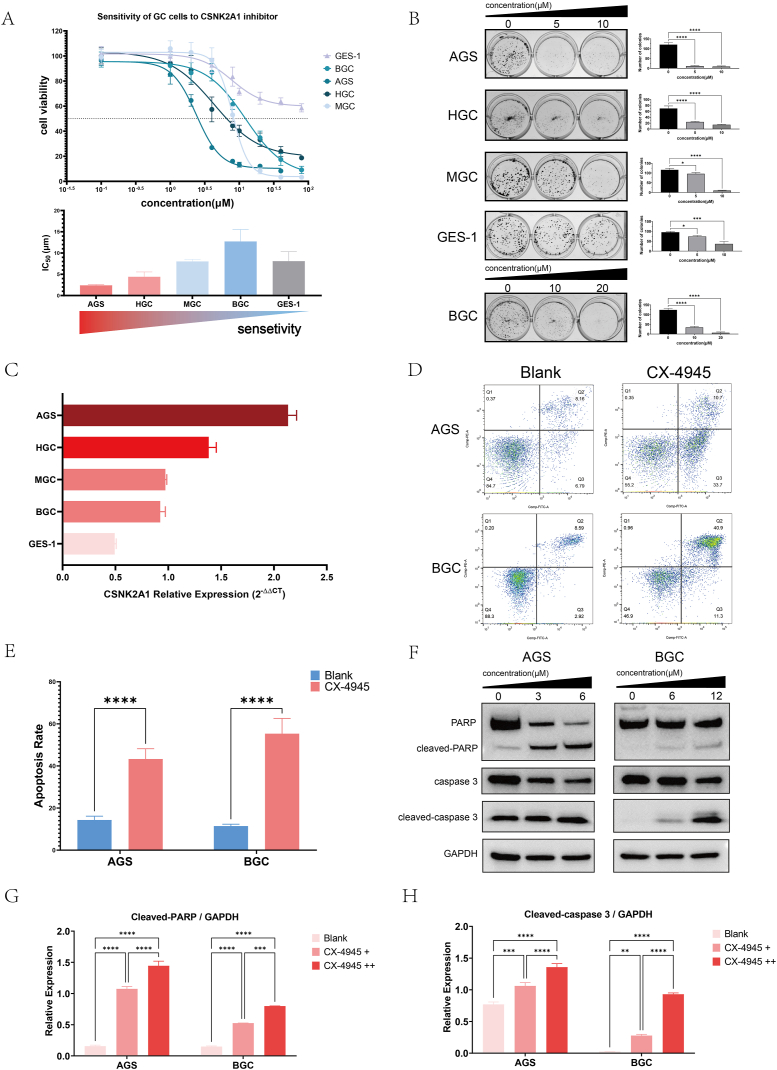
We performed in vitro experiments to identify CSNK2A1 as a potential therapeutic target. The cell proliferation assays revealed that five GC cells (AGS IC50 = 2.393 μM, BGC IC50 = 12.31 μM, MGC IC50 = 7.999 μM, HGC IC50 = 4.330 μM) were all sensitive to CSNK2A1 inhibitor CX-4945 while the normal mucosa cell GES-1 was insensitive (Fig. 5A). More than 50 % of the GES-1 cells survived when exposed to the maximum concentration of CX-4945 (80 μM). Among the GC cells, AGS was the most sensitive and BGC had the opposite effect (Fig. 5A). Colony formation assays showed that as the drug concentration increased (0, 5, 10 μM), the average number of colonies in AGS cells decreased from 120.70 to 11.33 and 8.67, HGC cells from 69.67 to 24.00 and 14.67, MGC cells from 116.30 to 95.67 and 10.00, and GES-1 cells from 95.33 to 74.00 and 36.67. For BGC cells, with the increase of drug concentration (0, 10, 20 μM), the average number of colonies decreased from 124.00 to 34.67 and 6.67. Statistically significant differences were observed in the number of cell colonies between various concentrations (Fig. 5B). Both assays indicated that CX-4945 inhibited GC cell proliferation. The expression of CSNK2A1 was then detected by qPCR. The sensitivity of GC cells to CX-4945 was positively associated with the expression of CSNK2A1 (Fig. 5C). The relative expression of CSNK2A1 in the most sensitive AGS cell line was significantly higher at 2.134, whereas the least sensitive GES-1 cell line exhibited the lowest relative expression value of 0.4936. Among the remaining cell lines, when ranked by sensitivity, the relative expression of CSNK2A1 was 1.384 in HGC, 0.9730 in MGC, and 0.9230 in BGC. Statistical analysis revealed significant differences in the relative expression of CSNK2A1 among all cell lines except for BGC VS MGC, which did not show any significant difference (P = 0.7918). The remaining comparisons displayed statistically significant variations (P < 0.0001).
