Abstract
Background
Cefiderocol exhibits potent in vitro activity against carbapenem-resistant Acinetobacter baumannii (CRAb), but this activity has not consistently translated to improved outcomes among patients. Cefiderocol heteroresistance, or the presence of a resistant subpopulation, has been proposed as one possible explanation. The objective of this study was to explore associations between heteroresistance and outcomes of patients with CRAb infections.
Methods
Baseline CRAb isolates were collected from 27 consecutive patients in the USA and Italy. Cefiderocol susceptibility was tested by broth microdilutions in triplicate. Heteroresistance was defined by population analysis profiling in duplicate. Resistance mechanisms and strain relatedness were evaluated through comparative genomic analysis.
Results
Overall, 59% of infecting CRAb isolates were identified as cefiderocol-heteroresistant; rates were higher among isolates from Italy (79%) than the USA (38%). The median Charlson Comorbidity and SOFA scores were 4 and 5, respectively; 44% of patients had pneumonia, which was the most common infection type. Rates of 28-day clinical success and survival were 30% and 73%, respectively. By broth microdilution, cefiderocol MICs ≥1 mg/L were associated with higher failure rates than MICs ≤0.5 mg/L (81% versus 55%). Rates of clinical failure were numerically higher among patients infected by cefiderocol-heteroresistant compared with susceptible CRAb (81% versus 55%). Whole-genome sequencing identified a premature stop codon in the TonB-dependent receptor gene piuA in six isolates, all of which were heteroresistant.
Conclusions
This pilot study supports the hypothesis that cefiderocol treatment failure may be associated with higher MICs and/or the presence of heteroresistance. Further studies are needed to confirm these findings.
Introduction
Antimicrobial resistance presents a significant threat to patient wellbeing and a burden to healthcare infrastructure.1 In 2019, carbapenem-resistant Acinetobacter baumannii (CRAb) was projected as the fourth leading cause of death among antimicrobial resistant pathogens globally.1 In 2020, an estimated 7500 CRAb cases with 700 associated deaths were identified in the USA alone.2 Worldwide, CRAb bloodstream and respiratory tract infections are associated with excessive morbidity and mortality as evidenced by recent randomized clinical trials.3–5 Bloodstream infections caused by CRAb have been associated with a 16% higher attributable mortality compared with those caused by carbapenem-susceptible Gram-negative bacilli.6 These high rates of mortality are due, in part, to the lack of safe and effective treatment options for CRAb infections.7
Cefiderocol, a novel siderophore cephalosporin, demonstrates promising in vitro activity against CRAb;8 however, patients randomized to treatment with cefiderocol for CRAb infections experienced higher mortality rates when compared with best available therapy in the CREDIBLE-CR clinical trial.5 Beyond baseline differences between patient cohorts, one possible explanation for the observed imbalance between treatment arms is a high rate of heteroresistance to cefiderocol among the infecting CRAb isolates.9 Broadly, heteroresistance describes a resistant subpopulation of cells that exist within a phenotypically susceptible majority that can be enriched upon selective pressure.10 Evidence to support the clinical impact of antibiotic heteroresistance largely stems from vancomycin heteroresistant Staphylococcus aureus;11,12 however, observations of antibiotic heteroresistance among A. baumannii have been reported.13–15 The objective of this study was to investigate the association between cefiderocol heteroresistance and clinical outcomes of patients treated with cefiderocol at two academic institutions in Italy and the USA.
