Abstract
Background
Foodborne and waterborne diseases and outbreaks are a neglected public health issue worldwide. In developing countries, diarrheal disease caused by foodborne and waterborne infections is a major cause of ill health. There is a lack of information on foodborne pathogens, their transmission routes, outbreaks, and related mortalities, due to the absence of a robust disease surveillance system and adequately equipped laboratories. Although hygiene practices are much better in Western countries, the widespread use of preserved and raw food items is a cause of concern. Consequently, the occurrence of foodborne diseases is not rare in these countries either. WHO has recently released the ‘Global Strategy for Food Safety 2022–2030’, addressing the emerging challenges, new technologies, and innovative approaches to strengthen food safety systems and enhance laboratory capacity for foodborne disease surveillance. Foodborne outbreaks are a huge challenge in India. Malnutrition, anemia, hookworm and enteric infections, are the predominant cryptic health conditions among children in rural and tribal areas, leading to severe consequences, including death, and posing a substantial threat to public health. Combating such events with adequate food safety and hygiene practices is achievable. Systematic collection of data can help to develop food safety policies that could reduce the burden of foodborne diseases.
Objective
This review aims to examine the current situation of foodborne and waterborne diseases, identification of the factors contributing to their occurrence and outbreaks, and defining the gaps in control measures, challenges, and potential solutions in improving the public health system.
Methods
Strengths, weaknesses, opportunities, and threats (SWOT) analysis was made based on the literature review of foodborne and waterborne infections to assess the current situation and to identify knowledge gaps.
Finding
SWOT analysis showed the strength and gaps in the different national initiatives analogous to the global programs. Though, Integrated Disease Surveillance Programme (IDSP), Food Safety and Standards Authority of India (FSSAI), the core Government missions, independently generate substantial information, sporadic and outbreak cases of diarrhea still prevail in the country due to the absence of a systematic national surveillance system. Recently, many government initiatives have been made through Sustainable Development Goals (SDGs), G20 goals, etc. However, potential threats such as risk of zoonotic disease transmission to humans, emerging infections and antimicrobial resistance (AMR), and unauthorized activities in the food sector pose a big challenge in safeguarding the public health.
Conclusion
Maintenance of global food safety requires a systematic analysis of present situations, identification of existing shortcomings, and targeted efforts toward prevention of infections. The ongoing G20 mission and the SDGs for 2030 represent significant strides in this direction. To have pathogen-free animals and supply of contamination-free raw foods is impractical, but, mitigating the prevalence of zoonotic diseases can be accomplished by rigorously enforcing hygiene standards throughout the food production chain. A crucial requirement at present is the implementation of integrated laboratory surveillance for foodborne and waterborne infections, as this will provide policymakers and stakeholders all the evidence based scientific information. This system will facilitate efforts in minimizing the risks associated with foodborne and waterborne infections.
Keywords: Foodborne diseases, Surveillance, Outbreaks, Hygiene practices, Public health
Highlights
-
•
Foodborne diseases remain a significant public health challenge in both developing and developed countries.
-
•
SWOT analysis reveals gaps in India's foodborne and waterborne disease surveillance systems.
-
•
Integrated laboratory surveillance is crucial for managing foodborne and waterborne infections.
-
•
National surveillance systems are needed to mitigate the rising threats of zoonotic and foodborne diseases.
-
•
High standards of hygiene throughout the food production chain can reduce zoonotic transmission.
1. Introduction
Food safety is an important component in Public Health management. It is estimated that 7.7 % of individuals all over the world suffer from foodborne illness every year with a mortality rate of 7.5 % [[1], [2], [3]]. Despite causing a large number of morbidity and mortality throughout the world [4], foodborne diseases remain a neglected subject. World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) regularly report the incidence of foodborne infections, causative pathogens, and outbreaks associated with contaminated food sources [5]. The pathogens that cause foodborne infections vary among different countries. The majority of cases in the United States are of Norovirus and Campylobacter, whereas Europe reports more Campylobacter and Salmonella infected cases. In Australia, the highest number of cases are due to Campylobacter, followed by Salmonella. In Korea, pathogenic Escherichia coli is followed by Norovirus. Campylobacter and Clostridium perfringens cases have been reported in Japan [6]. Southeast Asian countries are predispose to foodborne illness because of the climatic conditions, food habits, poverty, poor hygiene, inadequate sanitary facilities and low public awareness of food safety [7].
India faces a greater challenge with a high incidence of foodborne diseases, particularly diarrheal infections, resulting in considerable mortality and morbidity. More than 60 % of emerging and re-emerging pathogens have been identified from animals and the environment, which are transmitted by food and water [8]. However, the reported incidence of foodborne diseases is inaccurate in India, as they are not often reported and notified to the authority due to their sporadic and self-limiting nature. The Outbreak Surveillance data of Integrated Disease Surveillance Programme (IDSP) indicated a significant rise in both the frequency and diversity of outbreaks across India [9]. During 2009–2018, a total of 2688 foodborne disease outbreaks and 572 deaths were reported to the IDSP. An average of 269 outbreaks and 57 deaths were reported each year [10]. Outbreak investigations in India frequently lack essential comprehensive information needed for thorough analysis, monitoring, and evaluating response processes. Published reports often lack comprehensive investigative details, both in descriptive and analytical aspects. In view of the potential risk of foodborne disease outbreaks and to get the accurate picture of the extent of foodborne illness, there is a need for constant monitoring, data generation, analysis, and preparedness to manage future outbreaks.
This review focuses on the present state of foodborne illnesses in India, along with the awareness levels and existing infrastructure for reporting such infections. It also explores the efforts to meet global standards of food hygiene, emphasizing the current necessities for public health in comparison to global initiatives.
2. Methodology
Foodborne disease outbreaks are defined as the occurrence of 2 or more cases of a similar illness resulting from ingestion of a common food or when the observed number of cases of a particular disease exceeds the expected number [11]. These can be confirmed by identifying at least one causative agent or speculation-linked events (based on clinical and epidemiological information). Although most cases are sporadic, these diseases draw attention due to outbreaks, investigation of which can help in initiating control measures. This review covers the foodborne and waterborne disease outbreaks published between 1981 and 2022. A comprehensive literature review was performed by searching databases of scientific literature reporting the foodborne and waterborne disease outbreaks in India. Keywords used includes “India” AND (“food” OR “water”) AND (“pathogens” OR “Listeria” OR “Salmonella” OR “E. coli O157” OR “Campylobacter”) AND/OR “outbreak” AND/OR “foodborne infection,” “Food Network” AND/OR ‘NCDC annual report,’ AND/OR ‘WHO foodborne” to identify the published literature. The results of each search were filtered based on the relevance of the title and abstract/full text. Only peer-reviewed studies that reported outbreaks of foodborne or waterborne pathogens in India were included. In addition, for each selected publication, the reference section was examined to identify additional relevant publications. Information extracted from each report includes the onset date of illness, the state reporting the outbreak, the number of illnesses and fatalities, suspected sources of contaminated food and the identified pathogen(s). Exclusion criteria include (i) food poisoning outbreaks, (ii) articles with incomplete information or unmatched with our objectives, (iii) outbreaks with alternative modes of transmission, where the illness was not linked to foodborne or waterborne pathogens or suspected food items, or associated with person-to-person/animal contact.
The global foodborne illness outcomes were assessed, considering the current challenges to meet the emerging and re-emerging problems, including their root causes and influences. Strengths, weaknesses, opportunities, and threats analysis (SWOT) analysis was performed This analysis also includes qualitative and quantitative studies in India focusing on foodborne illness prevalence and existing infrastructure to tackle the problem. In addition, we have reviewed the IDSP, National Health Mission (NHM) diarrhea control measures, and other disease surveillance platforms in India.
2.1. Global scenario
The health burden of foodborne illnesses is substantial both in developed and developing countries [12]. Approximately 7.7 % of the global population (7.8 billion) suffer from foodborne illnesses annually, and 7.5 % of all deaths (56 million) are attributed to these diseases [3]. 30 % of reported foodborne deaths occur in children under the age of 5 [1]. As many cases are often not reported to health officials, the true impact of foodborne illness is not fully known.
A systematic review on emerging infectious disease outbreaks revealed that 76 % of infections are zoonotic resulting from heightened human-animal contact due to animal farming and deforestation, which elevates the risk of pathogens entering the food chain [8,13]. A large number of zoonotic pathogens thrive asymptomatically in the gastrointestinal tract of food animals (poultry, cattle, swine, sheep, and goats) shedding in significant quantities through their feces. These pathogens including Campylobacter spp, Salmonella spp., and Shiga toxin-producing Escherichia coli (STEC) are associated with many foodborne diseases and outbreaks [14]. Enteric pathogens are the third leading cause of infectious disease worldwide, accounting for almost two million deaths yearly [15]. Therefore, foods of animal origin are considered primary vehicles of foodborne infections. In addition, there are many other zoonotic pathogens associated with food transmission, like hepatitis E (HEV), A (HAV), paragonimus, etc [16].
In the CDC assessment of foodborne pathogens, HAV is considered a significant cause of the severe disease [17]. Contaminated clams caused an outbreak of HAV in China, which affected more than 300,000 people [18]. HEV is another important pathogen that has been recently considered for its transmission to humans through the food and water [19,20]. Epidemiological studies have provided evidence for the consumption of undercooked or raw pork as a risk factor for HEV infection, but only very few systematic studies have been reported [[21], [22], [23]]. Several studies across Europe indicate that pig herds are responsible for the transmission of HEV genotype G3 infections [[24], [25], [26], [27], [28]]. A survey conducted in France described a farm-level prevalence of HEV G3 (30 %) [29]. Similar findings in Canada [30] and Italy [31] have also been documented linking animal meat with HEV.
Norovirus is the other leading cause of acute gastroenteritis outbreaks. More than 7000 Norovirus outbreaks were reported to the National Outbreak Reporting System (NORS) by local, state, and territorial health departments in the US from 2009 to 2017 [32]. Foodborne Norovirus is estimated to cause more than 50 deaths per year in the UK [33]. Between 2012 and 2017, foodborne outbreak-associated Norovirus GI and GII were detected in foodborne outbreak investigations in France [34]. In Japan, recombinant GII virus with various genotypes has been reported in several foodborne outbreaks [35].
Among bacterial infections, STEC is estimated to be responsible for 2.8 million acute illnesses, resulting in 3890 cases of hemolytic uremic syndrome (HUS), among these cases 270 had end-stage renal disease, and 230 fatalities [36]. The primary source of this zoonotic pathogen is the intestinal tract of cattle, although other animals can also act as reservoirs [17]. In the US, E. coli O157:H7 emerged as a significant public health. Fifty-two (48 %) patients were hospitalized, and 13 (12 %) developed the HUS. This large multistate outbreak was due to eating at a national fast-food chain [37].
Campylobacter is one of the most common human enteric pathogens in both developed and developing countries. From 2014 to 2020, there was a constant upsurge in campylobacteriosis in France [38]. The reported incidence rose from 45.2 per 100,000 in 2014 to 57.5 in 2019 and further increased to 58.8 in 2020. Similarly, the number of foodborne outbreaks attributed to Campylobacter in Japan rose from 212 in 2014 to 278 in 2018 [38,39]. Approximately 15–28 % of Campylobacter infections were associated with the consumption of chicken [39]. In Japan, Campylobacter infections surpassed the rates of other foodborne bacterial pathogens like Salmonella and Vibrio parahaemolyticus [40].
