Abstract
A new family of monothiooxalamides derived from 2-aminobenzimidazole was synthesized, and their structures were confirmed by 1H and 13C one-dimensional and 2D NMR experiments (COSY, HSQC, and HMBC). The antioxidant capacity was evaluated by free radical scavenging assays: 1,1-diphenyl-2-picrylhydrazyl (DPPH•), 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation (ABTS•+), ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC), and the Fe(II) chelating ability. Our work group has previously reported the synthesis and antioxidant activity of monothiooxalamides derived from 2-aminopyridine (I). In this study, the in vitro hemolytic activity of compounds from the 2-aminopyridine (I) and 2-aminobenzimidazole (II) families was evaluated against human red blood cells (RBCs). The concentration at which monothiooxalamides showed no hemolytic activity was chosen to assess their ability to inhibit free radical-induced membrane damage in human RBCs, acute toxicity in brine shrimp, and in vivo toxicity against Drosophila melanogaster. Compounds with morpholine fragments (1g, 1h, 2g, and 2h) showed time- and concentration-dependent protective effects against radical-induced oxidative hemolysis. Moreover, they had the lowest acute toxicity in the brine shrimp lethality assay and a significant increase in chelating activity compared with the other molecules. In particular, monothiooxalamide 2g showed lower toxicity and can be considered for further biological screening and application trials.
Keywords: Monothiooxalamides, Pyridine, Aminobenzimidazole, Antioxidant, Toxicity, Drosophila melanogaster
Graphical abstract
Abbreviations
- RBCs
Red Blood Cells
- ASTM
American Society for Testing and Materials
- NMR
Nuclear Magnetic Resonance
- COSY
Two‐dimensional correlation spectroscopy
- HSQC
Heteronuclear Single Quantum Coherence
- HMBC
Heteronuclear Multiple Bond Correlation
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- ABTS
2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation
- FRAP
Ferric Reducing Antioxidant Power
- ORAC
Oxygen Radical Absorbance Capacity
- TEAC
Trolox Equivalent Antioxidant Capacity
- VCEAC
Vitamin C Equivalent Antioxidant Capacity
- EDTA
Ethylenediaminetetraacetic acid
- AAPH
2,2′-azobis(2-amidinopropane) dihydrochloride
- LC50
Median Lethal Concentration
1. Introduction
Drug attrition due to toxicity is one of the most serious problems facing the pharmaceutical industry. Approximately 30 % of clinical failures in drug development have been attributed to unmanageable toxicity [[1], [2], [3]]. In this context, drug design and development face challenges in increasing selectivity, enhancing therapeutic effects and predicting the likelihood of adverse effects associated with new drugs [[4], [5], [6]]. In drug design, sulfur-containing motifs have gained interest and have been used for various biological activities, such as antibacterial, antiviral, cytotoxic, antiallergic, and antimalarial [7,8]. Furthermore, a sulfur atom has different functionalities in different oxidation states, as illustrated by sulfamide, sulfone, sulfonamide, sulfamate, and sulfoxide functions [9]. Monothiooxalamides are structurally related to oxalamides and thiooxalamides and have been extensively studied for their biological activities, such as anti-inflammatory, antioxidant, and antiproliferative effects on human cancer cell lines [[10], [11], [12]]. In addition, monothiooxalamides and their analogs have shown applications in organic synthesis, asymmetric metal catalysis, and as ligands to form complexes with metals [[13], [14], [15], [16], [17]].
In drug design, sulfur-containing compounds are promising. By implementing strategic structural modifications in the early stages of drug discovery, toxic effects can be effectively minimized. Optimization of the drug candidate selection process relies on advancing predictive toxicity assays, both in vitro and in vivo. Therefore, an accurate assessment of toxicological risk helps to determine the optimal potency level of a substance while introducing structural features that improve metabolism and reduce toxicity [[18], [19], [20]].
Human Red blood cells (RBCs) are the most abundant cells in the blood; they carry oxygen to the tissues and remove urea and carbon dioxide. Currently, human RBCs are used as an in vitro model to screen and determine the toxicity of various compounds [[20], [21], [22]]. Oxidative damage to human RBCs caused by exposure to xenobiotics (metals, drugs, pesticides, and chemicals) can induce changes in the morphology, membrane protein conformation, protein crosslinking, lipid peroxidation, and release of intracellular components such as hemoglobin (hemolysis) [23,24]. A hemolysis percentage below 5 % is considered non-hemolytic according to ASTM (American Society for Testing and Materials) regulations [25,26]. Values above this limit are considered low (up to 10 %), and percentages above 10 % indicate marked hemolytic activity [[27], [28], [29], [30]].
Our work group previously reported the synthesis and antioxidant activity of monothiooxalamides derived from 2-aminopyridine (I) [13]. The present study focuses on the synthesis, characterization, antioxidant activity (DPPH•, ABTS•+, FRAP, and ORAC methods), and chelating capacity of ferrous ions in monothiooxalamides derived from 2-aminobenzimidazole (II). Furthermore, to determine the toxicity profile of the two groups of monothiooxalamides, hemolytic activity was evaluated using human RBCs. The concentration at which the compounds did not show significant hemolytic activity was then selected to assess the potential antioxidant capacity by inhibiting peroxyl radical-induced hemolysis. Monothiooxalamides were then evaluated for acute toxicity using a brine shrimp (Artemia salina) model [31]. This organism was chosen because it is sensitive to various toxic substances and compounds with diverse structures and modes of action. In addition, brine shrimp have been widely used as a feasible method to detect residues of pesticides, mycotoxins, anesthetics, and morphine-type compounds, among others [[31], [32], [33]]. In vivo, toxicity was determined in Drosophila melanogaster, also known as the fruit fly, an easily maintained organism with a relatively short lifespan and a well-established model for in vivo toxicity assays because D. melanogaster has almost 75 % functional homology with the disease-causing genes in humans [[34], [35], [36]].
2. Materials and methods
2.1. Equipment and materials
The reagents used for synthesis, antioxidant capacity, and toxicity of the compounds were purchased from commercial suppliers. They were used without further purification (see Supplementary material). Monothiooxalamides 1a-1h derived from 2-aminopyridine (I) (Fig. 1) were structurally confirmed and provided by Macías-Hernández et al. [13]. The solvents used for column chromatography were distilled before use. A 400 MHz Bruker NMR instrument with tetramethylsilane as an internal reference was used to obtain the NMR spectra; chemical shifts were reported in ppm and coupling constants in Hz. Melting points were obtained using a Melt-Temp II apparatus in the capillaries and were not corrected. A Biotek Powerwave XS microplate reader was used to read the absorbances.
Fig. 1.
Monothiooxalamides derived from 2-aminopyridine (I).
2.2. Procedure for the synthesis of chloroacetamide (2)
First, 10 mmol of 2-aminobenzimidazole (II) was placed in a 100 mL flask and dissolved in 50 mL of tetrahydrofuran. The reaction mixture was placed in an ice bath. Then 12 mmol of chloroacetyl chloride was added dropwise. After addition, the ice bath was removed, and the reaction was refluxed for 4 h. The white precipitate was collected and washed with tetrahydrofuran. The white solid was washed with NaHCO3 (10 %) aqueous solution (200 mL), and the solid was filtered and dried (yield, 78 %) (Scheme 1).
Scheme 1.
General method for monothiooxalamides synthesis from 2-aminobenzimidazole (II).
2.3. General procedure for the synthesis of monothiooxalamides (2c, 2e, 2g and 2h)
To obtain compounds 2c, 2e, 2g, and 2h, 1.1 equivalents of the corresponding amine (c, e, g, and h) and 10 mL of tetrahydrofuran with 1 mL of triethylamine were placed in a 100 mL ball flask. Then, 4.4 equivalents of elemental sulfur were added, and the mixture was stirred at room temperature for 30 min. Subsequently, 1.0 equivalent of chloroacetamide 2 was added slowly and stirred for 72 h. Monothiooxalamides were purified by column chromatography using a hexane/ethyl acetate mixture of suitable polarity (Scheme 1).
2.3.1. (S)–N-(1H-benzo[d]imidazole-2-yl)-2-((1-phenylethyl)amino)-2-thioxoacetamide (2c)
Yellow semisolid; yield 55 % (0.277 g); 1H NMR (400 MHz, DMSO) δ 12.17 (s, 1H, H10), 11.17 (d, 3J(H13-14) = 8.0 Hz, 1H, H13), 7.51–7.44 (dd, 3J(H4–H5) = 6.0 Hz, 3J(H7–H6) = 6.0 Hz, 4J(H4–H6) = 3.2 Hz, 4J(H7–H5) = 3.2 Hz, 1H, H4, H7), 7.43–7.39 (m, 1H, H18), 7.38–7.33 (m, 2H, H17), 7.30–7.25 (m, 1H, H19), 7.20–7.14 (dd, 3J(H5–H4) = 6.0 Hz, 3J(H6–H7) = 6.0 Hz, 4J(H6–H4) = 3.2 Hz, 4J(H5–H7) = 3.2 Hz,1H, H5, H6), 5.62 (p, 3J(H14–H15) = 8.0 Hz, 1H, H14), 1.56 (d, 3J(H15–H14) = 8.0 Hz, 3H, H15). 13C NMR (101 MHz, DMSO) δ 190.2 (C12), 164.9 (C11), 148.5 (C2), 142.2 (C16), 134.2 (C8, C9), 128.9 (C17), 127.8 (C19), 127.1 (C18), 122.6 (C5, C6), 113.9 (C4, C7), 54.3 (C14), 21.1 (C15). MS (TOF): Theoretical m/z = 324.1045 [M − H] −. Observed m/z = 324.1049 [M − H] −. Error: 1.43 ppm. HPLC purity: 94.99 %.
