Abstract
Objective
The aim of this study was to evaluate the effects of rapid maxillary expansion on the optic nerve sheath diameter and to examine its possible effects on intracranial pressure.
Design
20 patients with bilateral crossbite were selected. Hyrax Expander was applied and activated twice daily until the overcorrection was achieved. The optic nerve sheath diameter (ONSD) was measured via ultrasonography before the first activation (T0), then repeated after 1 (T1) and 10 min (T2). At the end of the expansion, ONSD was measured (T3) again, then the screw was activated for the last time, and measurements were repeated after 1 (T4) and 10 min (T5). The Friedman test was performed to compare the changes, and The Wilcoxon Signed-Rank test was done to determine the significant intergroup changes (p < 0.05).
Results
The ONSD increased significantly 1 min after the activations (T0-T1 and T3-T4) (P < 0.05). The ONSD values measured 10 min after the activations also increased significantly compared to the baseline values (T0-T2 and T3-T5) (P < 0.05).
Conclusion
The activation of maxillary expansion appliances increased the optic nerve sheath diameter in adolescents. Therefore, orthodontists should be careful with patients at risk of intracranial hypertension.
Keywords: Intracranial pressure, Optic nerve, Rapid maxillary expansion
1. Introduction
Widening the maxilla by separating the midpalatal suture with an expansion device has become a routine clinical procedure in treating maxillary transverse deficiency. With the maxillary expansion, dentoskeletal problems such as posterior crossbite, crowding, and narrow smile arch can be corrected [1]. It also contributes to the improvement of medical conditions such as nasal airway resistance [2], conductive hearing loss [3], nocturnal enuresis [4], sleep apnea [5], and halitosis [6]. Expansion forces applied to the maxilla cause changes in the circummaxillary sutures such as zygomaticofrontal, zygomaticomaxillary, frontomaxillary, zygomaticotemporal, nasomaxillary, frontonasal, and internasal sutures [7] and also cause widening in the spheno-occipital synchondrosis in children [8]. However, the effects of maxillary expansion on intracranial structures in adolescents are yet to be investigated.
The optic nerve is a cranial nerve covered by the meninges. Accordingly, the cerebrospinal fluid (CSF) between these meningeal layers also fills the subarachnoid space around the optic nerve [9]. An increase in intracranial pressure (ICP) to a certain degree can cause an increase in the volume of CSF around this space. As a result, the widening of the optic nerve sheath diameter (ONSD) occurs [10]. Intracranial or intraventricular microsensor devices are the gold standard methods for measuring ICP [11,12]. However, these may cause infections or haemorrhages. Ultrasonographic examination of the ONSD is first described by Helmke and Hansen [13] as a simple, accurate, safe, and non-invasive method for the evaluation of ICP. The reliability of this technique has been proven in many studies [10,[14], [15], [16]]. Geeraerts et al. reported that ONSD over 5.5 [17] mm reflects an increase in ICP over 20 mm Hg.
Children and adolescents are routinely treated with maxillary expansion in orthodontic clinical practice. However, children with medical conditions such as vasculitis, hydrocephalus, pseudotumor cerebri, and liver failure are at risk for increased ICP. Therefore, we conducted this study to investigate the possible ICP changes during maxillary expansion by measuring the ONSD using ultrasonography.
2. Materials and methods
The study was conducted in accordance with the Declaration of Helsinki and was approved by Bolu Abant Izzet Baysal University Clinical Researches Ethical Committee. Each patient joined the study voluntarily and each parent/legal guardian signed an informed consent form. 20 patients (11 females, 9 males) with bilateral maxillary crossbite are selected. Patients with neurological conditions, previous eye surgery, and eye diseases (glaucoma, diabetic retinopathy, papilledema) are excluded from the study.
Patients were treated with rapid maxillary expansion (RME) using a Hyrax expander. The expander was activated twice a day until the overcorrection of the maxillary expansion was achieved. The researcher did the first and the last activation of the screw to measure the ONSD. The first measurement of ONSD was performed just before the first activation of the expansion screw (T0) to determine the base value. Then, the expander was activated, and the measurements were repeated after 1 min (T1) and 10 min (T2). The same measurement protocol was followed on the last activation day as in the first measurements. The base value of the last activation was measured (T3). Then the expander was activated for the last time, and the measurements were repeated after 1 min (T4) and 10 min (T5). Mean arterial pressure (MAP), heart rate (BPM) and peripheral oxygen saturation levels (SpO2) were also measured at all times.
