Abstract
Heavy metal contamination in seafood is a developing concern due to the potential negative consequences on human health. Egypt's coastal regions are important for seafood production and consumption, making it critical to assess the safety of these aquatic resources. The current study examined toxic metal levels (Hg, Pb, Cd, and AS) in 96 samples of sardine and shrimp from four Egyptian coastal governorates (Alexandria, Kafr El-Sheikh, Damietta, and Port Said) from 2019 to 2021. Inductively coupled plasma-optical emission spectroscopy (ICP-OES) was used to investigate the four hazardous metals. The recovery percentages of the determined metals ranged between 97 % and 99 %. Limits of detection (LOD) and limit of quantification (LOQ) for the determined metals ranged from 0.001 to 0.0077 mg/l and from 0.0035 to 0.026 mg/l, respectively. Mercury (Hg) was not found in any of the samples tested. The concentrations of Pb and Cd in the sardines and shrimp samples were higher in the winter seasons than in the summer seasons. Meanwhile, the difference in seasons had no effect on the concentration of As in the sardines and shrimp samples. The highest concentrations of the other three metals in the sardine and shrimp samples were used to calculate the Estimated Daily Intake (EDI), Target Hazard Quotient (THQ), and Hazard Index (HI). The obtained THQ as well as the HI of Pb, Cd, and As were all less than 1.0 (with the exception of sardine samples from Kafr El-Sheikh Governorate, which recorded 1.262), indicating that there is no significant health risk to the consumer from consuming such sardines and shrimp from these governorates. The effect of different seasons on the concentrations of the metals under study does not have a specific behavior, but varies according to the governorate, the type of sample, and the type of contaminated metal. Due to the high level of contamination with heavy metals in sardine samples collected from Kafr El-Sheikh governorate (TTHQ = 1.26), an environmental study is required to determine the causes of contamination and control them.
Keywords: Risk assessment, Heavy metals, Sardine, Shrimp, Egypt
1. Introduction
Egypt is one of the most important countries in fish production due to its unique geographical location, as it occupies the northeastern corner of the African continent and has a coastline extending for approximately 2500 kilometers on the Mediterranean coast in the north and the Red Sea coast in the east, in addition to lakes and the Nile River and its tributaries [1], [2]. Egypt is not only one of the most important fish-producing countries, but it is also one of the countries with a high per capita intake of seafood, with an annual consumption of 22 kg, which is comparable to the European average [3].
The term "heavy metal" refers to any metallic element with a relatively high density that is poisonous or non-toxic even at low concentrations. Heavy metals are naturally occurring components of the Earth's crust that cannot be eliminated. The amount of naturally existing heavy metals in the environment, particularly the marine ecosystem, is considerably increased by human activities such as industry, agriculture, and mining. As a result, marine creatures (fish, shellfish, and crabs) can accumulate dangerous levels of these metals. Fish and other seafood are frequently among the most common causes of metal exposure in the general population. Toxic metals in food that exceed allowed levels are hazardous to human health and are forbidden by numerous national and international rules [4], [5], [6], [7], [8].
The IARC [9] (International Agency for Research on Cancer) classified cadmium (Cd) and arsenic (As) as carcinogenic to humans (Group 1), and lead as probably carcinogenic to humans (Group 2B). Heavy metals' harmful effects include impaired kidney function, as with lead (Pb), cadmium (Cd), and mercury (Hg), as well as liver damage, as with Pb and Cd. The same is true for Cd and Pb: too much Cd can cause high blood pressure, while Hg and Pb have opposing effects on neurological processes, with Hg causing teratogenic effects and malignancies [10], [11], [12]. Because of their ubiquitous presence in the environment, persistence, bioaccumulation properties, and toxicological potential, these three metallic elements, Pb, Cd, and Hg, are recognized to possibly influence marine ecosystems [13]. Arsenic is mostly found in fish and other shellfish, with an estimated daily intake of less than 0.35 mg. It is well understood that the marine environment has a substantial impact on As levels. Arsenic levels in sea fish were discovered to be approximately ten times greater than those in freshwater fish [14].
Shellfish, notably shrimp and clams, were popular due to their high nutritional content. However, trading processes in seafood marketplaces pose quality and safety challenges [15]. Most fish are easily contaminated with hazardous heavy metals like Cd, Pb, As, and Hg. Agricultural, industrial, and sanitary drainage, waste from unintentional chemical spills, and gasoline from fishing boats are all sources of pollution [16]. Heavy metals enter the flesh of fish in general by eating on benthic worms and crustaceans, which in turn feed on heavy metal-rich sediments [17]. Because toxic heavy metals are difficult to decompose, they persist and accumulate in environmental media such as water and sediment, and bioaccumulate in aquatic creatures such as fish to levels that are hazardous to human health. This bioaccumulation of heavy metals in fish and marine organisms is influenced by a number of factors, including the area and time of fishing, fish feeding habits, fish species, sex, age, size, levels of these heavy metals in the water, and duration of exposure, as well as other variables such as pH, water salinity, and temperature [18].
The various health benefits provided by fish intake may be harmed due to the presence of heavy metals such as Pb, Cd, As, and Hg, which can be harmful to human health if taken in hazardous amounts. As a result, monitoring and measuring the quantities of such metals in fish meat is critical for ensuring compliance with food safety specifications, laws, and regulations, as well as consumer protection. The toxicity of these heavy metals may be affected by polymorphisms and chemical composition, which necessitates the use of prediction modeling processes to determine toxic metal profiles from measured total metal concentrations [19], [20], [21], [22]. Some studies have found that heavy metal concentrations in fish and sediment samples, such as Hg, Cd, zinc (Zn), and Pb, are influenced and alter as the seasons change [23]. The purpose of this study is to assess the levels of heavy metals (Pb, Hg, As, and Cd) in shrimp and sardines in the coastal governorates markets of Alexandria, Kafr El-Sheikh, Damietta, and Port Said, Egypt. These markets are among the country's most significant for fish and seafood, and the study will also examine the impact of the winter and summer seasons on the quality of these products and assess the risk to human health.