Materials and methods
Consecutive adult patients who received cefiderocol for at least 48 hours to treat CRAb infections were included from institutions in Pisa, Italy (January 2022 to August 2022), and Pittsburgh, USA (July 2020 to May 2023). Cases were included only if the infecting CRAb isolate was identified as susceptible to cefiderocol by local susceptibility testing methods using disk diffusion or gradient strip testing at the time of treatment initiation. Infection types were defined according to the primary team and confirmed by study investigators. Patients without signs and symptoms of infection were excluded. The primary outcome of interest was clinical success at 28 days defined as a composite of survival, resolution of signs and symptoms of infection, and absence of recurrent infection or microbiologic failure following the onset of infection.16 Secondary outcomes included all-cause mortality and development of cefiderocol resistance following treatment. Cefiderocol resistance was determined by local susceptibility testing methods as any non-susceptible isolate according to CLSI breakpoints.17
Baseline isolates from patients who met the inclusion criteria were collected for further analysis. All cefiderocol minimum inhibitory concentrations (MICs) were determined in triplicate by broth microdilution using iron-depleted cation-adjusted Mueller–Hinton broth (ID-CAMHB) at a central laboratory. Cefiderocol concentrations that were tested ranged from 0.03–32 mg/L (Fetroja® for injection purchased from hospital pharmacy; LOT no. 0033); MICs were interpreted according to CLSI breakpoints and were only recorded when MICs for quality control strain Escherichia coli ATCC 25922 were within the reference range.17 Monoclonal population analysis profiling (PAP) was performed to identify heteroresistance as previously described.9,18 In brief, a single colony was selected at random for each clinical isolate and incubated overnight in MHB. The overnight culture growth (equivalent to ≥8 log10 cfu/mL) was diluted and 10 µL were plated on MH agar containing cefiderocol at 2, 4, 8, 16, 32 and 64 mg/L, and a drug-free control plate. Surviving colonies were enumerated after at least 24 hours of incubation. Isolates were considered susceptible if <0.001% of colonies grew in the presence of 32 mg/L relative to colonies growing on drug-free plates (equivalent to a >5-log10 decrease in cfu/mL). Isolates were categorized as heteroresistant if 0.001–50% of colonies grew in the presence of 32 or 64 mg/L of cefiderocol (equal to 2× and 4× the cefiderocol resistance breakpoint). Finally, isolates were considered resistant if >50% of colonies grew in the presence of cefiderocol at 16 mg/L or greater. All isolates were tested at least twice, each time with two technical replicates to ensure reproducibility. If the categorical interpretation of heteroresistance varied between the first two tests, a third test was conducted for adjudication and mean log-kills were used for analysis. Heteroresistance was also determined by disk diffusion using CLSI methods. Isolates that yielded colonies within the zone of inhibition were considered heteroresistant; zones that were clear were measured and classified by CLSI interpretative criteria.17
Isolates underwent whole-genome sequencing (WGS) on the Illumina platform, and genome assembly and multilocus sequence typing were performed as described previously.19,20 Sequence types were determined using the Oxford typing scheme.21 A core genome phylogenetic tree was constructed using snippy v4.6.0 for alignment and by RAxML v8.2.12 for the tree.22 Antibiotic-resistance genes were identified using ResFinder.9–11 Protein sequences of PirA, PiuA and its orthologue PiuD were compared with those of A. baumannii reference strain ACICU.23
Statistical analyses were performed in GraphPad Prism (version 10.2.3). Categorical variables were compared by Fisher’s exact test. Continuous variables were compared using a Wilcoxon rank-sum test. Using PAP profiles, area-under-the-curve (AUC) values were determined from PAP profiles.24 Statistical significance was defined as a P < 0.05.
Results
Isolates were collected from 27 patients with CRAb infections who were treated with cefiderocol for a median of 11 days (range, 2–50). The median age was 58 years (range, 21–87) and 44% of patients were men. Median Charlson comorbidity index, Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE-II) scores were 4 (range, 0–15), 5 (range, 0–15) and 20 (range, 2–31), respectively (Table 1). The primary infection sites were pneumonia (n = 12), skin/soft tissue infections including osteomyelitis (n = 6), bacteremia (n = 4), intra-abdominal infections (n = 3) and urinary tract infections (n = 2). Twenty-six (7/27) percent of patients received cefiderocol monotherapy; the remaining 74% (20/27) received cefiderocol in combination with at least one other antibiotic with in vitro activity against CRAb. Rates of 28-day clinical success and survival were 30% (8/27) and 63% (17/27), respectively. The emergence of cefiderocol resistance was documented in 26% (7/27) of patients during or following the initial treatment course.
Table 1.