Salmonella exists in the intestines of most livestock and many wild animals. Outbreaks caused by this pathogen is commonly associated with contaminated eggs, meat, and poultry products. The CDC estimates that approximately 1.35 million people in the US get infected with Salmonella that include 26,500 hospitalizations, and 420 deaths annually [41]. In 2023, five Salmonella outbreaks were documented linking 230 individuals, with 96 hospitalizations and 3 deaths [41]. The associated food sources include, flour (14 cases, 3 hospitalizations), raw cookie dough (26 cases, 4 hospitalizations), ground beef (18 cases, 7 hospitalizations), diced onions (73 cases, 15 hospitalizations), and cantaloupes [41].
Listeriosis is a serious infection with a high mortality rate (20–30 %) [42]. Listeria monocytogenes is considered an opportunistic pathogen that causes disease in older adults, pregnant women, newborns, and immunocompromised adults. Infections in pregnant women might cause miscarriages, stillbirths, and birth defects. Unlike many other foodborne pathogens, Listeria multiplies at lower temperatures and hence, refrigerated foods are a potential risk for this pathogen [42] . From January 2017 to July 2018, South Africa experienced the largest listeriosis outbreak with 1060 confirmed cases, and 216 deaths [43]. This outbreak was linked to ready-to-eat processed meat products contaminated with L. monocytogenes from a specific food production facility. A listeriosis outbreak in Spain, with 207 confirmed cases was reported between July and October 2019 [44]. About 68 % of the cases required hospitalization, and there were 3 fatalities and 5 suffered miscarriages. The source of the infection was identified as stuffed pork, a ready-to-eat product supplied by a single producer contaminated with L. monocytogenes sequence type 388 [44].
2.2. Indian scenario
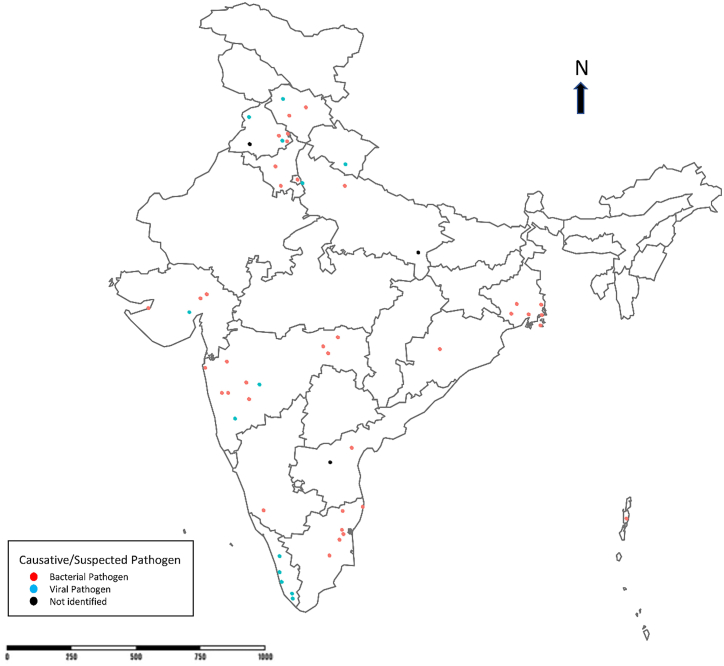
One of the frequent health risks that contribute to high morbidity in India is foodborne infections. The main cause of infection is due to the lack of public health infrastructure, economic problems, unstable health policies, poverty, unconstrained population displacement, rapid urbanization, inefficient disease control programs, and antimicrobial resistance [45]. Furthermore, globalisations of trade and travel have increased the emergence and re-emergence of foodborne pathogens [46]. Table 1 presents a list of foodborne and waterborne outbreaks reported in India during 1981–2022 with causative agents, sources, and impact across regions. The geographical distribution of these incidents in India has also been shown in Fig. 1.
Table 1.
A comprehensive analysis of foodborne and waterborne outbreaks in India (1981–2022) – Unraveling causative agents, sources, and regional impact.
| Causative/suspected pathogen | Year | Region/State | No. of cases | No of deaths | clinical symptoms | The event | Clinical and food samples investigated | Possible source | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Vibrio fluvialis | 1981 | Maharashtra | 14 | _ | Vomiting, abdominal pain | Religious ceremony | 9 stool samples + ve for V. fluvialis | Food | [54] |
| Salmonella enterica serovar Typhi | 1986 | Chandigarh | 54 | _ | Fever (100 %), nausea and vomiting (46 %), loose motions and abdominal pain (13 %), and palpable splenomegaly (63 %). | Picnic | Blood cultures 81 %, Widal (1:320 or more) in 43 % and suggestive (1:160) in 25 %. Bacteriological examination of water from the likely sources revealed gross faecal contamination. |
Contaminated water | [92] |
| Salmonella Paratyphi A var Durazzo | 1995 | Maharashtra | 33 | _ | Acute diarrhea and vomiting, high grade fever | Salmonella paratyphi A var durazzo isolated from 12 faecal samples | food | [58] | |
| Yersinia enterocolitica | 1997 | Tamil Nadu | 25 | _ | Fever, loose stool, abdominal cramps, with or without vomiting | Feast | Y. enterocolitica isolated from one stool sample and borewell water samples. Serology of first paired samples revealed high anti Y. enterocolitica antibody titre. Toxin was detected from the butter milk. | food (Buttermilk) | [55] |
| Salmonella enteritidis | 1998 | Himachal Pradesh | 78 | _ | Diarrhea, vomiting, fever, headache, vertigo and abdominal cramps of varying intensity | Food consumption in a common dining hall | Salmonella enteritidis was isolated from 6 stool samples and in one blood sample. | Food (Frozen fowl) | [56] |
| Vibrio cholerae O1 | 1999 | Chandigarh | 14 | _ | Diarrhea | Water from one of the hand pump | Microscopically confirmed cases of cholera, water was positive for V. cholerae O1 biotype El Tor serotype Ogawa. | Hand pump water contamination | [93] |
| Norwalk-like viruses | 2002 | New Delhi | 130 | _ | Diarrhea | Farewell party | All six stool samples were positive by RT-nested PCR. | Food (Salad sandwiches) | [94] |
| Vibrio cholerae O1 | 2002 | Andaman and Nicobar Islands | 16 | _ | Diarrhea | Vibrio cholerae O1 El Tor was isolated from 18 of the 67 patients tested. | Effluents from ships or poachers from neighboring countries where cholera is endemic. | [95] | |
| V. parahaemolyticus O3:K6 | 2003 | Kolkata | ∼200 | Outbreak of diarrhea | Blood donation camp. | V. parahaemolyticus was isolated from the stool samples of five patients. | Food (Rice with meat) | [96] | |
| Hepatitis A | 2004 | Pune | 123 | _ | Icterus, dark-colored urine, anorexia, vomiting/nausea, abdominal pain, fever, fatigue and diarrhea. | Large-scale outbreak of hepatitis among children | Serum samples, feces and sewage samples. | Contaminated water | [97] |
| Hepatitis A | 2005 | Kerala | 540 | 2 reported from Medical college area. | Fever, diarrhea, abdominal discomfort, | History of visit to medical college hospital area. | HAV RNA was present in the feces | Non-functional sewage treatment plant and the untreated sewage was constantly overflowing and getting mixed with water canal. | [73] |
| Vibrio cholerae O1 | 2005 | Kolkata | 89 | _ | Acute watery diarrhea | Emergency medical camp and health outposts as part of a routine surveillance programme at the area. | V. cholerae O1 biotype El Tor, serotype Ogawa was isolated as a sole pathogen from 15 (15.8 %) 89 stool samples screened. Water samples showed presence of coliform bacilli with high MPN count. | Leakage in the main pipeline supplying drinking water to the area. | [98] |
| Vibrio cholerae O1 | 2005 | Odisha | 62 cases in Chasapada 51 cases in Tentuliapatna |
_ | Diarrhea | Increase of incidence of watery diarrhea in these 2 villages | Stool samples and rectal swabs. Two stool samples (one from each village) were positive for V. cholerae El Tor O1 Ogawa. | 1 Foodborne due to contamination of the milk products by dirty hands. 2. Waterborne as water well contaminated by sewerage originating from the first village. |
[99] |
| Staphylococcus aureus and Bacillus cereus | 2007 | Maharashtra | 123 | _ | Loose motions, fever, pain abdomen and vomiting | Consumed meals in the mess | Samples of the chicken, environmental swabs from the kitchen, food handlers, Stool and blood showed no bacteriological growth. | Chicken | [100] |
| Salmonella enterica serovar Typhi | 2007 | West Bengal | 103 | _ | Occurrence of fever for ≥ one week | Slum of South Dumdum municipality, West Bengal reported an increase in fever cases. | Salmonella enterica Typhi was isolated from one of four blood specimens and 65 of 103 sera were ≥ 1:80 Widal positive. | The initial foodborne outbreak of typhoid led to the contamination of the water supply resulting in a secondary, waterborne wave | [101] |
| Hepatitis A | 2007 | Himachal Pradesh | 88 | _ | – | An outbreak of viral hepatitis | The hepatitis cases were 63.2 per cent (55/88) were positive for anti-HAV IgM. HAV-RNA present in serum, sewage and water samples showed 100 per cent sequence homology. |
Contaminated water | [102] |
| Staphylococcus aureus | 2007 | Uttar Pradesh | 94 | _ | Nausea, vomiting, abdominal pain, diarrhea, weakness and fever. | Dining in the catering establishment | Coagulase positive staphylococci were cultured from the vomitus and stool samples of cases. Nasal and fingernail swabs collected from food handlers also showed staphylococcal growth. | Food (Spoiled curd) | [103] |
| Hepatitis E | 2008 | Ahmedabad | 233 | _ | Jaundice or elevated serum aminotransferase levels | Clusters of jaundice cases were reported by a civic center, Girdharnagar ward, Ahmedabad. | A total of 16 out of 17 patients investigated were positive for the hepatitis E IgM antibody. | The sewage contamination of drinking water in the distribution system. | [104] |
| Salmonella enterica serotype Weltevreden | 2009 | Karnataka | 34 | _ | Vomiting, diarrhea, abdominal pains and fever | Consumption of a Fish dish from an outside caterer | Stool samples were cultured and identified biochemically similar to Salmonella and serotyping was confirmed as Salmonella Weltevreden | Food (Fish) | [64] |
| Vibrio cholerae O1 | 2009 | West Bengal | 1076 | 14 | Diarrhea | Increased number of diarrhea cases | Vibrio cholerae El Tor Ogawa was isolated from two of five probable case patients' stool specimens. Piped water specimens and stored drinking water were positive for faecal contamination. | Non-chlorinated piped water | [105] |
| Salmonella enterica serovar Weltevreden | 2010 | Maharashtra | 150 | _ | Acute watery diarrhea, fever, abdominal cramps, nausea and vomiting. | Lunch in hostel canteen | A total of nine isolates, provisionally identified as Salmonella spp. | Food from hostel canteen | [62] |
| Vibrio cholerae O1 | 2010–11 | Gujarat | 330 | _ | Loose rice watery diarrhea | – | Nineteen patients were found to be positive for Vibrio cholerae O1 serotype Ogawa. | leakages in the pipelines near riverbanks | [106] |
| E. coli | 2011 | Tamil Nadu | 53 | _ | Loose motions, fever, headache, vomiting and abdominal pain | Eating dinner in a Military establishment | With the given clinical picture and the incubation period the possible cause to be Salmonella spp. | Food (Potato-bitter gourd) | [107] |
| Vibrio cholerae O1 | 2012 | Pondicherry | 921 | 1 | Occurrence of diarrhea of more than three loose stools per day with or without vomiting | Ingestion of water contaminated by drainage following rains during cyclone. | Nine stool samples yielded V. cholerae O1 Ogawa. | Cases were clustered around two major leakages in the water supply system | [108] |
| Salmonella enterica serovar Typhi | 2013 | Chandigarh | 18 | _ | Continuous fever, abdominal pain with or without diarrhea, hepatosplenomegaly and bradycardia and neutropenia. | – | Twenty-seven strains of Salmonella Typhi were isolated from blood cultures. | Not identified | [109] |
| Salmonella enterica serovar Weltevreden and Vibrio fluvialis | 2013 | West Bengal | 278 | _ | Acute watery diarrhea | Food served at a funeral reception | Stool specimens were positive for V. parahaemolyticus. | Mutton-ghogni | [110] |