2.3.2. N-(1H-benzo[d]imidazole-2-yl)-2-(cyclohexylamino)-2-thioxoacetamide (2e)
Yellow solid; yield 59 % (0.293 g); 1H NMR (400 MHz, DMSO) δ 12.13 (s, 1H, H10), 10.69 (d, 3J(H13–H14) = 8 Hz, 1H, H13), 7.52–7.44 (dd, 3J(H4–H5) = 5.9 Hz, 3J(H7–H6) = 5.9 Hz, 4J(H4–H6) = 3.2 Hz, 4J(H7–H5) = 3.2 Hz, 1H, H4, H7), 7.20–7.12 (dd, 3J(H5–H4) = 5.9 Hz, 3J(H6–H7) = 5.9 Hz, 4J(H6–H4) = 3.2 Hz, 4J(H5–H7) = 3.2 Hz, 1H, H5, H6), 4.22 (qd, 3J(H14–H15) = 8 Hz, 1H, H14), 1.91 (d, 1H, H15), 1.75 (d, 1H, H16), 1.61 (d, 1H, H17), 1.41 (td, 1H, H15), 1.30 (d, 1H, H16), 1.22–1.09 (m, 1H, H17). 13C NMR (101 MHz, DMSO) δ 188.7 (C12), 164.1 (C11), 147.9 (C2), 134.8 (C8, C9), 122.4 (C5, C6), 114.1 (C4, C7), 54.5 (C14), 30.6 (C15), 25.5 (C16), 25.0 (C24). MS (TOF): Theoretical m/z = 302.1201 [M − H] −. Observed m/z = 302.1205 [M − H] −. Error: 1.08 ppm. HPLC purity: 100.00 %.
2.3.3. N-(1H-benzo[d]imidazole-2-yl)-2-((2-morpholinoethyl)amino)-2-thioxoacetamide (2g)
Yellow solid; yield 49 % (0.247 g); 1H NMR (400 MHz, DMSO) δ 12.12 (s, 1H, H12), 10.76 (s, 1H, H13), 7.49 (dd, 3J(H4–H5) = 5.9 Hz, 3J(H7–H6) = 5.9 Hz, 4J(H4–H6) = 3.2 Hz, 4J(H7–H5) = 3.2 Hz, 1H, H4, H7), 7.17 (dd, 3J(H5–H4) = 5.9 Hz, 3J(H6–H7) = 5.9 Hz, 4J(H6–H4) = 3.2 Hz, 4J(H5–H7) = 3.2 Hz, 1H, H5, H6), 3.73 (t, 3J(H14–H5) = 13.2 Hz, 2H, H14), 3.57 (t, 5H, H18), 2.61 (t, 3J(H15–H14) = 13.2 Hz, 1H, H15), 2.43 (s, 2H, H17). 13C NMR (101 MHz, DMSO) δ 188.9 (C7), 162.7 (C6), 147.8 (C2), 134.7 (C8, C9), 122.5 (C5, C6), 114.1 (C4, C7), 66.6 (C18), 55.2 (C15), 53.5 (C17), 42.7 (C14). MS (TOF): Theoretical m/z = 333.1259 [M − H] −. Observed m/z = 333.1263 [M − H] −. Error: 1.12 ppm. HPLC purity: 84.08 %.
2.3.4. N-(1H-benzo[d]imidazole-2-yl)-2-((3-morpholinopropyl)amino)-2-thioxoacetamide (2h)
Yellow solid; yield 45 % (0.224 g); 1H NMR (400 MHz, DMSO) δ 11.98 (s, 1H, H10), 11.15 (s, 1H, H13), 7.49 (dd, 3J(H4–H5) = 5.4 Hz, 3J(H7–H6) = 5.4 Hz, 4J(H4–H6) = 3.0 Hz, 4J(H7–H5) = 3.0 Hz, 1H, H4, H7), 7.17 (dd, 3J(H5–H4) = 5.4 Hz, 3J(H6–H7) = 5.4 Hz, 4J(H6–H4) = 3.0 Hz, 4J(H5–H7) = 3.0 Hz, 1H, H5, H6), 3.65 (dd, 2H, H14), 3.63–3.58 (m, 2H, H19), 2.44–2.32 (m, 3H, H16, H18), 1.81 (p, 1H, H15). 13C NMR (101 MHz, DMSO) δ 188.1 (C12), 162.0 (C11), 147.2 (C2), 135.3 (C8, C9), 122.4 (C5, C6), 114.3 (C4, C7), 66.6 (C19), 56.7 (C16), 53.7 (C18), 45.2 (C14), 23.7 (C2). MS (TOF): Theoretical m/z = 347.1416 [M − H] −. Observed m/z = 347.1416 [M − H] −. Error: 0-04 ppm. HPLC purity: 100.00 %.
2.4. Antioxidant activity
The in vitro antioxidant activity was determined using DPPH•, ABTS•+, FRAP, ORAC, and Fe(II) chelating ability assays. Our research group previously reported experimental procedures for evaluating the antioxidant activity of monothiooxalamides derived from 2-aminopyridine [13], which were used to assess compounds with a benzimidazole moiety. The experimental details and concentrations used for each assay are provided in the Supplementary material.
2.4.1. DPPH radical scavenging capacity assay
The reaction mechanism of the DPPH• radical involves either Hydrogen Atom Transfer (HAT) or single electron transfer (SET) [37]. The antioxidant capacity was determined according to reported procedures and was based on the measurement of the loss of DPPH• color at 515 nm after its reaction with the test compounds. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as a reference, and the results were expressed as Trolox equivalent antioxidant capacity (TEAC) with values based on micromoles of Trolox per liter (μmol ET/L) [13,38].
2.4.2. ABTS radical scavenging capacity assay
The scavenging ability of the cation ABTS•+ by monothiooxalamides was quantified by ABTS•+ discoloration at 754 nm. The cation radical was obtained from the ABTS(NH4)2 reaction with potassium persulfate until it reached an absorbance of A = 0.7 ± 0.1 at 754 nm. Ascorbic acid was used as a reference standard, and the outcomes were presented as vitamin C equivalent antioxidant capacity (VCEAC) in mg/100 mL [13,38].
2.4.3. Ferric-reducing antioxidant power (FRAP) assay
The FRAP test is based on a single electron transfer reaction and measures the antioxidant's ability to reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+) under acidic conditions at 700 nm. The assay was performed according to Hinneburg et al. (2006) with some modifications [39]. Ascorbic acid was used as a reference compound for the standard curve. The data were expressed as vitamin C equivalent antioxidant capacity (VCEAC) in mg/100 mL.
2.4.4. Oxygen radical absorbance capacity (ORAC) assay
The ORAC determination of monothiooxalamides was carried out using the method described by Huang et al. (2002) with some modifications. ORAC is a HAT-based assay that quantifies the peroxyl radical scavenging capacity. Thermal decomposition of AAPH was used as the peroxyl radical-generating system, and analyses were performed in phosphate buffer pH 7.0 at 37 °C [40]. The values were expressed as micromoles of Trolox per milliliter (μmol ET/mL) according to Equation (1). Where, AUC° is the area under the curve for the control, AUC is of the sample, AUCTrolox is for Trolox, and fd is the dilution factor for each monothiooxalamide [13,41,42].
| (Equation 1) |
2.4.5. Chelating activity of Fe(II)
The chelating activity of ferrous ions was determined by the ability of the monothiooxalamides to inhibit the formation of ferrozine-Fe2+ complexes. The reaction produces a blue-green color that absorbs at 562 nm, and ethylenediaminetetraacetic acid (EDTA) was used as a reference chelating agent. The data were expressed as millimoles EDTA equivalent per liter (mM EDTA) [13,43].
2.5. Hemolytic and antihemolytic activities
2.5.1. Ethics
The study was reviewed and approved by the State Bioethics Commission of the State of Nayarit, Mexico (Approval Number: CEBN/19/2024). Five volunteers participated, who gave their written informed consent to participate in the study.
2.5.2. Preparation of the human RBC suspension
Human RBC suspension was performed according to previously reported procedures [44,45]. Blood was collected by venipuncture from healthy, non-smoking, and fully informed volunteers (25–35 years). Samples were collected in tubes containing lithium heparin as an anticoagulant (BD Vacutainer®) and centrifuged at 3500 rpm for 4 min at 4 °C. The plasma and buffy coat were carefully removed. For the in vitro hemolysis assay, human RBCs were washed three times with Alsever's solution (0.116 M dextrose, 0.071 M NaCl, 0.027 M sodium citrate, and 2 mM citric acid) at pH 6.4 ± 0.4. The human RBC button was resuspended in Alsever's solution to give human RBCs 1 % (v/v) hematocrit suspension. For the hemolysis inhibition assay, the same extraction procedure was followed, and human RBCs were washed three times with PBS saline buffer (125 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, and 5 mM glucose) at a pH of 7.4 ± 0.2. After washing, a suspension of human RBCs at 2 % (v/v) hematocrit was prepared.
2.5.3. In vitro hemolysis assay
Twelve monothiooxalamides were tested for hemolytic activity in human RBCs, eight derived from 2-aminopyridine (1a-1h) and four from 2-aminobenzimidazole (2c, 2e, 2g and 2h). The experiments followed previously reported procedures [46]. Concentrations of 2.5, 5.0, 10, and 50 mg/mL in DMSO were evaluated for each compound; the concentration range was between 4 and 213 mM.