2. Materials and methods
2.1. Sample collection
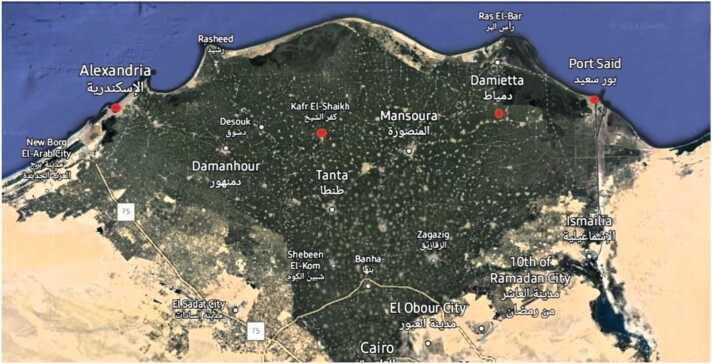
Sardine (Sardina pilchardus) and shrimp (Trachypenaeus curvirostris) samples (48 samples each) were collected from the fish markets of four governorates located on the Mediterranean Sea: Alexandria, Kafr El-Sheikh, Damietta, and Port Said. Samples were collected during the winter seasons (2019 and 2020) and summer seasons (2020 and 2021), 48 samples each. The collected samples were wrapped in polyethylene bags and immediately preserved in an ice box, then transferred to the laboratory and kept frozen at 20°C until analysis. Fig. 1
Fig. 1.
Sampling sites of the four coastal Egyptian governorates.
2.2. Heavy metals determination
2.2.1. Chemicals and reagents
All reagents were of analytical grade (Merck, Germany). Standard stock solutions (1000 mg/l) of the investigated heavy metals were obtained from Sigma and then diluted to the corresponding metal solution using 10 % HNO3. To avoid metal contamination and adhesion, no metal or glass equipment was used.
2.2.2. Sample preparation
A microwave reaction system (TOPwave Analytikjena GmbH) was used for the digestion of sardine fish and shrimp samples, according to the European Committee for Standardization [24]. Briefly, 0.5 g of homogenized sardine fish or shrimp sample was weighted and transferred into the PTFE vessels for microwave digestion. Subsequently, 9 ml of nitric acid (69 %) and 1 ml of H2O2 were added to the sample. The vessel was closed completely and excellently, and then transferred to the microwave until complete digestion. Digestion was performed in the microwave oven by a temperature-controlled program: heating to 200 °C for 15 min, holding for 15 min, and cooling to 85 °C for 15 min. After cooling to room temperature, the vessels was opened to evaporation the brown vapors resulting from the digestion of sample by nitric acid. The contents of the vessel were transferred to a volumetric flask (25 ml) and diluted with ultrapure water to the mark, then analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES).
2.2.3. Determination of heavy metals
The heavy metals under investigation were analyzed at the Water Pollution Department, National Research Centre, using the Agilent 5100 Synchronous Vertical Dual View (SVDV) ICP-OES according to APHA [25] equipped with the Agilent Vapor Generation Accessory VGA 77. For each series of measurements, a calibration curve of at least three or more points was constructed using different concentrations of heavy metal standards (Merck, Germany). The accuracy and precision of the Cd, Pb, As, and Hg ions measurements were confirmed using external reference standards from Merck, and a quality control sample from the National Institute of Standards and Technology (NIST) was used to confirm the instrument reading.
2.3. Human health risk assessment
2.3.1. Estimated daily intake (EDI)
The estimated daily intake (mg/kg bw/day) of the four heavy metals detected in the sardine and shrimp samples was calculated using the highest detected concentrations in each governorate in this study. The EDI was calculated using the following formula [23], [26], [27], [28]:
Where:
C: The heavy metal concentration in fish muscles (mg/kg).
FIR: The food ingestion rate (16 g/day for sardine and 9 g/day for shrimp).
BW: The average adult body weight (70 kg).
2.3.2. Target hazard quotient (THQ)
The health risk associated with consuming contaminated sardine and shrimp samples was measured on the basis of the Target Hazard Quotient (THQ). The THQ is the parameter that is considered in assessing the non-carcinogenic effects as described by Monier et al. [23] and USEPA [29]. The THQ is determined by the following formula:
Where:
ED: Exposure duration (70 years, average lifetime)
C: The heavy metal concentration in fish muscles (mg/kg)
FIR: The food ingestion rate (g/day) (16 g/day for sardine and 9 g/day for shrimp) [23].
EF: The exposure frequency (156 days/year for someone eating fish 3 times a week) [23].
RfD: The oral reference doses are 0.001, 0.0036, and 0.0003 mg/kg/day for Cd, Pb, and inorganic As respectively [30].
BW: The average adult body weight (70 kg);
AT: The average exposure time (156 days/year × exposure years (70 years) = 10,920 days)
The Hazard Index (HI) or Total Target Hazard Quotient (TTHQ) is a summation of the target hazard quotients (THQ) for the investigated heavy metals. To evaluate the risk of the combined metals, the following equation was used: [30], [31], [32].
| TTHQ = THQ Pb + THQ Cd + THQ As |
2.4. Quality control
All used glassware was soaked overnight in 10 % (v/v) nitric acid, rinsed with deionized water and dried in oven at 110°C before being used. All reagents were of analytical reagent grade. Deionized water was used for all dilutions. All experiments were carried out in triplicate. Relative standard deviations (RSD) were less than 5 % in all experiments. The recovery was within the accepted range between 97 % and 99 %. Also, limit of detection (LOD) and limit of quantification (LOQ) for each metal were determined.
2.5. Statistical analysis
The results were subjected to a one-way analysis of variance (ANOVA) of the general linear model (GLM) using the SAS statistical package [33]. The results were the average of three experiments (p≤0.05).
3. Results
3.1. Heavy metal levels in fish samples (wet weight)
Recovery percentage, limit of detection (LOD) and limit of quantification (LOQ) for each metal are presented in Table 1.
Table 1.
Recovery percentage, limit of detection (LOD) and limit of quantification (LOQ) for the determined metals.
| Metals | Recovery (%) | LOD (mg/l) | LOQ (mg/l) |
|---|---|---|---|
| Lead (Pb) | 97 | 0.0077 | 0.026 |
| Cadmium (Cd) | 98 | 0.002 | 0.0076 |
| Arsenic (As) | 99 | 0.001 | 0.0035 |
| Mercury (Hg) | 98 | 0.002 | 0.0065 |
LOD: Limits of Detection; LOQ: Limits of Quantification.