| Hepatitis A | 2013 | Kerala | 45 | _ | Acute illness with fever or loss of appetite followed by yellowish discoloration of sclera or urine | – | All eight blood samples tested were positive for IgM HAV. | Pipe water contamination | [111] |
| Hepatitis E | 2013 | Uttarakhand | 240 | _ | Jaundice, Dark urine, fever, pain in abdomen, vomiting, and loss of appetite | History of leakages in drinking water pipelines and overflowing drains in the area. | Out of 13 serum samples, 10 were found positive for HEV IgM antibodies and three cases had IgM antibodies for both HAV and HEV. | Sewage contamination of drinking water | [112] |
| Vibrio cholerae O1 | 2013 | Andhra Pradesh | 138 | 1 | Acute diarrhea as the occurrence of ≥ 3 loose stools in a da | Report of a cluster of diarrheal disease among residents of Medipally to the state surveillance unit. | Five rectal swabs were positive for V. cholerae O1 El Tor. Five water samples had the Most Probable Number (MPN) count higher than the permissible level. | Overhead water tank | [113] |
| Hepatitis E | 2013 | Punjab | 159 | 1 | Jaundice, anorexia, nausea, vomiting, abdominal pain, or fever | An increase in jaundice cases | All 14 were positive for anti-HEV IgM and negative for anti-HAV IgM. | Contamination of the municipal drinking water supply with HEV | [114] |
| Bacillus cereus | 2015 | Tamil Nadu | 36 | _ | Diarrhea (≥3 loose stools in 24 h) or vomiting | Political rally | Stool samples showed no growth of enteric pathogens. | Food (Lemon rice) | [115] |
| Hepatitis A | 2016 | Kerala | 53 | _ | Acute onset of illness with any of the symptoms of abdominal pain, vomiting, diarrhea with/without fever | An unusual occurrence of acute gastroenteritis in Ashramam | The water samples had shown growth of Escherichia coli. Stool samples had not shown any growth. | Pipe water contamination | [116] |
| Salmonella Paratyphi A var Durazzo | 2016 | Himachal Pradesh | 43 | _ | Fever, malaise and loss of appetite | Salmonellae were isolated from blood samples of clinical suspects of enteric fever or pyrexia of unknown origin during the next 7 months period. | Not identified | [117] | |
| Hepatitis A | 2016 | Kerala | 223 | _ | An acute illness with fever or loss of appetite followed by yellowish discoloration of sclera or urine | Hotel food | [74] | ||
| Shigella sonnei | 2016 | West Bengal | 25 | _ | Diarrhea/dysentery, abdominal pain, fever, and vomiting | Housewarming party | About 8 stool samples were positive for Shigella sonnei. | Food (Tomato salad) | [118] |
| Hepatitis A | 2016 | Kerala | 562 | 3 | Icterus or dark colored urine along with one or more symptoms such as fever, loss of appetite, fatigue, right upper quadrant tenderness and vomiting | Unusual rise in hepatitis cases | Blood samples from the suspected cases showed anti-HAV IgM positivity in 74.5 % (73/98). The water samples tested.negative for HAV and HEV RNA. | Well water | [75] |
| Vibrio cholerae O1 | 2016 | Maharashtra | 889 | _ | Person experiencing at least one loose stool | Acute diarrheal disease outbreak | Two stool samples tested positive. 16 samples from multiple sources were positive for fecal coliforms. | Contaminated groundwater | [119] |
| Hepatitis A and E | 2016–17 | Chandigarh | 141 | _ | Acute jaundice, dark urine, anorexia, malaise, extreme fatigue, and right upper quadrant tenderness. | Passive surveillance in Burail, Chandigarh | 141 cases were confirmed serologically for hepatitis A and E. | Leakage in drinking water pipeline | [120] |
| Rotavirus B | 2017 | Maharashtra | 258 | _ | Occurrence of ≥3 loose stools in a day | Cases of acute gastroenteritis were reported | All fecal specimens exhibited presence of RVB. The water samples were positive for RVB. | Drinking water | [121] |
| Not identified | 2017 | Andhra Pradesh | 191 | _ | ≥3 loose stools within 24 h | Medical camp in the village | Stool samples showed no growth of enteric pathogens. Four drinking water samples from bore-wells indicated faecal contamination. |
Shallow bore-wells water contamination | [122] |
| Not identified | 2017 | Uttar Pradesh | 70 | 2 infant deaths 2 infant deaths |
three or more loose stools or vomiting within 24 h Three or more loose stools or vomiting within 24 h. |
IDSP District Surveillance | All seven stool culture specimens were negative for V. cholerae | Contaminated well water | [123] |
| Vibrio cholerae O1 | 2018 | Maharashtra | 104 | _ | Loose stool with/out vomiting and fever | Cholera was detected in the stool sample of a 3-year-old child from a slum area | The water sample were found to be negative. Two stool samples were reported to be positive. | Well water | [124] |
| Vibrio cholerae O1 | 2018 | Delhi | 129 | _ | ≥3 loose stools within 24 h | North Delhi district surveillance unit of the IDSP identified a laboratory-confirmed cholera outbreak | 6 stool samples tested positive. | Untreated municipal water | [125] |
| Enterotoxigenic Staphylococcus aureus | 2018 | Maharashtra | 75 | _ | Vomiting or diarrhea (i.e., ≥3 loose stools within 24 h) | Wedding ceremony | No pathogens were isolated from the food samples. | Food (Carrot pudding) | [50] |
| Not identified | 2018 | Punjab | 116 | _ | Case as vomiting or ≥3 loose feces in 24 h plus abdominal pain and/or fever | An acute gastroenteritis outbreak | All three water samples showed coliform contamination. | Food (Mixed vegetables) | [126] |
| Salmonella enterica serotype Typhi | 2018 | Tamil Nadu | 7 | _ | Fever with abdominal pain, diarrhea or vomiting | Eating in the restaurant | About 7 blood culture cases confirmed ceftriaxone-resistant Salmonella Typhi cases. The serum samples of the restaurant workers were negative by Widal testing, and all stool samples showed no growth of Salmonella Typhi on culture. |
Roadside eatery Food (chicken gravy) | [127] |
| Vibrio cholerae O1 | 2018 | Maharashtra | 195 | _ | More than three loose stools occurring within a day | – | V. cholerae O1 Ogawa was isolated from five referred faecal samples. | Well water | [128] |
| Enteropathogenic E.coli/Salmonella spp | 2019 | Maharashtra | 291 | _ | Diarrhea, abdominal cramps, fever with chills, and vomiting. | Religious community lunch | Stool samples showed growth of I. | Food (Green gram) | [129] |
| Vibrio cholerae | 2019 | Haryana | 196 | 2 | Case as a person ≥1 year of age with a history of three or more loose stools within a 24-h period | Death due to acute diarrheal disease | Tested samples of water from tanks, sewage effluent and stool specimens were positive for V. cholerae. | Water from tanks | [130] |
| Salmonella enteritidis | 2019 | Puducherry | 33 | _ | Cases as those who presented with GI symptoms | Restaurant | The 10 stool samples and shawarma sample were positive by culture. | Food (Chicken shawarma) | [61] |
| Vibrio cholerae O1 | 2021 | Gujarat | 37 | _ | Three or more loose, watery stools (with or without vomiting) in the past 24 h | Unusual increase in cases of diarrhea was reported in slum area of Kalol town | Three stool samples were positive for the bacterium. | Leakage in drinking water supply | [131] |
| Vibrio cholerae O1 | 2022 | Chandigarh | 118 | _ | Cases as those who presented with GI symptoms | A large number of cases of diarrhea and a suspected death as reported through a leading local newspaper. | One of the stool sample grew Vibrio cholera O1(Ogawa) and twelve water samples had high coliform counts indicating fecal contamination. | Leakage in drinking water supply | [132] |
Fig. 1.
Geographic distribution of food and waterborne outbreaks from 1980 to 2022 in India.
Many outbreak investigation reports often lack essential information needed for systematic analysis, monitoring, and evaluation of outbreak response processes. The published outbreak reports frequently have incomplete investigation details, both in terms of descriptive and analytic findings, and notably lack data on antimicrobial resistance of the identified pathogens.
The outbreak investigations of bacterial foodborne diseases in India from 1980 to 2009 reported a total of 37 outbreaks involving 3485 persons [47]. The main outbreak-causing pathogens included Staphylococcus aureus, Salmonella, E. coli, Vibrio, and Yersinia enterocolitica. There is a lack of systematic studies to understand the types of foods involved and the etiological agents to create awareness among policymakers [47].
Action strategies for foodborne disease outbreaks applied between 2009 and 2018 have been recently reviewed [10]. Less number of outbreaks were reported in 2008 (n = 553) and 2009 (n = 799), which has increased in subsequent years. This increase in outbreak report is primarily attributed to the improved surveillance and reporting systems, enhanced infrastructure, increased investments, wider participation of states/union territories, the establishment of referral lab networks, and training of skilled manpower by the IDSP [48]. West Bengal has consistently recorded the highest number of outbreaks. The highest mortality rate was reported in Assam (14.5 %), despite having a lower percentage (6.4 %) of outbreaks [10]. Some of the states/union territories, including Arunachal Pradesh, Delhi, and Daman and Diu, have reported only one outbreak in ten years. Underestimation of outbreaks significantly impacts the planning, enhancement of strategies, and measures to control foodborne outbreaks [10].
Ten foodborne disease outbreaks involving 996 individuals have been investigated in Hyderabad [49]. The type of food responsible for these outbreaks includes ‘kaddu ka kheer’ (sweet made with bottle guard and desiccated milk), milkshake, chicken biryani (rice and chicken dishes), fruits, salad, juice, and ‘Gur wale chawal’ (jaggery rice). The etiological agents identified from these outbreaks include S. aureus and Salmonella spp. In Maharashtra, an outbreak was linked to consumption of ‘Gaajar Halwa’. During this outbreak, 42 cases (56 %) were hospitalized in 2018. The clinical presentation and incubation period of the patients aligned with enterotoxin-producing S. aureus [50].
Other studies indicated the incidences of foodborne diseases implicated with consumption of sandwiches, soybean milk, stale rice, chicken, bread, vegetable curry, and buttermilk [[51], [52], [53], [54], [55], [56]]. An outbreak of Salmonella food poisoning among soldiers was reported in the Western Himalayas during 1998, affecting 78 soldiers [56]. Six stool samples and one blood sample tested positive for Salmonella enteritidis during the investigation. Another food poisoning outbreak in a Tamil Nadu village affected 25 of 48 individuals who consumed buttermilk during a feast [55]. In 2021 acute gastroenteritis outbreak was reported in a school associated with the consumption of sweetened curd, affecting 204 students in Uttar Pradesh [57]. An outbreak of food poisoning was reported in Maharashtra in 1995, affecting 33 individuals after consuming vegetarian food contaminated with Salmonella Paratyphi A var Durazzo [58]. In 2023, a cluster of acute gastroenteritis outbreaks was reported following a marriage function in the Wayanad district of Kerala, linked to the consumption of chicken biriyani [59].