Characteristics and clinical outcomes of patients with CRAb infections treated with cefiderocol alone or in combination
| Patient | Age (Sex) | Primary infection | Charlson score | SOFA | APACHE II | Treatment regimen (duration in days) | 28d mortality | 28d response | Emergent Resistance | Isolate | FDC MIC (mg/L) | Heteroresistance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pitt-1 | 43 (F) | HAP/VAP | 0 | 15 | 25 | Cefiderocol (15), Ampicillin-sulbactam (8), Polymyxin B (12) | Yes | Failure | Yes | AB526 | 0.25 | Yes |
| Pitt-2 | 71 (M) | IAI | 8 | 8 | 25 | Cefiderocol (37), Tigecycline (37), Ampicillin-sulbactam (8) | No | Failure | Yes | AB555 | 2 | Yes |
| Pitt-3 | 38 (M) | HAP/VAP | 3 | 12 | 22 | Cefiderocol (8), Ampicillin-sulbactam (8), Inhaled colistin (8) | No | Failure | No | AB574 | 0.25 | Yes |
| Pitt-4 | 54 (M) | HAP/VAP | 4 | 6 | 23 | Cefiderocol (18), Ampicillin-sulbactam (19), Polymyxin B (12) | Yes | Failure | No | AB589 | 1 | Yes |
| Pitt-5 | 47 (M) | SSTI | 3 | 0 | 2 | Cefiderocol (50), Ampicillin-sulbactam (44) | No | Failure | Yes | AB648 | 2 | Yes |
| Italy-1 | 21 (M) | BSI | 0 | 10 | 20 | Cefiderocol (19), Tigecycline (11) | No | Failure | Yes | E0396 | 4 | Yes |
| Italy-2 | 61 (F) | IAI | 15 | 11 | 28 | Cefiderocol (8), Fosfomycin (11) | No | Failure | Yes | E0418 | 1 | Yes |
| Italy-3 | 71 (F) | HAP/VAP | 6 | 11 | 31 | Cefiderocol (3) | Yes | Failure | No | E0420 | 1 | Yes |
| Italy-4 | 87 (F) | BSI | 5 | 4 | 11 | Cefiderocol (13) | Yes | Failure | No | E0422 | 1 | Yes |
| Italy-5 | 53 (F) | SSTI | 2 | 7 | 15 | Cefiderocol (11), Tigecycline (16) | No | Success | No | E0423 | 0.5 | Yes |
| Italy-6 | 85 (F) | SSTI | 8 | 14 | 30 | Cefiderocol (4) | Yes | Failure | No | E0424 | 8 | Yes |
| Italy-7 | 80 (F) | HAP/VAP | 5 | 2 | 8 | Cefiderocol (10), Tigecycline (6) | No | Failure | Yes | E0425 | 2 | Yes |
| Italy-8 | 80 (F) | UTI | 7 | 4 | 15 | Cefiderocol (11), Ampicillin-sulbactam (11) | Yes | Failure | No | E0427 | 1 | Yes |
| Italy-9 | 72 (F) | HAP/VAP | 6 | 5 | 24 | Cefiderocol (8) | No | Success | No | E0428 | 0.5 | Yes |
| Italy-10 | 44 (F) | BSI | 0 | 12 | 27 | Cefiderocol (2), Tigecycline (2) | Yes | Failure | No | E0431 | 2 | Yes |
| Italy-11 | 25 (M) | HAP/VAP | 1 | 2 | 7 | Cefiderocol (10), Tigecycline (10) | No | Success | No | E0432 | 4 | Yes |
| Pitt-6 | 58 (M) | HAP/VAP | 3 | 9 | 25 | Cefiderocol (12), Polymyxin B (14), Minocycline (10), Inhaled colistin (14) | No | Success | No | AB536 | 1 | No |
| Pitt-7 | 48 (M) | HAP/VAP | 2 | 1 | 7 | Cefiderocol (15), Minocycline (15) | No | Success | No | AB545 | 1 | No |
| Pitt-8 | 60 (M) | IAI | 5 | 15 | 20 | Cefiderocol (6), Tigecycline (24), Ampicillin-sulbactam (24) | Yes | Failure | No | AB570 | 1 | No |
| Pitt-9 | 62 (F) | SSTI | 5 | 4 | 14 | Cefiderocol (6), Minocycline (27) | Yes | Failure | No | AB586 | 2 | No |
| Pitt-10 | 70 (M) | HAP/VAP | 10 | 11 | 27 | Cefiderocol (4), Tigecycline (4) | Yes | Failure | No | AB603 | 0.5 | No |
| Pitt-11 | 47 (F) | HAP/VAP | 1 | 5 | 21 | Cefiderocol (25), Minocycline (23), Polymyxin B (18) | No | Failure | No | AB605 | 0.5 | No |
| Pitt-12 | 79 (M) | SSTI | 7 | 0 | 9 | Cefiderocol (43) | No | Success | No | AB616 | 0.5 | No |
| Pitt-13 | 63 (F) | BSI | 3 | 2 | 6 | Cefiderocol (31) | No | Success | No | E0368 | 0.12 | No |
| Italy-12 | 44 (M) | SSTI | 7 | 4 | 8 | Cefiderocol (39), Tigecycline (42) | No | Failure | Yes | E0421 | 0.5 | No |