Fig. 5.
CX-4945 inhibited GC cells proliferation and induced apoptosis. (A) Cell viability of GC cells in cell proliferation assays and cell sensitivity to CX-4945. (B) Clone formation assay. (C) Relative CSNK2A1 expression of GC cells. (D) Apoptosis detection of AGS and BGC via flow cytometry. (E) Three-time repetition of apoptosis detection and the data was calculated. ****P < 0.0001. (F) AGS and BGC were cultured with different concentration of CX-4945 and apoptosis associated proteins were detected via Western blot. Full, non-adjusted images were shown in Supplementary Data 4. (G, H) Semiquantitative analysis of Western blot. Gray value of cleaved-caspase 3, cleaved-PARP, and GAPDH bands were analyzed via Image J software. For AGS, + and ++ represent 3 and 6 μM, respectively; for BGC, + and ++ represent 6 and 12 μM, respectively. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Flow cytometry was used to detect apoptosis in the GC cells. When exposed to 5 μM CX-4945, the apoptosis rate of AGS cells was 47.04 %, significantly higher than that of the non-drug group (10.61 %) (P < 0.0001); Similarly, when exposed to 10 μM CX-4945, the apoptosis rate of BGC cells was 51.61 %, significantly higher than that of the non-drug group (15.18 %) (P < 0.0001) (Fig. 5D and E). The expression of apoptosis-associated proteins was also detected. Western blot analysis showed that the precursor PARP and caspase 3 decreased and cleaved PARP and caspase 3 increased as the concentration of CX-4945 increased (Fig. 5F). A semi-quantitative analysis confirmed this tendency (Fig. 5G and H).
4. Discussion
Our data showed that GC-targeted therapy faces enormous challenges. The application of targeted therapy in GC is still in its infancy compared to its usage in other cancers (Supplementary Data 1, 2 and 3). However, our research could not conclude the factors behind the failure.
One representative phenomenon was that, although various cancers share commonality, enrolling one target in GC clinical trials briefly because of its success in another cancer would be very likely to fail, such as trials on EGFR and MET. For these reasons, it can be concluded that GC has extremely high heterogeneity. This characteristic was more definite with the development of single-cell sequencing technology [18]. High tumor heterogeneity would easily result in drug resistance [19]. To solve this problem, at least three aspects could be improved. The first aspect is screening for more specific and sensitive therapeutic target. Recent developments in detection technologies have successfully identified molecular subtypes of GC and various meaningful therapeutic targets for GC [[20], [21], [22]]. The second aspect is searching for reliable biomarkers. Patients were classified according to their biomarker features. According to our analysis, simple biomarker detection (detecting only the expression of targeted genes or proteins) did not improve the success of RCTs. Therefore, biomarkers should not be limited to the expression or mutations of therapeutic targets. Using MET as example, some researchers have suggested that the presence of neutrophils in the tumor microenvironment could influence the efficacy of MET inhibitors and that activation of the intracellular Src pathway is also associated with efficacy [16]. The third aspect is developing multitarget conjugated drugs or activating the immune system [23].