3.1.1. Lead
Table 2 shows that Pb values in samples obtained from Alexandria governorate in different seasons ranged from 0.104 to 0.442 mg/kg in sardine samples and between below detection limit (<d.l.) and 0.333 mg/kg in shrimp samples. According to the statistical research, there are significant differences between the four seasons. Samples collected from Kafr El-Sheikh governorate in different seasons contained Pb values in sardine samples ranged from 0.352 to 0.611 mg/kg, while shrimp samples ranged from 0.072 to 0.388 mg/kg. The statistical analysis revealed that there are substantial changes between the four seasons, with the exception of shrimp samples from the winter 2019 and winter 2020 seasons. Pb values in Damietta samples taken at different seasons ranged from 0.099 to 0.482 mg/kg in sardine samples and from 0.148 to 0.534 mg/kg in shrimp samples. Except for the summer 2020 and summer 2021 seasons in sardine and the summer 2020 and winter 2020 seasons in shrimp, the statistical analysis revealed significant variances between the four seasons. Pb values in Port-Said governorate samples ranged from <d.l. to 0.231 mg/kg in sardine samples, and between <d.l. and 0.170 mg/kg in shrimp samples. The statistical analysis revealed that there are significant differences between the four seasons, with the exception of shrimp samples from the winter 2019 and winter 2020 seasons, which were non-significant.
Table 2.
Lead concentrations (mg/kg) in sardine and shrimp samples collected from different Egyptian governorates through different seasons.
| Sample | Season | Governorate |
LSD | |||
|---|---|---|---|---|---|---|
| Alexandria | Kafr El-Sheikh | Damietta | Port Said | |||
| Sardine | Winter 2019 | 0.442d ± 0.016 | 0.611a ± 0.015 | 0.482c ± 0.017 | 0.231f ± 0.010 | 0.034 |
| Summer 2020 | 0.157h ± 0.005 | 0.352e ± 0.017 | 0.099i ± 0.009 | <d.l. | ||
| Winter 2020 | 0.373e ± 0.013 | 0.563b ± 0.014 | 0.253f ± 0.013 | 0.195g ± 0.007 | ||
| Summer 2021 | 0.104i ± 0.008 | 0.427d ± 0.012 | 0.115i ± 0.008 | 0.156h ± 0.011 | ||
| Shrimp | Winter 2019 | 0.333c ± 0.017 | 0.388b ± 0.017 | 0.534a ± 0.021 | 0.170e ± 0.014 | 0.044 |
| Summer 2020 | 0.086f ± 0.007 | 0.072f ± 0.008 | 0.236d ± 0.019 | <d.l. | ||
| Winter 2020 | 0.244d ± 0.016 | 0.385b ± 0.017 | 0.278d ± 0.013 | 0.165e ± 0.010 | ||
| Summer 2021 | <d.l. | 0.164e ± 0.011 | 0.148e ± 0.028 | 0.074f ± 0.015 | ||
Means followed by different subscripts are significantly different at the 5 % level; <dl: below detection limit
LOD and LOQ were 0.0077 and 0.026 mg/l, respectively. Recovery was 97 %.
The range of Pb concentrations in sardine samples ranged from <d.l. (in the summer 2020 season in Port Said governorate) to 0.611 mg/kg (in the winter 2019 season in Kafr El-Sheikh governorate) when Pb concentrations in shrimp samples were compared in the four governorates and four seasons. All of these readings are less than the FDA's maximum permitted threshold of 10 parts per million. The concentration of lead in the shrimp samples was found to vary between <d.l. (during the summer season of 2021 in Alexandria and Port Said governorates, respectively) and 0.534 mg/kg (during the winter season of 2019 in Damietta governorate). Notably, both of these concentrations fall below the maximum allowable limit of 10 ppm set by the FDA. Extrapolating the original data (raw data), it was discovered that the number of samples that surpassed the Egyptian permitted limit (0.3 mg/kg) in the case of sardine samples was 44 %, and this percentage was spread between summer samples (29 %), and winter samples (71 %). The percentage of shrimp samples that exceeded the Pb acceptable limit was 27 %. They were all in the winter season.
3.1.2. Cadmium
The samples collected from the studied governorates in different seasons contained Cd concentrations ranging from <d.l. to 0.377 mg/kg in sardine samples, while the range was between <d.l. and 0.331 mg/kg in shrimp samples (Table 3). The levels of Cd in sardine and shrimp samples were highly elevated in the winter season compared with those in the summer season, with significant differences between them. The obtained Cd levels were mostly less than the maximum allowable by the FDA (0.2 mg/kg), except for some samples in Kafr El-Sheikh governorate for the winter 2019, summer 2020, and winter 2020 seasons, as well as some samples in Damietta governorate for the winter 2019 and winter 2020 seasons. Also, it was noted that 50 % of the sardine’s samples exceeded the Egyptian permissible limit of Cd (0.05 mg/kg). In the case of the shrimp samples, they exceeded the permissible limit by 63 % (64 % in the winter and 36 % in the summer season).
Table 3.
Cadmium concentrations (mg/kg) in sardine and shrimp samples collected from different Egyptian governorates through different seasons.
| Sample | Season | Governorate |
LSD | |||
|---|---|---|---|---|---|---|
| Alexandria | Kafr El-Shaikh | Damietta | Port Said | |||
| Sardine | Winter 2019 | 0.042g ± 0.013 | 0.377a ± 0.017 | 0.200d ± 0.010 | 0.035g ± 0.008 | 0.029 |
| Summer 2020 | <d.l. | 0.275c ± 0.019 | 0.084f ± 0.005 | <d.l. | ||
| Winter 2020 | 0.033g ± 0.007 | 0.311b ± 0.015 | 0.194d ± 0.009 | <d.l. | ||
| Summer 2021 | <d.l. | 0.125e ± 0.014 | 0.078f ± 0.006 | <d.l. | ||
| Shrimp | Winter 2019 | 0.101e ± 0.008 | 0.331a ± 0.019 | 0.312a ± 0.014 | 0.073e ± 0.007 | 0.030 |
| Summer 2020 | 0.041f ± 0.005 | 0.171d ± 0.013 | 0.158d ± 0.008 | <d.l. | ||
| Winter 2020 | 0.079e ± 0.008 | 0.266b ± 0.016 | 0.233c ± 0.014 | <d.l. | ||
| Summer 2021 | 0.035f ± 0.004 | 0.165d ± 0.013 | 0.150d ± 0.010 | <d.l. | ||
Means followed by different subscripts are significantly different at the 5 % level; <dl: below detection limit
LOD and LOQ were 0.002 and 0.0076 mg/l, respectively; Recovery was 98 %.