| Italy-13 | 33 (F) | HAP/VAP | 0 | 4 | 8 | Cefiderocol (7), Tigecycline (7) | No | Failure | No | E0429 | 0.5 | No |
| Italy-14 | 31 (F) | UTI | 2 | 5 | 5 | Cefiderocol (11) | No | Success | No | E0430 | 0.25 | No |
APACHE II, acute physiology and chronic health evaluation II; BSI, bloodstream infection; F, female; FDC, cefiderocol; HAP, hospital-associated pneumonia; IAI, Intra-abdominal infection; M, male; MIC, minimum inhibitory concentration; SOFA, sequential organ failure assessment; SSTI, skin and soft tissue infection; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
Confirmatory broth microdilution and disk diffusion testing were performed on baseline isolates. By broth microdilution testing, the median cefiderocol MIC was 1 mg/L (range, 0.12–8 mg/L) and 96% (26/27) of isolates were categorized as susceptible using the CLSI a breakpoint of ≤4 mg/L. By comparison only 48% (13/27) of isolates were classified as susceptible by disk diffusion testing. For isolates classified as non-susceptible by disk diffusion, colonies were identified within the zone of inhibition for 79% [11/14; Figure S1 (available as Supplementary data at JAC-AMR Online)]. Heteroresistance defined by PAP was detected in 59% (16/27) of isolates. Among heteroresistant isolates, the proportion of colonies growing at 32 mg/L or 64 mg/L relative to drug-free control ranged from 0.0016%–0.165% and 0.0017%–0.2846%, respectively (Table S1 and Figure S2). The mean AUC for heteroresistant isolates was significantly higher than for susceptible isolates (260.1 versus 163.0; P < 0.001). Heteroresistance was numerically more common among isolates with cefiderocol MICs ≥1 mg/L [75% (12/16)] compared with isolates with MICs ≤0.5 mg/L [36% (4/11); P = 0.06, Figure S3].
Clinical success was achieved in 45% (5/11) of patients treated with cefiderocol-susceptible isolates (as determined by PAP) compared with 19% (3/16) for those infected with cefiderocol-heteroresistant isolates. When stratified by MIC, success was achieved in 45% (5/11) of patients infected with isolates demonstrating a cefiderocol MIC ≤0.5 mg/L compared with 19% (3/16) of patients infected with isolates demonstrating a cefiderocol MIC ≥1 mg/L (Figure S4). Rates of 28-day mortality did not vary significantly by the presence or the absence of heteroresistance (44% versus 27%). Subsequent development of cefiderocol resistance was identified in 38% (6/16) of patients infected by heteroresistant isolates compared with 9% (1/11) of susceptible isolates.
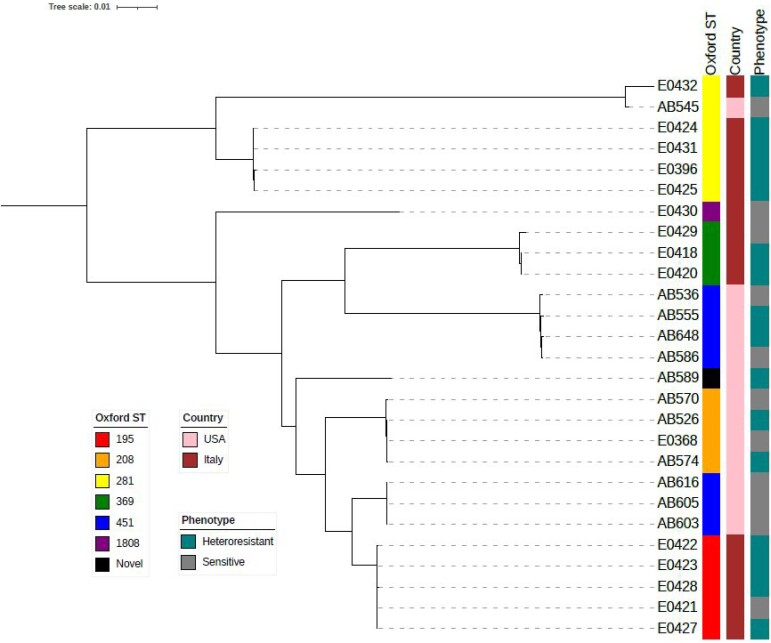
Whole-genome sequence analysis identified clonal clusters unique to each centre (Figure 1); yet, isolates were genetically diverse and represented seven unique sequence types (ST). The most common Oxford STs were ST281 (n = 6) and ST451 (n = 7). Rates of heteroresistance did not vary by ST or phylogenetic cluster; however, 79% (11/14) of isolates from Italy were classified as heteroresistant compared with 38% (5/13) of isolates from the USA (P = 0.05). Loss-of-function mutations in genes encoding TonB-dependent receptors (TBDR; pirA and piuA) were detected in 26% (7/27) of isolates. Eighty-six percent (6/7) of isolates with mutations in TBDR genes displayed a heteroresistant phenotype, including all six isolates with mutations encoding a premature stop codon in piuA (Table S2).