The high heterogeneity of GC did not mean that the targets successfully applied to other cancers could not be utilized to GC. Specific drugs should be adequately evaluated and tested before starting a GC clinical trial [24]. Uncovering the mechanism or discovering biomarkers would also contribute to successful applications [14,16]. We reanalyzed targets from other cancer therapies and screened them for GC. Most of the targets were protein kinase (PK). PK is a promising target that modulates cellular proliferation, metabolism, and differentiation via the phosphorylation of substrates [25,26]. It has been reported that the kinome consisting of 538 kinases is becoming the focus of targeted therapy research [27]. Meanwhile, the development of small-molecule inhibitors of PK will hopefully accelerate the coming era of individualized therapy [22,28].
We screened 13 potential therapeutic targets for gastric cancer from 11 common malignant tumor treatment targets, among which CSNK2A1 and PLK1/4 have not been used in clinical trials for gastric cancer. CSNK2A1 might play a more stable and independent role in GC progression (Fig. S1). Through literature retrieval, we found that CSNK2A1 is a widely present protein kinase in tumor tissues with a very wide range of substrate proteins [29], so we speculate that CSNK2A1 plays a more universal role in the occurrence and development of gastric cancer, and therefore selected CSNK2A1 for further in-depth analysis. CSNK2A1 is a member of protein serine/threonine kinase with the most abundant kinds of substrate proteins and is extensively involved in various life activities of cells, including cell growth, survival, apoptosis, DNA damage repair, differentiation, and metabolic regulation [29]. Based on current scientific literature, CSNK2A1 has been associated with the differentiation of B and T cells [30,31], yet its specific impact on gastric cancer cell differentiation remains unexplored. Our analysis indicates a potential correlation between CSNK2A1 and tumor differentiation, immune infiltration, as well as tumor stemness in gastric cancer. Nevertheless, the underlying molecular mechanisms necessitate further detailed and precise experimental investigations. Besides, we have also uncovered a profound association between CSNK2A1 and apoptosis in gastric cancer cells. To validate this function at the cellular level, we employed CX-4945 to inhibit the CSNK2A1 gene and observed successful induction of apoptosis in gastric cancer cells. Furthermore, our findings revealed a significant correlation between the expression level of the CSNK2A1 gene and the drug sensitivity of gastric cancer cells, suggesting the potential of CSNK2A1 expression as a biomarker for predicting the efficacy of CX-4945-induced apoptosis in gastric cancer. However, it is imperative to conduct further investigations into the underlying molecular mechanisms, animal models, and ultimately clinical trials to validate this observation.
5. Conclusion
In this comprehensive study, we have discovered a remarkably high failure rate in clinical trials for gastric cancer and identified CSNK2A1 as a potential therapeutic target for gastric cancer (GC). Furthermore, CX-4945 effectively induces apoptosis in GC cells. Notably, we observed a positive correlation between the sensitivity of GC cells to CX-4945 and the expression level of CSNK2A1. Given these promising results, further in-depth investigations into the underlying mechanisms as well as clinical trials evaluating the efficacy of CX-4945 in GC treatment are highly warranted.
Ethics approval
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University (No: 202204011). Additionally, informed consents were obtained from all patients in line with the Declaration of Helsinki.
Funding
This study was supported by grants from the National Natural Science Foundation of China [grant number 32070151], the Leading Talents in Scientific and Technological Innovation from Zhejiang Provincial Ten Thousand Talents Plan [grant numbers 2021R52024, 2019R52021], and the Key R&D Program of Zhejiang Province [grant numbers 2020C03029, 2021C03120], Zhejiang Medical Health Science and Technology Project [2019KY461], Zhejiang Public Welfare Technology Research Program and Social development Project [LGF20H070003].
Availability of data and material
The original contributions presented in the study are included in the article.
Consent for publication
All authors give consent for publication.