3.1.3. Arsenic
The results presented in Table 4 show that the lowest levels of As in sardines (0.006 mg/kg) and shrimp (0.083 mg/kg) samples were recorded in the winter 2019 season of Damietta and Port Said governorates, respectively. Meanwhile, the highest levels of As in sardines (0.475 mg/kg) and shrimp (0.965 mg/kg) samples were recorded in Kafr El-Sheikh (summer 2021 season) and Alexandria (winter 2019 season) governorates, respectively. The concentrations of As in the sardine samples in the four governorates at different seasons are below the level of 3 ppm specified by the FDA [34], except for the samples of the summer 2021 and winter 2020 seasons in Kafr El-Sheikh and Port Said Governorates, where they were above the permissible limit.
Table 4.
Arsenic concentrations (mg/kg) in different seafood samples collected from different Egyptian governorates through different seasons.
| Sample | Season | Governorate |
LSD | |||
|---|---|---|---|---|---|---|
| Alexandria | Kafr El-Shaikh | Damietta | Port Said | |||
| Sardine | Winter 2019 | 0.147e ± 0.012 | 0.225d ± 0.013 | 0.006g ± 0.002 | 0.020g ± 0.002 | 0.032 |
| Summer 2020 | 0.084f ± 0.005 | 0.174e ± 0.012 | 0.105f ± 0.014 | 0.288c ± 0.018 | ||
| Winter 2020 | 0.221d ± 0.013 | 0.277c ± 0.012 | 0.078f ± 0.006 | 0.380b ± 0.014 | ||
| Summer 2021 | 0.157e ± 0.005 | 0.475a ± 0.016 | 0.209d ± 0.013 | 0.035g ± 0.003 | ||
| Shrimp | Winter 2019 | 0.965a ± 0.016 | 0.362e ± 0.080 | 0.356e ± 0.015 | 0.083g ± 0.008 | 0.070 |
| Summer 2020 | 0.692c ± 0.020 | 0.338e ± 0.010 | 0.668c ± 0.016 | 0.106g ± 0.013 | ||
| Winter 2020 | 0.228f ± 0.011 | 0.843b ± 0.018 | 0.238f ± 0.012 | 0.360e ± 0.013 | ||
| Summer 2021 | 0.555d ± 0.017 | 0.210f ± 0.014 | 0.121g ± 0.014 | 0.255f ± 0.009 | ||
Means followed by different subscripts are significantly different at the 5 % level; Recovery was 99 %.
LOD and LOQ were 0.001 and 0.0035 mg/l, respectively;
Regarding the shrimp samples, the concentration of arsenic in Alexandria governorate in the winter 2020 season was less than the permissible limit, while the samples from other seasons were more than the maximum allowable limit. In Kafr El-Sheikh governorate, arsenic concentrations were higher than the permissible limit in shrimp samples, except in the samples of the summer 2021 season, where they were less than the permissible limits. In shrimp samples from Damietta Governorate, arsenic concentrations were higher than the permissible limit in the winter 2019 and summer 2020 seasons, while they were less than the permissible limit in the rest of the seasons. Finally, in Port Said governorate, the concentrations of As were less than the permissible limit, except for the samples from the winter 2020 season. In the case of As, the number of samples that exceeded the Egyptian permissible limit (2 mg/kg) amounted to 40 % in the sardine samples, and this percentage was distributed among summer samples with 42 % and winter samples with 58 %. In the shrimp samples, 79 % exceeded the permissible limit, distributed by 55 % in the winter and 45 % in the summer seasons.
3.1.4. Mercury
Regarding Hg, it was not detected in all samples collected from the four governorates, as the concentrations were less than the detection limits of the ICP-OES. Moreover, the detection limit was less than the maximum allowable limit in international and local specifications, which is estimated at a concentration of less than 0.5 mg/kg [35]. Generally, methyl mercury is more toxic than inorganic mercury.
3.2. Human health risk assessment of consuming sardine and shrimp
Heavy metals accumulate in fish muscles and can pose a potential health risk for humans after ingestion of such fish. EDI and THQ were used for the assessment of non-carcinogenic risk. In the current study, the highest concentrations of heavy metals (lead, cadmium, and arsenic) detected in the different governorates were used to calculate the EDI and THQ. The values of the EDI and THQ were calculated assuming that the Egyptian adult body weight was 70 kg and the food ingestion rate was 16 and 9 g/day for sardine and shrimp, respectively. The data presented in Table 5 illustrates the values of the EDI and THQ calculated from consumption of sardine and shrimp in four Egyptian governorates. The obtained data showed that all the calculated values for the EDI and THQ were below 1.0, which indicates the safety of consumption of such fish samples.
Table 5.
The Estimated Daily Intake (EDI) and Target Hazard Quotient (THQ) of heavy metals through consumption of sardine and shrimp samples collected from the four Egyptian governorates.
| Metal | Governorate | Sardine |
Shrimp |
||||
|---|---|---|---|---|---|---|---|
| Highest Concentration | EDI | THQ | Highest Concentration | EDI | THQ | ||
| Lead | Alexandria | 0.442 | 0.101 | 0.028 | 0.333 | 0.043 | 0.012 |
| Kafr El-Sheikh | 0.611 | 0.140 | 0.039 | 0.388 | 0.050 | 0.014 | |
| Damietta | 0.482 | 0.110 | 0.031 | 0.534 | 0.069 | 0.019 | |
| Port Said | 0.231 | 0.053 | 0.015 | 0.17 | 0.022 | 0.006 | |
| Cadmium | Alexandria | 0.042 | 0.010 | 0.096 | 0.101 | 0.013 | 0.130 |
| Kafr El-Sheikh | 0.377 | 0.086 | 0.862 | 0.331 | 0.043 | 0.426 | |
| Damietta | 0.2 | 0.046 | 0.457 | 0.312 | 0.040 | 0.401 | |
| Port Said | 0.035 | 0.008 | 0.080 | 0.073 | 0.009 | 0.094 | |
| Arsenic | Alexandria | 0.221 | 0.051 | 0.168 | 0.965 | 0.124 | 0.414 |
| Kafr El-Sheikh | 0.475 | 0.109 | 0.362 | 0.843 | 0.108 | 0.361 | |
| Damietta | 0.209 | 0.048 | 0.159 | 0.668 | 0.086 | 0.286 | |
| Port Said | 0.38 | 0.087 | 0.290 | 0.36 | 0.046 | 0.154 | |
The data presented in Table 6 showed the target total risk quotient values for the heavy metals studied in sardine and shrimp samples in four Egyptian governorates, and all the obtained values were less than 1.0, which indicates that there is no significant toxic effect from eating contaminated fish samples. The TTHQ values in Kafr El-Sheikh recorded the highest values of 1.262 and 0.801for sardines and shrimp, respectively, but were still less than 1.0 (except sardine samples in Kafr El-Sheikh governorate). It is known that if TTHQ values exceed 1.0, this indicates a potential adverse health risk of non-carcinogenic risks from consuming contaminated fish samples. Aissioui et al. [36] found that the calculated target hazard quotients (THQs) were < 1.0 and concluded that the consumption of S. pilchardus from Algerian coast was not likely to have adverse effect on human health.