Figure 1.
Phylogenetic tree and characterization of CRAb isolates from Pisa, Italy and Pittsburgh, USA.
Discussion
Heteroresistance is a poorly understood form of antibiotic resistance that may or may not be linked to poor clinical outcomes for patients.25 Unlike classic resistance, heteroresistance is not often detected by standard antibiotic susceptibility testing, and therefore represents a potentially underappreciated risk factor for treatment failure. Cefiderocol heteroresistance has now been documented in several reports,9,18,24,26–28 and is hypothesized to explain higher than anticipated rates of treatment failure for patients with CRAb infections.9 Against CRAb, reported rates of cefiderocol heteroresistance range from 47% to 59%,9,18 which is in agreement with the rate of 59% in the present study. Interestingly, we found that rates of heteroresistance varied from 79% of isolates collected in Italy to 38% of isolates collected at a single center in the USA. This variability could not be explained by a specific clonal cluster or mutations in specific antibiotic resistance-associated genes, suggesting that regional variability may be a key factor in the prevalence heteroresistance. Regional variability has also been described for cefiderocol heteroresistance against Pseudomonas aeruginosa.24 It is also possible that varying treatment approaches or clinical factors at each center contributes to the rate of heteroresistance.
Although experimental approaches and definitions used to identify cefiderocol heteroresistance vary across studies, it is clear that this is a common phenomenon among CRAb collected from infected patients. Herein, we attempted to associate the presence of cefiderocol heteroresistance with outcomes of patients treated with cefiderocol. In doing so, we found that rates of treatment failure were numerically higher among patients infected by CRAb isolates characterized as heteroresistant compared with patients infected by susceptible isolates according to PAP (81% versus 55%, respectively). Both heteroresistance and clinical failures were numerically more common among isolates with cefiderocol MICs ≥1 mg/L. While notable, these data should be interpreted cautiously given the small number of patients included in this study, a wide range of infectious syndromes evaluated and routine use of cefiderocol combination therapy. In addition, durations of cefiderocol exposure varied across patients from 2 to 50 days. Heteroresistant subpopulations are typically identified in the face of ongoing selective pressure and it is unclear if short exposures or the presence of heteroresistant subpopulations at baseline portend worse outcomes.9 Prolonged clinical exposures, on the other hand, have been reported in emergent heteroresistance during cefiderocol treatment for a complicated P. aeruginosa infection.26
The available data also suggest that heteroresistance could be an initial step in the evolution of cefiderocol resistance.29 In our experience, the emergence of cefiderocol resistance was identified in 38% of isolates classified as heteroresistant compared with 9% of susceptible isolates. The development of cefiderocol resistance against CRAb is mediated by multiple molecular mechanisms, including mutations in Acinetobacter-derived cephalosporinase, PBP3, and TBDR genes piuA and pirA.19,30,31 We identified pre-existing TBDR gene mutations in 26% of isolates prior to treatment with cefiderocol, among which 86% were classified as heteroresistant by PAP. It is plausible that TBDR mutations specifically confer an initial step towards heteroresistance, or more concerningly, outright resistance following treatment. Our findings corroborate a prior report linking mutations in TBDR genes to cefiderocol heteroresistance against P. aeruginosa.24 While the cumulative data of these two studies are limited to a relatively small number of isolates, the findings underscore a potentially important predictor of cefiderocol resistance. Moreover, the data highlight a disconnect between high rates of cefiderocol in vitro activity,8 and a growing number of reports describing clinical failures and/or emergence of resistance against CRAb.19,32–34 This disconnect is perpetuated by the technical challenges that have been described in testing cefiderocol susceptibility against A. baumannii,35 which may preclude MIC-based clinical decision making. Consistent with our findings, high error rates have been reported for cefiderocol disk diffusion testing against A. baumannii specifically.36 In fact, heteroresistance may contribute to this discordance when compared with the gold standard broth microdilution testing.37
Our findings contrast those recently reported from a post-hoc evaluation of isolates collected from patients enrolled in the CREDIBLE-CR trial, which did not identify associations between heteroresistance and clinical cure, microbiologic eradication, or mortality.18 In a post-hoc analysis, 38 CRAb isolates were available from patients treated with cefiderocol. By broth microdilution testing, 5% (2/38) were classified as resistant. Among the remaining 36 isolates, 19.4%, 50% and 30.6% were classified as susceptible, heteroresistant or resistant by PAP. Excluding a single patient with a complicated urinary tract infection, 100% (7/7) of patients infected by isolates classified as susceptible to cefiderocol by PAP died by the test-of-cure visit. Corresponding rates of death for those infected by cefiderocol-heteroresistant or resistant isolates were 22% (4/18) and 75% (8/12), respectively. It is worth noting that a significant proportion of patients in this trial are believed to have deteriorated due to underlying conditions and septic shock that was present prior to receipt of cefiderocol.5 Such clinical factors may mask potential associations between cefiderocol MIC, drug exposures, or the presence of heteroresistance and outcomes. Nonetheless, it is unclear why failure rates were numerically highest among isolates classified as susceptible to cefiderocol.