CRediT authorship contribution statement
Liang Zhang: Writing – review & editing, Writing – original draft, Project administration, Methodology, Conceptualization. Jiaqi Yang: Writing – review & editing, Writing – original draft, Project administration, Methodology, Conceptualization. Yingpeng Huang: Writing – review & editing, Software, Investigation. Tao You: Writing – review & editing, Software, Investigation. Qunjia Huang: Writing – review & editing, Validation, Data curation. Xian Shen: Writing – review & editing, Supervision, Funding acquisition. Xiangyang Xue: Writing – review & editing, Supervision, Funding acquisition. Shiyu Feng: Writing – review & editing, Supervision, Resources, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36205.
Contributor Information
Xiangyang Xue, Email: wzxxy001@163.com.
Shiyu Feng, Email: Plingzj1998@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. Epub 2021/02/05. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Park J.Y., Camargo M.C., Lunet N., Forman D., Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020 May;69(5):823–829. doi: 10.1136/gutjnl-2019-320234. Epub 2020 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng H., Chen W., Zheng R., Zhang S., Ji J.S., Zou X., et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Global Health. 2018;6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. Epub 2018/04/15. [DOI] [PubMed] [Google Scholar]
- 4.Wang F.H., Zhang X.T., Li Y.F., Tang L., Qu X.J., Ying J.E., et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. 2021;41(8):747–795. doi: 10.1002/cac2.12193. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. Epub 2010/08/24. [DOI] [PubMed] [Google Scholar]
- 6.Gradishar W.J., Anderson B.O., Abraham J., Aft R., Agnese D., Allison K.H., et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2020;18(4):452–478. doi: 10.6004/jnccn.2020.0016. Epub 2020/04/08. [DOI] [PubMed] [Google Scholar]
- 7.Benson A.B., Venook A.P., Al-Hawary M.M., Arain M.A., Chen Y.J., Ciombor K.K., et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021;19(3):329–359. doi: 10.6004/jnccn.2021.0012. Epub 2021/03/17. [DOI] [PubMed] [Google Scholar]
- 8.Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J.R., Bharat A., et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022;20(5):497–530. doi: 10.6004/jnccn.2022.0025. Epub 2022/05/12. [DOI] [PubMed] [Google Scholar]
- 9.Pirker R., Pereira J.R., Szczesna A., von Pawel J., Krzakowski M., Ramlau R., et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373(9674):1525–1531. doi: 10.1016/S0140-6736(09)60569-9. Epub 2009/05/05. [DOI] [PubMed] [Google Scholar]
- 10.Lordick F., Kang Y.K., Chung H.C., Salman P., Oh S.C., Bodoky G., et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–499. doi: 10.1016/S1470-2045(13)70102-5. Epub 2013/04/19. [DOI] [PubMed] [Google Scholar]
- 11.Andre F., O'Regan R., Ozguroglu M., Toi M., Xu B., Jerusalem G., et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(6):580–591. doi: 10.1016/S1470-2045(14)70138-X. Epub 2014/04/20. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsu A., Ajani J.A., Bai Y.X., Bang Y.J., Chung H.C., Pan H.M., et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J. Clin. Oncol. 2013;31(31):3935–3943. doi: 10.1200/JCO.2012.48.3552. Epub 2013/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajani J.A., D'Amico T.A., Bentrem D.J., Chao J., Cooke D., Corvera C., et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022;20(2):167–192. doi: 10.6004/jnccn.2022.0008. Epub 2022/02/08. [DOI] [PubMed] [Google Scholar]
- 14.Ahn D.H., Grothey A. Continued disappointments with targeted agents in first-line therapy of advanced gastric cancers. Lancet Oncol. 2017;18(11):1427–1428. doi: 10.1016/S1470-2045(17)30714-3. Epub 2017/09/30. [DOI] [PubMed] [Google Scholar]