The procedure involved mixing 0.1 mL of the human RBC suspension with 0.5 mL of Alsever's solution containing 1 % (v/v) of each previously prepared compound, resulting in final concentrations ranging from 0.07 to 2.13 mM (Table 5). A solution of human RBCs incubated with 1 % (v/v) or 40 mM of Triton X-100 was used to determine 100 % hemoglobin release. The 1 % (v/v) DMSO (141 mM) in Alsever's solution was used as a control to assess the spontaneous hemolysis of human RBCs and to compare it with the hemolysis induced by the testing compounds. The samples were incubated in a MultiTherm Benchmark shaker at 500 rpm and 37 °C for 1 h. The samples were centrifuged at 3000 rpm for 4 min. Finally, 100 μL aliquots of the supernatant were taken, and absorbance (A) at 410 nm was recorded. The percentage of hemolysis was calculated using Equation (2).
| (Equation 2) |
where, A410S is the area under the curve for the sample, A410NC is the area under the curve for the negative control, and A410PC is for the positive control [46].
Table 5.
Percentage of human RBC lysis obtained from the interaction of monothiooxalamides derived from 2-aminopyridine (I) and 2-aminobenzimidazole (II) with human RBCs, compared to the 100 % hemolysis.
| Compound | Hemolysis (%) |
|||
|---|---|---|---|---|
| 500 μg/mL (1.44–2.13 mM) | 100 μg/mL (0.30–0.43 mM) | 50 μg/mL (0.15–0.21 mM) | 25 μg/mL (0.07–0.11 mM) | |
| 1a | 47.59c ± 1.90 | 29.75b ± 1.78 | 10.6b ± 0.43 | 2.96b ± 0.27 |
| 1b | 10.45d ± 0.36 | 2.02c ± 0.61 | 1.03c ± 0.24 | NA |
| 1c | 13.72b ± 0.98 | 2.22c ± 0.12 | 0.72c ± 0.11 | NE |
| 1d | 62.95b ± 1.44 | 28.57b ± 0.44 | 12.97a ± 0.24 | 4.96b ± 0.69 |
| 1e | 70.85a ± 1.13 | 54.40a ± 2.35 | 12.50a ± 0.24 | 1.21b ± 0.27 |
| 1f | 3.31ef ± 0.49 | 1.26c ± 0.12 | 0.75c ± 0.14 | 0.63b ± 0.24 |
| 1g | 3.90e ± 0.27 | 1.66c ± 0.07 | 1.14c ± 0.12 | 0.20b ± 0.12 |
| 1h | 0.59fg ± 0.07 | 0.24c ± 0.38 | NA | NA |
| 2c | 34.50b ± 0.49 | 13.47c ± 0.51 | 12.31b ± 0.29 | 8.24b ± 0.76 |
| 2e | 39.53a ± 1.93 | 23.09a ± 0.34 | 21.25b ± 0.88 | 13.73a ± 0.79 |
| 2g | 20.19c ± 0.78 | 3.26c ± 0.65 | 2.58c ± 0.15 | 2.10c ± 0.20 |
| 2h | 13.85b ± 0.42 | 6.46b ± 0.45 | 4.34b ± 0.52 | 3.05b ± 0.42 |
| I | 0.42g ± 0.30 | 0.07c ± 0.15 | NA | NA |
| II | 1.55d ± 0.10 | 0.71d ± 0.24 | 0.58d ± 0.42# | 0.16d ± 0.11 |
NA: not active. NE: not evaluated. The letters (a-g) in each column refer to the pairwise comparisons between groups with a significant difference according to Tukey's HSD test; n = 3, p < 0.05.
2.5.4. In vitro oxidative hemolysis inhibition assay
The inhibition of oxidative hemolysis is based on the ability of antioxidants to inhibit free radicals and protect the human RBC membrane from damage. The aim of these experiments was to determine the resistance of human RBCs to oxidative destruction after short-term treatment with monothiooxalamides. The protective effect of monothiooxalamides on human RBCs was evaluated according to previously reported methods [[46], [47], [48]]. The concentrations used were those at which no hemolytic activity higher than 10 % was observed.
For this assay, 50 μL of the human RBC suspension was mixed with 50 μL of PBS buffer containing 5 % (v/v) of the compound at a concentration ranging from 4 to 213 mM and incubated at 37 °C for 10 min. Subsequently, 200 μL of the radical AAPH (50 mM) was added, and the reaction was incubated at 37 °C with shaking (500 rpm) for 1 h. The final concentration of each compound was in the range of 0.05–2.13 mM (see Table 6).
Table 6.
Inhibition of AAPH-induced human RBC hemolysis by monothiooxalamides derived from 2-aminopyridine (I) and 2-aminobenzimidazole (II). The concentrations at which the compounds did not show toxicity against human RBCs were evaluated.
| Compound | % Hemolysis inhibition |
||||
|---|---|---|---|---|---|
| 500 μg/mL (1.44–2.13 mM) | 100 μg/mL (0.30–0.43 mM) | 50 μg/mL (0.15–0.21 mM) | 25 μg/mL (0.07–0.11 mM) | 16.7 μg/mL (0.05–0.07 mM) | |
| 1a | – | – | – | 67.35d ± 0.18 | 54.45b ± 0.79 |
| 1b | – | 88.02b ± 0.48 | 82.27b ± 1.27 | 66.73d ± 0.73 | 47.74c ± 0.33 |
| 1c | NE | 74.13e ± 0.70 | 62.64d ± 0.97 | NE | NE |
| 1d | – | – | – | 77.49b ± 0.38 | 69.22a ± 0.33 |
| 1e | – | – | – | 68.28d ± 0.65 | 45.26d ± 0.57 |
| 1f | 91.46a ± 0.08 | 77.71d ± 0.64 | 61.12d ± 0.28 | 46.15e ± 1.23 | 38.83f ± 0.62 |
| 1g | 90.32b ± 0.14 | 82.94c ± 0.19 | 82.29b ± 0.20 | 79.93a ± 0.12 | 41.73e ± 0.44 |
| 1h | 86.09c ± 0.08 | 86.23b ± 0.17 | 79.80c ± 0.17 | 38.16f ± 1.27 | 30.88 f ± 1.39 |
| 2c | – | – | – | – | – |
| 2e | – | – | – | – | – |
| 2g | – | 90.32a ± 0.16 | 86.96a ± 0.17 | 71.48c ± 0.33 | NE |
| 2h | – | 48.0 a ± 1.0 | 40.67a ±1.53 | 39.33a ± 1.15 | 31.33a ± 2.08 |
| I | 19.09e ± 0.41 | 1.60 g ± 0.59 | NA | NA | NA |
| II | 80.04d ± 0.54 | 66.24f ± 0.41 | 38.67e ± 1.27 | 9.27g ± 0.95 | NE |
---Concentrations that resulted in hemolysis percentages greater than 5 % (Table 5).
NA: not active. NE: not evaluated. The letters (a-g) in each column refer to the pairwise comparisons between groups with a significant difference according to Tukey's HSD test; n = 3, p < 0.05.
Afterward, 1 mL of PBS buffer was added to the mixture and centrifuged at 3500 rpm for 10 min. Finally, 200 μL supernatant aliquots were taken, and absorbance was measured at 410 nm. As a control, 5 % (v/v) DMSO in PBS was used. The data were presented as a percentage of hemolysis inhibition using Equation (3). A410S is the area under the curve for the sample and A410C is the control.
| (Equation 3) |
The time dependence of the protective effect exerted by the tested compounds on human RBC oxidative damage was determined by mixing 100 μL of the human RBC suspension with 100 μL of PBS containing 5 % (v/v) of the compound at 14–22 mM and incubating the at 37 °C for 10 min. Subsequently, 400 μL of the radical AAPH (50 mM) was added and the reaction was incubated at 37 °C with shaking (500 rpm) for 3.5 h. The final concentration of each compound was between 0.14 and 0.21 mM. Aliquots of 75 μL were taken every 30 min, to which 250 μL of PBS buffer was added and the mixture was centrifuged at 3500 rpm for 10 min. After that, 100 μL from the supernatant was taken, and the absorbance was measured at 410 nm. The control was prepared using DMSO at 5 % (v/v) in PBS. The results were expressed as a percentage of hemolysis using Equation (4).
| (Equation 4) |
2.6. Brine shrimp (Artemia salina) lethality bioassay
Brine shrimp is a small crustacean with high adaptability to different temperatures, and salinities, distinguished by a short lifespan, and production of large offspring [49]. The brine shrimp lethality bioassay is valuable for conducting the general toxicity screenings of synthetic, semisynthetic compounds, and natural products. The test is based on the ability of the testing compounds to kill nauplii, that is, microcrustaceans in their larval stage [50,51].
Hatching was initiated by immersing 1 g of brine shrimp cysts in 50 mL of sodium hypochlorite (3 % v/v) for 10 min, promoting the dissolution of cyst chorion. Immediately after the cysts were washed with purified water and added to 1 L of seawater, they were incubated for 48 h under oxygenation with an aquarium pump, 3500 lumens at a distance of 40 cm, pH 7–8 and a temperature of 24 ± 2 °C [50]. The toxicity of the monothiooxalamides was performed according to previously reported procedures [[51], [52], [53], [54]]. 5 mg of each monothiooxalamide was dissolved in 100 μL of DMSO (144–213 mM). Aliquots were taken from this solution to obtain concentrations ranging from 0.02 to 2.13 mM in seawater. Then, 10 nauplii were placed in a Petri dish (60 × 15 mm) with 5 mL of the concentration to be evaluated. Seawater was used as the negative control in each experiment. In addition, different concentrations (34–680 μM) of potassium dichromate were assessed as a positive control, and DMSO activity was determined at 1 % and 2 % (v/v). Five replicates were analyzed for the different concentrations and samples evaluated, which were incubated at 22–26 °C for 24 h. Finally, the live and dead nauplii were counted and the lethality percentage (% L) was calculated using Equation (5).
| (Equation 5) |
where, LC is the living nauplii in the control and LT is the living nauplii with the tested compound after 24 h [55].