Finally, it is important to acknowledge the limitations of the current analysis. First, our study was limited by a small number of patients, its retrospective, observational nature and use of cefiderocol to treat various infection types. As such, we view these data as hypothesis-generating. Future, multicentre studies would be needed to identify definitive associations between cefiderocol heteroresistance, MICs and clinical outcomes of patients infected by CRAb. Secondly, we recognize that defining heteroresistance is subject to methodological variation and definitions applied. Here, we identified heteroresistance as a ≤5-log10 cfu/mL killing at a cefiderocol concentration of ≥32 mg/L, which is equal to 4× the non-susceptibility breakpoint for cefiderocol (MIC ≥8 mg/L). To account for potential differences in our definition compared with those that have been published previously,9,24 we derived AUC measurements from PAP studies. These analyses demonstrated clear differences between heteroresistant and susceptible isolates (Table S1). Lastly, we did not investigate mechanisms of cefiderocol heteroresistance beyond WGS analysis. Heteroresistance has been previously reported to be unstable and potentially overcome by partnering cefiderocol with β-lactamase inhibitors like avibactam.38 Consistent with current recommendations, cefiderocol may be best utilized in combination with other in vitro active antibiotics for treatment of CRAb infections.7,39 Despite these limitations, we have identified potential associations between elevated cefiderocol MICs and the presence of heteroresistance, both of which may be associated with higher rates of treatment failure for patients with CRAb infections.
Supplementary Material
Contributor Information
Ryan K Shields, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Center for Innovative Antimicrobial Therapy, University of Pittsburgh, Pittsburgh, PA, USA; Antibiotic Management Program, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Ava J Dorazio, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Giusy Tiseo, Department of Clinical and Experimental Medicine, Azienda Osperdaliero Universitaria Pisana, University of Pisa, Pisa, Italy.
Kevin M Squires, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Alessandro Leonildi, Microbiology Unit, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy.
Cesira Giordano, Microbiology Unit, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy.
Ellen G Kline, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Simona Barnini, Microbiology Unit, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy.
Alina Iovleva, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Center for Innovative Antimicrobial Therapy, University of Pittsburgh, Pittsburgh, PA, USA.
Marissa P Griffith, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Daria Van Tyne, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Center for Innovative Antimicrobial Therapy, University of Pittsburgh, Pittsburgh, PA, USA; Center for Evolutionary Biology and Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Yohei Doi, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Center for Innovative Antimicrobial Therapy, University of Pittsburgh, Pittsburgh, PA, USA; Departments of Microbiology and Infectious Diseases, Fujita Health University School of Medicine, Toyoake, Aichi, Japan.
Marco Falcone, Department of Clinical and Experimental Medicine, Azienda Osperdaliero Universitaria Pisana, University of Pisa, Pisa, Italy.
Funding
This project is supported by the National Institutes of Health through grant R21AI151363 awarded to R.K.S.
Transparency declarations
R.K.S. has served as a consultant for AbbVie, Biomerieux, Cidara, Entasis, GlaxoSmithKline, Melinta, Menarini, Merck, PfIzer, Shionogi, Utility and Venatorx, and has received investigator-initiated funding from Merck, Melinta, Roche, Shionogi and Venatorx. All other authors: none to declare.
Supplementary data
Figures S1 to S4 and Tables S1 and S2 are available as Supplementary data at JAC-AMR Online.