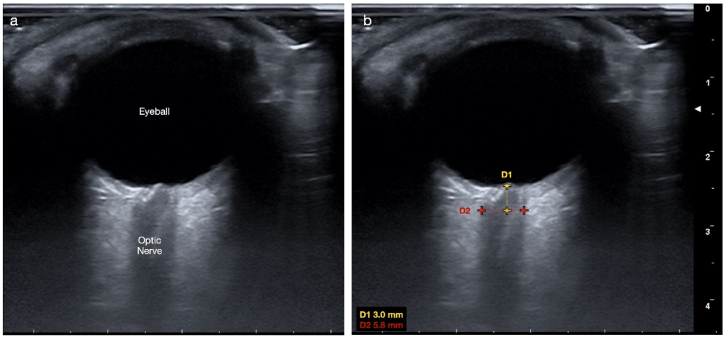
MyLab™ X7 model (Esaote SPA, Genova, Italy) ultrasonography device was used to measure the optic nerve sheath diameter with a linear probe at high frequency (>7.5 MHz). The patients were examined at a sitting position with their heads resting on the headrest of a dental unit and with their eyes closed. Then, thick conductive ultrasound gel was applied to the eyeballs, and the probe was placed gently. An experienced researcher (OD) performed all the ONSD measurements. Ultrasound settings were changed to get the best resolution between the retrobulbar echogenic adipose tissue and the vertical hypoechoic band. The ONSD was measured at the 3 mm posterior to the optic disc for both eyes (Fig. 1), and the arithmetic means of the measurements were calculated.
Fig. 1.
a) The ultrasonographic view of the eyeball and the optic nerve b) Optic nerve sheath diameter (ONSD) measurement was made 3 mm posterior to the optic disc.
3. Statistical analysis
The sample size was calculated using the G*power 3.1.9.7 program (Heinrich-Heine-University, Düsseldorf, Germany) to detect a difference of 1 mm or less in response to an alpha of 0.003 and a power of 80 %.the required number of patients was determined as 12 [18]. The data were not normally distributed (Shapiro Wilk test, α = 0.05). The Friedman test was performed to compare the changes in repeated measures (ONSD, SpO2, MAP and Heart rate). The Wilcoxon Signed-Rank test (with Bonferroni correction) was done to determine the significant intergroup changes. P values of <0.05 were statistically significant. SPSS v.26 (IBM, NY, USA) was used for all tests.
4. Results
The ONSD value measured before activation of the screw (T0) was significantly lower than the ONSD values at the 1 min (T1) and 10 min (T2) after activation of the screw (p < 0.05) (Fig. 2, Table 1).
Fig. 2.
Optic nerve sheath diameter (ONSD) values expressed as mean. T0: before the first activation, T1: 1 min after the first activation T2: 10 min after the first activation. T3: before the last activation, T4: 1 min after the last activation T5: 10 min after the last activation.
Table 1.
Optic nerve sheath diameter values associated with intracranial pressure at different time intervals.
| T0 |
T1 |
T2 |
T3 |
T4 |
T5 |
pa | Differenceb | |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| ONSD (mm) | 5.20 ± 0.44 | 5.67 ± 0.45 | 5.46 ± 0.47 | 5.29 ± 0.30 | 5.64 ± 0.35 | 5.48 ± 0.35 | 0.0004c | T0-T1 |
| 0.0119c | T0-T2 | |||||||
| 1.29 | T0-T3 | |||||||
| 0.0003c | T3-T4 | |||||||
| 0.0024c | T3-T5 |
Friedman Test.
Wilcoxon Signed-Rank Test.
p < 0.05.
When the measurements made at the end of the expansion were compared, The ONSD value measured before the last activation of the screw (T3) was significantly higher (p < 0.05) than the ONSD values at the 1st and 10th minutes after the last activation, T4 and T5 respectively. However, there is no significant difference between the baseline measurements made before the activations (T0-T3) (Fig. 2, Table 1).
No statistical significance was observed when the SpO2, MAP and Heart Rate values of T0, T1, T2, T3, T4 and T5 were compared (Table 2). Since there was no significant difference between these values in any of the time intervals, their relationship with ONSD values was not examined. The mean age for patients was 12.84 ± 1.99.
Table 2.
SpO2, heart rate and mean arterial pressure values at different time intervals.
| T0 |
T1 |
T2 |
T3 |
T4 |
T5 |
pa | Difference | |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Oxygen Saturation (%) | 96.65 ± 3.01 | 96.95 ± 2.03 | 97.30 ± 1.68 | 97.7 ± 1.62 | 97.65 ± 2.0 | 96.5 ± 1.98 | 0.178b | – |
| Heart Rate (beats per minute) | 81.95 ± 16.50 | 83.55 ± 13.28 | 81.95 ± 14.32 | 82.85 ± 18.11 | 80.40 ± 16.96 | 80.35 ± 17.25 | 0.614b | – |
| Mean Arterial Pressure (mmHg) | 77.45 ± 10.21 | 75.90 ± 10.83 | 76.50 ± 8.90 | 79.41 ± 15.30 | 73.16 ± 9.59 | 74.23 ± 11.34 | 0.255b | – |
Friedman Test.
p > 0.05.