2.7. Toxicity in Drosophila melanogaster
Based on the results of hemolytic activity and acute toxicity in brine shrimp, monothiooxalamides 1a, 1c, 1f, 1g, and 2g were selected to determine toxicity in D. melanogaster (0.08–0.11 mM). Each compound was first dissolved in DMSO (7–11 mM), then, 45 μL aliquots were taken from this solution and added directly to 4.5 mL of distilled water to give a final concentration of (0.08–0.11 mM). All solutions were prepared immediately before use, and the final pH was 6.5. As a negative control, 1 % (v/v) DMSO in distilled water was used. An in vivo toxicity test for monothiooxalamides was performed by crossing virgin females and males from the wild-type; 30 couples were placed in each tube and kept at 24 ± 1 °C and 60 % humidity. The standard culture medium was prepared with sugar, agar, cornmeal, yeast, propionic acid, and Nipagin (methyl-p-hydroxybenzoate) [56].
After 48 h, the flies were transferred to culture flasks containing standard medium to collect the eggs for 8 h, after which the adults were removed. When the larvae were 72 ± 4 h old (third instar), they were extracted with 20 % saccharose and washed with distilled water [57]. Between 50 and 100 larvae were then transferred to glass tubes with 1.0 g of Drosophila Instant Medium (Carolina Biological Supply Co., Burlington, NC, USA) and 4.5 mL of the solutions of each monothiooxalamide (0.08–0.11 mM). The larvae were fed on the medium for approximately 96 h until the appearance of the adults and three replicates were analyzed for each concentration [58]. During this time, larval motility and pupation phases were reviewed. Once hatching occurred, the physical characteristics of the adults (head, thorax, and abdomen) were examined, and the number of females and males in each tube was counted [56]. The survival index biomarker (SI) was used to identify the toxic compounds in D. melanogaster because it has demonstrated the organism's response to xenobiotics presence [56,[59], [60], [61]]. The SI was obtained as the average number of flies recovered in each series compared with the total number of flies in the control. Furthermore, the sexual ratio was used to determine whether the monothiooxalamides affect males and females differently.
2.8. Statistics
Samples were examined in triplicate, and the results are expressed as mean ± standard deviation. The data were assessed using one-way ANOVA with α = 0.05, performed in RStudio (version 4.1.4).In addition, Tukey's Honestly Significant Difference (HSD) test was applied. A p-value of less than 0.05 was considered statistically significant. The median lethal concentration (LC50) values were determined using the same software, and five replicates of each sample were analyzed.
3. Results and discussion
3.1. Synthesis
Macías-Hernández et al. previously reported a suitable and inexpensive method using elemental sulfur (S8) to synthesize monothiooxalamides with a pyridine scaffold. In this work, these conditions were used to synthesize monothiooxalamides containing the benzimidazole moiety (2c, 2e, 3g, and 2h) [13]. The synthesis was performed in two reaction steps as described in Scheme 1, and the physical properties of the compounds are shown in Table 1.
Table 1.
Physical properties and yields of the benzimidazole derivatives.
| Compound | Amine | State | Yield (%) |
|---|---|---|---|
| 2c | 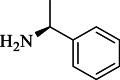 |
Yellow semisolid | 55 |
| 2e | 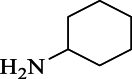 |
Yellow solid | 59 |
| 2g |  |
Yellow solid | 49 |
| 2h |  |
Yellow solid | 45 |
3.2. Chemistry
Compounds 2c, 2e, 2g, and 2h were characterized by 1D and 2D NMR experiments. The downfield signals corresponding to amide (11.98–12.21 ppm) and thioamide protons (10.69–11.42 ppm) in all compounds were confirmed by 1H NMR and 1H correlated spectroscopy (COSY). On compounds 2c and 2e, the coupling of H13 and H14 (3J13-14 = 8Hz) was observed; these hydrogens correspond to thioamide and aliphatic fragments, respectively (Fig. 2). This indicates a hydrogen bond between the thioamide proton (donor) and the carbonyl oxygen (acceptor). Therefore, H14 is a quadruplet in compound 2c and a triplet in compound 2e (3J14-13 = 8Hz). These intramolecular hydrogen bonds were previously reported by Padilla-Martinez et al. on oxamide and monothiooxalamides [13,62]. Without intramolecular hydrogen bonding, the signal for H13 should be broad because of the exchange rate.
Fig. 2.
A) Spin-spin splitting coupling for the thioamide proton. B) Correlation between the thioamide hydrogen and the neighboring proton. C) Structures of compounds 2c and 2e.
One-dimensional 13C NMR was performed, and the signals were confirmed by 2D13C and 1H Heteronuclear Single Quantum Coherence (HSQC) and Heteronuclear Multiple Bond Correlation (HMBC) experiments (see Supplementary material). In 13C NMR for compounds 2(c–h), the characteristic signal of the thiocarbonyl group, which is the most downfield (Table 2), appears between 188.1 and 191.5 ppm. On the other hand, the carbonyl signal is between 156.1 and 165.1 ppm. The shielding effect in the C=S group is more intense than in the C=O group, because the C=S bond is longer than the C=O bond [63].
Table 2.
Chemical shift in ppm (13C NMR) of monothiooxalamides 2(c–h).
| Compound | Chemical shift (δ in ppm) |
|
|---|---|---|
| C=O | C=S 2(c-h) or CH2 (2) | |
| 2 | 167.8 | 43.9 |
| 2c | 165.1 | 190.4 |
| 2e | 164.1 | 188.7 |
| 2g | 162.9 | 188.9 |
| 2h | 162.0 | 188.1 |
3.3. Toxicological and physicochemical properties prediction
For rational design, OSIRIS Property Explorer was used to estimate the physicochemical properties of the new active compounds. Macías-Hernández et al. previously reported physicochemical and toxicological parameter predictions for pyridine derivatives (1a-1h) [13]. Log P, an n-octanol/water partition coefficient, describes the hydrophobicity of the compounds. This parameter must be less than 5 to achieve a good absorption process in living organisms to avoid accumulation in tissue reservoirs [64]. All compounds have Log P values between −0.61 and 2.19. Thus, compounds with good absorption and bioavailability could be suitable for oral administration. Log S, which expresses drug solubility, is also a valid critical parameter for predicting the absorption process [65]. Most compounds have good solubility characteristics. However, the best compounds are 1g and 1h. However, the benzimidazole derivatives 2c and 2e, have Log S values of less than −4; consequently, they could present solubility problems in an aqueous medium.
OSIRIS Property Explorer was also used to estimate the toxicity risk. According to the toxicity risk prediction, pyridine derivatives are suitable. However, only compound 1f has a high risk of irritation due to the presence of the pyrrolidine group [13]. On the other hand, benzimidazole derivatives have a high risk of reproductive effects (Table 3). Nevertheless, the benzimidazole moiety is very common on existing drugs such as anthelmintics and anticancer drugs [66].
Table 3.
OSIRIS physicochemical and toxicological prediction for 2(c–h) and the starting material 2-aminobenzimidazole (II), low risk ( ), high risk (
), high risk ( ).
).
| Properties | Compound |
|||||
|---|---|---|---|---|---|---|
| 2c | 2e | 2g | 2h | II | ||
| Toxicity Risk | Mutagenic | |||||
| Tumorigenic | ||||||
| Irritant | ||||||
| Reproductive | ||||||
| Physicochemical Properties | cLogP | 2.19 | 2.04 | −0.15 | −0.61 | 0.95 |
| LogS (Solubility) | −4.24 | −4.42 | −2.33 | −2.6 | −2.17 | |
| Molecular Weight | 324 | 302 | 333 | 347 | 133 | |
| TPSA | 101.9 | 101.9 | 114.3 | 114.3 | 54.7 | |
| Druglikeness | 3.68 | −2.34 | 4.75 | 4.65 | 0.31 | |
| Drug Score | 0.46 | 0.25 | 0.54 | 0.53 | 0.27 | |
3.4. Antioxidant activity
The reducing power of Fe3+ ion, chelating capacity, and antioxidant activity were determined for 2c, 2e, 2g, and 2h (Table 4). The concentration used is found in Table S1 of the Supplementary material. Testing compounds showed antioxidant activity by DPPH•, ABTS•+, and FRAP methods. Only 2c and 2e could neutralize peroxyl radicals, whereas 2g and 2h showed free iron ions (Fe2+) chelating capacity.
Table 4.
The antiradical capability of monothiooxalamides by DPPH•, ABTS•+, FRAP, ORAC, and Fe(II) chelation methods.
| Compound | DPPH• (TEACa) | ABTS•+ (VCEACb) | FRAP (VCEACb) | ORAC (μmol TE/mL) | Chelating Activity (mM EDTA) |
|---|---|---|---|---|---|
| 2c | 2122.22ab ± 553.35 | 212.41d ± 5.74 | 233.97d ± 2.97 | 10647.81a ± 760.7 | NA |
| 2e | 2227.78a ± 145.61 | 304.47bc ± 9.13 | 233.22d ± 2.34 | 1633.12c ± 145.0 | NA |
| 2g | 2027.78ab ± 120.57 | 29.58ef ± 2.87 | 99.51e ± 7.79 | NA | 0.152e ± 0.07 |
| 2h | 1784.44ab ± 203.67 | 281.29c ± 5.3 | 231.55d ± 1.03 | NA | 5.407a ± 0.07 |
NA (Not Active).
TEAC: Trolox equivalent antioxidant capacity (μmol ET/L).
VCEAC: mg vitamin C/100 mL. The letters (a-f) in each column refer to the pairwise comparisons between groups with a significant difference according to Tukey's HSD test; n = 3, p < 0.05.