References
- 1. Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castanheira M, Mendes RE, Gales AC. Global epidemiology and mechanisms of resistance of Acinetobacter baumannii-calcoaceticus complex. Clin Infect Dis 2023; 76: S166–78. 10.1093/cid/ciad109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paul M, Daikos GL, Durante-Mangoni E et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18: 391–400. 10.1016/S1473-3099(18)30099-9 [DOI] [PubMed] [Google Scholar]
- 4. Kaye KS, Marchaim D, Thamlikitkul V et al. Colistin monotherapy versus combination therapy for carbapenem-resistant organisms. NEJM Evid 2023: 2: 10.1056/evidoa2200131. 10.1056/evidoa2200131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bassetti M, Echols R, Matsunaga Y et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21: 226–40. 10.1016/S1473-3099(20)30796-9 [DOI] [PubMed] [Google Scholar]
- 6. Falcone M, Tiseo G, Carbonara S et al. Mortality attributable to bloodstream infections caused by different carbapenem-resistant Gram-negative bacilli: results from a nationwide study in Italy (ALARICO Network). Clin Infect Dis 2023; 76: 2059–69. 10.1093/cid/ciad100 [DOI] [PubMed] [Google Scholar]
- 7. Tamma PD, Aitken SL, Bonomo RA et al. Infectious Diseases Society of America 2023 guidance on the treatment of antimicrobial resistant Gram-negative infections. Clin Infect Dis 2023. Online ahead of print. 10.1093/cid/ciad428 [DOI] [PubMed] [Google Scholar]
- 8. Yamano Y. In vitro activity of cefiderocol against a broad range of clinically important Gram-negative bacteria. Clin Infect Dis 2019; 69 Suppl 7: S544–51. 10.1093/cid/ciz827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choby JE, Ozturk T, Satola SW et al. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect Dis 2021; 21: 597–8. 10.1016/S1473-3099(21)00194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersson DI, Nicoloff H, Hjort K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat Rev Microbiol 2019; 17: 479–96. 10.1038/s41579-019-0218-1 [DOI] [PubMed] [Google Scholar]
- 11. Kang CK, Kim YK, Jung SI et al. Agr functionality affects clinical outcomes in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 2017; 36: 2187–91. 10.1007/s10096-017-3044-2 [DOI] [PubMed] [Google Scholar]
- 12. Claeys KC, Lagnf AM, Hallesy JA et al. Pneumonia caused by methicillin-resistant Staphylococcus aureus: does vancomycin heteroresistance matter? Antimicrob Agents Chemother 2016; 60: 1708–16. 10.1128/AAC.02388-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jo J, Ko KS. Tigecycline heteroresistance and resistance mechanism in clinical isolates of Acinetobacter baumannii. Microbiol Spectr 2021; 9: e0101021. 10.1128/Spectrum.01010-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karakonstantis S, Saridakis I. Colistin heteroresistance in Acinetobacter spp.: systematic review and meta-analysis of the prevalence and discussion of the mechanisms and potential therapeutic implications. Int J Antimicrob Agents 2020; 56: 106065. 10.1016/j.ijantimicag.2020.106065 [DOI] [PubMed] [Google Scholar]
- 15. Genteluci GL, de Souza PA, Gomes DBC et al. Polymyxin B heteroresistance and adaptive resistance in multidrug- and extremely drug-resistant Acinetobacter baumannii. Curr Microbiol 2020; 77: 2300–6. 10.1007/s00284-020-02064-6 [DOI] [PubMed] [Google Scholar]
- 16. Falcone M, Tiseo G, Nicastro M et al. Cefiderocol as rescue therapy for Acinetobacter baumannii and other carbapenem-resistant Gram-negative infections in intensive care unit patients. Clin Infect Dis 2021; 72: 2021–4. 10.1093/cid/ciaa1410 [DOI] [PubMed] [Google Scholar]
- 17. Clinical and Laboratory Standards Institute (CLSI) . Performance Standards for Antimicrobial Susceptibility Testing— Thirty Fourth Edition. CLSI Supplement M100 (ISBN 978-1-68440-220-5 [Print]). Clinical and Laboratory Standards Institute, 2024. [Google Scholar]
- 18. Longshaw C, Santerre Henriksen A, Dressel D et al. Heteroresistance to cefiderocol in carbapenem-resistant Acinetobacter baumannii in the CREDIBLE-CR study was not linked to clinical outcomes: a post hoc analysis. Microbiol Spectr 2023; 11: e0237123. 10.1128/spectrum.02371-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smoke SM, Brophy A, Reveron S et al. Evolution and transmission of cefiderocol-resistant Acinetobacter baumannii during an outbreak in the burn intensive care unit. Clin Infect Dis 2023; 76: e1261–5. 10.1093/cid/ciac647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heil EL, Claeys KC, Kline EG et al. Early initiation of three-drug combinations for the treatment of carbapenem-resistant A. baumannii among COVID-19 patients. J Antimicrob Chemother 2023; 78: 1034–40. 