5. Discussion
In this study, we observed that the appliances used for expansion caused an increase in the ICP measured ultrasonographically when they were first activated. However, 10 min after activation, a decrease in ICP was observed again. With the last activation of the expansion devices, the increase in ICP was repeated, and again a decrease was observed 10 min after the activation. This is the first article to show an increase in ICP independent of heart rate, mean arterial pressure and oxygen saturation in adolescents undergoing maxillary expansion.
In studies examining the effects of maxillary expansion on craniofacial complex, researchers found that many cranial structures are affected by the high forces that separate the midpalatal suture. Holberg and Rudzki-Janson stated that the highest stresses were presented at the pterygoid process of the sphenoid bone [19]. Iseri et al. stated that the highest stresses were observed in sphenoid and zygomatic bone [20]. In a similar finite element study, the maximum stresses were observed in the frontomaxillary, nasomaxillary, and frontonasal sutures [21]. These stresses affecting the cranial base cause an increase in intracranial pressure, which leads to an enlargement in ONSD.
Researchers reported that there is a correlation between ICP and ONSD in children. When the diameter of the optic nerve sheath is greater than 5.5 mm, ICP is increased by more than 20 mm Hg [10,17,22]. It is of great clinical importance as an increase in intracranial pressure greater than 15 mm Hg is defined as intracranial hypertension, and an increase of more than 20 mm Hg is the treatment threshold for traumatic brain injury [23]. In our study, we found that the ONSD was greater than 5.5 mm 1 min after the initial (T1) and final activation (T4) of the screw. When we measured the ONSD 10 min after the initial and final activation of the screw (T2 and T5, respectively), we found that the mean ONSD value was around 5.5 mm. This indicates an increase in ICP, but this escalation decreases to the threshold value within 10 min. Therefore, in patients with intracranial pathologies or risk factors associated with increased ICP, the complications that may occur due to the effect of RME on ICP should be considered during orthodontic treatment.
The baseline ONSD values (T0 and T3) prior to screw activation remain stable, indicating that the increased ICP is compensated and the ONSD value returns to normal over time after the screw is turned.
In a recent study, Baser et al. assessed the ONSD measurements of 15 patients who underwent micro implant-assisted rapid palatal expansion (MARPE) therapy and found no significant increase in ONSD [24]. However, their age group was older than ours. Therefore, their skeletal maturation was different from our study. In the mentioned study, bone-borne RME was performed in the adult patient group. In our study, tooth-borne RME was performed in the young age group, where the midpalatal suture can be opened easily and the resistance in the circummaxillary sutures is significantly lower. This may also be since the cranial base can absorb the forces produced by the RME. As the researchers pointed out, this may also be related to the autoregulation mechanisms of the brain in adulthood [24].
Zimring and Isaacson reported that a feeling of pressure around the eyes and dizziness occurs during the activation of the expansion screw [25]. This phenomenon may be due to increased ICP, and we may have found an answer more than 50 years later.
We used conventional RME device in this study. It has been reported that some side effects occur because the forces created by tooth-borne RME is transmitted to the maxilla through the dentition [[26], [27], [28]]. In this context, new studies are needed to evaluate the effect of bone-borne RME on ICP.
The limitation of this study is the need for precise quantitative values of intracranial pressure due to the invasive nature of the gold standard methods for measuring intracranial pressure. Also, we found no significant change in MAP values. However, we measured the mean arterial pressure with an electronic blood pressure monitoring device at the time intervals. We couldn't take continuous measurements because the continuous measurements require an intra-arterial cannulation. This method is very invasive and has it’s own risks especially in pediatric patients [29].
There is an increase in intracranial pressure after the activation of rapid maxillary expansion screw in adolescents. However, this increase in intracranial pressure decreases with time. In this study, we suggest that RME should be used more carefully in risk group patients. It is not necessary to measure ONSD in every patient who will undergo RME. However, it should be taken into consideration that RME may also increase ICP in children with previously increased ICP.
Funding
None.
Ethical approval
The Clinical Research and Ethics Committee of Bolu Abant Izzet Baysal University approved all study procedures (Decision No: 2022/330). This study was carried out in accordance with Declaration of Helsinki. Informed consent was obtained from all volunteers and/or legal guardian(s) for publication of their anonymised case details and images.