In India, poultry is the most common reservoir of Salmonella [60]. Recently, a foodborne outbreak due to non-typhoidal Salmonella was reported among the staff and students in a tertiary care hospital in southern India due to the consumption of chicken shawarma [61]. In 2015, the occurrence of S. Weltevreden associated foodborne outbreak was reported among hostel students in Pune, Maharashtra [62]. Environmental studies have reported the presence of Salmonella spp. from the hands of butchers as well as abattoir equipment from Punjab [11]. Nearly 8 % of eggs and 7 % of egg-storing trays from retail markets of Coimbatore, South India were reported to be contaminated with Salmonella enteritidis [63]. During 2008–2009, two separate food poisoning outbreaks due to Salmonella Weltevreden and Salmonella Wein in chicken and fish, respectively, were reported from Mangalore [64,65].
The prevalence of the parasitic protozoan Toxoplasma gondii has been reported in slaughtered pigs [66] in North India and goats [67] in Jharkhand. A study on dairy cattle and beef from Kolkata showed the presence of STEC O157:H7 serotype. Though this serotype was isolated from meat, no STEC was detected from the diarrheal cases [68]. The low prevalence of STEC-associated diarrhea in India may be due to prevalent cooking practices. Prevalence of E. coli O157:H7 has also been reported in the Ganga River in Varanasi [69].
The burden of viral hepatitis in India is not well described. IDSP has conducted surveillance across all states for epidemic-prone diseases, including foodborne and waterborne viral hepatitis. During 2011–2013, a total of 804,782 hepatitis cases and 291 outbreaks have been recorded. More than 7 % of the cases were positive for HAV, and 10.4 % were positive for HEV. Among the reported outbreaks, 56 % had known etiology. Of these positive cases, 48 % were caused by HEV, 33 % by HAV, 12 % by both HAV and HEV, and 7 % by hepatitis B (HBV) or hepatitis C (HCV). Contaminated drinking water was identified as the common source of most of the outbreaks [70].
The role of HEV infection associated with sporadic cases of acute viral hepatitis (AVH) and fulminant hepatic failure (FVF) has been investigated in northern India [71]. Confirmation of HEV infection was found in 22 AVH and 8 FVF cases. HEV was the sole cause in 44 % of cases with AVH and 43.7 % of cases with FHF. Prevalence of HEV and HAV was high in the northeast region (NER) [72], however, the transmission of HEV and HAV through food has not been thoroughly investigated. Several outbreaks of HAV have been reported in Kerala [[73], [74], [75], [76]]. However, the role of pork, poultry meat and milk in the transmission viral diseases are less investigated.
In Kerala, a cluster of acute jaundice among residents of Ernakulam district has been reported during 2016, involving 385 cases and 3 deaths [76]. This HAV outbreak was linked to the consumption of lime juice prepared by infected food handlers. The role of food handlers in HAV transmission has been reviewed to provide substantial evidence for minimizing transmission [77]. The knowledge and awareness of proper hygiene among food handlers has to be improved to prevent foodborne infections such as HAV [78].
In NER, it is a customary practice to store pork in chimneys for an extended period of time, and the smoked pork may not be well cooked before consumption [79]. Less than 10 % of households have refrigeration. The prolonged storage of meats and meat products, coupled with inconsistent electricity supply in the region, likely contributes to the contamination by enteric pathogens [80]. Moreover, the sequence of transmission from animal-human in the NER has to be investigated in detail, particularly in the areas where farming of pigs and the consumption of pork are very high. The majority of the people living in NER habitually consume fermented food and soybean products, which are frequently implicated in diarrheal outbreaks caused by Bacillus cereus, Proteus mirabilis and Clostridium botulinum [80]. Higher prevalence of S. aureus, C. perfringens, L. monocytogenes, and Yersinia enterocolitica were also detected in fermented soybean, bamboo shoot, fish, milk and pork products [80].
Norovirus gastroenteritis common among hospitalized children under five years of age in NER [81]. Paragonimiasis, a foodborne parasitic lung infection, has been reported in the NER, which spread through contaminated animal food products [[82], [83], [84]]. However, people infected with Paragonimus were erroneously diagnosed with pulmonary tuberculosis and treated incorrectly [83].
3. Result
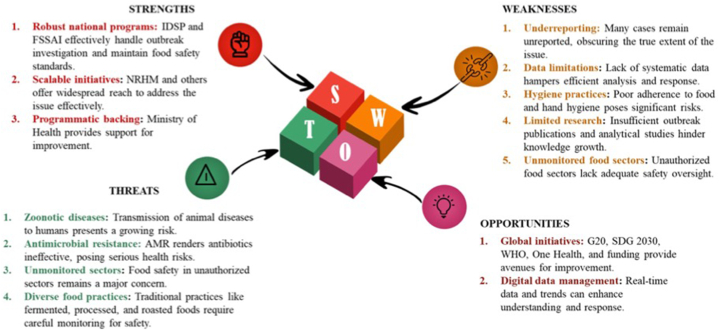
Fig. 2 illustrates the SWOT analysis performed in this study that presents an assessment of the strengths, weaknesses, opportunities, and threats associated with various global and national health programs and prevention strategies for ensuring food safety. In India, IDSP and the FSSAI are the important organisations, which play a crucial role in outbreak investigation and upholding the food safety standards. Additionally, the National Rural Health Mission (NRHM) and other mission-mode programs provide quality health care to the rural population, including vulnerable groups. However, accurate reporting of systematic data, food and hand hygiene practices, epidemiological and analytical outbreak reports, and monitoring of food standards in large unauthorized food sectors are the major aspects that need to be strengthened.
Fig. 2.
Strategic evaluation of current trends, situation, and control measures for foodborne and waterborne diseases in India: A SWOT analysis Unveiling risks and opportunities in outbreak response.
Despite these challenges, numerous opportunities exist for improvement, backed by programmatic support from the Ministry of Health. Global-level support through initiatives like G20 and SDGs 2030 goals, WHO guidance, the One Health initiative, and availability funds might resolve several complexities. The advent of digital data management holds significant promise in understanding data and trends in real-time. However, emerging threats, such as the transmission of zoonotic infections to humans, antimicrobial resistance, and unmonitored food sectors, pose hidden challenges. Additionally, the diverse food practices of various communities, including the consumption of fermented, processed, and roasted foods or mass food supply during festivals raise concerns.
4. Discussion
There is an urgent need to establish a comprehensive, long-term surveillance system in India in response to the escalating risk of foodborne infections and disease outbreaks. Evaluation of risk factors and contributors to contamination, and tracing the transmission of the suspected pathogen is crucial. Recommending measures to prevent future outbreaks and fortifying food safety policies is equally vital. This can be achieved through the acquisition of robust epidemiological findings, facilitating risk assessment of foodborne diseases. The lack of precise information on the full extent and financial burden of foodborne infections is an important barrier to address food safety concerns. The above discussed factors would help policymakers to decide on priorities for public health and utilize resources accordingly [1]. Robust epidemiological data on foodborne illnesses are still lacking, especially in underdeveloped nations.
5. Global Strategy for Food Safety
The WHO launched an initiative to estimate the Global Burden of Foodborne Diseases in 2006. In 2007,WHO Foodborne Disease Burden Epidemiology Reference Group (FERG) to lead the program was established. In 2015, WHO evaluated the ‘Global burden of foodborne diseases" and published a report on ‘Global burden of foodborne diseases’ for the first time [1]. Six task forces were commissioned systematic reviews and other published studies described the burden estimates. In addition, the burden of disease and pilot studies were conducted in four countries (Albania, Japan, Thailand, and Uganda) [1]. However, gaps in these studies are the major hurdle in estimating the foodborne disease burden, as some are country specific.
The global and regional estimates provided by FERG offer an interim solution. However, improved surveillance and laboratory capacity is important for the better management of outbreaks. The highest burden per population was observed in Africa, followed by South-East Asia. Detailed data on the economic costs of foodborne diseases in developing countries are unaccounted. The WHO Department of Food Safety, Zoonoses and Foodborne Diseases (FOS) took the initiative, enabling policy-makers and other stakeholders to set appropriate, evidence-based priorities in food safety [85]. In collaboration with multiple international partners, FOS launched a strategic framework to estimate the global burden of foodborne diseases. This strategy presents three principal goals; (i) to advocate risk-based food safety systems, (ii) to develop science-based measures to prevent exposure to hazards through food, and (iii) to assess and communicate foodborne risks [86].
WHO Global Foodborne Infections Network (GFN) promotes capacity building to detect, control, and prevent foodborne and other enteric infections from farm to table [87]. GFN also conducted several trainings for laboratory-based identification important foodborne pathogens, mostly in developing countries. Food and Agriculture Organization/WHO International Food Safety Authorities Network (INFOSAN) is a global network of national food safety authorities that was established in 2004 as a platform for sharing information about food safety issues and best practices. INFOSAN assists Member States in managing food safety risks, and sharing information during food safety emergencies to prevent the spread of contaminated food among countries [88].
The Foodborne Diseases Active Surveillance Network (FoodNet) is a collaborative mission among CDC, the US Department of Agriculture and the Food and Drug Administration (FDA), and the seven Emerging Infections Program (EIP) sites [89]. The EIP collects data from 10 US States relate to the spread of enteric pathogens through food. Available information in the CDC FoodNet showed Campylobacter (88 %) was the most frequently identified pathogen, followed by Salmonella, Shigella, E. coli O157 and Listeria [89]. A similar active surveillance network is needed in each country to have a true burden of foodborne pathogens so that appropriate intervention can be implemented at an early phase.
6. Food safety efforts in India
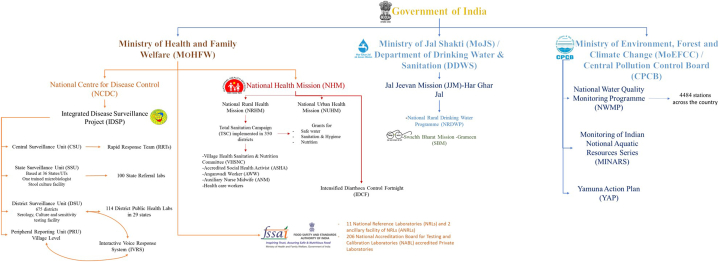
Government of India has three Ministries related to food and waterborne disease control programmes and missions; Ministry of health and family welfare (MoHFW), Ministry of drinking water and sanitation and Central pollution control board. IDSP, FSSAI and NRHP are functioning under the MoHFW. Fig. 3 illustrates the organogram of the structural framework of the disease control programs implemented by the Government of India.
Fig. 3.
Organogram of the structural framework of the disease control programs implemented by the Government of India.
FSSAI was established in 2006 under the MoHFW, is responsible for protecting and promoting public health by regulating and supervising food safety [90]. FSSAI functions as (i) to maintain science-based standards for different food items, (ii) regulation of manufacture, storage, distribution, import, and sale of food and (iii) expedites the safety of food in the country. Twenty referral food laboratories, in 72 State/UT food testing laboratories located throughout India and 206 National Accreditation Board for Testing and Calibration Laboratories (NABL) -accredited private laboratories are notified by the FSSAI. Apart from the policy decision for food safety, FSSAI also grants food safety licenses and certifications for food businesses. However, small manufacturers, retailers, and hawkers are not controlled by the FSSAI regulations. These segments might greatly contribute to the unorganised food supply and compromise the quality. Due to low sales price, population prefers and procure foods from these segments. There are only 377 products listed in the FSSAI regulations, whereas other countries have over 10,000 standards. FSSAI is not involved in any survey activities for the enforcement of the Act and does not have any database on food businesses.