- 15.Xu W., Yang Z., Lu N. Molecular targeted therapy for the treatment of gastric cancer. J. Exp. Clin. Cancer Res. 2016;35:1. doi: 10.1186/s13046-015-0276-9. Epub 2016/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzone M., Finisguerra V., Prenen H. Is there merit for MET-targeted therapies in gastroesophageal cancer? JAMA Oncol. 2018;4(1):131–132. doi: 10.1001/jamaoncol.2017.2725. Epub 2017/09/15. [DOI] [PubMed] [Google Scholar]
- 17.Strickler J.H. EGFR amplification as a target in gastroesophageal adenocarcinoma: do anti-EGFR therapies deserve a second chance? Cancer Discov. 2018;8(6):679–681. doi: 10.1158/2159-8290.CD-18-0191. Epub 2018/06/03. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M., Hu S., Min M., Ni Y., Lu Z., Sun X., et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut. 2021;70(3):464–475. doi: 10.1136/gutjnl-2019-320368. Epub 2020/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willyard C. Cancer therapy: an evolved approach. Nature. 2016;532(7598):166–168. doi: 10.1038/532166a. Epub 2016/04/15. [DOI] [PubMed] [Google Scholar]
- 20.Cristescu R., Lee J., Nebozhyn M., Kim K.M., Ting J.C., Wong S.S., et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449–456. doi: 10.1038/nm.3850. Epub 2015/04/22. [DOI] [PubMed] [Google Scholar]
- 21.Ge S., Xia X., Ding C., Zhen B., Zhou Q., Feng J., et al. A proteomic landscape of diffuse-type gastric cancer. Nat. Commun. 2018;9(1):1012. doi: 10.1038/s41467-018-03121-2. Epub 2018/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong M., Yu C., Shi J., Huang W., Ge S., Liu M., et al. Phosphoproteomics enables molecular subtyping and nomination of kinase candidates for individual patients of diffuse-type gastric cancer. iScience. 2019;22:44–57. doi: 10.1016/j.isci.2019.11.003. Epub 2019/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajani J.A. Challenges imposed by the complexity of cancer genome. Lancet Oncol. 2013;14(8):e291–e292. doi: 10.1016/S1470-2045(13)70224-9. Epub 2013/07/03. [DOI] [PubMed] [Google Scholar]
- 24.Apicella M., Corso S., Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget. 2017;8(34):57654–57669. doi: 10.18632/oncotarget.14825. Epub 2017/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farran B., Muller S., Montenegro R.C. Gastric cancer management: kinases as a target therapy. Clin. Exp. Pharmacol. Physiol. 2017;44(6):613–622. doi: 10.1111/1440-1681.12743. Epub 2017/03/09. [DOI] [PubMed] [Google Scholar]
- 26.Buljan M., Ciuffa R., van Drogen A., Vichalkovski A., Mehnert M., Rosenberger G., et al. Kinase interaction network expands functional and disease roles of human kinases. Mol Cell. 2020;79(3):504–520 e9. doi: 10.1016/j.molcel.2020.07.001. Epub 2020/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H., Cao X., Tang M., Zhong G., Si Y., Li H., et al. A subcellular map of the human kinome. Elife. 2021;10 doi: 10.7554/eLife.64943. Epub 2021/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson F.M., Gray N.S. Kinase inhibitors: the road ahead. Nat. Rev. Drug Discov. 2018;17(5):353–377. doi: 10.1038/nrd.2018.21. Epub 2018/03/17. [DOI] [PubMed] [Google Scholar]
- 29.Litchfield D.W. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003;369(Pt 1):1–15. doi: 10.1042/BJ20021469. Epub 2002/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson S.A., Yang W., Yan Z., Liu Y., Rowse A.L., Weinmann A.S., Qin H., Benveniste E.N. Protein kinase CK2 controls the fate between Th17 cell and regulatory T cell differentiation. J. Immunol. 2017 Jun 1;198(11):4244–4254. doi: 10.4049/jimmunol.1601912. Epub 2017 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei H., Yang W., Hong H., Yan Z., Qin H., Benveniste E.N. Protein kinase CK2 regulates B cell development and differentiation. J. Immunol. 2021 Aug 1;207(3):799–808. doi: 10.4049/jimmunol.2100059. Epub 2021 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article.