Availability of data and materials
The data used during the current study available from the corresponding author on reasonable request.
The use and declaration of AI and AI-assisted technologies in scientific writing
None.
CRediT authorship contribution statement
Yasin Hezenci: Writing – review & editing, Project administration, Methodology, Formal analysis, Conceptualization. Musa Bulut: Writing – original draft, Visualization, Methodology, Investigation. Oğuzhan Demirel: Writing – review & editing, Resources, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Dr. Abdullah Demirhan and Dr. Isa Yildiz for their guidance and mentoring.
References
- 1.McNamara J.A. Maxillary transverse deficiency. Am. J. Orthod. Dentofacial Orthop. 2000;117(5):567–570. doi: 10.1016/s0889-5406(00)70202-2. [DOI] [PubMed] [Google Scholar]
- 2.Basciftci F., Mutlu N., Karaman A., Malkoc S., Küçükkolbasi H. Does the timing and method of rapid maxillary expansion have an effect on the changes in nasal dimensions? Angle Orthod. 2002;72(2):118–123. doi: 10.1043/0003-3219(2002)072<0118:DTTAMO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Kilic N., Kiki A., Oktay H., Selimoglu E. Effects of rapid maxillary expansion on conductive hearing loss. Angle Orthod. 2008;78(3):409–414. doi: 10.2319/050407-217.1. [DOI] [PubMed] [Google Scholar]
- 4.Timms D.J. Rapid maxillary expansion in the treatment of nocturnal enuresis. Angle Orthod. 1990;60(3):229–233. doi: 10.1043/0003-3219(1990)060<0229:RMEITT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Villa M.P., Malagola C., Pagani J., Montesano M., Rizzoli A., Guilleminault C., et al. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med. 2007;8(2):128–134. doi: 10.1016/j.sleep.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Erhamza T.S., Ozdiler F.E. Effect of rapid maxillary expansion on halitosis. Am. J. Orthod. Dentofacial Orthop. 2018;154(5):702–707. doi: 10.1016/j.ajodo.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi R., Sicurezza E., Cutrera A., Barbato E. Early post-treatment changes of circumaxillary sutures in young patients treated with rapid maxillary expansion. Angle Orthod. 2011;81(1):36–41. doi: 10.2319/050910-250.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonardi R., Ronsivalle V., Lagravere M.O., Barbato E., Isola G., Lo Giudice A. Three-dimensional assessment of the spheno-occipital synchondrosis and clivus after tooth-borne and bone-borne rapid maxillary expansion. Angle Orthod. 2021;91(6):822–829. doi: 10.2319/013021-86.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith A.M., Czyz C.N. StatPearls. Treasure Island (FL; 2022. Neuroanatomy, cranial nerve 2 (optic) [Google Scholar]
- 10.Padayachy L.C., Padayachy V., Galal U., Gray R., Fieggen A.G. The relationship between transorbital ultrasound measurement of the optic nerve sheath diameter (ONSD) and invasively measured ICP in children : Part I: repeatability, observer variability and general analysis. Childs Nerv Syst. 2016;32(10):1769–1778. doi: 10.1007/s00381-016-3067-5. [DOI] [PubMed] [Google Scholar]
- 11.Lochner P., Czosnyka M., Naldi A., Lyros E., Pelosi P., Mathur S., et al. Optic nerve sheath diameter: present and future perspectives for neurologists and critical care physicians. Neurol. Sci. 2019;40(12):2447–2457. doi: 10.1007/s10072-019-04015-x. [DOI] [PubMed] [Google Scholar]
- 12.Padayachy L.C., Padayachy V., Galal U., Pollock T., Fieggen A.G. The relationship between transorbital ultrasound measurement of the optic nerve sheath diameter (ONSD) and invasively measured ICP in children. : Part II: age-related ONSD cut-off values and patency of the anterior fontanelle. Childs Nerv Syst. 2016;32(10):1779–1785. doi: 10.1007/s00381-016-3068-4. [DOI] [PubMed] [Google Scholar]
- 13.Helmke K., Hansen H. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. Pediatr. Radiol. 1996;26(10):701–705. doi: 10.1007/BF01383383. [DOI] [PubMed] [Google Scholar]
- 14.Dubourg J., Javouhey E., Geeraerts T., Messerer M., Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059–1068. doi: 10.1007/s00134-011-2224-2. [DOI] [PubMed] [Google Scholar]