Table 6.
The Total Target Hazard Quotient (TTHQ) for three detected heavy metals through consumption of sardine and shrimp samples collected from the four governorates.
| Governorate | HI (TTHQ) |
|
|---|---|---|
| Sardine | Shrimp | |
| Alexandria | 0.292 | 0.555 |
| Kafr El-Sheikh | 1.262 | 0.801 |
| Damietta | 0.647 | 0.707 |
| Port Said | 0.384 | 0.254 |
It is worth noting that the values obtained for EDI, THQ, and then TTHQ in the current study were greater than those obtained by Monier et al. [23] for Cd and Pb from sardine samples in Damietta Governorate. They were also greater than those values published by El-Sherbiny and Sallam [37] after analyzing the risk assessment of mercury, lead, and cadmium in mackerel and sardines from the Mediterranean coast of Egypt.
4. Discussion
Regarding the estimation of mercury concentrations in the current study samples, the results showed that the concentrations were less than the detection limit of the ICP-OES, which is characterized by its high accuracy. In addition, the detection limit was less than the permissible limit as the maximum limit for mercury, according to the Egyptian standard, which is 0.5 mg/kg. From this standpoint, it can be expected that the industrial pollution conditions that can cause mercury contamination may be limited; moreover, the small size (2–3 in.) of the collected sardine and shrimp samples helps in minimizing the Hg uptake. It is worth noting that this is the traditional size that is sold in the markets according to the consumption habits of the residents of these governorates. Metal bioaccumulation by fish and subsequent distribution in organs, according to the literature, is very inter-specific. Furthermore, several factors, like gender, age, size, reproductive cycle, swimming behaviors, feeding behavior, and living environment, can all influence metal uptake [38]. Methylmercury uptake in largemouth bass in 53 Florida lakes was shown to be positively related to fish age (strongest correlation) and fish size. They reported that fish less than 2.7 in. (collected from king mackerel sampled in 1999 from North Carolina, South Carolina, Georgia, and Florida, USA) contained mercury ranging from 0.14 to 0.36 mg/kg [39], [40].
Regarding some samples of raw sardines and shrimp that contain levels of the heavy metals under study (lead, cadmium, and arsenic), the cooking and grilling processes that will be followed in preparing them before eating them can reduce the concentration of these metals through various mechanisms. This has been studied through some studies related to this topic, which means reducing the risks to the health of consumers in some way, although this does not constitute a substitute for working to reduce pollution from the various sources of these heavy metals and limit their transmission through the food chain to the final product. In this regard, Atta et al. [41] found that cooking Nile tilapia by either steaming or grilling resulted in reduced cadmium and lead contents in the fish meat. Also, in Spain, Devesa et al. [42] reported that the cooking process, either by boiling or grilling, reduces the total arsenic contents of marketed fish and bivalves. Gheisari et al. [43] found that boiling reduced lead residues in shrimp and lobster meat collected from the Arabian Gulf by 35 % and 13 %, respectively.
In the same context, Sharafi et al. [32] recommended using the rinse cooking method to remove toxic metals from foods and attributed the decrease of metals to their dissolution and discharge in boiling water during cooking, which in turn will be disposed of after the completion of the cooking process with the dissolved toxic metals it contains. It has been reported that the decrease in mineral contents in fish meat during cooking may be attributed to the discharge of these minerals as free salts in the cooking water or in combination with soluble amino acids and uncoagulated proteins [41], [44], [45], [46]. In this regard, the effect of reducing cooking methods on the mineral content of seafood varies depending on the ingredient in question, its chemical specifications, and cooking conditions, including time, temperature, and cooking medium [41], [43].
Regarding seasonal variation, the results of the analysis of lead in the sardine and shrimp samples (Table 2, Table 3, Table 4) indicated that the average concentrations of lead and cadmium in the samples of both sardines and shrimp in general were higher in the winter seasons, to the point that they reached more than twice the average concentration in the summer in all samples collected from the four governorates. As for arsenic, the situation is different, as the results indicated that the average concentration of arsenic in sardine samples was higher in the summer than in the winter in three governorates (Kafr El-Sheikh, Damietta, and Port Said). While the opposite was the case in Alexandria Governorate samples, in the shrimp samples, there was no specific trend in the concentration of arsenic in the four governorates and the four seasons, but there was a fluctuation in the average concentrations. Januar et al. [47] noted that there was a significant difference (P < 0.05) in the seasonal variation of heavy metal concentration in seawater and sediment, and they reported that pollution in seawater was higher during the east monsoon season, while pollution in sediment was higher during the west monsoon season. The observation also revealed a difference in heavy metal accumulation in fish species. However, they did not find a significant relationship (P > 0.05) between the concentration of heavy metals in the environment and fish. In another study conducted by Saha et al. [48], they reported that fish caught during the summer accumulated more metals than in other seasons, which was attributed to the increased influx of agricultural waste, sewage, and sludge due to heavy rainfall and floods.