IDSP under the National Centre for Disease Control (NCDC), MoHFW, was launched in 2004 reports only a foodborne illness outbreak network [91]. Weekly surveillance data on 18 epidemic-prone diseases, including viral hepatitis, are being collected through this program. The block and district rapid response teams initiate a coordinated activity that includes sample collection, processing, and pathogen identification on the laboratory side; outbreak confirmation, field investigation, and identification of the epidemiologically-linked source of the outbreaks. IDSP portal also receives, disseminates and monitors continuous alerts about outbreaks of diarrheal diseases, including cholera and foodborne gastroenteritis outbreaks from different parts of the country. However, no systematic surveillance programme for foodborne illness is available in India.
Few mission mode national programs like the National Rural Drinking Water Programme (NRDWP), Total Sanitation Campaign, Swachh Bharat Mission, program etc., are part of several public health programs. Besides, the Indian Council of Medical Research (ICMR) assists the states in outbreak investigations and strategies for timely diagnosis and prevention of several diseases. National Water Quality Sub-Mission (NWQSM) under Ministry of Jal Shakti ensures the safety of drinking water. Water quality monitoring involves testing of water samples through IDSP laboratories (coliform count, test for arsenic and heavy metals) [https://jalshakti-ddws.gov.in/sites/default/files/JJM_Operational_Guidelines.pdf (Assessed on December 21, 2023)].
The NRHM, an umbrella programme launched by the MoHFW has a component in ensuring sanitation and hygiene i.e., “Total Sanitation Campaign”. This mission has been implemented in 350 districts of the country and the coverage will be completed in next 2 years. [https://nhm.gov.in/WriteReadData/l892s/nrhm-framework-latest.pdf. (Assessed on December 21, 2023)]. In 2014, the Swachh Bharat Abhiyan, program was commenced, to create awareness about sanitation and its linkage with public health. The other goal of this program is to eradicate the practice of open defecation in India, which causes major water contamination [https://www.pmindia.gov.in/en/major_initiatives/swachh-bharat-abhiyan/(Assessed on December 21, 2023)].
MoHFW & State Governments also carry out regular information, education & communication activities through various channels regarding prevention and control of diarrhea through the National Health Portal of India [https://nhm.gov.in/(Assessed on July 19, 2024)]. Intensified Diarrhea Control Fortnight/Defeat Diarrhea (D2) initiative was implemented during pre-monsoon/monsoon season with the aim of ‘zero child deaths due to childhood diarrhea’ since 2014. NHM promotes maintaining hygiene and cleanliness especially proper hand washing, household and environmental sanitation, proper disposal of wastes, safe and adequate drinking water, food safety, ORS and Zinc use for initial prevention of acute diarrhea, and seek timely care if someone falls sick. Such messages are also disseminated through the National Health Portal of India.
The National One Health Mission in India is an innovative endeavour recently undertaken by the Government of India to undertake health issues through a holistic and unified strategy. Its primary objective is to foster synergistic endeavours among different sectors, and to enhance the efficiency and adaptability of the healthcare system. The focus of the mission is to develop strong teams for medical, veterinarians, and other pertinent stakeholders through different training programs, including zoonotic disease management, environmental health and the One Health approaches so that emerging infections can be controlled effectively.
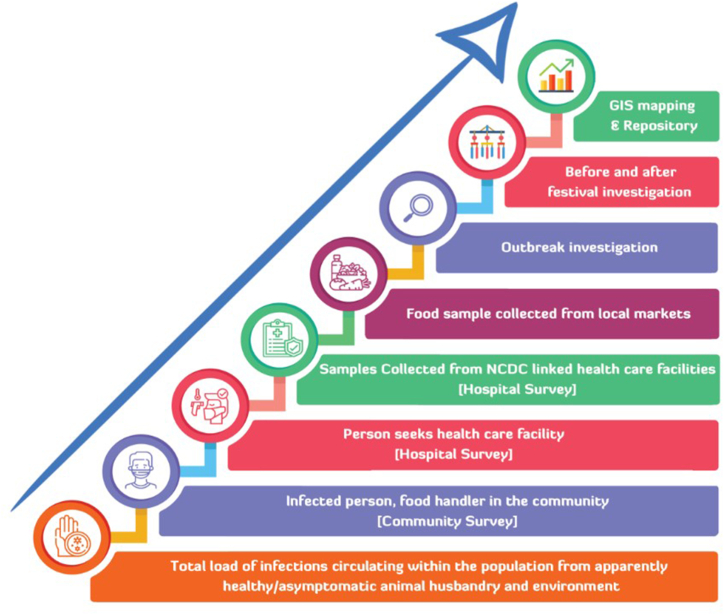
While it is challenging to fully comprehend the overall spread of infections within the livestock population and the environment, it is crucial to systematically collect data on foodborne infections. This entails implementing comprehensive surveillance across humans, animals, food, water, and the environment through hospital, animal husbandry, market, and community surveillance, along with outbreak investigations. This approach will help in the timely identification of pathogens originating from food, animals, and the environment. Assessing the burden of disease, including outbreaks, illnesses, hospitalizations, deaths, and annual incidence rates has to be focused as major components of the surveillance program. Fig. 4 illustrates the work plan for the survey, outlining the steps taken to systematically generate data on foodborne pathogens and enhance food safety practices in India.
Fig. 4.
Work plan for the Survey to capture the burden of illness.
7. Conclusion
Although different initiatives have been taken, the incidence of outbreaks as well as emerging pathogens are on the rise due to environmental changes, use of bio-fertilizers, trans boundary trades of food animals, antimicrobial resistance etc. Hence, a national surveillance system is a pressing need now, as this would provide strong data support to make appropriate policy changes to reduce foodborne diseases. The burden of zoonotic and other foodborne illnesses is significantly huge, yet remains as a neglected public health problem. It is not feasible to completely eliminate pathogenic organisms from animals and raw products. However, the occurrence of zoonosis can be minimized by applying high standards of hygiene in all the steps of the food production chain. Laboratory-based surveillance strategies can enhance the public health food safety infrastructure. For this, a higher degree of integration between medical and veterinary surveillance is needed. Integrated laboratory surveillance of foodborne pathogens would provide policymakers and other stakeholders with scientific information and recommendations in minimizing the risks of foodborne infections. Finally, implementing basic and applied research on the causative agents of foodborne infections is crucial for their prevention and control. Awareness programs, hand hygiene, and proper sanitation may complement future surveillance programs.
Data availability statement
The data that supports the findings of this study is publicly available research data as cited in the reference list.
Funding
Indian Council of Medical Research, India.
Funding Statement
This work was support by the Indian Council of Medical Research, New Delhi, India under the project “Surveillance of Foodborne Pathogens (FBP) from North-East India” Grant No. 5/8-1(3)/2019-ECD-II.
Disclosure statement
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is an original work and is not under review at any other publication.
CRediT authorship contribution statement
Venencia Albert: Writing – original draft, Data curation. Thandavarayan Ramamurthy: Writing – review & editing, Conceptualization. Samaresh Das: Writing – review & editing. Karma G Dolma: Writing – review & editing. Tapan Majumdar: Writing – review & editing. Pranjal Jyoti Baruah: Writing – review & editing. Suranjana Chaliha Hazarika: Writing – review & editing. Basumoti Apum: Writing – review & editing. Madhuchhanda Das: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
List of Standard Abbreviations
- IDSP
Integrated Disease Surveillance Programme
- SWOT
Strengths, Weaknesses, Opportunities, and Threats
- FSSAI
Food Safety and Standards Authority of India
- WHO
World Health Organization
- CDC
Centers for Disease Control and Prevention
- SDGs
Sustainable Development Goals
- AMR
Antimicrobial Resistance
- NHM
National Health Mission
- HEV
Hepatitis E Virus
- HAV
Hepatitis A Virus
- STEC
Shiga Toxin-Producing Escherichia coli
- NORS
National Outbreak Reporting System
- HUS
Hemolytic Uremic Syndrome
- HBV
Hepatitis B Virus
- HCV
Hepatitis C Virus
- AVH
Acute Viral Hepatitis
- FVF
Fulminant Hepatic Failure
- NER
Northeast Region
- FERG
Foodborne Disease Burden Epidemiology Reference Group
- FOS
Foodborne Diseases
- GFN
Global Foodborne Infections Network
- INFOSAN
International Food Safety Authorities Network
- FoodNet
Foodborne Diseases Active Surveillance Network
- FDA
Food and Drug Administration
- EIP
Emerging Infections Program
- MoHFW
Ministry of Health and Family Welfare
- NCDC
National Centre for Disease Control
- NRDWP
National Rural Drinking Water Programme
- ICMR
Indian Council of Medical Research
- NWQSM
National Water Quality Sub-Mission
Contributor Information
Venencia Albert, Email: albertvenencia@gmail.com.
Thandavarayan Ramamurthy, Email: rama1murthy@yahoo.com.
Samaresh Das, Email: samaresh.cdac@gmail.com.
Karma G Dolma, Email: kgdolma@outlook.com.
Tapan Majumdar, Email: drtapan1960@gmail.com.
Pranjal Jyoti Baruah, Email: pjbaruah.rmrcne@gov.in.
Suranjana Chaliha Hazarika, Email: suranjanachalihahazarika@yahoo.com.
Basumoti Apum, Email: apumbasu@gmail.com.
Madhuchhanda Das, Email: drmadhuaiims@gmail.com, dasm.hq@icmr.gov.in.
References
- 1.World Health Organization . World Health Organization; Geneva: 2015. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007-2015.https://iris.who.int/handle/10665/199350 [Google Scholar]
- 2.D. Dewey-Mattia, H. Kisselburgh, K. Manikonda, R. Silver, S. Subramhanya, P. Sundararaman, H. Whitham, S. Crowe, Surveillance for Foodborne Disease Outbreaks, United States, 2017 Annual Report.
- 3.Dattani S., Spooner F., Ritchie H., Roser M. Causes of death, our world in data. 2023. https://ourworldindata.org/causes-of-death
- 4.Glavin M.O. A single microbial sea: food safety as a global concern. SAIS Rev. 2003;23:203–220. doi: 10.1353/sais.2003.0012. [DOI] [Google Scholar]
- 5.Mead P.S., Slutsker L., Dietz V., McCaig L.F., Bresee J.S., Shapiro C., Griffin P.M., Tauxe R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H., Yoon Y. Etiological agents implicated in foodborne illness world wide. Food Sci Anim Resour. 2021;41:1–7. doi: 10.5851/kosfa.2020.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization [WHO] Framework for action on food safety in the WHO South-East asia region. https://www.who.int/docs/default-source/searo/indonesia/framework-for-action-on-food-safety.pdf?sfvrsn=912dce4b_2 New Delhi.