Compounds 2c and 2e showed the highest values for the ability to donate electrons to ferric ions and ABTS•+ radical. In addition, monothiooxalamides showed the ability to donate hydrogen atoms to neutralize oxygen radicals in the ORAC test. These compounds can also react with DPPH• by hydrogen and electron transfer mechanisms. The 2-aminobenzimidazole moiety in monothiooxalamides has a higher antioxidant capacity than previously reported 2-aminopyridine derivatives [13]. However, the trend in antioxidant capacity was similar for both groups of monothiooxalamides. Compounds with the highest activity were those with benzylamine (a), 1-phenylethylamine (c), and cyclohexylamine (e) in their structure. Regarding chelating activity, the highest values were obtained for monothiooxalamides with the morpholine group (1h and 2h). Similar to what was observed in compounds 1a-1h, increasing the size of the ligand by adding a methylene group decreases the activity against the DPPH• radical from 2027.78 ± 120.57 (2g) to 1784.44 ± 203.67 μM TE (2h).
3.5. Hemolytic activity assay
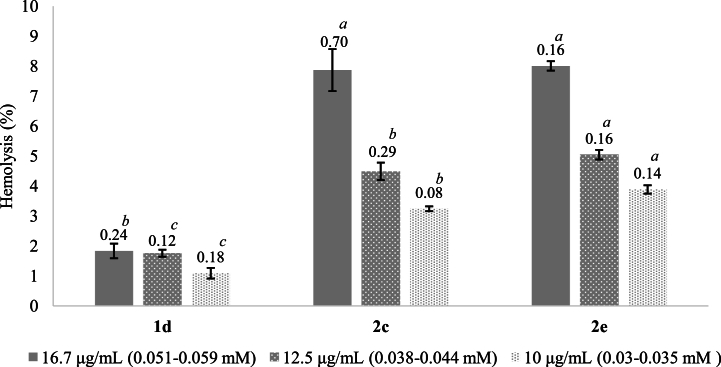
The hemolytic activity of the monothiooxalamides (1a-1h, 2c, 2e, 2g, and 2h) was determined to find the concentrations at which these compounds are not toxic to human RBCs (Table 5). In addition, it allowed the selection of monothiooxalamides suitable for use in future in vivo assays. The results are expressed as a percentage of hemolysis; when this value is less than 10 %, it is considered potentially safe for cell membrane integrity [[25], [26], [27],67]. Compounds 1d, 2c, and 2e showed the highest percentage of hemolysis at 0.08–0.09 mM and were subsequently evaluated at three lower concentrations, ranging from 0.03 to 0.05 mM.
Monothiooxalamides 1f, 1g, and 1h and raw materials (I and II) showed a hemolysis percentage of less than 5 % at the maximum concentrations evaluated. In contrast, compound 1b slightly exceeded 10 % (10.45 % ± 0.36) of hemolysis compared to the positive control (Triton X-100 at 1 %).
On the other hand, the monothiooxalamides that showed a greater capacity to damage human RBCs at 1.44–2.13 mM were 1a, 1d, 1e, 2c, and 2e, with values between 34.5 % and 70.85 %. At the same concentration, the remaining molecules 1c, 2g, and 2h presented a hemolysis percentage of 13.72 %, 20.19 %, and 13.85 %, respectively. Although, compound 2e exerted the highest toxicity at 0.08 mM (13.73 % ± 0.79) when it was evaluated at lower concentrations, it showed similar activity to the monothiooxalamide 2c, and both compounds can be used for subsequent studies at concentrations below 0.03 mM, see Fig. 3 (Table S2, Supplementary material). The results indicated that the hemolytic activity depended on the concentration of all monothiooxalamides.
Fig. 3.
Hemolysis percentages for monothiooxalamides 1d, 2c, and 2e. The letters (a-c) in each column refer to the pairwise comparisons between groups with a significant difference according to Tukey's HSD test; n = 3, p < 0.05.
Błaszczak-Świątkiewicz et al. (2016) reported the hemolytic activity of compounds containing benzimidazole. They found an increase in the hemolysis percentage with increasing concentration, and at 0.17 to 0.18 mMthese compounds showed a hemolysis percentage of less than 10 %. This agrees with the results observed for the monothiooxalamides 2g and 2h, which at concentrations between 0.30 and 0.43 mM did not show a hemolytic activity higher than 10 % [47]. Nandwana et al. (2018) reported another group of molecules derived from benzimidazole, where the hemolytic activity is also concentration-dependent, and at 0.10 mM the molecules caused 3–28 % hemolysis, whereas the monothiooxalamides exhibited 2.6–22 % hemolysis at a concentration of 0.14–0.17 mM [25].
Substitution of 2-aminobenzimidazole by 2-aminopyridine increases the hemolytic activity of all monothiooxalamides at 0.07–0.11 mM. It was also observed that the compounds with the highest toxicity against human RBCs were those with the cyclohexyl group (1e and 2e) and aromatic rings (a, c, and d) in the structure.
In contrast, compounds with the morpholine group (1g, 1h, 2g, and 2h) and 1f had the lowest percentages of hemolysis, which is why they are proposed as candidates for future research. The morpholine group and its derivatives have shown antimicrobial potential and enzyme inhibition activity. Therefore, they are important in designing and developing new therapeutically potent compounds in pharmaceutics [68].
3.5.1. Oxidative hemolysis inhibition assay
The protective effect of monothiooxalamides on human RBCs was evaluated based on the results of previously analyzed hemolytic activity; concentrations were used at which no toxicity was observed. Trolox at various concentrations (25–500 μM) was used as a reference compound to protect human RBCs against oxidative damage. Trolox at 400 μM achieves 72.42 % ± 0.62 inhibition of hemolysis and does not significantly increase the protective effect at higher concentrations. (Table S3, Supplementary material). This agrees with what has been reported in the literature for this antioxidant [69].
The inhibition of oxidative hemolysis induced by peroxyl radicals for the monothiooxalamides and the raw materials depends on the concentration, as shown in Table 6. Some compounds reach values between 80 % and 90 % of inhibition when the concentration increases. The protective effect at the lowest concentration used (0.05–0.07 mM) was greater than 50 % for 1d (69.22 %) and 1a (54.45 %). The remaining compounds are between 30 % and 40 % as follows: 1b (47.74 %) > 1e (45.26 %) > 1g (41.73 %) > 1f (38.83 %) > 1h (30.88 %). These monothiooxalamides exceeded the antihemolytic activity observed for Trolox, which showed a percentage of 17.74 % ± 0.83 at 0.08 mM.
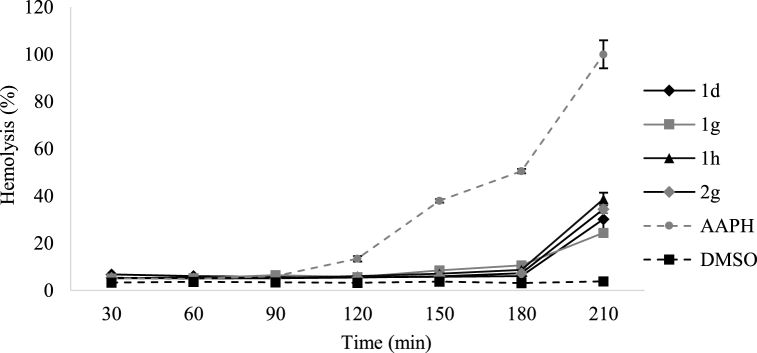
To assess the protective capacity of the compounds over a specific time period, 50 μL samples were taken every 30 min for 3.5 h at a concentration of 0.15–0.21 mM. The results are shown in Fig. 4 (Table S4, Supplementary material).
Fig. 4.
Effect of monothiooxalamides on the kinetics of human RBCs hemolysis induced by 50 mM AAPH at 37 °C in buffered saline. These compounds exerted a significant protective effect against AAPH-induced oxidative hemolysis; n = 3, p < 0.05.
During the first 3 h, the hemolytic activity was greater than 10 % for RBCs treated with 1d, 1h, and 2g compounds. At this time, the hemolysis percentage for 1g was 10.6 %, indicating that the protection provided by monothiooxalamides against oxidative damage to human RBCs is time- and concentration-dependent. The human RBCs protection assay showed that the monothiooxalamides effectively protected these cells and their components against oxidative damage induced by the AAPH radical. An increase in hemolytic activity was observed in the last 30 min of analysis for all compounds. Free radicals can damage the morphological components of blood. In particular, free radicals can cause protein aggregation, peroxidation, and increased membrane permeability [[70], [71], [72]]. The sample containing only the AAPH free radical's initiator presents a hemolytic activity of 13.4 % at 2 h of exposure, increasing to 50.5 % at 3 h, as reported [47,73].
3.6. Brine shrimp lethality bioassay
The percentage lethality (% L) is related to the acute toxicity of the compounds tested and allows the determination of the median lethal concentration (LC50). LC50 was determined by plotting the mean percentage mortality versus the logarithm of the concentration [74,75]. The concentrations chosen for the evaluation of each monothiooxalamide were those at which the hemolytic activity against human RBCs was less than 10 %. The values obtained are shown in the Supplementary material, Table S5.
Potassium dichromate toxicity was evaluated at concentrations of 34, 85, 170, 255, 340, and 680 μM; this compound reached the highest lethality at 340 μM with an LC50 of 70.67 ± 3.75 μM (20.79 ± 1.11 μg/mL) at 24 h after hatching. The effect of potassium dichromate on nauplii changes along the different stages of its development was reported by Ocaranza-Joya et al., in 2019, where they found that potassium dichromate showed the highest LC50 (LC50 = 21 μg/mL) in the first 24 h after hatching, which is consistent with the value reported in the present work [76]. Among the monothiooxalamides, 1f and 1g showed the lowest lethality percentages with LC50 values of 1.393 ± 77 and 5.955 ± 33.8 mM, respectively. As shown in Fig. 5, compound 1f has a lethality of less than 3 % when used at concentrations less than 0.85 mM, whereas 1g reaches a lethality percentage of 70 % from 0.65 mM.