Fluctuations in heavy metal levels are mostly caused by phytoplankton blooms and especially by the reproductive cycle, which showed a certain interannual shift in the period of gametogenesis. Lower concentrations were observed and recorded in the summer months for the majority of elements, while a different seasonal cycle was observed for arsenic, and it was not related to the development of gonads or to other elements. Chemical species of arsenic have been identified to distinguish compounds of natural origin from those likely to reflect human influence [49]. The many health benefits attributed to fish consumption may be negatively affected by the presence of heavy metals such as lead, cadmium, arsenic, and mercury, which can have adverse effects on human health if consumed in toxic concentrations. Therefore, monitoring and estimating the concentrations of these metals in fish meat is of utmost importance to ensure compliance with food safety specifications, laws, and regulations and the resulting consumer protection. The toxicity of these heavy metals may depend on their polymorphism and chemical composition, which requires predictive modeling processes in order to determine toxic metal profiles from the total measured metal concentrations [19], [20], [21], [22].
According to the estimated daily intake (EDI), target risk quotient (THQ), total target risk quotient (TTHQ), and permissible safety limits set by various agencies, consumption of the investigated fish species should be considered safe for human health, except for sardine samples in Kafr El-Sheikh governorate [48]. Leite et al., [50] study exposure to Toxic Metals and Health Risk Assessment through Ingestion of Canned Sardines Sold in Brazil and concluded that all tested samples contains heavy metals although most of them with limits (Al, Ni, Cr, Cu and Zn) while Ba, Fe, and Se exceeded the threshold set for children in at least one sample. The obtained results indicate that it is not safe to consume canned sardine in Brazil especially on continued basis concerning the toxic elements content. Mohiuddin et al. [51] studied the exposure to heavy metals in finfish and shellfish and concluded that THQ was conducted on both adults and children and concluded that none of them had a non-carcinogenic effect on health. Patrick-Iwuanyanwu et al. [52] reported that even though the calculated THQ values of the metals were< 1 for all the samples, 30 % of the fish species showed calculated HI values> 1. This suggests that the exposed population may be at risk of heavy metals contamination over time due to the consumption of fishes from Ka-Bangha River contaminated with heavy metals.
5. Conclusion
In conclusion, the human health risk assessment of heavy metal contaminants, namely Mercury (Hg), Cadmium (Cd), Lead (Pb), and Arsenic (As), in sardines and shrimp from four Egyptian coastal governorates has provided valuable insights into the safety of seafood consumption in these regions. The results of our assessment have revealed that Hg, Cd, Pb, and As levels in the sardine and shrimp samples generally fell within permissible limits set by international standards such as the Food and Drug Administration (FDA) and the Egyptian Organization for Standardization and Quality (EOS). However, some samples did exceed the Egyptian permissible limits, particularly for Cd and As, emphasizing the need for continued vigilance and regulatory measures. In addition, the obtained THQ as well as the HI of Pb, Cd, and As were below 1, indicating no significant health risk to the consumer associated with the consumption of such sardines and shrimp from these governorates (except sardine samples in Kafr El-Sheikh governorate). Furthermore, it is evident that the health risks associated with the consumption of contaminated seafood are not uniform across different governorates, suggesting the importance of localized monitoring and intervention strategies. Ultimately, this study contributes to the ongoing efforts to ensure the safety of seafood in Egypt and serves as a reminder of the importance of maintaining stringent quality control measures to protect the health and well-being of the population. Continuous research and monitoring will be crucial in addressing and mitigating the potential health risks associated with heavy metal contamination in seafood from Egyptian coastal governorates.
Funding
This research was funded by National Research Centre, Egypt, project number 12050308”.
CRediT authorship contribution statement
Mohamed A. Embaby: Methodology, Investigation, Formal analysis, Data curation. Ahmed M. Ayesh: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology. Salah H. Salem: Validation, Formal analysis, Data curation, Conceptualization. Gomaa N. Abdel-Rahman: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof. L.H. Lash
Data availability
No data was used for the research described in the article.
References
- 1.Bird E. Springer Science & Business Media; Netherlands: 2010. Encyclopedia of the World’s Coastal Landforms. [Google Scholar]
- 2.GAFRD (General Authority for Fish Resources Development), 2014. Fisheries Statistics Yearbook. Cairo. GAFRD. Ministry of Agriculture and Land Reclamation, Egypt.
- 3.FAO, 2019. Food Supply-Livestock and Fish Primary Equivalent, Food and Agriculture Organization of the United Nations. Available at: http://www.fao. org/faostat/en/#data/CL.
- 4.Tanhan P., Lansubsakul N., Phaochoosak N., Sirinupong P., Yeesin P., Imsilp K. Human health risk assessment of heavy metal concentration in seafood collected from Pattani Bay, Thailand. Toxics. 2022;11(1):18. doi: 10.3390/toxics11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S., Zheng N., Sun S., An Q., Li P., Li X., Li Z., Zhang W. Trends and health risk of trace metals in fishes in Liaodong Bay, China, From 2015 to 2020. Front. Mar. Sci. 2022;8 doi: 10.3389/fmars.2021.789572. [DOI] [Google Scholar]
- 6.Liu Y., Xu J., Wang Y., Yang S. Trace metal bioaccumulation in oysters (Crassostrea gigas) from Liaodong Bay (Bohai Sea, China) Environ. Sci. Pollut. Res. 2021;28:20682–20689. doi: 10.1007/s11356-020-11968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaher H.A., Mohamed A.H., Hamed S.E., Khateeb A.E. Risk assessment of heavy metal bioaccumulation in raw crab and prawn flesh market in Egypt. J. Hum. Environ. Health Promot. 2021;7(1):6–14. doi: 10.52547/jhehp.7.1.6. [DOI] [Google Scholar]