- 15.Geeraerts T., Merceron S., Benhamou D., Vigué B., Duranteau J. Noninvasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Crit. Care. 2008;12(2):1–2. doi: 10.1007/s00134-008-1149-x. [DOI] [PubMed] [Google Scholar]
- 16.Hansen H.-C., Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J. Neurosurg. 1997;87(1):34–40. doi: 10.3171/jns.1997.87.1.0034. [DOI] [PubMed] [Google Scholar]
- 17.Karali E., Demirhan A., Gunes A., Ural A. Evaluation of the effect of Boyle-Davis mouth gag on intracranial pressure in patients undergoing adenotonsillectomy by using ultrasonographic optic nerve sheath diameter measurement. Int. J. Pediatr. Otorhinolaryngol. 2020;131 doi: 10.1016/j.ijporl.2019.109856. [DOI] [PubMed] [Google Scholar]
- 18.Pangrazio-Kulbersh V., Jezdimir B., de Deus Haughey M., Kulbersh R., Wine P., Kaczynski R. CBCT assessment of alveolar buccal bone level after RME. Angle Orthod. 2013;83(1):110–116. doi: 10.2319/030712-198.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holberg C., Rudzki-Janson I. Stresses at the cranial base induced by rapid maxillary expansion. Angle Orthod. 2006;76(4):543–550. doi: 10.1043/0003-3219(2006)076[0543:SATCBI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Iseri H., Tekkaya A.E., Oztan O., Bilgic S. Biomechanical effects of rapid maxillary expansion on the craniofacial skeleton, studied by the finite element method. Eur. J. Orthod. 1998;20(4):347–356. doi: 10.1093/ejo/20.4.347. [DOI] [PubMed] [Google Scholar]
- 21.Gautam P., Valiathan A., Adhikari R. Stress and displacement patterns in the craniofacial skeleton with rapid maxillary expansion: a finite element method study. Am. J. Orthod. Dentofacial Orthop. 2007;132(1):5 e1–e11. doi: 10.1016/j.ajodo.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Altiparmak B., Korkmaz Toker M., Uysal A.I., Koseoglu S., Gumus Demirbilek S. Evaluation of the effect of the mouth gag use on optic nerve sheath diameter of pediatric patients undergoing tonsillectomy or Adenotonsillectomy: an observational study. BMC Anesthesiol. 2020;20(1):163. doi: 10.1186/s12871-020-01079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Group UKPTBIS. Morris K.P., Forsyth R.J., Parslow R.C., Tasker R.C., Hawley C.A., et al. Intracranial pressure complicating severe traumatic brain injury in children: monitoring and management. Intensive Care Med. 2006;32(10):1606–1612. doi: 10.1007/s00134-006-0285-4. [DOI] [PubMed] [Google Scholar]
- 24.Baser B., Bolukbasi M., Uzlu D., Ozbay A.D. Does MARPE therapy have effects on intracranial pressure? a clinical study. BMC Oral Health. 2022;22(1):450. doi: 10.1186/s12903-022-02482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimring J.F., Isaacson R.J. Forces produced by rapid maxillary expansion III. Forces present during retention. Angle Orthod. 1965;35:178–186. doi: 10.1043/0003-3219(1965)035<0178:FPBRME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi R., Ronsivalle V., Barbato E., Lagravere M., Flores-Mir C., Lo Giudice A. External root resorption (ERR) and rapid maxillary expansion (RME) at post-retention stage: a comparison between tooth-borne and bone-borne RME. Prog. Orthod. 2022;23(1):45. doi: 10.1186/s40510-022-00439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo Giudice A., Leonardi R., Ronsivalle V., Allegrini S., Lagravere M., Marzo G., et al. Evaluation of pulp cavity/chamber changes after tooth-borne and bone-borne rapid maxillary expansions: a CBCT study using surface-based superimposition and deviation analysis. Clin Oral Investig. 2021;25(4):2237–2247. doi: 10.1007/s00784-020-03539-3. [DOI] [PubMed] [Google Scholar]
- 28.Ronsivalle V., Isola G., Lo Re G., Boato M., Leonardi R., Lo Giudice A. Analysis of maxillary asymmetry before and after treatment of functional posterior cross-bite: a retrospective study using 3D imaging system and deviation analysis. Prog. Orthod. 2023;24(1):41. doi: 10.1186/s40510-023-00494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King M.A., Garrison M.M., Vavilala M.S., Zimmerman J.J., Rivara F.P. Complications associated with arterial catheterization in children. Pediatr. Crit. Care Med. 2008;9(4):367–371. doi: 10.1097/PCC.0b013e318172d94f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used during the current study available from the corresponding author on reasonable request.