Fig. 5.
Percentages of lethality for compounds 1g, 1f, and potassium dichromate; n = 5, p < 0.05.
For the concentrations evaluated, 2-aminobenzimidazole derivatives 2e and 2g showed the lowest lethality percentages between 0 and 4 %. 2h exhibited a lethality of 8.0 % at 0.14 mM, lower than those reported in the literature for various benzimidazole derivatives [77]. According to the hemolytic activity induced by the monothiooxalamides, 2c was evaluated at 0.08 mM and showed a lethality percentage of 22 %. The most toxic compounds were 1c, 1d, and 1e in all concentrations evaluated (0.03–0.06 mM), which exceeded the potassium dichromate toxicity. Compound 1b had a lethality of 68 % at 0.09 mM, and it showed greater toxicity than 1a, 1f, 1g, and 1h.
The previously described behavior can be related to the structural differences in the monothiooxalamides (Fig. 1). The compounds derived from 2-aminobenzimidazole had less acute toxicity to brine shrimp than those derived from 2-aminopyridine, which was confirmed by comparing the structure of the molecules with groups a, c, e, g, or h. In addition, the substituents such as aromatic rings and cyclohexyls increase the lethality, whereas substituents with heteroatoms such as the morpholine group decrease the toxicity (1f, 1g, and 2g). The presence of the methyl group in 1a and 1c increased the toxicity of compounds.
Regarding the use of methylene or ethylene in structural modifications, the presence of ethylene in 1d increases the lethality percentage by 80 % compared with that in 1a. A similar trend was observed for 1g, 1h, 2g, and 2h, with toxicity increasing as the number of methylenes increases.
3.7. Toxicity in Drosophila melanogaster
To determine toxicity, the survival index (SI) was obtained as the average number of flies recovered in each series compared with the total number of flies in the control (Fig. 6). The response of D. melanogaster larvae to monothiooxalamides was reflected in the number of adult hatched flies. The SI decreased for all the compounds evaluated and was less than 0.5, indicating a high toxicity level. The most toxic compounds were 1a and 1g with a total SI of 0.083 and 0.043, respectively. 1c and 1f showed survival values of 0.22; the molecule with the lowest toxicity was 2g, with a survival value of 0.435, as shown in Fig. 6.
Fig. 6.
Survival of flies treated with monothiooxalamides, E: 72 × 48 h.
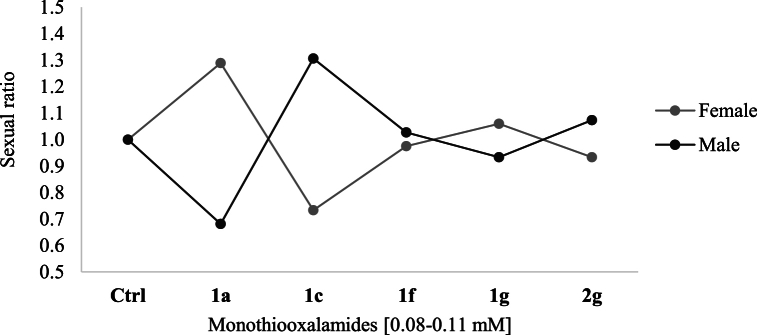
The variation in this toxicity study was related to the structural differences between compounds. The presence of 2-aminobenzimidazole in 2g reduced the toxicity about ten-fold compared with 1g, which is derived from 2-aminopyridine. It was subsequently reported that the morpholine ring can inhibit important enzymes such as kinases, to which it is attributed [67]. Similarly, the methyl group of 1c increased the survival of D. melanogaster compared to 1a. On the other hand, monothiooxalamides 1a and 1c exerted the greatest effect on the sexual ratio, while 1f, 1g, and 2g showed no preferential effect on females or males. When the third stage larvae were exposed to 1a, the proportion of females was greater than that of males, whereas the opposite was observed for 1c (Fig. 7).
Fig. 7.
Sexual index of flies treated with monothiooxalamides, E: 72 × 48 h.
According to the literature, some endocrine disruptors are carcinogenic compounds that cause different effects in mammalian females and males, mainly related to the hormonal regulation of the treated organisms [61].
In the future, it will be necessary to conduct more in vivo studies to complement the findings of this work. In vitro studies have limitations, such as difficulties in accounting for xenobiotic metabolism, problems in simulating the secondary effects of long-term exposure in vitro, and difficulties in extrapolating from biomarkers in vitro to adverse effects in vivo, among others [77].
4. Conclusions
The presence of 2-aminobenzimidazole in the monothiooxalamides structure increases the in vitro antioxidant capacity and decreases the toxicity against brine shrimp and Drosophila melanogaster, all concerning 2-aminopyridine derivatives. The toxicity of monothiooxalamides against human RBCs was concentration-dependent and lower in the 2-aminopyridine group. In addition, these compounds exhibited a time- and concentration-dependent protective effect against radical-induced oxidative hemolysis.
In the synthesis of monothiooxalamides, besides 2-aminobenzimidazole and 2-aminopyridine, different organic groups were used for structural modifications. The morpholine group exerted the lowest toxicity to the compounds evaluated in vitro and in vivo and showed a significant increase in chelating activity compared to the other groups.
The monothiooxalamides with morpholine (1g, 1h, 2g, and 2h) showed a hemolysis percentage of less than 5 % at a concentration of 0.30–0.43 mM. They also showed the most significant protective effect against radical-induced oxidative hemolysis. This means that these compounds can efficiently quench radicals to protect human RBCs from free radical-induced hemolysis and exceed the protective effect observed with Trolox. In this study, Drosophila melanogaster was used to verify the toxic effects of monothiooxalamides with the survival index and sexual ratio as biomarkers. These dipterans showed sensitivity to 2-aminobenzimidazole and 2-aminopyridine derivatives; however, the lowest toxicity was observed with 2g, with a survival of 0.435 compared to untreated flies. Therefore, compound 2g was selected for further research due to its antioxidant capacity and exhibited the lowest toxicity in the in vitro and in vivo models.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
María M. Romero-Chávez: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Carlos Eduardo Macías-Hernández: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Angel Ramos-Organillo: Validation, Supervision, Project administration, Funding acquisition, Formal analysis. Edgar Iván Jiménez-Ruiz: Supervision, Resources, Funding acquisition. Marcela Robles-Machuca: Methodology, Investigation. Victor Manuel Ocaño-Higuera: Resources, Funding acquisition. María Teresa Sumaya-Martínez: Validation, Supervision, Project administration, Funding acquisition, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the Cuerpo Académico de Biotecnología de Alimentos y Productos Funcionales (UAN-CA-255) and Red Temática de Bioproductos y Bioprocesos for their participation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36182.
Contributor Information
Angel Ramos-Organillo, Email: aaramos@ucol.mx.