- 8.Han J.L., Pan X.D., Chen Q., Huang B.F. Health risk assessment of heavy metals in marine fish to the population in Zhejiang, China. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-90665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IARC I. IARC monographs on the evaluation of carcinogenic risks to humans: arsenic, metals. Fibres, Dusts A Rev. Hum. Carcinog. 2012:100. [PMC free article] [PubMed] [Google Scholar]
- 10.Taghizadeh S.F., Davarynejad G., Asili J., Nemati S.H., Rezaee R., Goumenou M.…Karimi G. Health risk assessment of heavy metals via dietary intake of five pistachio (Pistacia vera L.) cultivars collected from different geographical sites of Iran. Food Chem. Toxicol. 2017;107:99–107. doi: 10.1016/j.fct.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Taghizadeh S.F., Karimi G., Tzatzarakis M., Tsakiris I., Ahmadpourmir H., Azizi M., Afshari A., Ghorani V., Yarmohammadi F., Tsatsakis A., Rezaee R. Probabilistic risk assessment of exposure to multiple metals and pesticides through consumption of fruit juice samples collected from Iranian market. Food Chem. Toxicol. 2022;170 doi: 10.1016/j.fct.2022.113493. [DOI] [PubMed] [Google Scholar]
- 12.Taghizadeh S.F., Rezaee R., Badibostan H., Karimi G. Probabilistic carcinogenic and non-carcinogenic risk assessment of heavy metal ingestion through consumption of different walnut cultivars: an Iranian study. Environ. Monit. Assess. 2020;192:1–15. doi: 10.1007/s10661-020-08551-4. [DOI] [PubMed] [Google Scholar]
- 13.Diop M., Howsam M., Diop C., Goossens J.F., Diouf A., Amara R. Assessment of trace element contamination and bioaccumulation in algae (Ulva lactuca), mussels (Pernaperna), shrimp (Penaeuskerathurus), and fish (Mugilcephalus, Saratherondonmelanotheron) along the Senegalese coast. Mar. Pollut. Bull. 2016;103(1-2):339–343. doi: 10.1016/j.marpolbul.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Jiao Y., Yang L., Kong Z., Shao L., Wang G., Ren X., Liu Y. Evaluation of trace metals and rare earth elements in mantis shrimp Oratosquillaoratoria collected from Shandong Province, China, and its potential risks to human health. Mar. Pollut. Bull. 2021;162 doi: 10.1016/j.marpolbul.2020.111815. [DOI] [PubMed] [Google Scholar]
- 15.Amin A.N., Ahemd A.M., Ahmed O.M. Chemical and bacteriological risks of shrimp and clams (Gandofly) from Suez Gulf. Food Res. 2021;5(3):281–288. doi: 10.26656/fr.2017.5(3).635. [DOI] [Google Scholar]
- 16.Satheeshkumar P., Senthilkumar D. Identification of heavy metals contamination by multivariate statistical analysis methods in Pondicherry mangroves, India. J. Environ. Earth Sci. 2011;1(1):30–48. [Google Scholar]
- 17.Burgos M.G., Rainbow P.S. Availability of cadmium and zinc from sewage sludge to the flounder, Platichthys flesus, via a marine food chain. Mar. Environ. Res. 2001;51(5):417–439. doi: 10.1016/S0141-1136(00)00249-X. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Wong M.H. Environmental mercury contamination in China: sources and impacts. Environ. Int. 2007;33:108–121. doi: 10.1016/j.envint.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Kamaruzzaman B.Y., Rina Z., John B.A., Jalal K.C.A. Heavy metal accumulation in commercially important fishes of South West Malaysian coast. Res. J. Environ. Sci. 2011;5(6):595–602. doi: 10.3923/rjes.2011.595.602. [DOI] [Google Scholar]
- 20.Heidarieh M., Maragheh M.G., Shamami M.A., Behgar M., Ziaei F., Akbari Z. Evaluate of heavy metal concentration in shrimp (Penaeussemisulcatus) and crab (Portunuspelagicus) with INAA method. Springer. 2013;2(1):1–5. doi: 10.1186/2193-1801-2-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djedjibegovic J., Marjanovic A., Tahirovic D., Caklovica K., Turalic A., Lugusic A.…Caklovica F. Heavy metals in commercial fish and seafood products and risk assessment in adult population in Bosnia and Herzegovina. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-70205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farag M.A., Mansour S.T., Nouh R.A., Khattab A.R. Crustaceans (shrimp, crab, and lobster): a comprehensive review of their potential health hazards and detection methods to assure their biosafety. J. Food Saf. 2023;43(1) doi: 10.1111/jfs.13026. [DOI] [Google Scholar]
- 23.Monier M.N., Soliman A.M., Al-Halani A.A. The seasonal assessment of heavy metals pollution in water, sediments, and fish of grey mullet, red seabream, and sardine from the Mediterranean coast, Damietta, North Egypt. Reg. Stud. Mar. Sci. 2023;57 doi: 10.1016/j.rsma.2022.102744. [DOI] [Google Scholar]
- 24.European Committee for standardization (2014). Foodstuffs-determination of Trace Elements-pressure Digestion, (EN 13805:2014).
- 25.APHA (2017). American Public Health Association, AWWA (American Water Works Association), WEF (Water Environment Federation), E.W. Rice, R.B. Baird, A.D. Eaton, L.S. Clesceri (Eds.), Standard Methods for the Examination of Water and Wastewater, twenty third ed., Washington DC.
- 26.Khan S., Cao Q., Zheng M.Y., Huang Z.Y., Zhu G.Y. (2008) Health risks of potential toxic elements in contaminated soils and food crops irrigated with treated wastewater in Beijing, China, 152, Department of Environmental Sciences, University of Peshawar Environmental, Peshawar, pp 686–692. DOI: 10.1016/j.envpol.2007.06.056. [DOI] [PubMed]
- 27.Yaacob A., Yap C.K., Nulit R., Omar H., Al-Shami S.A., Bakhtiari A.R. Assessment of health risks of the toxic Cd and Pb between leafy and fruit vegetables collected from selected farming areas of Peninsular Malaysia. Integr. Food Nutr. Metab. 2018;5(3):1–9. doi: 10.15761/IFNM.1000215. [DOI] [Google Scholar]
- 28.Hussain A., Priyadarshi M., Dubey S. Experimental study on accumulation of PTEs in vegetables irrigated with treated wastewater. Appl. Water Sci. 2019;9(5):1–11. doi: 10.1007/s13201-019-0999-4. [DOI] [Google Scholar]
- 29.USEPA Risk assessment guidance for superfund. Human health evaluation manual Part A, Interim Final. U. S. Environ. Prot. Agency 1 (Part A) 1989:300. [Google Scholar]
- 30.USEPA, 2000. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories. Volume 2: Risk Assessment and Fish Consumption Limits Third Edition. US Environmental Protection Agency. Office of Science and Technology, Office of Water, Washington, DC.