10.1093/jac/dkad042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bartual SG, Seifert H, Hippler C et al. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 2005; 43: 4382–90. 10.1128/JCM.43.9.4382-4390.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30: 1312–3. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iacono M, Villa L, Fortini D et al. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob Agents Chemother 2008; 52: 2616–25. 10.1128/AAC.01643-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egge SL, Rizvi SA, Simar SR et al. Cefiderocol heteroresistance associated with mutations in TonB-dependent receptor genes in Pseudomonas aeruginosa of clinical origin. Antimicrob Agents Chemother 2024; 68:e0012724. 10.1128/aac.00127-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tiseo G, Galfo V, Falcone M. What is the clinical significance of ‘heteroresistance’ in nonfermenting Gram-negative strains? Curr Opin Infect Dis 2023; 36: 555–63. 10.1097/QCO.0000000000000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teran N, Egge SL, Phe K et al. The emergence of cefiderocol resistance in Pseudomonas aeruginosa from a heteroresistant isolate during prolonged therapy. Antimicrob Agents Chemother 2024; 68: e0100923. 10.1128/aac.01009-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moon SH, Udaondo Z, Jun SR et al. Cefiderocol heteroresistance in Klebsiella pneumoniae is linked to mutations in the siderophore receptor cirA and beta-lactamase activities. Int J Antimicrob Agents 2022; 60: 106635. 10.1016/j.ijantimicag.2022.106635 [DOI] [PubMed] [Google Scholar]
- 28. Witt LS, Steed DB, Burd EM et al. Bacteraemia with an MBL-producing Klebsiella pneumoniae: treatment and the potential role of cefiderocol heteroresistance. J Antimicrob Chemother 2022; 77: 2569–71. 10.1093/jac/dkac197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Band VI, Weiss DS. Heteroresistance to beta-lactam antibiotics may often be a stage in the progression to antibiotic resistance. PLoS Biol 2021; 19: e3001346. 10.1371/journal.pbio.3001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tiseo G, Giordano C, Leonildi A et al. Salvage therapy with sulbactam/durlobactam against cefiderocol-resistant Acinetobacter baumannii in a critically ill burn patient: clinical challenges and molecular characterization. JAC Antimicrob Resist 2023; 5: dlad078. 10.1093/jacamr/dlad078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik S, Kaminski M, Landman D et al. Cefiderocol resistance in Acinetobacter baumannii: roles of beta-lactamases, siderophore receptors, and penicillin binding protein 3. Antimicrob Agents Chemother 2020; 64: e01221-20. 10.1128/AAC.01221-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Findlay J, Bianco G, Boattini M et al. In vivo development of cefiderocol resistance in carbapenem-resistant Acinetobacter baumannii associated with the downregulation of a TonB-dependent siderophore receptor, PiuA. J Antimicrob Chemother 2024; 79: 928–30. 10.1093/jac/dkae018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. VanNatta M, Grier L, Khan MH et al. In vivo emergence of pandrug-resistant Acinetobacter baumannii strain: comprehensive resistance characterization and compassionate use of sulbactam-durlobactam. Open Forum Infect Dis 2023; 10: ofad504. 10.1093/ofid/ofad504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falcone M, Tiseo G, Leonildi A et al. Cefiderocol- compared to colistin-based regimens for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2022; 66: e0214221. 10.1128/aac.02142-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simner PJ, Patel R. Cefiderocol antimicrobial susceptibility testing considerations: the Achilles’ heel of the Trojan horse? J Clin Microbiol 2020; 59: e00951-20. 10.1128/JCM.00951-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simner PJ, Palavecino EL, Satlin MJ et al. Potential of inaccurate cefiderocol susceptibility results: a CLSI AST subcommittee advisory. J Clin Microbiol 2023; 61: e0160022. 10.1128/jcm.01600-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ozturk T, Weiss DS. Heteroresistance is a cause of discrepant antibiotic susceptibility testing results. Lancet Microbe 2024; 5: e312. 10.1016/S2666-5247(23)00374-9 [DOI] [PubMed] [Google Scholar]
- 38. Stracquadanio S, Bonomo C, Marino A et al. Acinetobacter baumannii and cefiderocol, between cidality and adaptability. Microbiol Spectr 2022; 10: e0234722. 10.1128/spectrum.02347-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shields RK, Paterson DL, Tamma PD. Navigating available treatment options for carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex infections. Clin Infect Dis 2023; 76: S179–93. 10.1093/cid/ciad094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.