María Teresa Sumaya-Martínez, Email: teresa.sumaya@uan.edu.mx.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dowden H., Munro J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019;18:495–496. doi: 10.1038/d41573-019-00074-z. [DOI] [PubMed] [Google Scholar]
- 2.Harrison R.K. Phase II, and III failures: 2013-2015. Nat. Rev. Discov. 2016;15:817–818. doi: 10.1038/nrd.2016.184. [DOI] [PubMed] [Google Scholar]
- 3.Sun D., Gao W., Hu H., Zhou S. Why 90 % of clinical drug development fails and how to improve it? Acta Pharm. Sin. B. 2022;12(7):3049–3062. doi: 10.1016/j.apsb.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassar A.E., Kamel A.M., Clarimont C. Improving the decision-making process in the structural modification of drug candidates: enhancing metabolic stability. Drug Discov. Today. 2004;9(23):1020–1028. doi: 10.1016/S1359-6446(04)03280-5. 1. [DOI] [PubMed] [Google Scholar]
- 5.Fleming T.R., Powers J.H. Biomarkers and surrogate endpoints in clinical trials. Stat. Med. 2012;31(25):2973–2984. doi: 10.1002/sim.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drews J. Drug discovery: a historical perspective. Science. 2000;287(5460):1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- 7.Guo H., Sun B., Gao H., Chen X., Liu S., Yao X., Liu X., Che Y. Diketopiperazines from the cordyceps-colonizing fungus Epicoccum nigrum. J. Nat. Prod. 2009;72:2115–2119. doi: 10.1021/np900654a. [DOI] [PubMed] [Google Scholar]
- 8.Wang J.M., Ding G.Z., Fang L., Dai J.G., Yu S.S., Wang Y.H., Chen X.G., Ma S.G., Qu J., Xu S., Du D. Thiodiketopiperazines produced by the endophytic fungus Epicoccum nigrum. J. Nat. Prod. 2010;73:1240–1249. doi: 10.1021/np1000895. [DOI] [PubMed] [Google Scholar]
- 9.Feng M., Tang B., Liang S.H., Jiang X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016;16:1200–1216. doi: 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mustafa M., Winum J.Y. The importance of sulfur-containing motifs in drug design and discovery. Expet Opin. Drug Discov. 2022;17(5):501–512. doi: 10.1080/17460441.2022.2044783. [DOI] [PubMed] [Google Scholar]
- 11.Aleksanyan D.V., Churusova S.G., Brunova V.V., Yu E., Yu O., Peregudov A.S., Klemenkova Z.S., Denisov G.L., Kozlov V.L. Synthesis, characterization, and cytotoxic activity of N -metallated rhenium (I) pincer complexes with (thio) phosphoryl pendant arms. J. Organomet. Chem. 2020;926 doi: 10.1016/j.ica.2022.121369. [DOI] [Google Scholar]
- 12.El-sharief M.A.M.S., Abbas S.Y., El-sharief A.M.S., Sabry N.M., Moussa Z., El-messery S.M., Elsheakh A.R., Hassan G.S., El M.T. 5-Thioxoimidazolidine-2-one derivatives: synthesis, anti-inflammatory activity, analgesic activity, COX inhibition assay, and molecular modeling study. Bioorg. Chem. 2019;87:679–687. doi: 10.1016/j.bioorg.2019.03.075. [DOI] [PubMed] [Google Scholar]
- 13.Macías-Hernández C.E., Romero-Chávez M.M., Mojica-Sánchez J.P., Pineda-Urbina K., Martínez M.T.S., Jiménez-Ruiz E.I., Via L.D., Ramos-Organillo Á. Synthesis and characterization of new monothiooxalamides containing pyridine nuclei with promising antiproliferative and antioxidant activity. J. Mol. Struct. 2022;1265 doi: 10.1016/j.molstruc.2022.133360. [DOI] [Google Scholar]
- 14.Myannik K.A., Beloglazkina E.K., Moiseeva A.A., Baryshnikova T.K., Yarovenko N., Krayushkin M.M. Synthesis and electrochemical study of 2-carbamoyl-4, 5-dihydro- diazole-containing ligands and their complexes with Cu ii, Co ii and Ni ii. Mendeleev Commun. 2018;28:79–80. doi: 10.1016/j.mencom.2018.01.026. [DOI] [Google Scholar]
- 15.Gavrilov K.N., Mikhel I.S., Zheglov S.V., Gavrilov V.K., Chuchelkin I.V., Firsin I.D., Birin K.P., Pytskii I.S., Paseshnichenko K.A., Tafeenko V.A., Chernyshev V.V., Shiryaev A.A. Oxalamide-Based bisdiamidophosphites: synthesis, coordination, and application in asymmetric metallocatalysis. Org. Chem. Front. 2019;6:1637–1648. doi: 10.1039/C9QO00237E. [DOI] [Google Scholar]
- 16.Pilia L., Espa D., Concas G., Congiu F., Marchiò L., Mercuri M.L., Serpe A., De-plano P. Tuning the magnetic, oxidation state and coordination behavior of Iron and Cobalt Complexes on O/S variation in mono-thio and dithiooxamide chelating ligands. New J. Chem. 2015;39:4716–4725. doi: 10.1039/b000000x. [DOI] [Google Scholar]
- 17.Krasavin M., Lukin A., Zhurilo N., Kovalenko A., Zahanich I., Zozulya S., Moore D., Tikhonova I.G. Novel free fatty acid receptor 1 (GPR40) agonists based on 1,3,4-thiadiazole-2-carboxamide scaffold. Bioorg. Med. Chem. 2016;24:2954–2963. doi: 10.1016/j.bmc.2016.04.065. [DOI] [PubMed] [Google Scholar]
- 18.Ma X., Deng S., Liang J., Chen J., Su J., Huang H., Song Q. Unsymmetric monothiooxalamides from S8, bromodifluoro reagents and anilines: synthesis and applications. Tetrahedron Chem. 2022;3 doi: 10.1016/j.tchem.2022.100026. [DOI] [Google Scholar]
- 19.Olaharski A.J., Uppal H., Cooper M., Platz S., Zabka T.S., Kolaja K.L. In vitro to in vivo concordance of a high throughput assay of bone marrow toxicity across a diverse set of drug candidates. Toxicol. Lett. 2009;188(2):98–103. doi: 10.1016/j.toxlet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Pereira C.V., Nadanaciva S., Oliveira P.J., Will Y. The contribution of oxidative stress to drug-induced organ toxicity and its detection in vitro and in vivo. Expet Opin. Drug Metabol. Toxicol. 2012;8(2):219–237. doi: 10.1517/17425255.2012.645536. [DOI] [PubMed] [Google Scholar]
- 21.Podsiedlik M., Markowicz-Piasecka M., Sikora J. Erythrocytes as model cells for biocompatibility assessment, cytotoxicity screening of xenobiotics and drug delivery. Chem. Biol. Interact. 2020;332 doi: 10.1016/j.cbi.2020.109305. [DOI] [PubMed] [Google Scholar]
- 22.Farag M.R., Alagawany M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem. Biol. Interact. 2018;279:73–83. doi: 10.1016/j.cbi.2020.109305. [DOI] [PubMed] [Google Scholar]
- 23.Magalhães A.S., Silva B.M., Pereira J.A., Andrade P.B., Valentão P., Carvalho M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem. Toxicol. 2009;47(6):1372–1377. doi: 10.1016/j.fct.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Shiva Shankar Reddy C.S., Subramanyam M.V., Vani R., Asha-Devi S. In vitro models of oxidative stress in rat erythrocytes: effect of antioxidant supplements. Toxicol. Vitro. 2007;21:1355–1364. doi: 10.1016/j.tiv.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Błaszczak-Świątkiewicz K., Sikora J., Szymański J., Danilewicz M., Mikiciuk-Olasik E. Biological evaluation of the toxicity and the cell cycle interruption by some benzimidazole derivatives. Tumor Biol. 2016;37(8):11135–11145. doi: 10.1007/s13277-016-4828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbán P., Liptrott N.J., Bremer S. Overview of the blood compatibility of nanomedicines: a trend analysis of in vitro and in vivo studies. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(3) doi: 10.1002/wnan.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luna-Vázquez-Gómez R., Arellano-García M.E., García-Ramos J.C., Radilla-Chávez P., Salas-Vargas D.S., Casillas-Figueroa F., Ruiz-Ruiz B., Bogdanchikova N., Pestryakov A. Hemolysis of human erythrocytes by Argovit™ AgNPs from healthy and diabetic donors: an in vitro study. Materials. 2021;14(11):2792. doi: 10.3390/ma14112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer D., Li Y., Ahlemeyer B., Krieglstein J., Kissel T. In vitro cytotoxicity testing 686 of polycations: influence of polymer structure on cell viability and hemolysis. Q6 687 Biomaterials. 2003;24(7):1121–1123. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 29.Markowicz-Piasecka M., Mikiciuk-Olasik E., Joanna S. Stability of erythrocyte membrane and overall hemostasis potential - a biocompatibility study of mebrofenin and other iminodiacetic acid derivatives. Pharmacol. Rep. 2015;67(6):1230–1239. doi: 10.1016/j.pharep.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Cimen M.Y. Free radical metabolism in human erythrocytes. Clin. Chim. Acta. 2008;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobsen L.B., Nichols D.E., McLaughlin J.L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45(5):31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- 32.Montes-Ávila J., Sarmiento-Sánchez J.I., Delgado-Vargas F., Rivero I.A., Díaz-Camacho S.P., Uribe-Beltrán M. Antioxidant activity and antimicrobial evaluation of 1-benzyl-1,2,3-triazole. Acta Univ. 2016:63–67. doi: 10.15174/au.2016.937. [DOI] [Google Scholar]
- 33.Bustos-Obregon E., Vargas A. Chronic toxicity bioassay with populations of the crustacean Artemia salina exposed to the organophosphate diazinon. Biol. Res. 2010;43(3):357–362. [PubMed] [Google Scholar]
- 34.Pandey U.B., Nichols C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011;63(2):411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spieth H.T. Courtship behavior in Drosophila. Annu. Rev. Entomol. 1974;19:385–405. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- 36.Demir E., Babur O., Rodchenkov I., A Aksoy B., Fukuda K.I., Gross B., Sümer O.S., Bader G.D., Sander C. Using biological pathway data with paxtools. PLoS Comput. Biol. 2013;9(9) doi: 10.1371/journal.pcbi.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 38.Pérez D.J., Díaz-reval M.I., Zakai U.I., Gómez Z., Razo-Hernández R.S., Sumaya-Martínez M.T., Ramos-Organillo Á. Silicon containing ibuprofen derivatives with antioxidant and anti-inflammatory activities: an in vivo and silico study. Eur. J. Pharmacol. 2017;814:18–27. doi: 10.1016/j.ejphar.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 39.Hinneburg I., Damien Dorman H.J., Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–129. doi: 10.1016/j.foodchem.2005.03.028. [DOI] [Google Scholar]