- 8.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health & Family Welfare Government of India Integrated disease surveillance programme. 2017. https://www.idsp.nic.in/WriteReadData/l892s/36105768501527248161.pdf
- 10.Bisht A., Kamble M.P., Choudhary P., Chaturvedi K., Kohli G., Juneja V.K., Sehgal S., Taneja N.K. A surveillance of food borne disease outbreaks in India: 2009–2018. Food Control. 2021;121 doi: 10.1016/j.foodcont.2020.107630. [DOI] [Google Scholar]
- 11.Foodborne disease. CD alert. https://ncdc.mohfw.gov.in/WriteReadData/linkimages/Dec_091047732317.pdf
- 12.Elbehiry A., Abalkhail A., Marzouk E., Elmanssury A.E., Almuzaini A.M., Alfheeaid H., Alshahrani M.T., Huraysh N., Ibrahem M., Alzaben F., Alanazi F., Alzaben M., Anagreyyah S.A., Bayameen A.M., Draz A., Abu-Okail A. An overview of the public health challenges in diagnosing and controlling human foodborne pathogens. Vaccines. 2023;11:725. doi: 10.3390/vaccines11040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe N.D., Daszak P., Kilpatrick A.M., Burke D.S. Bushmeat hunting, deforestation, and prediction of zoonotic disease. Emerg. Infect. Dis. 2005;11(12):1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coker R., Rushton J., Mounier-Jack S., Karimuribo E., Lutumba P., Kambarage D., Pfeiffer D.U., Stärk K., Rweyemamu M. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect. Dis. 2011;11:326–331. doi: 10.1016/S1473-3099(10)70312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard M.P., Steele D., Chaignat C.-L., Kieny M.P. A review of vaccine research and development: human enteric infections. Vaccine. 2006;24:2732–2750. doi: 10.1016/j.vaccine.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Tauxe R.V. Emerging foodborne pathogens. Int. J. Food Microbiol. 2002;78:31–41. doi: 10.1016/s0168-1605(02)00232-5. [DOI] [PubMed] [Google Scholar]
- 17.Scallan E., Griffin P.M., Angulo F.J., Tauxe R., Hoekstra R.M. Foodborne illness acquired in the United States-unspecified agents. Emerg. Infect. Dis. 2011;17:16–22. doi: 10.3201/eid1701.P21101. https://10.3201/eid1701.091101p2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO-food safety fact sheet. Pdf. https://foodhygiene2010.files.wordpress.com/2010/06/who-food_safety_fact-sheet.pdf
- 19.Aggarwal R., Jameel S. Hepatitis E. Hepatology. 2011;54:2218–2226. doi: 10.1002/hep.24674. [DOI] [PubMed] [Google Scholar]
- 20.Izopet J., Tremeaux P., Marion O., Migueres M., Capelli N., Chapuy-Regaud S., Mansuy J.-M., Abravanel F., Kamar N., Lhomme S. Hepatitis E virus infections in Europe. J. Clin. Virol. 2019;120:20–26. doi: 10.1016/j.jcv.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Colson P., Borentain P., Queyriaux B., Kaba M., Moal V., Gallian P., Heyries L., Raoult D., Gerolami R. Pig liver sausage as a source of Hepatitis E virus transmission to humans. J. Infect. Dis. 2010;202:825–834. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- 22.Lewis H.C., Wichmann O., Duizer E. Transmission routes and risk factors for autochthonous hepatitis E virus infection in Europe: a systematic review. Epidemiol. Infect. 2010;138:145–166. doi: 10.1017/S0950268809990847. [DOI] [PubMed] [Google Scholar]
- 23.Wichmann O., Schimanski S., Koch J., Kohler M., Rothe C., Plentz A., Jilg W., Stark K. Phylogenetic and case-control study on Hepatitis E virus infection in Germany. J. Infect. Dis. 2008;198:1732–1741. doi: 10.1086/593211. [DOI] [PubMed] [Google Scholar]
- 24.Berto A., Backer J.A., Mesquita J.R., Nascimento M.S., Banks M., Martelli F., Ostanello F., Angeloni G., Di Bartolo I., Ruggeri F.M., Vasickova P., Diez-Valcarce M., Hernandez M., Rodriguez-Lazaro D., van der Poel W.H. Prevalence and transmission of Hepatitis E virus in domestic swine populations in different European countries. BMC Res. Notes. 2012;5:190. doi: 10.1186/1756-0500-5-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breum S.Ø., Hjulsager C.K., de Deus N., Segalés J., Larsen L.E. Hepatitis E virus is highly prevalent in the Danish pig population. Vet. Microbiol. 2010;146:144–149. doi: 10.1016/j.vetmic.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Jiménez de Oya N., de Blas I., Blázquez A.-B., Martín-Acebes M.A., Halaihel N., Gironés O., Saiz J.-C., Escribano-Romero E. Widespread distribution of Hepatitis E virus in Spanish pig herds. BMC Res. Notes. 2011;4:412. doi: 10.1186/1756-0500-4-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose N., Lunazzi A., Dorenlor V., Merbah T., Eono F., Eloit M., Madec F., Pavio N. High prevalence of Hepatitis E virus in French domestic pigs. Comparative Immunology, Microbiol Infect Dis. 2011;34:419–427. doi: 10.1016/j.cimid.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Rutjes S.A., Bouwknegt M., Van Der Giessen J.W., De Roda Husman A.M., Reusken C.B.E.M. Seroprevalence of Hepatitis E virus in pigs from different farming systems in The Netherlands. J Food Prot. 2014;77:640–642. doi: 10.4315/0362-028X.JFP-13-302. [DOI] [PubMed] [Google Scholar]
- 29.Walachowski S., Dorenlor V., Lefevre J., Lunazzi A., Eono F., Merbah T., Eveno E., Pavio N., Rose N. Risk factors associated with the presence of Hepatitis E virus in livers and seroprevalence in slaughter-age pigs: a retrospective study of 90 swine farms in France. Epidemiol. Infect. 2014;142:1934–1944. doi: 10.1017/S0950268813003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leblanc D., Poitras E., Gagné M.-J., Ward P., Houde A. Hepatitis E virus load in swine organs and tissues at slaughterhouse determined by real-time RT-PCR. Int. J. Food Microbiol. 2010;139:206–209. doi: 10.1016/j.ijfoodmicro.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Di Bartolo I., Ponterio E., Castellini L., Ostanello F., Ruggeri F.M. Viral and antibody HEV prevalence in swine at slaughterhouse in Italy. Vet. Microbiol. 2011;149:330–338. doi: 10.1016/j.vetmic.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Steele M.K., Wikswo M.E., Hall A.J., Koelle K., Handel A., Levy K., Waller L.A., Lopman B.A. Characterizing Norovirus transmission from outbreak data, United States. Emerg. Infect. Dis. 2020;26(8):1818–1825. doi: 10.3201/eid2608.191537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland D., Thomson L., Mahmoudzadeh N., Khaled A. Estimating deaths from foodborne disease in the UK for 11 key pathogens. BMJ Open Gastroenterol. 2020;7 doi: 10.1136/bmjgast-2020-000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hennechart-Collette C., Martin-Latil S., Fraisse A., Niveau F., Perelle S. Virological analyses in collective catering outbreaks in France between 2012 and 2017. Food Microbiol. 2020;91 doi: 10.1016/j.fm.2020.103546. [DOI] [PubMed] [Google Scholar]
- 35.Motoya T., Umezawa M., Saito A., Goto K., Doi I., Fukaya S., Nagata N., Ikeda Y., Okayama K., Aso J., Matsushima Y., Ishioka T., Ryo A., Sasaki N., Katayama K., Kimura H. Variation of human Norovirus GII genotypes detected in Ibaraki, Japan, during 2012–2018. Gut Pathog. 2019;11:26. doi: 10.1186/s13099-019-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majowicz S.E., Scallan E., Jones-Bitton A., Sargeant J.M., Stapleton J., Angulo F.J., Yeung D.H., Kirk M.D. Global incidence of human Shiga toxin–producing Escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodb. Pathog. Dis. 2014;11:447–455. doi: 10.1089/fpd.2013.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stager C. Notes from the field: multistate outbreak of Escherichia coli O157:H7 infections linked to a national fast-food chain-United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023;72 doi: 10.15585/mmwr.mm7226a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F., Lee S.A., Xue J., Riordan S.M., Zhang L. Global epidemiology of campylobacteriosis and the impact of COVID-19. Front. Cell. Infect. Microbiol. 2022 Nov 28;12 doi: 10.3389/fcimb.2022.979055. https://doi:10.3389/fcimb.2022.979055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshikura H. Declining Vibrio parahaemolyticus and Salmonella, Increasing Campylobacter and persisting Norovirus food poisonings: inference derived from food poisoning statistics of Japan. Jap J Infect Dis. 2020;73:102–110. doi: 10.7883/yoken.JJID.2019.247. [DOI] [PubMed] [Google Scholar]
- 40.Vetchapitak T., Misawa N. Current status of Campylobacter food poisoning in Japan. Food Saf. 2019;7:61–73. doi: 10.14252/foodsafetyfscj.D-19-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC . Centers for Disease Control and Prevention; 2023. Foodborne Illnesses and Germs.https://www.cdc.gov/foodsafety/foodborne-germs.html [Google Scholar]
- 42.Allerberger F., Wagner M. Listeriosis: a resurgent foodborne infection. Clin. Microbiol. Infect. 2010;16:16–23. doi: 10.1111/j.1469-0691.2009.03109.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaptchouang Tchatchouang C.-D., Fri J., De Santi M., Brandi G., Schiavano G.F., Amagliani G., Ateba C.N. Listeriosis outbreak in South Africa: a comparative analysis with previously reported cases worldwide. Microorganisms. 2020;8:135. doi: 10.3390/microorganisms8010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-Martínez N.F., Ruiz-Montero R., Briones E., Baños E., Rodríguez-Alarcón L.G.S.M., Chaves J.A., Abad R., Varela C. Listeriosis outbreak caused by contaminated stuffed pork, Andalusia, Spain, July to October 2019. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.43.2200279. on behalf of the L. Team, N. Lorusso. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhalla T.C., Monika Sheetal, Savitri . In: Food Safety and Human Health. Singh R.L., Mondal S., editors. Academic Press; 2019. Chapter 12 - international laws and food-borne illness; pp. 319–371. [DOI] [Google Scholar]
- 46.Tauxe R.V., Doyle M.P., Kuchenmüller T., Schlundt J., Stein C.E. Evolving public health approaches to the global challenge of foodborne infections. Int. J. Food Microbiol. 2010;139(Suppl 1):S16–S28. doi: 10.1016/j.ijfoodmicro.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Rao Vemula S., Naveen Kumar R., Polasa K. Foodborne diseases in India – a review. British Food J. 2012;114:661–680. doi: 10.1108/00070701211229954. [DOI] [Google Scholar]
- 48.IDSP achievements: integrated disease surveillance programme (IDSP) https://idsp.mohfw.gov.in/index1.php?lang=1&level=1&sublinkid=5769&lid=3701
- 49.Sudershan R.V., Naveen Kumar R., Kashinath L., Bhaskar V., Polasa K. Foodborne infections and intoxications in Hyderabad India. Epidemiol Res Int. 2014;2014 doi: 10.1155/2014/942961. [DOI] [Google Scholar]