- 31.Zhuang P., McBride M.B., Xia H., Li N., Li Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009;407:1551–1561. doi: 10.1016/j.scitotenv.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 32.Sharafi K., Yunesian M., Nodehi R.N., Mahvi A.H., Pirsaheb M.A. A systematic literature review for some toxic metals in widely consumed rice types (domestic and imported) in Iran: human health risk assessment, uncertainty and sensitivity analysis. Ecotoxicol. Environ. Saf. 2019;176:64–75. doi: 10.1016/j.ecoenv.2019.03.072. [DOI] [PubMed] [Google Scholar]
- 33.SAS (1999). Statistical Analysis System, SAS / STAT User’s Guide. Release 6.03 Edn. SAS Institute, Cary, NC 1028.
- 34.Food and Drug Administration (FDA) (2009). US Food and Drug Administration, Internet Publication FDA.
- 35.Egyptian Standards (2010). Maximum levels for certain contaminants in foodstuffs. ES7136/2010. Egyptian Organization for Standardization and Quality.
- 36.Aissioui S., Laurence Poirier B., Rachid Amara C., Zouhir Ramdane (2022) Concentrations of lead, cadmium and mercury in sardines, Sardina pilchardus (Walbaum, 1792) from the Algerian coast and health risks for consumers. Journal of Food Composition and Analysis 109 (2022) 104490 https://doi.org/10.1016/j.jfca.2022.104490.
- 37.El-Sherbiny H.M.M., Sallam K.I. Residual contents and health risk assessment of mercury, lead and cadmium in sardine and mackerel from the Mediterranean Sea Coast, Egypt. J. Food Compos. Anal. 2021;96 doi: 10.1016/j.jfca.2020.103749. [DOI] [Google Scholar]
- 38.Zhao S., Feng C., Quan W., Chen X., Niu J., Shen Z. Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Mar. Pollut. Bull. 2012;64(2012):1163–1171. doi: 10.1016/j.marpolbul.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Lange T.R., Royals H.E., Connor L.L. Influence of water chemistry on mercury concentration in largemouth bass from Florida lakes. Trans. Am. Fish. Soc. 1993;122(1):74–84. 10.1577/1548-8659(1993)122<0074:IOWCOM>2.3.CO;2. [Google Scholar]
- 40.Moore C.J. (2000). A review of mercury in the environment (Its occurrence in marine fish), Technical Report No. 88. Office of Environmental Management, Marine Resources Division, South Carolina Marine Resources Division, Charleston. http://hdl.handle.net/10827/25363.
- 41.Atta M.B., El-Sebaie L.A., Noaman M.A., Kassab H.E. The effect of cooking on the content of heavy metals in fish (Tilapia nilotica) Food Chem. 1997;58(1-2):1–4. doi: 10.1016/0308-8146(95)00205-7. [DOI] [Google Scholar]
- 42.Devesa Vicenta, Macho Mari, Jalon Mercedes, Urieta Inés, Muñoz Ociel, Súñer María, López Fernando, Vélez Dinoraz, Montoro Rosa. Arsenic in cooked seafood products: study on the effect of cooking on total and inorganic arsenic contents. J. Agric. Food Chem. 2001;49:4132–4140. doi: 10.1021/jf010274l. [DOI] [PubMed] [Google Scholar]
- 43.Gheisari E., Raissy M., Rahimi E. The effect of different cooking methods on lead and cadmium contents of shrimp and lobster. J. Food Biosci. Technol. 2016;6(2):53–58. [Google Scholar]
- 44.Ganjavi M., Ezzatpanah H., Givianrad M.H., Shams A. Effect of canned tuna fish processing steps on lead and cadmium contents of Iranian tuna fish. Food Chem. 2010;118(3):525–528. doi: 10.1016/j.foodchem.2009.05.018. [DOI] [Google Scholar]
- 45.Hajeb P., Sloth J.J., Shakibazadeh S.H., Mahyudin N.A., Afsah-Hejri L. Toxic elements in food: occurrence, binding, and reduction approaches. Compr. Rev. Food Sci. Food Saf. 2014;13(4):457–472. doi: 10.1111/1541-4337.12068. [DOI] [PubMed] [Google Scholar]
- 46.Wang C., Duan H.Y., Teng J.W. Assessment of microwave cooking on the bioaccessibility of cadmium from various food matrices using an in vitro digestion model. Biol. Trace Elem. Res. 2014;160:276–284. doi: 10.1007/s12011-014-0047-z. [DOI] [PubMed] [Google Scholar]
- 47.Januar H.I., Dwiyitno, Hidayah I. Seasonal variation of heavy metal accumulation in environment and fishes from the Cirebon coast, Indonesia. Aquat. Ecosyst. Health Manag. 2021;24(2):121–129. doi: 10.14321/aehm.024.02.16. [DOI] [Google Scholar]
- 48.Saha N., Mollah M.Z.I., Alam M.F., Rahman M.S. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016;70:110–118. doi: 10.1016/j.foodcont.2016.05.040. [DOI] [Google Scholar]
- 49.Fattorini D., Notti A., Di Mento R., Cicero A.M., Gabellini M., Russo A., Regoli F. Seasonal, spatial and inter-annual variations of trace metals in mussels from the Adriatic Sea: a regional gradient for arsenic and implications for monitoring the impact of off-shore activities. Chemosphere. 2008;72(10):1524–1533. doi: 10.1016/j.chemosphere.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 50.Leite L.C.S., de Lima N.V., Melo E.S.D.P., Cardozo C.M.L., do Nascimento V.A. Exposure to toxic Metals and Health Risk Assessment through ingestion of canned sardines sold in Brazil. Int. J. Environ. Res. Public Health. 2022;19(13):7678. doi: 10.3390/ijerph19137678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohiuddin M., Hossain M.B., Ali M.M., Hossain M.K., Habib A., Semme S.A., Rakib M.R.J., Rahman M.A., Yu J., Al-Sadoon M.K., Gulnaz A. Human health risk assessment for exposure to heavy metals in finfish and shellfish from a tropical estuary. J. King Saud. Univ. -Sci. 2022;34(4) doi: 10.1016/j.jksus.2022.102035. [DOI] [Google Scholar]
- 52.Patrick-Iwuanyanwu K.C., et al. Health risk assessment of hazardous metals in seafood from Ka-Bangha River, Khana, Rivers State, Nigeria. Egypt. J. Aquat. Biol. Fish. 2022;26(3):499–511. doi: 10.21608/ejabf.2022.243662. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.