- 40.Apak R., Güçlü K., Demirata B., Özyürek M., Çelik S.E., Bekta s¸ o glu B., Berker K.I., Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang D., Ou B., Hampsch-Woodill M., Flanagan J.A., Prior R.L. Highthroughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 42.Marcela M., Santiago Z., Tania R.J., Stephania R., Andrés F.A., Maria E.M., Pedro Z., Benjamín A.R. Mangiferin content, carotenoids, tannins and oxygen radical absorbance capacity (ORAC) values of six mango (Mangifera indica) cultivars from the Colombian Caribbean. J. Med. Plants Res. 2017;11:144–152. doi: 10.5897/jmpr2017.6335. [DOI] [Google Scholar]
- 43.Gulcin I., Buyukokuroglu M.E., Kufrevioglu O.I. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 2003;34:278–281. doi: 10.1034/j.1600-079X.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 44.Tolwinski N.S. Introduction: drosophila-A model system for developmental biology. J. Dev. Biol. 2017;20(5):3–9. doi: 10.3390/jdb5030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manna C., Galletti P., Cucciolla V., Montedoro G., Zappia V. Olive oil hydroxytyrosol protects human erythrocytes against oxidative damages. J. Nutr. Biochem. 1999;10:159–165. doi: 10.1016/s0955-2863(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 46.López-Nahuatt G., Sumaya-Martínez M.T., Jiménez-Ruiz E.I., Sánchez-Herrera L.M., Bautisa-Rosales P.U., Medina-Carrillo R.E., Guzmán-Ceferino J. Hemolytic, antimicrobial and antioxidant activity of aqueous extracts of calyx from roselle. Rev. Biociencias. 2022;7:e995. doi: 10.15741/revbio.07.e995. [DOI] [Google Scholar]
- 47.Nandwana N.K., Singh R.P., Patel O.P.S., Dhiman S., Saini H.K., Jha P.N., Kumar A. Design and synthesis of imidazo/benzimidazo[1,2- c]quinazoline derivatives and evaluation of their antimicrobial activity. ACS Omega. 2018;3:16338–16346. doi: 10.1021/acsomega.8b01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko F.N., Hsiao G., Kuo Y.H. Protection of oxidative hemolysis by demethyldiisoeugenol in normal and β-thalassemic red blood cells. Free Radic. Biol. Med. 1997;22:215–222. doi: 10.1016/S0891-5849(96)00295-X. [DOI] [PubMed] [Google Scholar]
- 49.Balalakshmi C., Gopinath K., Govindarajan M., Lokesh R., Arumugam A., Alharbi N.S., Kadaikunnan S., Khaled J.M., Benelli G. Green synthesis of gold nanoparticles using a cheap Sphaeranthus indicus extract: impact on plant cells and the aquatic crustacean Artemia nauplii. J. Photochem. Photobiol., B. 2017;173:598–605. doi: 10.1016/j.jphotobiol.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 50.Santos Filipe M., Isca V.M.S., Ntungwe N.E., Princiotto S., Díaz-Lanza A.M., Rijo P. Lethality bioassay using Artemia salina L. J. Vis. Exp. 2022;188 doi: 10.3791/64472. [DOI] [PubMed] [Google Scholar]
- 51.Sarah Q.S., Anny F.C., Misbahuddin M. Brine shrimp lethality assay, Bangladesh. J. Pharmacol. 2017;12:186–189. doi: 10.3329/bjp.v12i2.32796. [DOI] [Google Scholar]
- 52.Htun H.H., Mandalay M., San H.H., Taungoo M., Swe Z.M. Effects of salinity on the hatching efficiency of Artemia cysts decapsulation. Int. J. Sci. Eng. Appl. 2019;8(8):341–344. doi: 10.7753/IJSEA0808.1019. [DOI] [Google Scholar]
- 53.Michael A.S., Thompson C.G., Abramovitz M. Artemia salina as a test organism for bioassay. Science. 1956;123(3194):464. doi: 10.1126/science.123.3194.464. [DOI] [PubMed] [Google Scholar]
- 54.Raka S.C., Rahman A., Hussain F., A Rahman S.M. Synthesis, characterization and in vitro, in vivo, in silico biological evaluations of substituted benzimidazole derivatives. Saudi J. Biol. Sci. 2022;29(1):239–250. doi: 10.1016/j.sjbs.2021.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nieto R I.J., Salama A.M., Cataño P J.E., Chegwin C.A. Determinación de la toxicidad de Pleurotus ostreatus, Pleurotus pulmonarius y Paxillus involutus sobre Artemia salina. Rev. Iberoam. De. Micol. 2008;25(3):186–187. doi: 10.1016/S1130-1406(08)70044-5. [DOI] [PubMed] [Google Scholar]
- 56.Anaya-Gil J., Ramos-Morales P., Muñoz-Hernandez A., Bermúdez A., Gomez-Estrada H H. In vivo evaluation of the toxic activity and genotoxicity of the Hymenaea courbaril L.'s resin in Drosophila melanogaster. Saudi J. Biol. Sci. 2022;29(1):480–488. doi: 10.1016/j.sjbs.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jenkins J.B., Tompkins L. Effects of amiloride on taste responses of Drosophila melanogaster adults and larvae. J. Insect Physiol. 1990;36(9):613–618. doi: 10.1016/0022-1910(90)90064-M. [DOI] [Google Scholar]
- 58.Graf U., van Schaik N. Improved high bioactivation cross for the wing somatic mutation and recombination test in Drosophila melanogaster. Mutat. Res. 1992;271(1):59–67. doi: 10.1016/0165-1161(92)90032-h. [DOI] [PubMed] [Google Scholar]
- 59.Bauer J.H., Goupil S., Garber G.B., Helfand S.L. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 2004;101(35):12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamra O.P., Gollapudi B. Mutagenic effects of sodium azide in Drosophila melanogaster. Mutat. Res. 1979;66(4):381–384. doi: 10.1016/0165-1218(79)90049-1. [DOI] [PubMed] [Google Scholar]
- 61.Cederroth C.R., Auger J., Zimmermann C., Eustache F., Nef S. Soy, phytoestrogens, and male reproductive function: a review. Int. J. Androl. 2010;33(2010):304–316. doi: 10.1111/j.1365-2605.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- 62.Padilla-martínez F.J., Brito M.A., Geniz E.D., Rojas R.C., Saavedra J.B.R., Höp H., Tlahuextl M., Contreras R., Ј N.N. Three-center intramolecular hydrogen bonding in oxamide derivatives . NMR and X-ray diffraction study. J. Chem. Soc., Perkin Trans. 1998;2:401–406. doi: 10.1039/A704640E. [DOI] [Google Scholar]
- 63.Wiberg K.B., Wang Y., Box P.O., Haven N. A comparison of some properties of C = O and C = S bonds. ARKIVOC (Gainesville, FL, U. S.) 2011;2011:45–56. doi: 10.3998/ark.5550190.0012.506. [DOI] [Google Scholar]
- 64.Ayati A., Falahati M., Irannejad H., Emami S. Synthesis, in vitro antifungal evaluation and in silico study of 3-azolyl-4-chromanone phenylhydrazones. DARU J. Pharm. Sci. 2012:1–7. doi: 10.1186/2008-2231-20-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee Y.T., Tan Y.J., Oon C.E. Benzimidazole and its derivatives as cancer therapeutics: the potential role from traditional to precision medicine. Acta Pharm. Sin. B. 2023;13:478–497. doi: 10.1016/j.apsb.2022.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.V. Padmavath, G. S Reddy, A. Padmaja, P. Kondaiah, A. Shazia, Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4- triazoles, Eur. J. Med. Chem. 44 (200) 2106-2112, 10.1016/j.ejmech.2008.10.012. [DOI] [PubMed]
- 67.Gul S., Aziz-ur-Rehman, Athar-Abbasi M., Khan K.M., Nafeesa K., Siddiqa A., Nadeem-Akhtar M., Shahid M., Subhani Z. Synthesis, antimicrobial evaluation and hemolytic activity of 2-[[5-alkyl/aralkyl substituted-1,3,4-Oxadiazol-2-yl]thio]-N-[4-(4-morpholinyl)phenyl]acetamide derivatives. Saudi Chem. Soc. 2017;21(1):S425–S433. doi: 10.1016/j.jscs.2014.04.005. [DOI] [Google Scholar]
- 68.Takebayashi J., Chen J., Tai A. A method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. Methods Mol. Biol. 2010;594:287–296. doi: 10.1007/978-1-60761-411-1_20. [DOI] [PubMed] [Google Scholar]
- 69.Clemens M.R., Waller H.D. Lipid peroxidation in erythrocytes. Chem. Phys. Lipids. 1987;45:251–268. doi: 10.1016/0009-3084(87)90068-5. [DOI] [PubMed] [Google Scholar]
- 70.Stolze K., Nohl H. Free radical formation and erythrocyte membrane alterations during MetHb formation induced by the BHA metabolite, tert-butylhydroquinone. Free Radic. Res. 1999;30:295–303. doi: 10.1080/10715769900300321. [DOI] [PubMed] [Google Scholar]
- 71.Sinha A., Chu T., Dao M., Chandramohanadas R. Single-cell evaluation of red blood cell bio-mechanical and nano-structural alterations upon chemically induced oxidative stress. Sci. Rep. 2015;5:9768. doi: 10.1038/srep09768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aswathanarayanappa C., Bheemappa E., Bodke Y.D., Krishnegowda P.S., Venkata S.P., Ningegowda R. Synthesis and evaluation of antioxidant properties of novel 1,2,4-triazole-based Schiff base heterocycles. Arch. Pharm. (Weinheim) 2013;346(12):922–930. doi: 10.1002/ardp.201300202. [DOI] [PubMed] [Google Scholar]
- 73.Cyboran-Mikołajczyk S., Męczarska K., Solarska-Ściuk K., Ratajczak-Wielgomas K., Oszmiański J., Jencova V., Bonarska-Kujawa D. Protection of erythrocytes and microvascular endothelial cells against oxidative damage by Fragaria vesca L. and Rubus idaeus L. leaves extracts - the Mechanism of Action. Molecules. 2022;27:5865. doi: 10.3390/molecules27185865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Syahmi A.R.M., Vijayarathna S., Sasidharan S., Latha L.Y., Kwan Y.P., Lau Y.L., Shin L.N., Chen Y. Acute oral toxicity and brine shrimp lethality of Elaeis guineensis Jacq., (oil palm leaf) methanol extract. Molecules. 2010;15:8111–8121. doi: 10.3390/molecules15118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ocaranza-Joya V., Manjarrez-Alcivar I., Ruiz-González L., Guerrero-Galván S., Vega-Villasante F. Sensitivity of different stages of Artemia franciscana to potassium dichromate. Pan Am. J. Aquat. Sci. 2019;14:8–12. [Google Scholar]
- 76.Özkay Y., Tunalı Y., Karaca H., Işıkdağ I. Antimicrobial activity of a new series of benzimidazole derivatives. Arch Pharm. Res. (Seoul) 2011;34(9):1427–1435. doi: 10.1007/s12272-011-0903-8. [DOI] [PubMed] [Google Scholar]
- 77.Tice R.R., Austin C.P., Kavlock R.J., Bucher J.R. Improving the human hazard characterization of chemicals: a Tox21 update. Environ. Health Perspect. 2013;121(7):756–765. doi: 10.1289/ehp.1205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.