- 50.Vardhan V., Dikid T., Yadav R., Patil R., Awate P. Epidemic intelligence service programme working group, foodborne disease outbreak associated with eating gaajar halwa at a wedding – palghar district, Maharashtra, India. 2018. Indian J. Publ. Health. 2021;65:10. doi: 10.4103/ijph.IJPH_1099_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lalitha M.K., Walter N.M., Jesudason M., Mathan V.I. An outbreak of gastroenteritis due to Vibrio parahaemolyticus in Vellore. Indian J. Med. Res. 1983;78:611–615. [PubMed] [Google Scholar]
- 52.Kulshrestha S.B., Kapoor K.N., Malik S.V., Khanna P.N. Enterotoxigenic Escherichia coli from an outbreak with cholerigenic syndromes of gastroenteritis. J Commun Dis. 1989;21:313–317. [PubMed] [Google Scholar]
- 53.Choudhary S.P., Narayan K.G., Saxena S.N., Mago M.L., John P.C. Isolation of Salmonella Bornum (6, 7, 14: Z38) for the first time in India. Indian J. Med. Sci. 1985;39:45–46. [PubMed] [Google Scholar]
- 54.Thekdi R.J., Lakhani A.G., Rale V.B., Panse M.V. An outbreak of food poisoning suspected to be caused by Vibrio fluvialis. J. Diarrhoeal Dis. Res. 1990;8:163–165. [PubMed] [Google Scholar]
- 55.Abraham M., Pai M., Kang G., Asokan G.V., Magesh S.R., Bhattacharji S., Ramakrishna B.S. An outbreak of food poisoning in Tamil Nadu associated with Yersinia enterocolitica. Indian J. Med. Res. 1997;106:465–468. [PubMed] [Google Scholar]
- 56.Singh M., Kalghatgi A.T., Narayanan K., Rao K.S., Nagendra A. Outbreak of Salmonella food poisoning at high altitude. Med. J. Armed Forces India. 1998;54:96–98. doi: 10.1016/S0377-1237(17)30490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahu R., Ray A.L., Yadav A.K., Kunte R., Faujdar D.S., Working Group* Acute gastroenteritis outbreak in a school associated with religious ceremony in Mirzapur District, Uttar Pradesh, India. Indian J. Publ. Health. 2021;65:S18–S22. doi: 10.4103/ijph.IJPH_1045_20. [DOI] [PubMed] [Google Scholar]
- 58.Fule R.P., Ingole K.V., Jalgaonkar S.V., Moon B.U. Outbreak of food poisoning due to Salmonella Paratyphi A var Durazzo (2,12:a:-) in yavatmal (Maharashtra) in may 1995. Indian J. Med. Res. 1996;103:74–76. [PubMed] [Google Scholar]
- 59.Vaman R.S., Mathew S.S. An outbreak investigation of a cluster of gastroenteritis following a marriage function in Wayanad district, Kerala, India. Indian J. Clin. Med. 2023;13:34–38. doi: 10.1177/26339447221143212. [DOI] [Google Scholar]
- 60.Murugkar H.V., Rahman H., Kumar A., Bhattacharyya D. Isolation, phage typing and antibiogram of Salmonella from man and animals in northeastern India. Indian J. Med. Res. 2005;122:237–242. [PubMed] [Google Scholar]
- 61.Deepanjali S., Jharna M., Chanaveerappa B., Sarumathi D., Gopichand P., Anupriya K. An outbreak of Salmonella Enteritidis food poisoning following consumption of chicken shawarma: a brief epidemiological investigation. F1000Res. 2022;10:851. doi: 10.12688/f1000research.54410.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jain P., Nandy S., Bharadwaj R., Niyogi S.K., Dutta S. Salmonella enterica serovar Weltevreden ST1500 associated foodborne outbreak in Pune, India. Indian J. Med. Res. 2015;141:239–241. doi: 10.4103/0971-5916.155595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suresh T., Hatha A.A.M., Sreenivasan D., Sangeetha N., Lashmanaperumalsamy P. Prevalence and antimicrobial resistance of Salmonella Enteritidis and other salmonellas in the eggs and egg-storing trays from retails markets of Coimbatore, South India. Food Microbiol. 2006;23:294–299. doi: 10.1016/j.fm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Antony B., Dias M., Shetty A.K., Rekha B. Food poisoning due to Salmonella enterica serotype Weltevreden in Mangalore. Indian J. Med. Microbiol. 2009;27:257–258. doi: 10.4103/0255-0857.53211. [DOI] [PubMed] [Google Scholar]
- 65.Antony B., Scaria B., Dias M., Pinto H. Salmonella Wien from gastroenteritis cases encountered in Mangalore, India: a report of 10 cases and review of the literature. Indian J. Med. Sci. 2009;63:195–197. [PubMed] [Google Scholar]
- 66.Thakur R., Sharma R., Aulakh R.S., Gill J.P.S., Singh B.B. Prevalence, molecular detection and risk factors investigation for the occurrence of Toxoplasma gondii in slaughter pigs in North India. BMC Vet. Res. 2019;15:431. doi: 10.1186/s12917-019-2178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bachan M., Deb A.R., Maharana B.R., Sudhakar N.R., Sudan V., Saravanan B.C., Tewari A.K. High seroprevalence of Toxoplasma gondii in goats in Jharkhand state of India. Vet Parasitol Reg Stud Reports. 2018;12:61–68. doi: 10.1016/j.vprsr.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Dutta S., Deb A., Chattopadhyay U.K., Tsukamoto T. Isolation of Shiga toxin-producing Escherichia coli including O157:H7 strains from dairy cattle and beef samples marketed in Calcutta, India. J. Med. Microbiol. 2000;49:765–767. doi: 10.1099/0022-1317-49-8-765. [DOI] [PubMed] [Google Scholar]
- 69.Hamner S., Broadaway S.C., Mishra V.B., Tripathi A., Mishra R.K., Pulcini E., Pyle B.H., Ford T.E. Isolation of potentially pathogenic Escherichia coli O157:H7 from the ganges river. Appl. Environ. Microbiol. 2007;73:2369–2372. doi: 10.1128/AEM.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar T., Shrivastava A., Kumar A., Laserson K.F., Narain J.P., Venkatesh S., Chauhan L.S., Averhoff F. Viral hepatitis surveillance--India, 2011-2013. MMWR Morb. Mortal. Wkly. Rep. 2015;64(28):758–762. doi: 10.15585/mmwr.mm6428a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madan K., Gopalkrishna V., Kar P., Sharma J.K., Das U.P., Das B.C. Detection of Hepatitis C and E virus genomes in sera of patients with acute viral hepatitis and fulminant hepatitis by their simultaneous amplification in PCR. J. Gastroenterol. Hepatol. 1998;13:125–130. doi: 10.1111/j.1440-1746.1998.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 72.Kumar S., Ratho R.K., Chawla Y.K., Chakraborti A. The incidence of sporadic viral hepatitis in North India: a preliminary study. Hepatobiliary Pancreat. Dis. Int. 2007;6:596–599. [PubMed] [Google Scholar]
- 73.Arankalle V.A., Sarada Devi K.L., Lole K.S., Shenoy K.T., Verma V., Haneephabi M. Molecular characterization of Hepatitis A virus from a large outbreak from Kerala, India. Indian J. Med. Res. 2006;123:760–769. [PubMed] [Google Scholar]
- 74.Rakesh P.S., Mainu T.T.C.R., Raj A., Babu D., Rajiv M., Mohandas K.S., Das A., Balasubramanian A. Investigating a community wide outbreak of Hepatitis A in Kerala, India. J. Fam. Med. Prim. Care. 2018;7:1537. doi: 10.4103/jfmpc.jfmpc_127_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurav Y.K., Babu G.R., Vinu K.P., Lole K.S. Suspected spread of hepatitis A virus from a restaurant among adults in rural area of the Kerala state, India. Epidemiol. Infect. 2019;147:e210. doi: 10.1017/S0950268819000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurup K.K., Manickam P., Gurav Y. Infected food handlers led to an outbreak of Hepatitis A in Ernakulam district, Kerala, Southern India, 2016. Clin Epidemiol Glob Health. 2020;8:308–312. doi: 10.1016/j.cegh.2019.08.001. [DOI] [Google Scholar]
- 77.Shenoy B., Andani A., Kolhapure S., Agrawal A., Mazumdar J. Endemicity change of Hepatitis A infection necessitates vaccination in food handlers: an Indian perspective. Hum. Vaccines Immunother. 2022;18 doi: 10.1080/21645515.2020.1868820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattacharya S., Talati S., Gupta A.K., Malhotra S., Singh A. Implementing a skill development program among food handlers in tertiary care hospital to improve their personal hygiene: a pilot study. J. Educ. Health Promot. 2019;8:129. doi: 10.4103/jehp.jehp_452_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fahrion A.S., Jamir L., Richa K., Begum S., Rutsa V., Ao S., Padmakumar V.P., Deka R.P., Grace D. Food-safety hazards in the pork chain in Nagaland, North East India: implications for human health. Int J Environ Res Public Health. 2014;11:403–417. doi: 10.3390/ijerph110100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keisam S., Tuikhar N., Ahmed G., Jeyaram K. Toxigenic and pathogenic potential of enteric bacterial pathogens prevalent in the traditional fermented foods marketed in the Northeast region of India. Int. J. Food Microbiol. 2019;296:21–30. doi: 10.1016/j.ijfoodmicro.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Borkakoty B., Bali N.K., Jakaria A., Hazarika R., Temsu T., Gohain M., Kaur H. Norovirus gastroenteritis in children under-five years hospitalized for diarrhea in two cities of northeast India: a retrospective study. Indian J. Med. Microbiol. 2023;45 doi: 10.1016/j.ijmmb.2023.100397. [DOI] [PubMed] [Google Scholar]
- 82.Singh T.S., Sugiyama H., Rangsiruji A. Paragonimus & paragonimiasis in India. Indian J. Med. Res. 2012;136:192–204. [PMC free article] [PubMed] [Google Scholar]
- 83.Das M., Doleckova K., Shenoy R., Mahanta J., Narain K., Devi K.R., Konyak T., Mansoor H., Isaakidis P. Paragonimiasis in tuberculosis patients in Nagaland, India. Glob. Health Action. 2016;9 doi: 10.3402/gha.v9.32387. 10.3402/gha.v9.32387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roy J.S., Das P.P., Borah A.K., Das J.K. Paragonimiasis in a child from Assam, India. J. Clin. Diagn. Res. 2016;10:DD06–DD07. doi: 10.7860/JCDR/2016/18160.7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.World Health Organization, The global burden of foodborne diseases : taking stock and charting the way forward : WHO Consultation to Develop a Strategy to Estimate the Global Burden of Foodborne Diseases. https://iris.who.int/bitstream/handle/10665/43635/9789241595292_eng.pdf?sequence=1 (Assessed on December 21, 2023).
- 86.FAO/WHO Food safety risk analysis - a guide for national food safety authorities. https://www.fao.org/sustainable-food-value-chains/library/details/ar/c/265860/ [PubMed]
- 87.Centers for Disease Control and Prevention (CDC) 2011. Global Foodborne Infections Network.https://www.cdc.gov/ncezid/dfwed/pdfs/gfn.pdf [Google Scholar]
- 88.World Health Organization FAO/WHO international food safety authorities network (INFOSAN) https://www.who.int/groups/fao-who-international-food-safety-authorities-network-infosan/about
- 89.Centers for Disease Control and Prevention (CDC) Foodborne diseases active surveillance network (FoodNet) 2023. https://www.cdc.gov/foodnet/index.html
- 90.Food Safety and Standards Authority of India (FSSAI). SoFTeL Strengthening of Food Testing Laboratories. https://fssai.gov.in/cms/strengthening-of-food-testing-system.php (accessed December 20, 2023).
- 91.Suresh K. Integrated diseases surveillance project (IDSP) through a consultant's lens. Indian J. Publ. Health. 2008;52:136–143. [PubMed] [Google Scholar]
- 92.Gupta V., Kaur U., Singh G., Prakash C., Sharma M., Aggarwal K.C. An outbreak of typhoid fever in Chandigarh, North India. Trop. Geogr. Med. 1986;38:51–54. [PubMed] [Google Scholar]
- 93.Thakur J.S., Swami H.M., Dutt R., Mehta M., Gupta V. Epidemiological investigation of cholera outbreak in a periurban slum colony in Chandigarh. Indian J. Med. Sci. 2001;55:429–433. [PubMed] [Google Scholar]
- 94.Girish R., Broor S., Dar L., Ghosh D. Foodborne outbreak caused by a Norwalk-like virus in India. J. Med. Virol. 2002;67:603–607. doi: 10.1002/jmv.10145. [DOI] [PubMed] [Google Scholar]
- 95.Sugunan A.P., Ghosh A.R., Roy S., Gupte M.D., Sehgal S.C. A cholera epidemic among the Nicobarese tribe of Nancowry, Andaman, and Nicobar, India. Am. J. Trop. Med. Hyg. 2004;71:822–827. [PubMed] [Google Scholar]
- 96.Sen B., Dutta B., Chatterjee S., Bhattacharya M.K., Nandy R.K., Mukhopadhyay A.K., Gangopadhyay D.N., Bhattacharya S.K., Ramamurthy T. The first outbreak of acute diarrhea due to a pandemic strain of Vibrio parahaemolyticus O3:K6 in Kolkata, India. Int. J. Infect. Dis. 2007;11:185–187. doi: 10.1016/j.ijid.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 97.Chadha M.S., Lole K.S., Bora M.H., Arankalle V.A. Outbreaks of Hepatitis A among children in western India. Trans. R. Soc. Trop. Med. Hyg. 2009;103:911–916. doi: 10.1016/j.trstmh.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 98.Sur D., Sarkar B.L., Manna B., Deen J., Datta S., Niyogi S.K., Ghosh A.N., Deb A., Kanungo S., Palit A., Bhattacharya S.K. Epidemiological, microbiological & electron microscopic study of a cholera outbreak in a Kolkata slum community. Indian J. Med. Res. 2006;123:31–36. [PubMed] [Google Scholar]
- 99.Das A., Manickam P., Hutin Y., Pattanaik B., Pal B.B., Chhotray G.P., Kar S.K., Gupte M.D. Two sequential outbreaks in two villages illustrate the various modes of transmission of cholera. Epidemiol. Infect. 2009;137:906–912. doi: 10.1017/S0950268808001611. [DOI] [PubMed] [Google Scholar]
- 100.Jadhav S., Sinha A., Banerjee A., Chawla P. An outbreak of food poisoning in a military establishment. Med. J. Armed Forces India. 2007;63:130–133. doi: 10.1016/S0377-1237(07)80055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhunia R., Hutin Y., Ramakrishnan R., Pal N., Sen T., Murhekar M. A typhoid fever outbreak in a slum of South Dumdum municipality, West Bengal, India, 2007: evidence for foodborne and waterborne transmission. BMC Publ. Health. 2009;9:115. doi: 10.1186/1471-2458-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chobe L.P., Arankalle V.A. Investigation of a hepatitis A outbreak from shimla Himachal Pradesh. Indian J. Med. Res. 2009;130:179–184. [PubMed] [Google Scholar]
- 103.Mustafa M., Jain S., Agrawal V. Food poisoning outbreak in a military establishment. Med. J. Armed Forces India. 2009;65:240–243. doi: 10.1016/S0377-1237(09)80013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chauhan N.T., Prajapati P., Trivedi A.V., Bhagyalaxmi A. Epidemic investigation of the jaundice outbreak in Girdharnagar, Ahmedabad, Gujarat, India, 2008. Indian J. Community Med. 2010;35:294–297. doi: 10.4103/0970-0218.66864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhunia R., Ghosh S. Waterborne cholera outbreak following Cyclone Aila in Sundarban area of West Bengal, India, 2009. Trans. R. Soc. Trop. Med. Hyg. 2011;105:214–219. doi: 10.1016/j.trstmh.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Shah H.D., Shah V.P., Desai A.N. An epidemic outbreak of Vibrio cholerae El Tor O1 serotype Ogawa biotype in a Lalpur town, Jamnagar, India. J. Postgrad. Med. 2012;58:14–18. doi: 10.4103/0022-3859.93247. [DOI] [PubMed] [Google Scholar]
- 107.Kunwar R., Singh H., Mangla V., Hiremath R. Outbreak investigation: Salmonella food poisoning. Med. J. Armed Forces India. 2013;69:388–391. doi: 10.1016/j.mjafi.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fredrick T., Ponnaiah M., Murhekar M.V., Jayaraman Y., David J.K., Vadivoo S., Joshua V. Cholera outbreak linked with lack of safe water supply following a tropical cyclone in Pondicherry, India, 2012. J. Health Popul. Nutr. 2015;33:31–38. [PMC free article] [PubMed] [Google Scholar]
- 109.Singla N., Bansal N., Gupta V., Chander J. Outbreak of Salmonella Typhi enteric fever in sub-urban area of North India: a public health perspective. Asian Pac. J. Tropical Med. 2013;6:167–168. doi: 10.1016/S1995-7645(13)60017-6. [DOI] [PubMed] [Google Scholar]
- 110.Chowdhury G., Ghosh S., Pazhani G.P., Paul B.K., Maji D., Mukhopadhyay A.K., Ramamurthy T. Isolation and characterization of pandemic and nonpandemic strains of Vibrio parahaemolyticus from an outbreak of diarrhea in North 24 Parganas, West Bengal, India. Foodb. Pathog. Dis. 2013;10:338–342. doi: 10.1089/fpd.2012.1340. [DOI] [PubMed] [Google Scholar]
- 111.Rakesh P., Sherin D., Sankar H., Shaji M., Subhagan S., Salila S. Investigating a community-wide outbreak of hepatitis A in India. J. Global Infect. Dis. 2014;6:59–64. doi: 10.4103/0974-777X.132040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Awsathi S., Rawat V., Rawat C.M.S., Semwal V., Bartwal S.J. Epidemiological investigation of the jaundice outbreak in lalkuan, nainital district, uttarakhand. Indian J. Community Med. 2014;39:94–97. doi: 10.4103/0970-0218.132725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uthappa C.K., Allam R.R., Nalini C., Gunti D., Udaragudi P.R., Tadi G.P., Murhekar M.V. An outbreak of cholera in Medipally village, Andhra Pradesh, India, 2013. J. Health Popul. Nutr. 2015;33:7. doi: 10.1186/s41043-015-0021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumar T., Shrivastava A., Bhatia D., Mitra Y., Kumar A., Hussain S., Chauhan L.S., Laserson K.F., Narain J.P., Kumar R., Francisco A. Jaundice outbreak likely caused by HEV in Amritsar, Punjab, India, 2013. BMC Publ. Health. 2019;19:464. doi: 10.1186/s12889-019-6786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patil A.A., Velayudhan A., Durairaj G.K., Khasnobis P., Sodha S.V., Working Group Outbreak investigation of foodborne illness among political rally attendees, Cuddalore, Tamil Nadu, India. Indian J. Publ. Health. 2021;65:S55–S58. doi: 10.4103/ijph.IJPH_1069_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rakesh P.S., Narayanan V., Pillai S.S., Retheesh R., Dev S. Investigation of an outbreak of acute gastroenteritis in kollam, Kerala, India. J Prim Care Community Health. 2016;7:204–206. doi: 10.1177/2150131916641286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Verma S., Sharma V., Mokta K., Thakur C., Angrup A., Singh D., Kanga A. Outbreak of enteric fever due to Salmonella Paratyphi A variety Durazzo (2,12:a:-) in a hilly region of North India: a report of 43 cases. Indian J. Med. Microbiol. 2016;34:387–389. doi: 10.4103/0255-0857.188366. [DOI] [PubMed] [Google Scholar]
- 118.Debnath F., Mukhopadhyay A.K., Chowdhury G., Saha R.N., Dutta S. An Outbreak of foodborne infection caused by Shigella sonnei in West Bengal, India. Jpn. J. Infect. Dis. 2018;71:162–166. doi: 10.7883/yoken.JJID.2017.304. [DOI] [PubMed] [Google Scholar]
- 119.Sheoran P., Rammayyan A., Shukla H.K., Dikid T., Yadav R., Sodha S.V. An outbreak investigation of acute diarrheal disease, Nagpur District, Maharashtra, India. Indian J. Publ. Health. 2021;65:S14–S17. doi: 10.4103/ijph.IJPH_962_20. [DOI] [PubMed] [Google Scholar]
- 120.Kankaria A., Gupta M., Bashar M.A., Aggarwal S., Murugan S., Bhag C., Kumar S., Chaudhary K., Sandha K.S., Jain R. Epidemiological investigation of an outbreak of acute viral Hepatitis A and E in a semi-urban locality in Chandigarh, North Indian Union Territory, 2016–17. J Family Med Prim Care. 2020;9:1856. doi: 10.4103/jfmpc.jfmpc_1244_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joshi M.S., Lole K.S., Barve U.S., Salve D.S., Ganorkar N.N., Chavan N.A., Shinde M.S., Gopalkrishna V. Investigation of a large waterborne acute gastroenteritis outbreak caused by group B Rotavirus in Maharashtra state, India. J. Med. Virol. 2019;91:1877–1881. doi: 10.1002/jmv.25523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maramraj K.K., Subbalakshmi G., Ali M.S., Dikid T., Yadav R., Sodha S.V., Jain S.K., Singh S.K. A community-wide acute diarrheal disease outbreak associated with drinking contaminated water from shallow bore-wells in a tribal village, India, 2017. BMC Publ. Health. 2020;20:231. doi: 10.1186/s12889-020-8263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gupta G., Singh A., Dikid T., Saroha E., Sodha S.V. Acute diarrheal disease outbreak in muzaffarpur village, chandauli district, Uttar Pradesh, India. Indian J. Publ. Health. 2021;65:S34–S40. doi: 10.4103/ijph.IJPH_1111_20. [DOI] [PubMed] [Google Scholar]
- 124.Goswami S., Jha A., Sivan S.P., Dambhare D., Gupta S.S. Outbreak investigation of cholera outbreak in a slum area of urban Wardha, India: an interventional epidemiological study. J Family Med Prim Care. 2019;8:1112–1116. doi: 10.4103/jfmpc.jfmpc_308_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh A., Gupta R., Dikid T., Saroha E., Sharma N.C., Sagar S., Gupta S., Bindra S., Khasnobis P., Jain S.K., Singh S. Cholera outbreak investigation, bhadola, Delhi, India, april-may 2018. Trans. R. Soc. Trop. Med. Hyg. 2020;114:762–769. doi: 10.1093/trstmh/traa059. [DOI] [PubMed] [Google Scholar]
- 126.Kumar A., Grover G.S., Dikid T., Kaur S., Patil A., Working Group Foodborne illness outbreak linked to a rural community kitchen in a rural area of Patiala District, Punjab, India, 2018. Indian J. Publ. Health. 2021;65:S41–S45. doi: 10.4103/ijph.IJPH_1112_20. [DOI] [PubMed] [Google Scholar]
- 127.Dzeyie K.A., Dhanapaul S., Rubeshkumar P., Desing S., Vignesh M.S., Raveendran I., Kumar P., Kathuria S., Choudhary S., Saroha E., Siromany V., Raju M., Ganeshkumar P., Ponnaiah M., Sodha S.V., Laserson K., Bhatnagar T., Kapoor L., Bahl A., Jain S.K., Gupta S., Murhekar M.V., Singh S.K. Outbreak of ceftriaxone-resistant Salmonella enterica serotype typhi-tiruchirappalli, Tamil Nadu, India, june 2018. IJID Reg. 2021;1:60–64. doi: 10.1016/j.ijregi.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumar A., Barve U., Gopalkrishna V., Tandale B.V., Katendra S., Joshi M.S., Salve D., Viswanathan R. Outbreak of cholera in a remote village in western India. Indian J. Med. Res. 2022;156:442–448. doi: 10.4103/ijmr.IJMR_2440_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bajaj S., Dudeja P. Food poisoning outbreak in a religious mass gathering. Med. J. Armed Forces India. 2019;75:339–343. doi: 10.1016/j.mjafi.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jain A., Choudhary S., Saroha E., Bhatnagar P., Harvey P. Cholera outbreak in an informal settlement at shahpur huts, panchkula district, Haryana state, India. Indian J. Publ. Health. 2021;65(Supplement):S51–S54. doi: 10.4103/ijph.IJPH_970_20. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shah H.D., Desai B., Jadav P., Shah N., Kadikar R., Singh A.J. An epidemiological investigation of a cholera outbreak in peri-urban slum settlements of Gujarat, India. J Family Med Prim Care. 2022;11:6061–6066. doi: 10.4103/jfmpc.jfmpc_133_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abu Bashar M., Soundappan K. Outbreak investigation of acute watery diarrhea in a village of North India: timely action saved lives. J Infect Dev Ctries. 2022;16:843–849. doi: 10.3855/jidc.13113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study is publicly available research data as cited in the reference list.